Abstract
Introduction and aims
A review was undertaken on the occurrence, toxicity, and degradation of triclosan (TCS; 5-chloro-2,4-dichlorophenoxy)phenol) in the environment. TCS is a synthetic, broad-spectrum antibacterial agent incorporated in a wide variety of household and personal care products such as hand soap, toothpaste, and deodorants but also in textile fibers used in a range of other consumer products (e.g., toys, undergarments and cutting boards among other things).
Occurrence
Because of its partial elimination in sewage treatment plants, most reports describe TCS as one of the most commonly encountered substances in solid and water environmental compartments. It has been detected in a microgram per liter or microgram per kilogram level in sewage treatment plants (influents, effluents, and sludges), natural waters (rivers, lakes, and estuarine waters), and sediments as well as in drinking water.
Toxicity
Moreover, due to its high hydrophobicity, TCS can accumulate in fatty tissues and has been found in fish and human samples (urine, breast milk, and serum). TCS is known to be biodegradable, photo-unstable, and reactive towards chlorine and ozone.
Discussion
As a consequence, it can be transformed into potentially more toxic and persistent compounds, such as chlorinated phenols and biphenyl ethers after chlorination, methyl triclosan after biological methylation, and chlorinated dibenzodioxins after photooxidation. The toxicity of TCS toward aquatic organisms like fish, crustaceans, and algae has been demonstrated with EC50 values near TCS environmental concentrations. It has even been shown to produce cytotoxic, genotoxic, and endocrine disruptor effects.
Conclusion
Furthermore, the excessive use of TCS is suspected to increase the risk of emergence of TCS-resistant bacteria and the selection of resistant strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction and aims
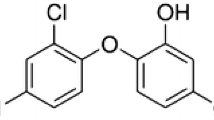
Triclosan (TCS; see chemical structure Fig. 2) or 2,4,4′-trichloro-2′-hydroxy-diphenyl ether is an antibacterial ingredient also known under the trade name Irgasan DP300, Microban, and Biofresh (Adolfsson-Erici et al. 2002; Glaser 2004). It has been used in consumer products since 1968 as an antiseptic, disinfectant, and preservative in clinical settings; various consumer products including cosmetics, household cleaning products and toys; and it has also been incorporated on the surface of medical devices, plastic materials, textiles, and kitchen utensils. However, TCS was removed in 2010 from the UE list of additives for use in plastic use food-contact materials (Commission decision 2010/169/EU). A detailed list of products containing TCS was provided by the US Environmental Protection Agency (EPA; McMahon et al. 2008) and by the Environmental Working Group, a US NGO (http://www.ewg.org/node/26752). It has been estimated that the annual usage of TCS is higher than 300 t in the USA and 350 t in Europe (Halden and Paull 2005; Singer et al. 2002). This amount reached approximately 450 t (as 100% active) in the year 2006 in Europe (SCCS 2010), and Dye et al. (2007) estimated TCS production in the EU to be 10–1000 t per year. On the other hand, about 85% of the total volume of TCS is used in personal care products, compared to 5% for textiles and 10% for plastics and food contact materials (SCCS 2010). As shown in Table 1, the regulation of cosmetic products differs according to the country where the product is marketed. TCS is listed in a positive list (authorized ingredient) as a preservative for cosmetic products by the 76/768/EEC European Union council directive, the Japanese standards for cosmetics and Canadian authorities. Triclosan is regulated by both the EPA and Food Drug Administration (FDA). Basically, the EPA regulates the pesticide uses of triclosan while the FDA is concerned with its nonpesticide usage (see Table 1).
TCS presents low water solubility (12 mg/L; Reiss et al. 2002). However, it is much more soluble in ethanol (Aragon et al. 2008) and in fat, with a high octanol–water partitioning coefficient (log Kow = 3.5–4.8 at neutral pH; Halden and Paull 2005) and high organic carbon–water partitioning coefficients \( \left( {{\text{log Koc}} = {3}.{8}-{4}.0} \right) \) (Lindström et al. 2002). The log Kow depends on the pH of water because of the presence of the hydroxyl group. Because of its high hydrophobicity, TCS would be expected to adsorb to particulate materials (Chu and Metcalfe 2007).
During the last decade, and even quite recently (Brausch and Rand 2011), TCS has been the target of several studies focused on the evaluation of its fate and distribution in the environment (all types of water, living organisms, soil, sludge, sediment, etc.), indoor environment (dust), and human samples. Because of the complex nature of these different matrices and the low related concentrations (ng/L), advanced analytical techniques such as gas chromatography/mass spectrometry (GC/MS), GC/MS/MS or liquid chromatography/mass spectrometry (LC/MS), LC/MS/MS are used (Hua et al. 2005; Trenholm et al. 2008; González-Mariño et al. 2009). In the last 5 years, some 60 articles were published about TCS analytical methods. For GC analyses, the polarity of TCS required a derivatization step, N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide being often used to improve the quality of the GC separations (Regueiro et al. 2009). But new developments in gas chromatography avoided this step (Chau et al. 2008). The detection is usually by mass spectrometry, but UV detection is also used. The analytical sensitivity was greatly improved in LC/MS/MS by derivatisation with dansyl chloride (Yu et al. 2011). Also, high-resolution mass spectrometry allowed reaching lower detection, especially for by-products of TCS like dioxins (Chau et al. 2008). Selective and efficient extraction procedures are also required, especially for analysis in very complex matrices, such as wastewater, sludge, and sediment and human matrices. For liquid samples, liquid–liquid extraction has been applied, but solid-phase extraction provided a more selective extraction, consumed less solvent, and could be easily automated (Agüera et al. 2003) or coupled with liquid chromatography (Ye et al. 2008). The octanol–water partition coefficient of TCS allows using absorption extraction techniques like solid phase micro-extraction (SPME), stir bar sorption extraction, and quite recently microextraction by packed sorbent especially suitable for large volume injection gas chromatography–mass spectrometry, improving the limit of detection to 20 ng/L in the most complex samples such as raw wastewater (González-Mariño et al. 2011). Introducing all the extract in GC improves the detection limit. Another recent micro-extraction is dispersive liquid–liquid micro-extraction (Montes et al. 2009). This technique allowed fast and sensitive determination of TCS.
With its widespread use and mostly “down-the-drain” disposal, and due to improvements in analytical methods and environmental distribution studies, TCS has been found in many environmental samples. The first objective of this literature review is the identification of contamination pathways of environment and of humans by analyzing the TCS occurrence. Meanwhile, because its transformation products are not yet fully understood, the fate of TCS is studied in order to achieve a better knowledge of the degradation rate and to identify the nature of the generated by-products. The TCS degradation pathways which are process-dependant produce a variety of by-products examined in the present review. Studies of chemical and biological conversions of TCS are important for risk assessments of environmental ecosystems and human health. The last part shows that not only is the occurrence and toxicity of TCS are important issues, but also the formation of persistent and toxic by-products is especially relevant and should be considered for risk assessment on human health.
2 Occurrence
TCS is considered as a ubiquitous pollutant, detected in all types of environmental compartments (Fig. 1) including aquatic environments (lakes and rivers, coastal and estuarine waters, wastewater treatment plants (WWTP)), domestic and drinking water, soils and biosolids (sediments and sludge), indoor dust, living organisms (fish) and humans (Chu and Metcalfe 2007; Chalew and Halden 2009; Reiss et al. 2009). Data detailed below and in Table 2 show the wide variability of TCS concentrations in function of sample nature and location. In average, concentration range looses 1 log of magnitude between raw wastewater and effluent of WWTP as well as between this last and surface water with values around 1,000–100 and 10 ng/L, respectively. In some case, it is possible to observe concentration 10–100 times higher than these average ones. Moreover, it is not possible to show a substantial evolution of concentration over the years. One important point, as mentioned at the end of the chapter, is the bioaccumulation of TCS and thus human impregnation from foodstuff exposure (in particular fish) and likely risk for human population.
Life cycle of triclosan (TCS) in the aquatic environment. Incorporated in a wide variety of household and personal care products, TCS is partially transformed in WWTPs, one part sorbed onto sludge (Biosolid) while the last fraction reminded in the treated effluent reaches waterworks or accumulates in live organisms
2.1 Wastewater
Given its wide use in personal care products and consumer products, TCS, which is fully retained in WWTP, enters the environment either through treated wastewater (effluent) or raw influent directly discharged (by sewage overflows) or adsorbed to sludge or biosolids. In a study on the occurrence of organic wastewater contaminants in 139 American sites across 30 states during 1999 and 2000, the US Geological Survey detected TCS in 57.6% of the sites studied at a maximum level of 2,300 ng/L with a median level of 140 ng/L (Kolpin et al. 2002). More generally, high levels of TCS have been shown in WWTP influents in USA, Europe, and Asia with reported values of 608–86,200 ng/L, 52–21,900 idem, and 140–2,301 ng/L, respectively. In effluents, TCS was detected with concentrations varying from 50 to 5,370, 10 to 2,210, and 11 to 360, respectively. The highest level reported for influents was found in Georgia with total TCS concentrations ranging from 13,700 to 86,200 ng/L (Kumar et al. 2010).
2.2 Rivers, lakes, and coastal waters
Many water bodies are the receiving medium of treated wastewaters, explaining why TCS has been detected in 75–100% of estuarine waters, rivers, and lakes in North America, Europe, Oceania, and Asia, with values ranging from 1 to 34.9, 0.2 to 285, 3 to 75, and 18 to 1023 ng/L, respectively (see references in Table 2). High levels were found in South China in the Pearl River system, where the TCS concentration levels reached a maximum of 1,023 ng/L (Peng et al. 2008) and 478 ng/L (Zhao et al. 2010). Several factors like such as population density, household wastewater, and illegal residential wastewater outlets contribute to the high levels of TCS (Wu et al. 2007; Chau et al. 2008; Zhao et al. 2010), with seasonal variations shown in Hong Kong (Wu et al. 2007; Chau et al. 2008). In the USA, TCS was detected in the Hudson River Estuary (Wilson et al. 2009), Key Largo Harbor (Singh et al. 2010), Georgia (Kumar et al. 2010), Mississippi River (Kolpin et al. 2002), and in the Charleston South Carolina estuary (Fair et al. 2009) at a maximum concentration varying from 10 to 35 ng/L. These levels were similar to the ones found in Japan (Nishi et al. 2008) and in Canada (Hua et al. 2005). The highest concentrations of TCS in river water were quite recently reported in the Kaveri, Vellar, and Tamiraparani rivers in India, with values of 3,800 ng/L, 5,160, and 944 ng/L, respectively (Ramaswamy et al. 2011). TCS concentration may vary in different years by consumption variation. For example, in Charleston Harbor (DeLorenzo et al. 2008), the median TCS concentration in 2006 was 0.6 ng/L compared to a mean concentration of 7.5 ng/L in 2008 (Fair et al. 2009).
