Abstract
Today, more than 3,000 pharmaceutical and personal care products (PPCPs) are used and released into the environment at low doses but they are barely degraded in wastewater treatment plants. One of the potential alternatives to effectively degrade PPCPs is based on the use of white-rot fungi (WRF) and involves the oxidative action of extracellular fungal enzymes. The aim of this work is to study the potential ability of three WRF strains, an anamorph species of Bjerkandera sp. R1, Bjerkandera adusta and Phanerochaete chrysosporium, to degrade PPCPs belonging to different therapeutic groups: anti-depressants (citalopram and fluoxetine), antibiotics (sulfamethoxazole), anti-inflammatory drugs (diclofenac, ibuprofen and naproxen), anti-epileptics (carbamazepine), tranquilizers (diazepam) and fragrances (celestolide, galaxolide and tonalide). The results reported complete degradation of all the PPCPs except for fluoxetine and diazepam, which were partially removed in percentages from 23 to 57%. In the case of fragrances, these compounds were neither detected in the fungal cultures nor in the abiotic controls, indicating the possibility of volatilization during the experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceutical and personal care products (PPCPs) represent a wide group of chemicals used for human and veterinary medicine and as fragrances in perfumes and other household products. These types of compounds are considered to be emerging pollutants due to their recalcitrant nature (Kosjek et al. 2007; Ternes et al. 2006). Most PPCPs cannot be easily removed at wastewater treatment plants (WWTPs) (Carballa et al. 2004). Previous studies detected PPCP concentrations in the environment in the range of nanograms per liter (ng/l) to micrograms per liter (μg/l) (Clara et al. 2005; Ikehata et al. 2006; Kim et al. 2009; Larsson et al. 2007; Reif et al. 2008; Suárez et al. 2008; Zucatto et al. 2006). Risk for acute toxic effects is unlikely, but chronic environmental toxic effects cannot be excluded (Péry et al. 2008).
Several treatments have been proposed for PPCP removal. Conventional physicochemical processes, such as coagulation-flocculation and flotation, significantly degraded certain compounds, including fragrances (i.e., galaxolide and tonalide), tranquilizers (i.e., diazepam) and anti-inflammatory drugs (i.e., diclofenac), whereas other compounds, such as anti-epileptics (i.e., carbamazepine) or certain anti-inflammatory drugs (i.e., ibuprofen and naproxen), showed poor degradation (Ikehata et al. 2006). In recent years, the development and implementation of advanced oxidation processes (AOPs), nitrifying-denitrifying plants, membrane filtration and sorption on activated carbon have been used to solve the problem of micropollutants (Carballa et al. 2005; Clara et al. 2005; Esplugas et al. 2007; Ikehata et al. 2006; Reif et al. 2008; Suárez et al. 2005; Von-Gunten et al. 2006). Ternes et al. (2003) showed that a low dose of ozone (5–15 mg/l) was sufficient to oxidize pharmaceuticals, musk fragrances and estrogens. However, ozonation is still rather expensive, leading to economical limitation for the feasibility of this method (Larsen et al. 2004; Ternes et al. 2003). Membrane filtration and activated carbon demonstrated remarkable removal efficiencies for compounds including ibuprofen, naproxen and bisphenol A; however, degradation was limited for other compounds, such as carbamazepine and diclofenac (<9%), and for lipophilic compounds, such as fragrances (Clara et al. 2005; Reif et al. 2008; Von-Gunten et al. 2006).
An emerging technology for the effective degradation of PPCPs involves the application of white-rot fungi. These microorganisms are capable of degrading lignin and several persistent pollutants, such as synthetic dyes, PAHs and PCBs (Ding et al. 2008; Eibes et al. 2005; López et al. 2004; Quintero et al. 2007; Rubilar et al. 2007; Valentin et al. 2006; Valentin et al. 2007). This ability is related to the secretion of oxidative enzymes, such as lignin peroxidase (LiP), manganese peroxidase (MnP), versatile peroxidase (VP) and laccase (Eibes et al. 2005; Feijoo et al. 2008; López et al. 2004; Marco-Urrea et al. 2009; Rubilar et al. 2007; Valentin et al. 2006).