In Europe, the presence of TCS in surface water was investigated in Germany, Italy, Greece, Slovenia, Spain, Romania, Switzerland, and the UK, showing concentrations of up to 285 ng/L. In Germany, the TCS concentration in the Ruhr river catchments area, which sustains the treated wastewater inflow of approximately 2 million inhabitants, ranged from 3 to 10 ng/L (Bester 2005). High values were also detected for water samples contaminated by sewage treatment plant effluents. TCS was detected in Italian and Swiss lakes at concentrations up to 14 ng/L. In Switzerland, TCS was found in lakes and in a river with concentrations ranging from 1.4 to 74 ng/L (Lindström et al. 2002). In the UK, TCS concentrations in a river ranged from 19 to 80 ng/L (Sabaliunas et al. 2003). The highest values of TCS were shown in rivers in Spain, with levels up to 285 ng/L in the Campredo area (Kantiani et al. 2008).
2.3 Drinking water
Despite the detection of TCS in rivers, very few studies have been reported for drinking water catchments or tap water. The highest level of TCS, ranging from 0.6 to 9.7 ng/L, was detected in bottled and tap water in Guangzhou, China (Li et al. 2010). In 2007, the presence of TCS in 22 drinking water samples from the Barcelona city area was evaluated and the concentrations measured were near the limit of detection (Kantiani et al. 2008). These results were similar to those reported by Kuster et al. (2008) and Benotti et al. (2009) in the USA. It has been reported that chlorination used during treatment produces chlorophenols (Rule et al. 2005), which are more persistent and highly toxic compounds. Because of these compounds, which are not currently analyzed, the total concentration of TCS and related by-products in drinking water was underestimated.
2.4 Soils and biosolids
Due to its physical and chemical properties, TCS is accumulated onto sludge and biosolids at about 0.4–30 mg/kg (Singer et al. 2002; Bester 2003; Morales et al. 2005; Heidler et al. 2006; Heidler and Halden 2007; Chu and Metcalfe 2007; Ying and Kookana 2007; Cha and Cupples 2009; Behera et al. 2010; Langdon et al. 2011). With triclocarban (another antiseptic), TCS were the most abundant hormone-like personal care products detected in the dewatered sludge in the Pearl River system at a level of 5,088 and 1,188 ng/g, respectively (Yu et al. 2011). The Targeted National Sewage Sludge Survey (USEPA 2009) reported concentration value of 133 mg/kg. Finally, a very recent review of “emerging” organic contaminants in biosolids, including TCS, found that the primary route of human exposure to TCS is due to the domestic environment and not to land applying biosolids (Clarke and Smith 2011).
Varying concentrations of TCS were measured in soil receiving biosolids (Lapen et al. 2008; Topp et al. 2008; Edwards et al. 2009). These concentrations were significant for short times after application and reached low levels 16 months after application (Lozano et al. 2010). On the other hand, studies reported TCS soil half-life between 18 and 58 days (Ying et al. 2007; Wu et al. 2009) and between 2.5 and 35 days (unpublished data as in Reiss et al. 2009) under aerobic conditions, while it was stable under anaerobic conditions. Moreover, Cha and Cupples (2010) showed a very low leaching potential for TCS in amended agricultural soil and concluded that the risk of contamination of groundwater (Kd 33–55 L/kg) was unlikely. Two studies reported the presence of TCS leached from agricultural areas amended with biosolids into tile drainage water (Lapen et al. 2008) and runoff (Topp et al. 2008). TCS is predicted to have a half-life estimated at 540 days (Halden and Paull 2005), but information on its long-term fate is limited. However, some studies have found measurable levels of TCS present in a dated lake and estuarine sediment core, suggesting long-term preservation may be occurring (Singer et al. 2002; Miller et al. 2008; Cantwell et al. 2010).
2.5 Living organisms
TCS accumulation has been described for freshwater aquatic organisms mainly in lower trophic-level organisms such as algae, crustaceans, and fish. High levels of TCS (0.24–4.4 mg/kg) were reported in the bile of fish, one sample being as high as 120 mg/kg in Rainbow trout (Oncorhynchus mykiss) exposed to downstream WWTP discharges (Adolfsson-Erici et al. 2002), and in blood plasma of fish in the Detroit River of North America (Valters et al. 2005). Lower amounts were determined in Bluegill tissues (Mottaleb et al. 2009), in the bile of wild living fish (Adolfsson-Erici et al. 2002; Ramirez et al. 2009), and in plasma of wild Atlantic bottlenose dolphins (Tursiops truncates) in US estuaries (Fair et al. 2009), in which TCS was found to be between 0.025 and 0.27 ng/g wet weight. Methyltriclosan was also found in fish at concentrations ranging from 30 to 600 ng/g (Balmer et al. 2004; Leiker et al. 2009).
The bioaccumulation factor (BAF) of TCS has been calculated for some animal species. BAF values ranged from 27 in earthworm tissues (Kinney et al. 2008), 44 in stage 66 Xenopus laevis larvae and 740 in Bufo woodhousii (Palenske et al. 2010), up to around 500 for snails (Helisoma trivolvis; Coogan and La Point 2008). For algal species (Cladophora spp.), BAF values ranged from 900 to 1200 (Coogan et al. 2007).
2.6 Humans
A number of human biomonitoring studies have reported occurrence of TCS in biological fluids (Dann and Hontela 2011). Concentrations in urine ranged between 1 and 3,790 μg/L with a mean around 12 μg/L, with around 70% of tested samples being positive (Wolff et al. 2007; Kawaguchi et al. 2008; Calafat et al. 2008). Lower concentrations, between 0.52, 0.07–16 and 0.03–0.54 μg/L were respectively found in serum (Allmyr et al. 2008; Dirtu et al. 2008), plasma (Allmyr et al. 2006), and in human breast milk (Adolfsson-Erici et al. 2002; Allmyr et al. 2006; Dayan 2007). According to the USEPA, ingestion of dust is the most likely exposure route for infants due to their hand-to-mouth activities (USEPA 2007). The presence of TCS in homes is most likely associated with spills of liquid personal care products, use of aerosols, dead skin cells, and diffusion from textiles treated with this bactericide (Geens et al. 2009). TCS is reported in domestic dust at homes and offices from 25 to 1,828 ng/g (medium = 220 ng/g) in Flanders, Belgium (Geens et al. 2009) and from 240 to 2,200 ng/g (medium = 702 ng/g) in Santiago de Compostela, Galicia-Spain (Canosa et al. 2007a, b).
3 Environmental fate
From the sewage network to the different environmental compartments, TCS can undergo various transformation processes. High rates of chemical, photochemical, sonochemical, or biological transformation have been described in the literature, but always leading to the formation of by-products (Table 3). As usual, the efficiency of the treatment is depending on the initial concentration, pH, matrix, and experimental conditions. Interesting are the by-products generated. While chlorophenols are the main by-products of chemical treatments, methyltriclosan is generated in biological ones. Considering TCS concentrations in WWTP influents, the ones of by-products are expected to be non-negligible, some of them presenting higher toxicity than TCS.
3.1 Degradation
Photodegradation is the most documented degradation process for TCS while very few studies have reported on chemical or sonochemical treatment. Chemical treatment (chlorination and ozonolysis), photodegradation (including photocatalytic oxidation), and sonolysis conversions were described as pseudo first-order reaction kinetics of TCS degradation. Degradation kinetics is evaluated by the calculated half-life time t 1/2 shown in Table 3.
Chlorination and ozonation of TCS containing solutions showed the greatest degradation kinetics (t 1/2, 0.9 ms to 2.2 min, pH 7.0–7.3; Canosa et al. 2005; Fiss et al. 2007; Suarez et al. 2007) followed by sonolysis (3–110 min, pH 7.0–8.0) (Sanchez-Prado et al. 2008), photo-SPME degradation (irradiation of the absorbed TCS fiber coating; 6.3–8.0 min, pH 7.0; Sanchez-Prado et al. 2006), photocatalytic oxidation (15 min to 1 day; Yu et al. 2006) and finally photolysis (2.7 min to 8 days, pH 7.0–8.0; Aranami and Readman 2007; Ferrer et al. 2004; Son et al. 2007; Latch et al. 2005; Sanchez-Prado et al. 2006; Mezcua et al. 2004; Buth et al. 2009). Physical and chemical factors like the pH of the aqueous solution, salinity, the presence of metal ions and/or humic/fulvic acids (in wastewater) and irradiation conditions (UV wavelength or sunlight) had a large influence on TCS degradation. Chlorination and ozonolysis efficiencies were correlated with the pH value, the TCS phenolate form being the most reactive species. This activating group stabilizes the cationic “Wheland intermediate” (or arenium ion) during the substitution reaction pathway by resonance effect (Canosa et al. 2005; Fiss et al. 2007; Suarez et al. 2007).
There are discrepancies concerning the role of organic matter on TCS photosensitive degradation (Aranami and Readman 2007; Mezcua et al. 2004) because of its scavenger properties (Sanchez-Prado et al. 2006). Organic matter could also be a competitive target compound for ozonolysis (Suarez et al. 2007). Irradiation by solar light of spiked wastewater led to calculated half-life times varying from 2.7 min (Sanchez-Prado et al. 2006) to 23 h (Ferrer et al. 2004). This variability could be related to the different sampling parameters, but TCS degradation occurred with UV or sunlight irradiation. The shortest half-lives (<5 min) were obtained in ultrapure (Sanchez-Prado et al. 2006) or deionized (Son et al. 2007) water. For Buth et al. (2009), indirect photoreactions via photosensitization of dissolved organic matter show negligible half-life values varying between 8 and 9–10 min from buffered water to river water. Sonolysis has been recently reported by Sanchez-Prado et al. (2008) and the presence of salts and solids could have a beneficial effect on the removal of TCS.
3.2 Biodegradation
TCS could be biodegraded under aerobic conditions in laboratory activated sludge systems (Federle et al. 2002; Stasinakis et al. 2007, 2010; Svenningsen et al. 2011). In WWTP using activated sludge as a secondary treatment, a removal rate higher than 90% was generally observed (McAvoy et al. 2002; Bester 2003; Kanda et al. 2003; Sabaliunas et al. 2003; Heidler and Halden 2007). In fact, both biodegradation and sorption/storage on solids or cells may explain the removal rate of organics in WWTP and the partial transfer to biosolids (resulting from the treatment of excess activated sludge and primary sludge removed during the treatment), as between 50% to 75% of TCS was recovered in sludge (Heidler and Halden 2007; Ying and Kookana 2007). Singer et al. (2002) showed that around 79% of degradation was due to aerobic or anaerobic degradation in WWTP while 15% was attributed to sorption on activated sludge.
Little is known about the microorganisms responsible for TCS biodegradation in wastewater and WWTP. Indeed, as an antibacterial agent, the efficiency of TCS is attributed to the inhibition of a lipid synthesis enzyme occurring in Gram-negative and -positive bacteria, as well as in mycobacteria (Heath et al. 1999; Levy et al. 1999; Gomez-Escalada et al. 2005; Dann and Hontela 2011). However, some bacteria are able to inactivate TCS. Hay et al. (2001) produced a bacterial consortium, enriched from WWTP-activated sludge that was able to degrade 35% of a 500 mg/L TCS solution in 13 days. Sphingomonas sp was isolated from this consortium as being especially efficient. Pseudomonas putida and Alcaligenes xylosoxidans isolated from soil have been reported as resistant to TCS and able to use it as a carbon source (Meade et al. 2001). Roh et al. (2009) showed that both ammonia-oxidizing bacteria and non ammonia-oxidizing bacteria were able to biodegrade TCS (2 mg/L in 5 days). But this degradation also involved a competitive inhibition of ammonia oxidation (decrease of the nitrification yield).
Fungi strains were also studied. Cajthaml et al. (2009) showed that almost all the fungi (eight strains tested) were able to degrade TCS (3 or 5 mg/L in 14 days), but not to remove the total estrogenic activity. In spite of the inhibition of their growth by TCS, even at low concentrations, the fungi Trametes versicolor and Pycnoporus cinnabarinus have been reported by Hundt et al. (2000) as able to decrease the cytotoxic and microbicidal effects of TCS by converting it to methylated and glycosyl conjugated forms. However, they also generated toxic chlorophenols such as 2,4-dichlorophenol.
Recently, biodegradation of TCS in soil has also been described (Table 3). Its half-life ranges from 4 to 60 days depending on the initial concentration and the environmental factors. In these conditions, methyltriclosan (MTCS) has been found as the main transformation product of TCS (DeLorenzo et al. 2008; Al-Rajab et al. 2009; Wu et al. 2009; Xu et al. 2009)
3.3 By-products
Transformation products or by-products formed during TCS degradation can be classified into five categories depending on the process used and the chemical structure (Table 3 and Fig. 2).
Chlorinated TCS derivatives: 4,5-dichloro-2-(2,4-diclorophenoxy)phenol (4-Cl-TCS); 5,6-dichloro-2-(2,4-diclorophenoxy)phenol (6-Cl-TCS); and 4,5,6-trichloro-2-(2,4-diclorophenoxy)phenol (4,6-Cl-TCS). The formation of polychlorodibenzo-p-dioxin and 2,8-dichloro-p-dioxin is the result of the photodegradation of the Cl-TCS products. 2,4-DCP and 2,4,6-TCP result from the degradation of the Cl-TCS products. 2,4-Dichlorophenol (2,4-DCP) can be transformed into the 6-nitro-2,4-DCP and chloroform is formed by degradation of the 2,4 DCP et 2,4,6 TCP. (Buth et al. 2009; Kanetoshi et al. 1987; Chiron et al. 2007; Menoutis and Parisi 2002; Canosa et al. 2005; Brun et al. 2008; Fiss et al. 2007)
The first class corresponds to chlorinated diphenyl ether (tetraclosan and pentaclosan). Upon exposure to chlorine, TCS was transformed to 4,5 dichloro-2-(2,4-dichlorophenoxy)phenol (4-Cl-TCS), 5,6 dichloro-2-(2,4-dichlorophenoxy)phenol (6-Cl-TCS), and 4,5,6 trichloro-2-(2,4-dichlorophenoxy)phenol (4,6-Cl-TCS; Rule et al. 2005). Those three chlorinated triclosan derivatives (CTDs) resulted from the chlorine aromatic electrophilic substitution (Canosa et al. 2005; Fiss et al. 2007; Buth et al. 2009). During chlorination of TCS, tetraclosan (4-Cl-TCS, 6-Cl-TCS) and pentaclosan (4,6-Cl-TCS) were detected as precursors of more stable by-products (2,4-dichlorophenol and 2,4,6-trichlorophenol) (Canosa et al. 2005; Fiss et al. 2007), potential precursors of polychlorodibenzo-p-dioxins (PCDDs) and polychlorodibenzofurans (Buth et al. 2009). The latter, known to be persistent environmental contaminants, form the second class. They were characterized in several studies as by-products of TCS photodegradation, the most cited PCDDs being 2,8 and 2,7 dichlorodibenzo-p-dioxins (Aranami and Readman 2007; Mezcua et al. 2004; Latch et al. 2005; Son et al. 2007; Yu et al. 2006; Sanchez-Prado et al. 2006). 2,8 and 2,7 Dichlorodibenzo-p-dioxins (DCDDs) were detected in a yield of 1–12% of photodegraded TCS. As shown by Aranami and Readman (2007), TCS in natural water irradiated with natural light was partially converted into 2,8 DCDD. In this study, the half-life of photolabile DCDD was estimated at around 122 days in seawater and 26 days in fresh water under low intensity white light source versus 4 days for TCS. A series of photolysis experiments with 1,2,7,8 TCDD and 1,2,7,8 TCDF in pure water in sunlight showed half-lives of 6.4 and 8.3 h, respectively (Kim and O’Keefe 2000). Yu et al. (2006) suggested that the ring closure of TCS is wavelength-dependent. In another study, Son et al. (2007) showed that DCDDs and PCDDs were found in samples irradiated at 365 nm. 2,8 Dichlorodibenzo-p-dioxin, 2,3,7 and 1,2,8 trichlorodibenzo-p-dioxins and 1,2,3,8 tetrachlorodibenzo-p-dioxin were also detected in sediment cores as a consequence of the phototransformation (solar irradiation) of TCS and of its CTDs (Buth et al. 2010).
The third class corresponds to chlorinated hydroxylated diphenyl ether (DCHDPE and DCDHDPE; Ferrer et al. 2004; Sanchez-Prado et al. 2006), hydroxyquinone, and quinone triclosan. Generated by a reductive dechlorination reaction, DCHDPE and DCDHDPE were detected during a photo-SPME degradation (Sanchez-Prado et al. 2006) or by photolysis of TCS in wastewater samples (Ferrer et al. 2004; Rule et al. 2005). Hydroxyquinone and quinone triclosan resulted from photocatalytic oxidation (Yu et al. 2006). These organic compounds were also found to be unstable in wastewater samples irradiated with natural sunlight and were precursors of 2,4-dichlorophenol and monochlorophenol (Yu et al. 2006).
The fourth category is chlorophenol derivatives (CPs) and chloroform. 2,4,6 trichlorophenol (TCP) and chloroform appeared during chlorination when monochlorophenol was detected during photolysis. 2,4 Dichlorophenol (DCP) is a major by-product of photocatalytic oxidation, photo-SPME degradation, photolysis, and chlorination (see references in Table 3). The half-lives of the CPs have been measured in different conditions (Benitez et al. 2000; Pera-Titus et al. 2004). Ozonation showed the greatest degradation kinetics (t 1/2: 3.1 min for 2,4,6 TCP and 3.3 min for 2,4 DCP, pH 9.0) compared to UV irradiation (t 1/2: 25.2 min for 2,4,6 TCP and 17.5 min for 2,4 DCP, 300 nm; Benitez et al. 2000). These values are much more higher than those determined for TCS in similar conditions in particular for ozonation (0.9–3.0 ms for TCS; Table 3).
The last category of transformation is methylation producing the lipophilic derivative of TCS, MTCS (Lindström et al. 2002; Balmer et al. 2004). This compound is found under biodegradation conditions (Wu et al. 2009; Chen et al. 2009; Al-Rajab et al. 2009; Xu et al. 2009) and creates the opposite scenario from many anthropogenic compounds, which become more hydrophilic and less likely to bioaccumulate when mineralized (Lindström et al. 2002). Although MTCS is generally less prevalent in the environment than TCS, its mechanism of action is similar and can occur at measurable levels even when TCS is below the limit of detection (Lindström et al. 2002). In activated sludge, MTCS was formed concomitantly with the removal of triclosan under aerobic conditions (Chen et al. 2011). Of further concern is the tendency of MTCS to resist photodegradation at common surface water pH (Lindström et al. 2002) and it has also a higher potential to bioaccumulate (DeLorenzo and Fleming 2008).
4 Toxicity
Toxicity and ecotoxicity studies show a variety of organisms sensitive to TCS exposure (Table 4). The effects can be evaluated by using representative organisms, bacteria, plants, fish, birds, protozoa, and mammals (Capdevielle et al. 2008; Chalew and Halden 2009; Lyndall et al. 2010; Fuchsman et al. 2010). Considering this variability of EC50 values, the risk assessment cannot clearly be assessed. Concerning aquatic and terrestrial organisms, data show clearly that the risk should be more related to chronic effect (due to bioaccumulation) than acute impact. However, the sensitivity of some species (namely microorganisms and aquatic plants) even at environmental concentrations shows that the ecosystems can be disturbed.
4.1 Plants
The effect of TCS on the growth and reproduction inhibition of Dunaliella tertiolecta, Scenedesmus subspicatus, Selenastrum capricornutum, and Navicula pelliculosa was examined and the EC50 was 3.5, 0.7–2.8, 1.2–4.5, and 19 μg/L, respectively. Diatoms and duckweed Lemna gibba are less sensitive to TCS than green algae. The lowest observed effect concentration (LOEC) values for the phytoplankton taxa ranged between 0.1 and 1.2 μg/L, whereas no effect concentration (NOEC) ranged from 0.5 to 8.3 μg/L. Among green algae, S. capricornutum seems to be the least sensitive to TCS with a NOEC of 8.3 μg/L, confirmed by the highest EC50 value (4.5 μg/L) or IC50 value (4.7 μg/L). Finally S. subspicatus appears to be the most sensitive with a NOEC of 0.5 μg/L, confirmed by the lowest EC50 value (0.7 μg/L; DeLorenzo and Fleming 2008; DeLorenzo et al. 2008; Orvos et al. 2002; Harada et al. 2008; Wilson 2003; Tatarazako et al. 2004). These values correspond to the levels of TCS found in surface river water (see Section 2). The negative effects of TCS on these organisms may impact higher trophic levels.
Oryza sativa, Triticum aestivum, Sesbania herbacea, and Cucumis sativus are angiosperm taxa used to assess the phytotoxicity of TCS (Liu et al. 2009; Stevens et al. 2009; An et al. 2009). Seed germination and plant growth tests were monitored during 20–28 days with different concentrations of TCS. The LOEC values showed that root elongation was inhibited in soils treated with TCS at 10 and 30 mg/kg for rice and cucumber, respectively, and shoot growth was altered at concentrations higher than 30 mg/kg. Root elongation was more sensitive than shoot growth for the two species with EC50 values of 57 and 243 mg/kg, respectively for O. sativa and 108 and 277 mg/kg for C. sativus. These results were confirmed by the NOEC values. The plant growth tests showed that plant species varied in their sensitivity to TCS in soil, with rice being more sensitive. The investigation of the degradation of TCS in soil under aerobic and anaerobic conditions showed persistence in the anaerobic soil after 70 days (Ying et al. 2007). These studies suggest that biosolids from treated WWTP sludge containing a relatively high concentration of TCS, for example 55 mg/kg reported by Heidler and Halden (2007), may cause negative effects on plant growth when applied on agricultural land.