Specifically for ligninolytic fungi, Marco-Urrea et al. (2009) studied the ability of four white-rot fungi, Trametes versicolor, Irpex lacteus, Ganoderma lucidum and Phanerochaete chrysosporium, to degrade carbamazepine, ibuprofen and clofibric acid. This study demonstrated that after 7 days of incubation, ibuprofen was degraded by all four strains, while carbamazepine and clofibric acid were much more recalcitrant; only T. versicolor attained significant degradation of both of these compounds. The use of oxidative enzymes to oxidize PPCPs in vitro has been demonstrated to remove the estrogenic activities from genistein, bisphenol A, nonylphenol, estrone (E1), 17β-estradiol (E2), estriol (E3) and ethinyl estradiol (EE2) (Cabana et al. 2007; Hirano et al. 2000; Sei et al. 2008; Soo-Min et al. 2005; Tamagawa et al. 2005, 2006; Tsutsumi et al. 2001).
The aim of this work was to study the potential ability of three WRF strains, an anamorph of Bjerkandera sp. R1, Bjerkandera adusta and Phanerochaete chrysosporium, to degrade a wide range of PPCPs belonging to different therapeutic groups: anti-depressants (citalopram and fluoxetine), antibiotics (sulfamethoxazole), anti-inflammatory drugs (diclofenac, ibuprofen and naproxen), anti-epileptics (carbamazepine), tranquilizers (diazepam) and fragrances (celestolide, galaxolide and tonalide). In this work, we aim to search for new highly active fungal species with a broader and more efficient application for PPCP degradation.
Materials and methods
PPCPs and chemicals
The PPCPs used in this work were citalopram (CTL), fluoxetine (FLX), sulfamethoxazole (SMX), diclofenac (DCF), ibuprofen (IBP), naproxen (NPX) and carbamazepine (CBZ), all of the previous PPCPs were purchased from Sigma–Aldrich as pure grade; the other PPCPs used were diazepam (DZP; Roche Pharma, pure), celestolide (ADBI; LGC-Promochem, 98%), galaxolide (HHCB; LGC-Promochem, 79%) and tonalide (AHTN; LGC-Promochem, 98%). The main characteristics of these compounds, including their Henry coefficients, dissociation constants, octanol–water partition coefficients and pseudo first-order degradation constants, are shown in Table 1. The following solvents were used: acetone (J.T. Baker, 99.5%), ethyl acetate (J.T. Baker, 99.5%), acetonitrile (J.T. Baker, 99.8%), methanol (J.T. Baker, HPLC grade, 99.8% and Panreac, 99.5%) and n-hexane (J.T. Baker, 95%).
Microorganisms
The white-rot fungi used in this work were Bjerkandera adusta (ATTC 90940), Phanerochaete chrysosporium (ATTC 24725) and an anamorph of Bjerkandera sp. R1, a fungus isolated in a Chilean forest. The strains were grown in slants with malt extract agar at 30°C for 7 days and then transferred to plates with agar (15 g/l), glucose (10 g/l) and malt extract (3.5 g/l) and incubated at 30°C for 7 days before the start of the degradation experiments.
Mycelium growth inhibition by different PPCP concentrations
Fungal resistance to different concentrations of PPCPs was studied. According to the protocol from Soo-Min et al. (2005), each fungus was inoculated on malt extract plates prepared as it was mentioned above and also containing mixtures of several PPCPs. One group of plates contained 1 and 2 mg/l of CTL, FLX and SMX, while another group contained DCF, IBP, NPX, CBZ, DZP, ADBI, HHCB and AHTN at identical concentrations. Also, plates with no PPCPs were used as controls. The plates were incubated at 30°C for 6 days in dark and the hyphal extension of each fungus was measured daily from the center of the colony to the edge of the plate, considering that the maximal growth corresponded to a hyphal extension of 4 cm.
PPCP removal assays
The experiments used to study the PPCP removal were performed in Erlenmeyer flasks (100 ml) containing 15 ml of modified Kirk medium (Tien and Kirk 1988). Two mixtures of PPCPs were prepared for the PPCPs removal assays; these solutions were based on the available methods to determine each of the compounds and also to avoid interferences between the different compounds. A first solution containing CTL, FLX and SMX was analyzed by HPLC; meanwhile DCF, IBP, NPX, CBZ, DZP, ADBI, HHCB and AHTN were analyzed by GC–MS. Each flask was inoculated with three plugs (6 mm) of agar with active fungus, and the PPCP solution was added from the two mixtures mentioned previously to provide the desired concentration (~1 mg/l). This experiment was performed in triplicate. In addition, abiotic controls were used to verify any possible adsorption or evaporation of the PPCPs. The flasks were statically incubated at 30°C for 2 weeks and samples were taken after 2 h and then after 4, 7 and 14 days. Enzymatic activity was measured by the oxidation of dimethoxyphenol (DMP) as it was reported by Field et al. (1992).