4.2 Microorganisms
A recent study by Ricart et al. (2010) on the short-term effects of TCS (from 0.05 to 500 μg/L) on biofilm algae and bacteria showed that environmental concentrations of TCS caused an increase of bacterial mortality with an NOEC of 0.21 μg/L. At the highest concentration, 85% of the total bacterial dies. Finally, the toxicity of TCS was higher for bacteria than algae.
For the cyanobacteria Anabaena flosaquae, the EC50 value for a 96-h exposure test was 1.6 μg/L and the NOEC was 0.8 μg/L approaching the sensitivity of phytoplankton taxa (Orvos et al. 2002). When sludge containing TCS was applied on agricultural land as fertilizer, it was found to significantly inhibit soil respiration and phosphatase activity during the first 7 days of incubation, before recovering the microbial and enzyme activities afterwards (Liu et al. 2009). In experiments with heterotrophic and autotrophic biomass, higher sensitivity of nitrifying bacteria to TCS was shown (Stasinakis et al. 2008). Among the methods used to monitor effluent wastewater toxicity, in vitro bioassay with luminescent bacterium Vibrio fischeri was obviously applied. This bioassay, while not strictly relevant to the activated sludge process, was selected because of its suitability as an established standardized screening test for acute toxicity (ISO 11348). EC50 values for V. fischeri ranged from 220 to 520 μg/L according to the duration of exposure (Farré et al. 2007; Stasinakis et al. 2008; Harada et al. 2008).
4.3 Fish
LC50 values for Danio rerio (zebrafish), Lepomis macrochirus, O. mykiss, Oryzias latipes (medaka), and Pimephales promelas range from 270 to 602 μg/L. As usual, the sensitivity of each organism is dependent on the time of exposure. L. macrochirus values of TCS LC50 decrease with the exposure duration, with values of 440, 410, and 370 μg/L for 24, 48, and 96 h, respectively (Orvos et al. 2002). The same phenomenon is observable for P. promelas with 96 and 24 h LC50 values of 360 and 500 μg/L, respectively (Mayer and Ellersieck 1986).
The age or the development stage is also an important sensitivity parameter. The 96-h exposure LC50 values for 24-h-old larvae and embryos less than 24 h post-fertilization of O. latipes were 602 and 399 μg/L (Ishibashi et al. 2004). The early life stage is more sensitive than later ones. The LOEC and NOEC of this taxon ranged between 31.6 and 313 μg/L and from 15 to 162 μg/L, respectively, all species, ages, and exposure times taken together. The results also showed that male fish were more sensitive to TCS than female fish, at least for O. latipes (Ishibashi et al. 2004).
4.4 Other aquatic organisms
The different species used for aquatic toxicity of TCS on animals are listed in Table 4. The LC50 for Chironomus tentans and for Hyalella azteca were, respectively, 400 and 200 μg/L for an exposure of 10 days to TCS with pH fixed at 8.4 (Dussault et al. 2008). The EC50 for Ceriodaphnia dubia and for Daphnia magna were 0.22 and 260 μg/L for an exposure of 7 days and 48 h, respectively (Orvos et al. 2002). For C. dubia (neonate organisms of less than 1 day old), EC50 values ranged between 0.12 and 0.55 μg/L depending on exposure time and pH, the sensitivity of C. dubia being increased with the decrease of exposure duration (Orvos et al. 2002; Tatarazako et al. 2004). For a pH of 7, survival and reproduction NOEC values were 50 and 6 μg/L, respectively, for an exposure time of 7 days (Orvos et al. 2002). For a higher pH of 8.5 and the same duration of exposure time, the survival and reproduction NOEC values was 339 and 182 μg/L, respectively, showing that the molecular form of TCS is more toxic than the dissociated one at higher pH (Orvos et al. 2002). For amphibians, the 7-day NOEC value obtained with X. laevis was 400 and the 96-h growth inhibition LC50 was 820 μg/L (Harada et al. 2008; Matsumura et al. 2005).
4.5 Terrestrial animals
The toxicity of TCS on Eisenia fetida (earthworm) has been assessed by Lin et al. (2010). The results showed that the activity of catalase (CAT) and glutathione-S-transferase (GST) at the highest tested dose could be stimulated after a 2-day exposure, reaching 148% and 123% of that in the control with prolonged exposure, while the activity of CAT and GST was inhibited at the highest tested dose, falling to 47% and 33% of that in the control, respectively. Furthermore, the comet assay demonstrated that TCS treatments led to dose-dependent DNA damage of E. fetida after exposures of 7 and 14 days suggesting that TCS has sublethal effects on E. fetida. For rats, TCS was found to alter circulating serum concentrations of total thyroxine in rats (Crofton et al. 2007). For mammals, TCS does not seem to be acutely toxic (Bhargava and Leonard 1996), but may interact with specific enzymes (cytochrome P450-dependent enzymes, UDP-glucuronosyltransferases) and nuclear receptors (human pregnane X receptor; Hanioka et al. 1996; Jacobs et al. 2005; Wang et al. 2004). For humans, studies evaluating the safety and tolerability of TCS did not show evidence of toxicity associated with human exposure to levels currently used in personal care, although only kidney effects were considered for human exposure assessment (Rodricks et al. 2010).
Finally, another recent study based on the use of a stochastic water quality model for TCS contamination prediction in rivers from wastewater discharges (Price et al. 2010) showed that total TCS concentrations exceed a conservative predicted no effect concentration of 69 ng/L in almost 15% of rivers. However, considering the pH of water, a significant fraction of TCS is under phenolate form, which has much lower toxicity than the molecular form of TCS, reducing the associated risks.
5 Discussion, risk assessment, and potential effects on human health
5.1 Human exposure
As previously mentioned in this paper, TCS is widely used in various products. The exposure pathways reported were oral, dermal, and inhalation, although the latter was estimated to be negligible (Rodricks et al. 2010; Dann and Hontela 2011). One third of the TCS used in Europe appeared to reach consumers in oral care products and a similar amount in skin care products (European Commission 2002). The uptake of TCS through the skin has been shown to be low in vitro (Geens et al. 2009) as absorption was less than 10% when the products were applied according to recommended use (Rodricks et al. 2010). However, this absorption rate could be not negligible considering the maximum content of 0.3% TCS in EU cosmetics (Table 1). Oral use of care products is probably the most significant route of exposure to TCS for adults (Allmyr et al. 2008; Dann and Hontela 2011). Studies have shown fairly rapid and complete absorbance of TCS in animals and humans, and urine appears to be the preferred excretion pathway (Rodricks et al. 2010). To our knowledge, no data is available on concentration levels of TCS in foodstuffs. The first evidence is a direct exposure regarding the widespread use of TCS in personal care products. The detection of TCS in human breast milk raises questions of potential adverse effects to babies (Dayan 2007). The question of the passage of TCS from the human breast or from the foodstuffs has to be investigated as well as the passage of TCS by-products like MTCS, DCDDs, or chlorophenols. Another possible contamination pathway for food (except for UE since 2011, see Commission decision 2010/169/EU) could be related to contact with polymeric materials containing TCS additives to prevent microorganism growth and prevention the formation of stains and odors, thereby extending the polymer’s shelf life. One study was carried out on the assessment of the diffusion coefficient of TCS into and within foodstuffs (Sanches-Silva et al. 2010).
After absorption, TCS is eliminated under unchanged form in urines and feces. However, no study monitored TCS concentration, diffusion, and degradation in human tissues and the presence of by-products has not been explored. TCS elimination after intravenous injection to the rat followed partition kinetics with a big part (42% in weight), what suggests a quick transfer of the substance of blood towards the tissues where it is able of stocking. Half-life time of TCS in blood was evaluated near 9 h (US FDA 2008). The question of the detection of the toxic TCS by-products in blood is raised.
5.2 Toxicity and potential effects on human health
Very few works are available on chronic toxicity, some being reviewed by Dann and Hontela (2011). The first study concerning TCS safety was realized by Da Salva et al. (1989). Data from recent works mainly concern ecotoxicity and the development of antibiotic resistance in bacteria (Levy 2001; Sandborgh-Englund et al. 2006; Birosová and Mikulásová 2009). In this last mentioned study, TCS-resistant mutants of Salmonella enterica serovar Typhimurium with reduced antibiotic susceptibility were selected. In addition, longer-term or higher-dose exposure of bacteria to TCS in the community setting increased bacterial resistance (Chuanchuen et al. 2001; Aiello et al. 2004; Maillard 2007). Tolerant-TCS Staphylococcus aureus strain with increased resistance to penicillin, gentamicin, and quinolones (Seaman et al. 2007; Tkachenko et al. 2007) and tolerant TCS Mycobacterium smegmatis strain with increased resistance to isoniazid have been detected (Parikh et al. 2000).
TCS has been included in the list of endocrine disruptors because of its similar chemical structure with known nonsteroidal estrogens. A study to the mice showed a hypothermia and a depressant effect on the central nervous system (Miller et al. 1983) and recent reports showed that it can cause endocrine disruption in aquatic species (Foran et al. 2000; Ishibashi et al. 2004; Matsumura et al. 2005), reduce sperm production in treated male rats (Kumar et al. 2009) and the LH-induced testosterone production in rat testicular Leyding cells (Kumar et al. 2008). Other studies have shown that TCS can bind to thyroid hormone receptor (Veldhoen et al. 2006). The anti-androgenic and anti-estrogenic capacities have been demonstrated in vitro (Chen et al. 2007; Gee et al. 2008). A mechanism has been proposed where TCS moves the link of the œstradiol in its receiver (ER) on cells of human breast cancer (MCF-7), inhibits the induction of estrogenic answer and growth stimulation by 17-β-oestradiol. It also moves 3 (H) testosterone of its receiver (AR), inhibits the induction of androgenic answer of testosterone in cells of mammary tumors of mouse and in the cells of human breast cancer (T47D; Gee et al. 2008).
A study of cytotoxicity in vitro on descendants of human gingival cells showed that TCS could lead to cell death by impairment of the plasma membrane and by apoptosis. Cytotoxic effect could be enhanced in the presence of zinc citrate or sodium fluoride currently found in toothpastes (Zuckerbraun et al. 1998).
Epidemiology studies and animal models have shown that specific endocrine-disrupting compounds may influence the development of prostate cancer (Hess-Wilson and Knudsen 2006; Mink et al. 2008). Studies in animal models also showed augmentation of prostate carcinogenesis with several other environmental estrogenic compounds including UV filters and bisphenol A especially during utero and neonatal time as well as during puberty (Prins 2008). Thus children may be considered a highly susceptible population for endocrine-disrupting compounds exposure because it may increase risk of prostate cancers with aging (Prins 2008). Nevertheless, human health impacts of TCS use has not been investigated enough and there is significant interest in uncovering the potential effects of TCS (and of its by-products) exposure on the development of prostate cancer.
6 Conclusion
This literature review shows that TCS is widely detected in various environmental compartments from nanograms per liter to several micrograms per liter and is accumulated in biota and in humans. Because of its partial removal in WWTP, TCS enters the aquatic environments directly through wastewater (treated or not) and indirectly by runoff from biosolids for amended agricultural soils. Occurrence studies are beginning to be consistent in the USA, Europe, and Asia but some further questions need to be considered, such as the influence of the removal process in WWTP on the fate of TCS in natural matrices. The large majority of the studies reviewed focused only on TCS, with very few considerations on the nature and amount of transformation products or by-products that can persist in effluents or biosolids. Contaminated sludge spreading on agricultural soils can pose several important agro-ecosystem disturbances. TCS accumulation in aquatic organisms has at least two negative impacts, the first being the selection of resistant species and the second being the possible contamination by TCS or MTCS of the organisms from higher trophic levels. But to our knowledge, no works have examined the effects on higher trophic level organisms and subsequent effects on aquatic ecosystems. Meanwhile, different degradation processes have been monitored in laboratories, with an emphasis on kinetic degradation and by-products. It was clearly shown that TCS is chemically unstable and is degraded under aerobic or anaerobic conditions. Among all the TCS metabolites, chlorophenols (2 CP, 2,4 DCP, and 2,4,6 TCP) belong to the list of priority toxic pollutants established by the USEPA (http://water.epa.gov/scitech/methods/cwa/pollutants.cfm). CPs half-life has been shown to be much more higher than TCS in similar conditions and hardly biodegradable. Their half-life in water can reach 3.5 months in aerobic waters and some years in organic sediments (Abe and Tanaka 1997). Moreover, it was shown that the experimental conditions largely influence the efficiency, the half-life time of TCS and the type of by-products. There is a strong need to strengthen the control of TCS transformation occurring in natural matrices where organic matter could play a major role. Furthermore, there is a lack of data concerning the co-occurrence of TCS and by-products in natural compartments.
The toxicity of TCS has been studied by using several types of organisms, including microalgae and fish, leading to very low EC50 approaching the amount of TCS detected in natural aquatic environments. The fact that TCS showed relatively short half-life time under certain conditions raises the question of the origin of the toxicity, because by-products could also interact with organisms. Finally, very few studies have been reported either on the ecotoxicity of these by-products or on the effect of a mixture of these by-products with TCS in order to detect potential synergistic or antagonist effects. Very recently, affection of thyroid hormone-response gene transcripts and alteration of stress indicators of amphibian and mammalian cells were detected with 10 nM MTCS exposure (Hinther et al. 2011). These results not only show the effects of MTCS at very low concentrations but also raise the question of the mode of action of TCS in the organisms because of its degradation in MTCS which is more persistent in the environment (Lindström et al. 2002). Brausch and Rand (2011) have shown that all data published till now cannot be conducted to determine potential effect of MTCS because we have incomplete data about environmental concentration and no data of acute and chronic toxicity of MTCS. Effects of environmental pollution on human health are still poorly reported. Despite, it possesses endocrine-disrupting properties and it alters reproduction, the TCS chronic effects have not been extensively studied. Because toxic by-products are rapidly formed, those have to be monitored in all experiment. The incomplete data include the environmental concentrations of TCS transformation products and by-products and bioaccumulation capacity, toxicity, and ecotoxicity studies of these compounds and mixture of them, endocrine disruption and effects on fetus. These studies should help to identify potential risk of TCS release into the environment.
References
Abe KI, Tanaka K (1997) Fe3+ and UV-enhanced ozonation of chlorophenolic compounds in aqueous medium. Chemosphere 35:2837–2847
Adolfsson-Erici M, Petterson M, Parkkonen J, Sturve J (2002) Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 46:1485–1489
Agüera A, Fernández-Alba AR, Piedra L, Mézcua M, Gómez MJ (2003) Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas chromatography mass spectrometry and liquid chromatography mass spectrometry. Anal Chim Acta 480:193–205
Aiello AE, Marshall B, Levy SB, Della-Latta P, Larson E (2004) Relationship between triclosan and susceptibilities of bacteria isolated from hands in the community. Antimicrob Agents Chemother 48:2973–2979
Allmyr M, Adolfsson-Erici M, McLachlan MS, Sandborgh-Englund G (2006) Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci Total Environ 372:87–93
Allmyr M, Harden F, Toms LML, Mueller JF, McLachlan MS, Adolfsson-Erici M, Sandborgh-Englund G (2008) The influence of age and gender on triclosan concentrations in Australian human blood serum. Sci Total Environ 393:162–167
Al-Rajab AJ, Sabourin L, Scott A, Lapen DR, Topp E (2009) Impact of biosolids on the persistence and dissipation pathways of triclosan and triclocarban in an agricultural soil. Sci Total Environ 407:5978–5985
An J, Zhou Q, Sun Y, Xu Z (2009) Ecotoxicological effects of typical personal care products on seed germination and seedling development of wheat (Triticum aestivum L.). Chemosphere 76:1428–1434
Antoniou CV, Koukouraki EE, Diamadopoulos E (2009) Analysis of selected pharmaceutical compounds and endocrine disruptors in municipal wastewater using solid-phase microextraction and gas chromatography. Water Res 81:664–669
Aragon DM, Ruidiaz MA, Vargas EF, Bregni C, Chiappetta DA, Sosnik A, Martinez F (2008) Solubility of the antimicrobial agent triclosan in organic solvents of different hydrogen bonding capabilities at several temperatures. J Chem Eng Data 53:2576–2580
Aranami K, Readman JW (2007) Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 66:1052–1056
Balmer ME, Poiger T, Droz C, Romanin K, Bergqvist PA, Müller MD, Buser HR (2004) Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ Sci Technol 38:390–395
Behera SK, Oh SY, Park HS (2010) Sorption of triclosan onto activated carbon, kaolinite and montmorillonite: effects of pH, ionic strength, and humic acid. J Hazard Mater 179:684–691
Benitez FJ, Beltran-Heredia J, Acero JL, Rubio FJ (2000) Contribution of free radicals to chlorophenols decomposition by several advanced oxidation processes. Chemosphere 41:1271–1277
Benotti MJ, Trenholm RA, Vanderford BJ, Holady JC, Stanford BD, Snyder SA (2009) Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ Sci Technol 43:597–603
Bester K (2003) Triclosan in a sewage treatment process—balances and monitoring data. Water Res 37:3891–3896
Bester K (2005) Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch Environ Contam Toxicol 49:9–17
Bhargava HN, Leonard PA (1996) Triclosan: applications and safety. Am J Infect Control 24:209–218
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009a) Cytotoxic and genotoxic effects of in vivo exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp Biochem Physiol C 150:50–56
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009b) In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in zebra mussel hemocytes. Aquat Toxicol 91:238–244
Birosová L, Mikulásová M (2009) Development of triclosan and antibiotic resistance in Salmonella enterica serovar Typhimurium. J Med Microbiol 58:436–441
Brausch JM, Rand GM (2011) A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere 82:1518–1532
Brun EM, Bonet E, Puchades M, Maquieira A (2008) Selective enzyme-linked immunosorbent assay for triclosan. Application to wastewater treatment plant effluents. Environ Sci Technol 42:1665–1672
Buth JM, Grandbois M, Vikesland PJ, McNeill K, Arnold WA (2009) Aquatic photochemistry of chlorinated triclosan derivatives: potential source of polychlorodibenzo-p-dioxins. Environ Toxicol Chem 28:2555–2563
Buth JM, Steen PO, Sueper C, Blumentritt D, Vikesland PJ, Arnold WA, McNeill K (2010) Dioxins photoproducts of triclosan and its chlorinated derivatives in sediment cores. Environ Sci Technol 44:4545–4551
Cajthaml T, Kresinova Z, Svobodova K, Moder M (2009) Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 75:745–750
Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL (2008) Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect 116:303–307
Canosa P, Morales S, Rodríguez I, Rubí E, Cela R, Gómez M (2005) Aquatic degradation of triclosan and formation of toxic chlorophenols in presence of low concentrations of free chlorine. Anal Bioanal Chem 383:1119–1126
Canosa P, Pérez-Palacios D, Garrido-Lopez A, Tena MT, Rodriguez I, Rubi E, Cela R (2007a) Pressurized liquid extraction with in-cell clean-up followed by gas chromatography-tandem mass spectrometry for the selective determination of parabens and triclosan in indoor dust. J Chromatogr A 1161:105–112
Canosa P, Rodríguez I, Rubí E, Cela R (2007b) Determination of parabens and triclosan in indoor dust using matrix solid-phase dispersion and gas chromatography with tandem mass spectrometry. Anal Chem 79:1675–1681
Cantwell MG, Wilson BA, Zhu J, Wallace GT, King JW, Olsen CR, Burgess RM, Smith JP (2010) Temporal trends of triclosan contamination in dated sediment cores from four urbanized estuaries: evidence of preservation and accumulation. Chemosphere 78:347–352