Adsorption assays were carried out in Erlenmeyer flasks (100 ml) containing 15 ml of Kirk medium and three plugs of agar with the individual strains. Besides, abiotic controls were prepared containing 15 ml of sterile distilled water. All the flasks were incubated in static at 30°C for 7 days and thereafter the fungal mycelium was sterilized in autoclave at 110°C for 10 min to inactivate the fungal biomass. A pulse of the pharmaceutical compounds used in this work were added (~1 mg/l) and then samples were taken from the liquid phase and analyzed by HPLC and GC–MS.
Extraction of PPCPs
Before sampling, 15 ml of acetonitrile were added to each flask for the extraction of the PPCPs. The flasks were sealed with Teflon and agitated for 2 h at 180 rpm in a shaker (Ika Labortechnik, HS 501 Digital, Germany). From the supernatant of each flask, samples were withdrawn according to a protocol specific for each determination analysis.
High performance liquid chromatography analysis
Residual concentrations of CTL, FLX and SMX were measured by High Performance Liquid Chromatography—Diode Array Detection (HPLC–DAD). After extraction with acetonitrile, 2 ml were taken from each flask and centrifuged in an Eppendorf centrifuge for 15 min at 7,000 rpm (Alresa, mod Digicen, Spain). Thereafter, 1 ml was withdrawn from the supernatant to determine the concentration of the selected PPCPs in a HPLC–DAD by using a Lichrosphere column (100 RP-18 5 μ Lichrocart 250-4, Merck). The elution conditions were 40:60 acetonitrile:phosphate buffer (50 mM, pH 2.2), flow of 0.6 ml/min, retention time of 30 min and wavelength of 226, 240 and 205 nm for each compound, respectively.
Gas chromatography–mass spectrometry analysis
Residual concentrations of DCF, IBP, NPX, CBZ, DZP, ADBI, HHCB and AHTN were measured by Gas chromatography-mass spectrometry (GC–MS). After agitation, 10 ml from each flask were placed in glass tubes sealed with Teflon and centrifuged at 7,000 rpm for 15 min. A sample of 4 ml was taken from each tube, diluted in 100 ml water and then extracted using 60 mg OASIS HLB cartridges (Waters closet, Milford, MA, USA) to allow for the determination of the soluble fraction of these compounds (Reif et al. 2008; Rodríguez et al. 2003).
Results and discussion
Resistance to different PPCP concentrations. Effects on fungal growth
Initial experiments were performed to determine the PPCPs concentration that did not effect on the optimal growth of the fungi. In the assays with CTL, FLX and SMX, no significant differences were observed between the control and experimental plates at PPCP concentrations below 2 mg/l (Fig. 1a, c, e). The mixture of eight PPCPs (DCF, IBP, NPX, CBZ, DZP, ADBI, HHCB and AHTN) had a significant effect on the growth of the anamorph of Bjerkandera sp. R1 and B. adusta (Fig. 1b, d), which showed slower extension, whereas P. chrysosporium reached the maximum hyphal extension after 4 and 5 days at concentrations of 1 and 2 mg/l, respectively (Fig. 1f). Once checked that the concentration of 1 mg/l of the PPCPs did not affect fungal growth, the degradation assays were performed in flasks.
Inhibition of the fungal growth of the anamorph of Bjerkandera sp. R1 (a, b), B. adusta (c, d) and P. chrysosporium (e, f) with different concentrations of two mixtures of PPCPs: (1) CTL, FLX and SMX; (2) DCF, IBP, NPX, CBZ, DZP, ADBI, HHCB and AHTN. Symbols Control 0 mg/l (filled circle), 1 mg/l (open square), 2 mg/l (open triangle). Vertical bars represent the standard deviation
Degradation of selected PPCPs
During the degradation assays, MnP activity was measured and 20 U/l was detected after 2 weeks for B. adusta and P. chrysosporium; however, after 11 days of static incubation, activities up to 900 U/l were detected for the anamorph of Bjerkandera sp. R1 (data not shown). Also, adsorption assays were carried out and after the analysis of the samples by HPLC and GC–MS. The results show similar range of concentrations in the flasks with inactivated biomass and in the abiotic controls. Thus, adsorption of the pharmaceutical compounds on the fungal mycelium was excluded (data not shown).