Capdevielle M, Egmond RV, Whelan M, Versteeg D, Hofmann-Kamensky M, Inauen J, Cunningham V, Voltering D (2008) Consideration of exposure and species sensitivity of triclosan in the freshwater environment. Integr Environ Assess Manage 4:15–23
Cha J, Cupples AM (2009) Detection of the antimicrobials triclocarban and triclosan in agricultural soils following land application of municipal biosolids. Water Res 43:2522–2530
Cha J, Cupples AM (2010) Triclocarban and triclosan biodegradation at field concentrations and the resulting leaching potentials in three agricultural soils. Chemosphere 81:494–499
Chalew ET, Halden RU (2009) Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J Am Water Resour Assoc 45:4–13
Chau WC, Wu JL, Cai Z (2008) Investigation of levels and fate of triclosan in environmental waters from the analysis of gas chromatography coupled with ion trap mass spectrometry. Chemosphere 73:S13–S17
Chen J, Ahn KC, Gee NA, Gee SJ, Hammock BD, Lasley BL (2007) Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol 221:278–284
Chen Z, Song Q, Cao G, Chen Y (2008) Photolytic degradation of triclosan in the presence of surfactants. Chemical Papers 62:608–615
Chen X, Pauly U, Rehfus S, Bester K (2009) Removal of personal care compounds from sewage sludge in reed bed container (lysimeter) studies—effects of macrophytes. Sci Total Environ 407:5743–5749
Chen Z, Cao G, Song Q (2010) Photo-polymerization of triclosan in aqueous solution induced by ultraviolet radiation. Environ Chem Lett 8:33–37
Chen X, Nielsen JL, Furgal C, Liu Y, Lolas IB, Bester K (2011) Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere 84:452–456
Chiron S, Minero C, Vione D (2007) Occurrence of 2,4-dichlorophenol and of 2,4-dichloro-6-nitrophenol in the Rhone River Delta (Southern France). Environ Sci Technol 41:3127–3133
Chu S, Metcalfe CD (2007) Simultaneous determination of triclocarban and triclosan in municipal biosolids by liquid chromatography tandem mass spectrometry. J Chromatogr A 1164:212–218
Chuanchuen R, Beinlich K, Hoang TT, Becher A, Karkhoff-Schweizer RR, Schweizer HP (2001) Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overewpressing MexCD-OprJ. Antimicrob Agents Chemother 45:428–432
Clarke BO, Smith SR (2011) Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ Int 37:226–247
Coogan MA, La Point TW (2008) Snail bioaccumulation of triclocarban, triclosan, and methyltriclosan in a North Texas, USA stream affected by wastewater treatment plant runoff. Environ Toxicol Chem 27:1788–1793
Coogan MA, Edziyie RE, La Point TW, Venables BJ (2007) Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67:1911–1918
Crofton KM, Paul KB, DeVito MJ, Hedge JM (2007) Short-term in vivo exposure to the water contaminant triclosan: evidence for disruption of thyroxine. Environ Toxicol Pharmacol 24:194–197
Cuderman P, Heath E (2007) Determination of UV filters and antimicrobial agents in environmental water samples. Anal Bioanal Chem 387:1343–1350
Da Salva SJ, King BM, Lin YJ, Lin YJ (1989) Triclosan: a safety profile. Am J Dent 2:185–196
Dann AB, Hontela A (2011) Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31:285–311
Dayan AD (2007) Risk assessment of triclosan [Irgasan®] in human breast milk. Food Chemical Toxicol 45:125–129
DeLorenzo ME, Fleming J (2008) Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch Environ Contam Toxicol 54:203–210
DeLorenzo ME, Keller JM, Arthur CD, Finnegan MC, Harper HE, Winder VL, Zdankiewicz DL (2008) Toxicity of the antimicrobial compound triclosan and formation of the metabolite methyl-triclosan in estuarine systems. Environ Toxicol 23:224–232
Dirtu AC, Roosens L, Geens T, Gheorge A, Neels H, Covaci A (2008) Simultaneous determination of bisphenol A, triclosan, and tetrabromobisphenol A in human serum using solid-phase extraction and gas chromatography-electron capture negative-ionization mass spectrometry. Anal Bioanal Chem 391:1175–1181
Dussault E, Balakrishnan V, Sverko E, Solomon K, Sibley P (2008) Toxicity of human pharmaceutical and personal care products to benthic invertebrates. Environ Toxicol Chem 27:425–432
Dye C, Schlabach M, Green J, Remberger M, Kaj L, Palm-Cousins A, Brorström-Lundén E (2007) Bronopol, resorcinol, m-cresol and triclosan in the Nordic environment. Nordic Council of Ministers Copenhagen.TemaNord 585:83 pp. http://www.nordicscreening.org/index.php
Edwards M, Topp E, Metcalfe CD, Li H, Gottschall N, Bolton P (2009) Pharmaceutical and personal care products in tile drainage following surface spreading and injection of dewatered municipal biosolids to an agricultural field. Sci Tot Environ 407:4220–4230
European Commission (2002) Opinion on: Triclosan resistance. Adopted by the SSC at its meeting of 27–28 June 2002. http://ec.europa.eu/food/fs/sc/sccp/out182_en.pdf
Fair PA, Lee HB, Adams J, Darling C, Pacepavicius G, Alaee M, Bossart GD, Henry N, Muir D (2009) Occurrence of triclosan in plasma of wild Atlantic bottlenose dolphins (Tursiops truncatus) and in their environment. Environ Pollut 157:2248–2254
Farré M, Asperger D, Kantiani L, Gonzales S, Petrovic M, Barcelo D (2007) Assessment of the acute toxicity of triclosan and methyl triclosan in wastewater based on the bioluminescence inhibition of Vibrio fischeri. Anal Bioanal Chem 390:1999–2007
Federle TW, Kaiser SK, Nuck BA (2002) Fate and effects of triclosan in activated sludge. Environ Toxicol Chem 21:1330–1337
Ferrer I, Mezcua M, Gomez MJ, Thurman EM, Aguera A, Hernando MD, Fernandez-Alba AR (2004) Liquid chromatography/time-of-flight mass spectrometric analyses for the elucidation of the photodegradation products of triclosan in wastewater samples. Rapid Commun Mass Spectrom 18:443–450
Fiss EM, Rule KL, Vikesland PJ (2007) Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing antibacterial products. Environ Sci Technol 41:2387–2394
Foran CM, Bennett ER, Benson WH (2000) Developmental evaluation of a potential non-steroidal estrogen: triclosan. Marine Environ Res 50:153–156
Franz S, Altenburger R, Heilmeier H, Schmitt-Jansen M (2008) What contributes to the sensitivity of microalgae to triclosan? Aquat Toxicol 90:102–108
Fuchsman P, Lyndall J, Bock M, Lauren D, Barber T, Leigh K, Perruchon E, Capdevielle M (2010) Terrestrial ecological risk evaluation for triclosan in land-applied biosolids. Integr Environ Assess Manage 6:405–418
Gee RH, Charles A, Taylor N, Darbre PD (2008) Oestrogenic and androgenic activity of triclosan in breast cancer cells. J Appl Toxicol 28:78–91
Geens T, Roosens L, Neels H, Covaci A (2009) Assessment of human exposure to bisphenol-A, triclosan and tetrabromobisphenol-A through indoor dust intake in Belgium. Chemosphere 76:755–760
Glaser A (2004) The ubiquitous triclosan, a common antibacterial agent exposed. Pesticides and You 24:12–17. http://www.beyondpesticides.org/pesticides/factsheets/Triclosan%20cited.pdf
Gomez MJ, Martinez Bueno MJ, Lacorte S, Fernandez-Alba AR, Agüera A (2007) Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 66:993–1002
Gomez-Escalada MG, Harwood JL, Maillard JY, Ochs D (2005) Triclosan inhibition of fatty acids synthesis and its effect on growth of Escherichia coli and Pseudomonas aeruginosa. J Antimicrob Chemother 55:879–882
González-Mariño I, Quintana JB, Rodriguez I, Cela R (2009) Simultaneous determination of parabens, triclosan and triclocarban in water by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 23:1756–1766
González-Mariño I, Benito Quintana J, Rodríguez I, Schrader S, Moeder M (2011) Fully automated determination of parabens, triclosan and methyl triclosan in wastewater by microextraction by packed sorbents and gas chromatography–mass spectrometry. Anal Chim Acta 684:59–66
Guo JH, Li XY, Cao XL, Li Y, Wang XZ, Xu XB (2009) Determination of triclosan, triclocarban and methyl-triclosan in aqueous samples by dispersive liquid–liquid microextraction combined with rapid liquid chromatography. J Chromatogr A 1216:3038–3043
Halden RU, Paull DH (2005) Co-occurrence of triclocarban and triclosan in US water resources. Environ Sci Technol 39:1420–1426
Hanioka N, Omae E, Nishimura T, Jinno H, Onodera S, Yoda R, Ando M (1996) Interaction of 2,4,4′-trichloro-2′-hydroxydiphenyl ether with microsomal cytochrome P450-dependent monooxygenases in rat liver. Chemosphere 33:265–276
Harada A, Komori K, Nakada N, Kitamura K, Suzuki Y (2008) Biological effects of PPCPs on aquatic lives and evaluation of river waters affected by different wastewater treatment level. Water Sci Technol 58:1541–1546
Hay AG, Dees PM, Sayler GS (2001) Growth of a bacterial consortium on triclosan. FEMS Microbiol Ecol 36:105–112
Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO (1999) Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem 274:11110–11114
Heidler J, Halden RU (2007) Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 66:362–369
Heidler J, Sapkota A, Halden RU (2006) Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ Sci Technol 40:3634–3639
Hess-Wilson JK, Knudsen KE (2006) Endocrine disrupting compounds and prostate cancer. Cancer Lett 241:1–12
Hinther A, Bromba CM, Wulff JE, Helbing CC (2011) Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ Sci Technol 45:5395–5402
Hua W, Bennett ER, Letcher RJ (2005) Triclosan in waste and surface waters from the upper Detroit River by liquid chromatography–electrospray–tandem quadrupole mass spectrometry. Environ Int 31:621–630
Hundt K, Martin D, Hammer E, Jonas U, Kindermann MK, Schauer F (2000) Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl Environ Microbiol 66:4157–4160
Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K (2004) Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol 67:167–179
Jacobs MN, Nolan GT, Hood SR (2005) Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR). Toxicol Appl Pharmacol 209:123–133
James MO, Li W, Summerlot DP, Rawland-Faux L, Wood CE (2010) Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ Int 36:942–949
Johnson DR, Czechowska K, Chèvre N, Roelof van der Meer J (2009) Toxicity of triclosan, penconazole and metalaxyl on Caulobacter crescentus and a freshwater microbial community as assessed by flow cytometry. Environ Microbiol 11:1682–1691
Kanda R, Griffin P, James HA, Fothergill J (2003) Pharmaceutical and personal care products in sewage treatment works. J Environ Monit 5:823–830
Kanetoshi A, Ogawa H, Katsura E, Kaneshima H (1987) Chlorination of Irgasan and formation of dioxins from its chlorinated derivatives. J Chromatogr 389:139–153