Anti-depressants (citalopram and fluoxetine)
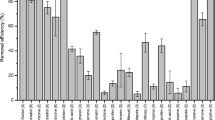
Due to their physicochemical properties (Table 1), CTL and FLX are very persistent and significant concentrations have been detected in the effluents of wastewater treatment plants in the order of ng/l for CTL and μg/l for FLX (Kwon and Armbrust 2005, 2006). Despite the fact that these compounds are used in large amounts, there are few studies about their degradation in WWTPs (Kwon and Armbrust 2005, 2006). In this study, the anamorph of Bjerkandera sp. R1 started to degrade CTL faster than the other fungal species, since after 4 days of incubation, the degradation of CTL by this fungus was 58%, whereas for B. adusta and P. chrysosporium, the percentage of degradation was much lower, up to 10%. However, at the end of the experiment, this compound was not detected in any of the fungal cultures. These degradation levels are higher compared with the results obtained by Kwon and Armbrust (2005) who reported the photodegradation of CTL in alkaline conditions (pH 9) after more than 60 days of treatment, while at pH 5–7, the degradation was negligible. On the other hand, the degradation of FLX was not significant since none of the fungi were able to totally degrade this pharmaceutical compound (Fig. 2b); partial degradations were obtained, from 23 to 46%. Other authors have reported only limited removal percentages, lower than 10% for FLX after 60 days of treatment in aqueous phase and soil matrices (Redshaw et al. 2008). FLX is highly recalcitrant to hydrolysis, photolysis and microbial degradation, and its total degradation is still a challenge to attain (Kwon and Armbrust 2006).
Antibiotics (sulfamethoxazole)
Figure 2c shows the kinetics of degradation of SMX and it can be observed that after 4 days of incubation, up to 32% of the initial concentration was degraded in the experiments with P. chrysosporium; however, at the end of the experiment, this compound was not detected in any of the fungal cultures. Previous studies reported degradations of 60% after treatment in WWTPs and 52% in a membrane bioreactor (Carballa et al. 2004; Reif et al. 2008). In the case of advanced oxidation processes (AOPs), Dantas et al. (2008) reported that the nearly complete abatement of SMX by means of an ozonation process at a dose of 0.4 g of ozone/l.
Anti-inflammatory drugs (diclofenac, ibuprofen and naproxen)
This group of compounds is one of the major classes of PPCPs used worldwide, and residues of these drugs have been found in treated wastewater at concentrations ranging from 42 to 2,556 ng/l (Gagnon et al. 2008). In this study, anti-inflammatory drugs were degraded after 14 days of incubation in all the fungal cultures (Fig. 2d–f). DCF was degraded up to 99% by the anamorph of Bjerkandera sp. R1 at day 4; whereas after 7 days of incubation, DCF was still present in the flasks with B. adusta and P. chrysosporium, although at very low levels (9 and 12%, respectively). IBP was degraded rapidly after 4 days in the presence of the three fungus showing residual concentrations of IBP below of 26%. In the case of NPX, this compound was totally degraded by P. chrysosporium at day 4th with degradation rates higher than those of the other two species. Figure 2d and 2f shows the kinetics of degradation of DCF and NPX; it can be observed the same behavior for both compounds since the fungus P. chrysosporium reached a complete elimination after 4 days, while for the rest of the fungi, total removal was reached after 7 days. Contrarily to this, IBP was easily removed by the three fungi after 4 days (Fig. 2e). In previous research studies, Carballa et al. (2004) reported degradations of 55 and 70% for NPX and IBP, respectively, after a secondary treatment in biological reactors using the conventional activated sludge process in a sewage treatment plant (STP); while Kosjek et al. (2007) reported degradations between 59 and 87% in a pilot wastewater treatment plant. Reif et al. (2008) attained high levels of degradation for IBP and NPX after 2 months in a membrane bioreactor (MBR), while only 10% DCF degradation was achieved. Suarez et al. (2009) applied coagulation–flocculation and flotation processes for the pre-treatment of wastewater from a hospital, and limited degradation (23–46%) was attained. Recently, Gagnon et al. (2008) studied the degradation of various PPCPs, including DCF, IBP and NPX, using different disinfection processes, such as UV irradiation, ozonation and injection of a performic acid mixture. Removal efficiencies of 70% for these three compounds were achieved when an optimal ozone concentration was used. Our results compare favorably with these previous findings and higher degradation rates were achieved in this work.