Kantiani L, Farré M, Asperger D, Rubio F, Gonzalez S, Lopez de Alda MJ, Petrovic M, Shelver WL, Barcelo D (2008) Triclosan and methyl triclosan monitoring study in the northeast of Spain using a magnetic particle enzyme immunoassay and confirmatory analysis by gas chromatography–mass spectrometry. J Hydrol 361:1–9
Kawaguchi M, Ito R, Honda H, Endo N, Okanouchi N, Saito K, Seto Y, Nakazawa H (2008) Determination of urinary triclosan by stir bar sorptive extraction and thermal desorption–gas chromatography–mass spectrometry. J Chromatogr B 875:577–580
Kim MK, O’Keefe PW (2000) Photodegradation of polychlorinated dibenzo-p-dioxins and dibenzofurans in aqueous solutions and in organic solvents. Chemosphere 41:793–800
Kim JW, Ishibashi H, Yamauchi R, Ichikawa N, Takao Y, Hirano M, Koga M, Arizono K (2009) Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes). J Toxicol Sci 34:227–232
Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Zaugg SD, Werner SL, Bossio JP, Benotti MJ (2008) Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ Sci Technol 42:1863–1870
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kookana RS, Ying GG, Waller NJ (2011) Triclosan: its occurrence, fate and effects in the Australian environment. Water Sci Technol 63:598–604
Kumar V, Balomajumder C, Roy P (2008) Disruption of LH-induced testosterone biosynthesis in testicular leydig cells by triclosan: probable mechanism of action. Toxicology 250:124–131
Kumar V, Chakraborty A, Kural MR, Roy P (2009) Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reprod Toxicol 27:177–185
Kumar KS, Priya SM, Peck AM, Sajwan KS (2010) Mass loadings of triclosan and triclocarban from four wastewater treatment plants to three rivers and landfill in Savannah, Georgia, USA. Arch Environ Contam Toxicol 58:275–285
Kuster A, Pohl K, Altenburger R (2007) A fluorescence-based bioassay for aquatic macrophytes and its suitability for effect analysis of non-photosystem II inhibitors. Env Sci Pollut Res 14:377–383
Kuster M, Lopez de Alda MJ, Hernando MD, Petrovic M, Martin-Alonso J, Barcelo D (2008) Analysis and occurrence of pharmaceuticals, estrogens, protestogens and polar pesticides in sewage treatment plant effluents, river water and drinking water in the Llobregat river basin (Barcelona, Spain). J Hydrol 358:112–123
Langdon KA, Warne MS, Smernik RJ, Shareef A, Kookana RS (2011) Selected personal care products and endocrine disruptors in biosolids: an Australia-wide survey. Sci Total Environ 409:1075–1081
Lapen DR, Topp E, Metcalfe CD, Li H, Edwards M, Gottschall N (2008) Pharmaceutical and personal care products in tile drainage following land application of municipal biosolids. Sci Total Environ 399:50–65
Latch DE, Packer JL, Stender BL, VanOverbeke J, Arnold WA, McNeill K (2005) Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products. Environ Toxicol Chem 24:517–525
Leiker TJ, Abney SR, Goodbred SL, Rosen MR (2009) Identification of methyl triclosan and halogenated analogues in male common carp (Cyprinus carpio) from Las Vegas Bay and semipermeable membrane devices from Las Vegas Wash, Nevada. Sci Total Environ 407:2102–2114
Levy SB (2001) Antibacterial household products: cause for concern. Emerg Infect Dis 7:512–515
Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB (1999) Molecular basis of triclosan activity. Nature 398:383–384
Li X, Ying GG, Su HC, Yang XB, Wang L (2010) Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ Int 36:557–562
Lin D, Zhou Q, Xie X, Liu Y (2010) Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida). Chemosphere 81:1328–1333
Lindström A, Buerge IJ, Poiger T, Bergqvist PA, Müller MD, Buser HR (2002) Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ Sci Technol 36:2322–2329
Liu F, Ying GG, Yang LH, Zhou QX (2009) Terrestrial ecotoxicological effects of the antimicrobial triclosan. Ecotoxicol Environ Saf 72:86–92
Loos R, Wollgast J, Huber T, Hanke G (2007) Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around Lake Maggiore in Northern Italy. Anal Bioanal Chem 387:1469–1478
Lores M, Llompart M, Sanchez-Prado L, Garcia-Jares C, Cela R (2005) Confirmation of the formation of dichlorodibenzo-p-dioxin in the photodegradation of triclosan by photo-SPME. Anal Bioanal Chem 381:1294–1298
Lozano N, Rice CP, Ramirez M, Torrents A (2010) Fate of triclosan in agricultural soils after biosolid applications. Chemosphere 78:760–766
Lyndall J, Fuchsman P, Bock M, Barber T, Lauren D, Leigh K, Perruchon E, Capdevielle M (2010) Probabilistic risk evaluation for triclosan in surface water, sediments, and aquatic biota tissues. Integr Environ Assess Manage 6:419–440
Maillard JY (2007) Bacterial resistance to biocides in the healthcare environment: shall we be concerned? J Hosp Infect 65:S60–S72
Matsumura N, Ishibashi H, Hirano M, Nagao Y, Watanabe N, Shiratsuchi H, Kai T, Nishimura T, Kashiwagi A, Arizono K (2005) Effects of nonylphenol and triclosan on production of plasma vitellogenin and testosterone in male South African clawed frogs (Xenopus laevis). Biol Pharm Bull 28:1748–1751
Mayer FL Jr, Ellersieck MR (1986) Manual of acute toxicity: interpretation and data base for 410 chemicals and 66 species of freshwater animals. Resour Publ 160, U.S. Dep. Interior, Fish Wildl. Serv., Washington, DC
McAvoy D, Schatowitz B, Jacob M, Hauk A, Eckhoff W (2002) Measurement of triclosan in wastewater treatment systems. Environ Toxicol Chem 21:1323–1329
McClellan K, Halden RU (2010) Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Res 44:658–668
McMahon T, Shamim N, Gowda S, Angle G, Leighton T (2008) 5-Chloro-2-(2,4-dichlorophenoxy) phenol (triclosan): risk assessment for the Registration Eligibility Decision (RED) Document. USEPA, Washington DC. http://www.epa.gov/oppsrrd1/REDs/2340red.pdf
Meade MJ, Waddell RL, Callahan TM (2001) Soil bacteria Pseudomonas putida and Alcaligenes xylosoxidans subsp. denitrificans inactivate triclosan in liquid and solid substrates. FEMS Microbiol Lett 204:45–48
Menoutis J, Parisi I (2002) Testing for dioxin and furan contamination in triclosan. Cosmetics Toiletries 117:75–78
Mezcua M, Gomez MJ, Ferrer I, Aguera A, Hernando MD, Fernandez-Alba AR (2004) Evidence of 2,7/2,8-dibenzodichloro-p-dioxin as a photodegradation product of triclosan in water and wastewatersamples. Anal Chim Acta 524:241–247
Miller TL, Lorusso DJ, Walsh ML, Deinzer ML (1983) The acute toxicity of penta-, hexa-, and heptachlorohydroxydiphenyl ethers in mice. J Toxicol Environ Health 12:245–253
Miller TR, Heidler J, Chillrud SN, Delaquil A, Ritchie JC, Mihalic JN, Bopp R, Halden RU (2008) Fate of triclosan and evidence of reductive dechlorination of triclocarban in estuarine sediments. Environ Sci Technol 42:4570–4576
Mink PJ, Adami HO, Trichopoulos D, Britton NL, Mandel JS (2008) Pesticides and prostate cancer: a review of epidemiologic studies with specific agricultural exposure information. Eur J Cancer Prev 17:97–110
Montes R, Rodriguez I, Rubi E, Cela R (2009) Dispersive liquid–liquid microextraction applied to the simultaneous derivatization and concentration of triclosan and methyltriclosan in water samples. J Chromatogr A 1216:205–210
Morales S, Canosa P, Rodriguez I, Rubi E, Cela R (2005) Microwave assisted extraction followed by gas chromatography with tandem mass spectrometry for the determination of triclosan and two related chlorophenols in sludge and sediments. J Chromatogr A 1082:128–135
Morrall N, McAvoy D, Schatowitz B, Inauen J, Jacob M, Hauk A, Eckhoff W (2004) A field study of triclosan loss rates in river water (Cibolo Creek, TX). Chemosphere 54:653–660
Mottaleb MA, Usenko S, O’Donnell JG, Ramirez AJ, Brooks BW, Chambliss CK (2009) Gas chromatography–mass spectrometry screening methods for select UV filters, synthetic musks, alkylphenols, an antimicrobial agent, and an insect repellent in fish. J Chromatogr A 1216:815–823
Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H (2006) Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res 40:3297–3303
Nassef M, Kim SG, Seki M, Kang IJ, Hano T, Shimasaki Y, Oshima Y (2010a) In ovo nanoinjection of triclosan, diclofenac and carbamazepine affects embryonic development of medaka fish (Oryzias latipes). Chemosphere 79:966–973
Nassef M, Matsumoto S, Seki M, Khalil F, Kang IJ, Shimasaki Y, Oshima Y, Honjo T (2010b) Acute effects of triclosan, diclofenac and carbamazepine on feeding performance of Japanese medaka fish (Oryzias latipes). Chemosphere 80:1095–1100
Neumegen RA, Fernandez-Alba AR, Chisti Y (2005) Toxicities of triclosan, phenol, and copper sulfate in activated sludge. Environ Toxicol 20:160–164
Nieto A, Borrull F, Marce RM, Pocurull E (2009) Determination of personal care products in sewage sludge by pressurized liquid extraction and ultra high performance liquid chromatography–tandem mass spectrometry. J Chromatogr A 1216:5619–5625
Nishi I, Kawakami T, Onodera S (2008) Monitoring of triclosan in the surface water of the Tone Canal, Japan. Bull Environ Contam Toxicol 80:163–166
Oliveira R, Domingues I, Koppe Grisolia C, Soares AMVM (2009) Effects of triclosan on zebrafish early-life stages and adults. Environ Sci Pollut Res 16:679–688
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21:1338–1349
Palenske NM, Nallani GC, Dzialowski EM (2010) Physiological effects and bioconcentration of triclosan on amphibian larvae. Comp Biochem Physiol Part C 152:232–240
Parikh SL, Xiao G, Tong PJ (2000) Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 39:7645–7650
Pedrouzo M, Borrull F, Marcé RM, Pocurull E (2010) Stir-bar-sorptive extraction and ultra-high-performance liquid chromatography–tandem mass spectrometry for simultaneous analysis of UV filters and antimicrobial agents in water samples. Anal Bioanal Chem 397:2833–2839
Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397:158–166
Pera-Titus M, Garcia-Molina V, Baños MA, Giménez J, Esplugas S (2004) Degradation of chlorophenols by means of advanced oxidation processes: a general review. Appl Catal B: Environ 47:219–256
Pothitou P, Voutsa D (2008) Endocrine disrupting compounds in municipal and industrial wastewater treatment plants in Northern Greece. Chemosphere 73:1716–1723
Price OR, Williams RJ, van Egmond R, Wilkinson MJ, Whelan MJ (2010) Predicting accurate and ecologically relevant regional scale concentrations of triclosan in rivers for use in higher-tier aquatic risk assessments. Envir Int 36:521–526
Prins GS (2008) Endocrine disruptors and prostate cancer risk. Endocr Relat Canc 15:649–656
Ramaswamy BR, Shanmugam G, Velu G, Rengarajan B, Larsson DGJ (2011) GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J Hazard Mater 186:1586–1593