Anti-epileptics (carbamazepine) and tranquilizers (diazepam)
Two of the most persistent pharmaceuticals in the environment are carbamazepine (CBZ) and diazepam (DZP). CBZ is a carboxamide-type anti-convulsant and has been found ubiquitously in the aquatic environment at 1–2 μg/l. DZP is a benzodiazepine-type anti-anxiety agent used for the treatment of many other neurological and psychiatric disorders and motion sickness (Ikehata et al. 2006). In this study, CBZ was not detected after 14 days in presence of the three fungi strains (Fig. 2g). However, a slow removal was observed during the first week of assay demonstrating eliminations percentages below of 33% for the three fungi. In the case of DZP, none of the fungi were able to completely degrade this compound, finding residual concentrations in the range of 43–61% (Fig. 2h). In previous investigations, degradation rates from 9 to 35% have been reported for CBZ and DZP using biological methods, such as anaerobic digestion, nitrifying-denitrifying plants, conventional activated sludge (CAS) and membrane bioreactor (MBR), as well as physicochemical processes, including coagulation-flocculation and flotation (Carballa et al. 2005; Clara et al. 2005; Joss et al. 2006; Reif et al. 2008; Suárez et al. 2008). High degradation percentages (47–58%) for CBZ have been reported by Marco-Urrea et al. (2009). Gagnon et al. (2008) attained CBZ degradation as high as 70% using an optimal dose of ozone. In contrast, other authors have reported negligible degradation of CBZ and DZP using different methods (Clara et al. 2005; Redshaw et al. 2008). Comparing our results with the ones reported, higher degradation percentages were obtained in this work for CBZ, whereas in the case of DZP, further research on the fungal action is still necessary to attain more positive results.
Fragrances (celestolide, galaxolide and tonalide)
Celestolide (ADBI), galaxolide (HHCB) and tonalide (AHTN) belong to a group of synthetic musk fragrances and are found in household detergents, soaps, perfumes, shampoos, air fresheners and cosmetics (Peck et al. 2006). In both, the abiotic controls and experimental flasks, it was not possible to detect ADBI, HHCB or AHTN after 14 days (data not shown), which could have resulted from volatilization due to the high Henry coefficients of these fragrances. These compounds are characterized by their low solubility in water and strong lipophilic character (Table 1).
Conclusions
In this work the degradation of 11 pharmaceutical and personal care products (PPCPs) was investigated using three ligninolytic fungi. The results reported total degradation of CTL, SMX, DCF, IBP, NPX and CBZ after 14 days of incubation with the three fungal strains. Lower degradation percentages were obtained for highly recalcitrant compounds, such as FLX and DZP, which were partially removed in percentages from 23 to 57%. In the case of ADBI, HHCB and AHTN, these compounds were neither detected in the fungal cultures nor in the abiotic controls, indicating the possibility of volatilization during the experiment. The degradation process of pharmaceutical compounds using ligninolytic fungi can be considered an alternative of advanced oxidation processes.
References
Cabana H, Jones JP, Agathos SN (2007) Elimination of endocrine disrupting chemicals using white rot fungi and their lignin modifying enzymes: a review. Eng Life Sci 7(5):429–456
Carballa M, Omil F, Lema JM, Llompart M, García-Jares C, Rodríguez I, Gómez M, Ternes T (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38:2918–2926
Carballa M, Omil F, Lema JM (2005) Removal of cosmetic ingredients and pharmaceuticals in sewage primary treatment. Water Res 39:4790–4796
Clara M, Strenn B, Gans O, Martínez E, Kreuzinger N, Kroiss H (2005) Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res 39:4797–4807
Dantas RF, Contreras S, Sans C, Esplugas S (2008) Sulfamethoxazole abatement by means of ozonation. J Hazar Mater 150:790–794
Ding J, Cong J, Zhou J, Gao S (2008) Polycyclic aromatic hydrocarbon biodegradation and extracellular enzyme secretion in agitated and stationary cultures of Phanerochaete chrysosporium. J Environ Sci 20:88–93
Eibes G, Lú-Chau T, Feijoo G, Moreira MT, Lema JM (2005) Complete degradation of anthracene by manganese peroxidase in organic solvent mixtures. Enzym Microb Tech 37:365–372
Esplugas S, Bila DM, Krause LGT, Dezotti M (2007) Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J Hazar Mater 149:631–642
Feijoo G, Moreira MT, Álvarez P, Lú-Chau T, Lema JM (2008) Evaluation of the Enzyme manganese peroxidase in an industrial sequence for the lignin oxidation and bleaching of eucalyptus kraft pulp. J Appl Polymer Sci 109:1319–1327
Field JA, De Jong E, Feijoo Costa G, De Bont JAM (1992) Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white rot fungi. Appl Environ Microbiol 58(7):2219–2226
Gagnon C, Lajeunesse A, Cejka P, Gagné F, Hausler R (2008) Degradation of selected acidic and neutral pharmaceutical products in a primary-treated wastewater by disinfection processes. Ozone Sci Eng 30:387–392
Hirano T, Honda Y, Watanabe T, Kuwahara M (2000) Degradation of bisphenol A by the lignin-degrading enzyme, Manganese Peroxidase, produced by white rot fungi basidiomycete, Pleurotus ostreatus. Biosci Biotechnol Biochem 64(9):1958–1962
Ikehata K, Naghashkar N, El-Din MG (2006) Degradation of aqueous pharmaceuticals by ozonation and advanced oxidation processes: a review. Ozone Sci Eng 28(6):353–414
Joss A, Zabczynski S, Göbel A, Hoffmann B, Löffler D, McArdell CS, Ternes TA, Thomsen A, Siegrist H (2006) Biological degradation of pharmaceuticals in municipal wastewater treatment: proposing a classification scheme. Water Res 40:1686–1696
Kim I, Yamashita N, Tanaka H (2009) Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 77:518–525
Kosjek T, Heath E, Kompare B (2007) Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal Bioanal Chem 387:1379–1387
Kwon JW, Armbrust K (2005) Degradation of citalopram by simulated sunlight. Environ Toxicol Chem 24(7):1618–1623
Kwon JW, Armbrust K (2006) Laboratory persistence and fate of fluoxetine in aquatic environments. Environ Toxicol Chem 25(10):2561–2568
Larsen TA, Lienert J, Joss A, Siegrist H (2004) How to avoid pharmaceuticals in the aquatic environment? J Biotechnol 113:295–304
Larsson JDG, De Pedro C, Paxeus N (2007) Effluent from drug manufactures contains extremely high levels of pharmaceuticals. J Hazar Mater 148:751–755
López C, Moreira MT, Feijoo G, Lema JM (2004) Dye decolorization by manganese peroxidase in an enzymatic membrane bioreactor. Biotechnol Prog 20:74–81
Marco-Urrea E, Pérez-Trujillo M, Vicent T, Caminal G (2009) Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 74:765–772
Peck AM, Linebaugh EK, Hornbuckle KC (2006) Synthetic musk fragrances in Lake Erie and Lake Ontario sediment cores. Environ Sci Technol 40:5629–5635
Péry ARR, Gust M, Vollat B, Mons R, Ramil M, Fink G, Ternes T, Garric J (2008) Fluoxetine effects assessment on the life cycle of aquatic invertebrates. Chemosphere 73:300–304
Quintero JC, Lú-Chau TA, Moreira MT, Feijoo G, Lema JM (2007) Bioremediation of HCH present in soil by the white rot fungi Bjerkandera adusta in a slurry batch reactor. Int Biodeterior Biodegrad 60:319–326
Redshaw CH, Cooke MP, Talbot HM, McGrath S, Rowland SJ (2008) Low biodegradability of fluoxetine HCl, diazepam and their human metabolites in sewage sludge-amended soil. J Soils Sediments 8:217–230