Ramirez AJ, Brain RA, Usenko S, Mottaleb MA, O’Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt JL, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK (2009) Occurrence of pharmaceuticals and personal care products (PPCPs) in fish: results of a national pilot study in the U.S. Environ Toxicol Chem 25:1–10
Regueiro J, Becerril E, Garcia-Jares C, Llompart M (2009) Trace analysis of parabens, triclosan and related chlorophenols in water by headspace solid-phase microextraction with in situ derivatization and gas chromatography–tandem mass spectrometry. J Chromatogr A 1216:693–702
Reiss R, Mackay N, Habig C, Griffin J (2002) An ecological risk assessment for triclosan in lotic systems following discharge from wastewater treatment plants in the United States. Environ Toxicol Chem 21:2483–2492
Reiss R, Lewis G, Griffin J (2009) An ecological risk assessment for triclosan in the terrestrial environment. Environ Toxicol Chem 28:1546–1556
Ricart M, Guasch H, Alberch M, Barceló D, Bonnineau C, Geiszinger A, Farré M, Ferrer J, Ricciardi F, Romaní AM, Morin S, Proia L, Sala L, Sureda D, Sabater S (2010) Triclosan persistence through wastewater treatment plants and its potential toxic effects on river biofilms. Aquat Toxicol 100:346–353
Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM (2010) Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol 40:422–484
Roh H, Subramanya N, Zhao F, Yu CP, Sandt J, Chu KH (2009) Biodegradation potential of wastewater micropollutants by ammonia-oxidizing bacteria. Chemosphere 77:1084–1089
Rule KL, Ebbett VR, Vikesland PJ (2005) Formation of chloroform and chlorinated organics by free-chlorine-mediated oxidation of triclosan. Environ Sci Technol 39:3176–3185
Sabaliunas D, Webb SF, Hauk A, Jacob M, Eckhoff WS (2003) Environmental fate of triclosan in the River Aire Basin, UK. Water Res 37:3145–3154
Sanches-Silva A, Cruz-Freire JM, Paseiro-Losada P (2010) Study of the diffusion coefficients of diphenylbutadiene and triclosan into and within meat. Eur Food Res Technol 230:957–964
Sanchez-Prado L, Llompart M, Lores M, Garcia-Jares C, Bayona JM, Cela R (2006) Monitoring the photochemical degradation product of triclosan in wastewater by UV light and sunlight usinf solid-phase microextraction. Chemosphere 65:1338–1347
Sanchez-Prado L, Barro R, Garcia-Jarès C, Llompart M, Lores M, Petrakis C, Kalogerakis N, Mantzavinos D, Psillakis E (2008) Sonochemical degradation of triclosan in water and wastewater. Ultrason Sonochem 15:689–694
Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J (2006) Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health A 69:1861–1873
SCCS (2010) Scientific Committee on Consumer Safety. Opinion on: Triclosan antimicrobial, resistance (SCCP/1251/09). Adopted by the SCCP during the 7th plenary on 22 June 2010
Seaman PF, Ochs D, Day MJ (2007) Comment on: triclosan resistance in methicillin-resistant Staphylococcus aureus expressed as small colony variants: a novel mode of evasion of susceptibility to antibiotics. J Antimicrob Chemother 60:175–176
Singer H, Müller S, Tixier C, Pillonel L (2002) Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36:4998–5004
Singh SP, Azua A, Chaudhary A, Khan S, Willett KL, Gardinali PR (2010) Occurrence and distribution of steroids, hormones and selected pharmaceuticals in South Florida coastal environments. Ecotoxicology 19:338–350
Son HS, Choi SB, Zoh KD, Khan E (2007) Effects of ultraviolet intensity and wavelength on the photolysis of triclosan. Water Sci Technol 55:209–216
Stasinakis AS, Petalas AV, Mamais D, Thomaidis NS, Gatidou G, Lekkas TD (2007) Investigation of triclosan fate and toxicity in continuous-flow activated sludge systems. Chemosphere 68:375–381
Stasinakis AS, Mamais D, Thomaidis NS, Danika E, Gatidou G, Lekkas TD (2008) Inhibitory effect of triclosan and nonylphenol on respiration rates and ammonia removal in activated sludge systems. Ecotoxicol and Environ Saf 70:199–206
Stasinakis AS, Kordoutis CI, Tsiouma VC, Gatidou G, Thomaidis NS (2010) Removal of selected endocrine disrupters in activated sludge systems: effect of sludge retention time on their sorption and biodegradation. Bioresource Technol 101:2090–2095
Stevens KJ, Kim SY, Adhikari S, Vadapalli V, Venables BJ (2009) Effect of triclosan on seed germination and seedling development of three wetland plants. Environ Toxicol Chem 28:2598–2609
Suarez S, Dodd MC, Omil F, von Gunten U (2007) Kinetics of triclosan oxidation by aqueous ozone and consequent loss of antibacterial activity: relevance to municipal wastewater ozonation. Water Res 41:2481–2490
Svenningsen H, Henriksen T, Prieme A, Johnsen AR (2011) Triclosan affects the microbial community in simulated sewage-drain-field soil and slows down xenobiotic degradation. Environ Pollut 159:1599–1605
Tatarazako N, Ishibashi H, Teshima K, Kishi K, Arizono K (2004) Effects of triclosan on various aquatic organisms. Environ Sci 11:133–140
Tkachenko O, Shepard J, Aris VM et al (2007) A triclosan-ciprofloxacin cross-resistant mutant strain of Staphylococcus aureus displays an alteration in the expression of several cell membrane structural and functional genes. Res Microbial 158:651–658
Topp E, Monteiro SC, Beck A, Coelho BB, Boxall AB, Duenk PW (2008) Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Sci Tot Environ 396:52–59
Trenholm RA, Vanderford BJ, Drewes JE, Snyder SA (2008) Determination of household chemicals using gas chromatography and liquid chromatography with tandem mass spectrometry. J Chromatogr A 1190:253–262
USEPA (United States Environmental Protection Agency) (2007) An analysis of laboratory and observational field data characterizing cumulative exposure to pesticides, EPA 600/R-07/013. Environmental Protection Agency, U.S. Research Triangle Park, NC, 27711, USA
USEPA (United States Environmental Protection Agency) (2009) Targeted national sewage sludge survey and analysis technical report, EPA-822-R-08-016. U.S. Washington, USA: Environmental Protection Agency
USFDA (United States Food Drug Administration) (2008) Supporting information for toxicological evaluation by the national toxicology program http://ntp.niehs.nih.gov/ntp/htdocs/Chem_Background/ExSumPdf/triclosan_508.pdf
Valters K, Li H, Alaee M, D’Sa I, Marsh G, Bergman A, Letcher RJ (2005) Polybrominated diphenyl ethers and hydroxylated and methoxylated brominated and chlorinated analogues in the plasma of fish from the Detroit River. Environ Sci Technol 39:5612–5619
Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC (2006) The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquatic Toxicol 80:217–227
Villaverde-de-Sáa E, González-Mariňo I, Quintana JB, Rodil R, Rodriguez I, Cela R (2010) In-sample acetylation-non-porous membrane-assisted liquid-liquid extraction for the determination of parabens and triclosan in water samples. Anal Bioanal Chem 397:2559–2568
Waller NJ, Kookana RS (2009) Effect of triclosan on microbial activity in Australian soils. Environ Toxicol Chem 28:65–70
Wang LQ, Falany CN, James MO (2004) Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos 32:1162–1169
Weigel S, Berger U, Jensen E, Kallenborn R, Thoresen H, Hühnerfuss H (2004) Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere 56:583–592
Wert EC, Rosario-Ortiz FL, Snyder SA (2009) Effect of ozone exposure on the oxidation of trace organic contaminants in wastewater. Water Res 43:1005–1014
Wilson BA (2003) Effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. Environ Sci Technol 37:1713–1719
Wilson BA, Zhu J, Cantwell MG, Olsen CR (2008) Short-term dynamics and retention of triclosan in the lower Hudson River estuary. Mar Pollut Bull 56:1215–1233
Wilson B, Chen RF, Cantwell M, Gontz A, Zhu J, Olsen CR (2009) The partitioning of triclosan between aqueous and particulate bound phases in the Hudson River estuary. Mar Pollut Bull 59:207–212
Wind T, Werner U, Jacob M, Hauk A (2004) Environmental concentrations of boron, LAS, EDTA, NTA and triclosan simulated with GREAT-ER in the river Itter. Chemosphere 54:1135–1144
Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, Godbold J, Biro F, Kushi LH, Pfeiffer CM, Calafat AM (2007) Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect 115:116–121
Wu JL, Lam NP, Martens D, Kettrup A, Cai Z (2007) Triclosan determination in water related to wastewater treatment. Talanta 72:1650–1654
Wu C, Spongberg AL, Witter JD (2009) Adsorption and degradation of triclosan and triclocarban in soils and biosolids-amended soils. J Agric Food Chem 57:4900–4905
Xie Z, Ebinghaus R, Flöser G, Caba A, Ruck W (2008) Occurrence and distribution of triclosan in the German Bight (North sea). Environ Pollut 156:1190–1195
Xu J, Wu L, Chang AC (2009) Degradation and adsorption of selected pharmaceuticals and personal care products (PPCPs) in agricultural soils. Chemosphere 77:1299–1305
Ye X, Bishop AM, Needham LL, Calafat AM (2008) Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta 622:150–156
Ying GG, Kookana RS (2007) Triclosan in wastewaters and biosolids from Australian, wastewater treatment plants. Environ Int 33:199–205
Ying GG, Yu XY, Kookana RS (2007) Biological degradation of triclocarban and triclosan in a soil under aerobic and anaerobic conditions and comparison with environmental fate modelling. Environ Pollut 150:300–305
Yu CP, Chu KH (2009) Occurrence of pharmaceuticals and personal care products along the West Prong Little Pigeon River in east Tennessee, USA. Chemosphere 75:1281–1286
Yu JC, Kwong TY, Luo Q, Cai Z (2006) Photocatalytic oxidation of triclosan. Chemosphere 65:390–399
Yu Y, Huang Q, Wang Z, Zhang K, Tang C, Cui J, Feng J, Peng X (2011) Occurrence and behavior of pharmaceuticals, steroid hormones, and endocrine-disrupting personal care products in wastewater and the recipient river water of the Pearl River Delta, South China. J Environ Monit 13:871–878
Zhang S, Zhang Q, Darisaw S, Ehie O, Wang G (2007) Simultaneous quantification of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and pharmaceuticals and personal care products (PPCPs) in Mississippi river water, in New Orleans, Louisiana, USA. Chemosphere 66:1057–1069
Zhao JL, Ying GG, Liu YS, Chen F, Yang JF, Wang L (2010) Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: from source to the receiving environment. J Hazard Mater 179:215–222
Zuckerbraun HL, Babich H, May R, Sinensky MC (1998) Cytotoxicity, mode of action, and induction of apoptosis in human gingival cells in vitro. Eur J Oral Sci 106:628–636
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Bedoux, G., Roig, B., Thomas, O. et al. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res 19, 1044–1065 (2012). https://doi.org/10.1007/s11356-011-0632-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0632-z