Reif R, Suárez S, Omil F, Lema JM (2008) Fate of pharmaceuticals and cosmetic ingredients during the operation of a MBR treating sewage. Desalination 221:511–517
Rodríguez I, Quintana JB, Carpinteiro J, Carro AM, Lorenzo RA, Cela R (2003) Determination of acidic drugs in sewage by gas chromatography-mass spectrometry as tert.-butylmethylsilyl derivatives. J Chrom 985:265–274
Rubilar O, Feijoo G, Diez C, Lu-Chau TA, Moreira MT, Lema JM (2007) Biodegradation of pentachlorophenol in soil slurry cultures by Bjerkandera adusta and Anthracophyllum discolor. Ind Eng Chem Res 46:6744–6751
Sei K, Takeda T, Soda SO, Fujita M, Ike M (2008) Removal characteristics of endocrine-disrupting chemicals by laccase from white rot fungi. J Environ Sci Health, Part A 43(1):53–60
Soo-Min L, Bon-Wook K, Joon-Weon C, Don-Ha C, Beum-Soo A, Eui-Bae J, In-Gyu C (2005) Degradation of bisphenol A by white-rot fungi, Stereum hirsutum and Heterobasidium insulare, and reduction of its estrogenic activity. Biol Pharm Bull 28(2):201–207
Suarez S, Lema JM, Omil F (2009) Pre-treatment of hospital wastewater by coagulation-flocculation and flotation. Bioresour Technol 100:2138–2146
Suárez S, Ramil M, Omil F, Lema JM (2005) Removal of pharmaceutically active compounds in nitrifying-denitrifying plants. Water Sci Technol 52(8):9–14
Suárez S, Carballa M, Omil F, Lema JM (2008) How are pharmaceutical and personal care products (PPCPs) removed from urban wastewaters? Rev Environ Sci Biotechnol 7:125–138
Tamagawa Y, Hirai H, Kawai S, Nishida T (2005) Removal of estrogenic activity of endocrine-disrupting genistein by ligninolytic enzymes from white rot fungi. FEMS Microbiol Lett 244:93–98
Tamagawa Y, Yamaki R, Hirai H, Kawai S, Nishida T (2006) Removal of estrogenic activity of natural steroidal hormone estrone by ligninolytic enzymes from white rot fungi. Chemosphere 65:97–101
Ternes TA, Herrmann N, McDowell D, Ried A, Kampmann M, Teiser B (2003) Ozonation: a tool for removal of pharmaceuticals, contrast media and musk fragrances from wastewater? Water Res 37:1976–1982
Ternes TA, Giger W, Joss A (2006) Chapter 1: Introduction. In: Ternes TA, Joss A (eds) Human pharmaceuticals, hormones and fragrances: the challenge of micro pollutants in urban water management. lWA Publishing, London, pp 1–5. ISBN 1843390930
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. In: Wood WA, Klogg ST (eds) Methods in enzymology-biomass, part b, lignin, pectin, and chitin, vol 161. Academic Press Inc, San Diego, pp 238–249
Tsutsumi Y, Haneda T, Nishida T (2001) Removal of estrogenic activities of bisphenol A by oxidative enzymes from lignin-degrading basidiomycetes. Chemosphere 42:271–276
Valentin L, Feijoo G, Moreira MT, Lema JM (2006) Biodegradation of polycyclic aromatic hydrocarbon in forest and salt marsh soils by white rot fungi. Int Biodeterior Biodegrad 58:15–21
Valentín L, Lu-Chau TA, López C, Feijoo G, Moreira MT, Lema JM (2007) Biodegradation of dibenzothiophene, fluoranthene, pyrene and chrysene in a soil slurry reactor by the white-rot fungus Bjerkandera sp. BOS55. Process Biochem 42:641–648
Von-Gunten U, Janex-Habibi ML, Ternes TA, Weber L (2006) Chapter 7: removal of PPCP during drinking water treatment. In: Ternes TA, Joss A (eds) Human pharmaceuticals, hormones and fragrances: the challenge of micropollutants in urban water management. London, UK, pp 308–311. ISBN 1843390930
Zucatto E, Castiglioni S, Fanelli R, Reitano G, Bagnati R, Chiabrando C, Pomati F, Rossetti C, Calamari D (2006) Pharmaceuticals in the environment in Italy: causes, occurrence, effects and control. Environ Sci Pollut Res 13(1):15–21
Acknowledgments
This work was funded by Xunta de Galicia (PGIDIT06PXIB265088PR) and the Spanish Ministry of Education and Science (CICYT-CTQ2007-66788/PPQ and CTQ2010-20258). The author A.I. Rodarte-Morales would like to express her gratitude to CONACYT (Consejo Nacional de Ciencia y Tecnología) from México for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodarte-Morales, A.I., Feijoo, G., Moreira, M.T. et al. Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J Microbiol Biotechnol 27, 1839–1846 (2011). https://doi.org/10.1007/s11274-010-0642-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0642-x