Abstract
In the present study, an analysis of antimicrobial activity; and an assessment of genomic biocontrol attributes of the recently described radioresistant strain Kocuria rhizophila PT10 were conducted. PT10, a Gram-positive and yellow pigmented actinobacterial strain, was isolated from the roots of xerophyte Panicum turgidum collected from Ksar Ghilane in the south of Tunisia and its genome was sequenced. In order to assess the potential capacity of this strain to adapt to its environment, a genomic and functional characterization of key enzymes involved in polysaccharides/protein degradation, chelation of iron and production of secondary metabolites was done and revealed an interesting potential. Precisely, Cazy, antiSMASH and BAGEL analyses of the genome showed the potential of K. rhizophila PT10 to synthesize specialized metabolites (bacteriocin, linocin M18, terpenes, siderophores, etc.) and enzymes (amylase, chitinase, protease, etc.). PT10 also possesses genes potentially involved in the biosynthesis of molecules with antifungal and antimicrobial activities, such as bacilysin and cycloserine. Biocontrol assays were thus done and showed an effective antagonism under in vitro conditions against phytopathogenic fungi, Botrytis cinerea BC21 and Fusarium graminearum g1, with percents of growth inhibition of 98 and 42%, respectively. Strain PT10 also showed moderate antibacterial activity against the Gram+ Staphylococcus pasteuri PT2 and the Gram− Acinetobacter baumannii PT6. These results suggest a multivariate mode of antagonistic activity of strain PT10 against microbial pathogens through the production of hydrolytic enzymes, secondary metabolites, siderophores and other antimicrobial molecules, the characterization of which is underway.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
African regions have a wide variety of ecosystems, marked by two rainy seasons with relatively sparse, dry and arid vegetation. The agro-diversity is large, which offers immense potential in terms of agricultural products (sorghum, wheat, etc.). This diversity also represents a potential source of antimicrobial and antifungal compounds to sustain agricultural development globally (AFDB 2019). These agro zones are highly conducive to the emergence of plant pathogens in addition to adverse environmental conditions (such as hydric stress, desiccation), which cause a large array of diseases (Agrios 2009). In order to control these damages related to plant diseases, there is a large use of fungicides, pesticides and insecticides.
There are multiple drawbacks associated with the use of chemical products, for example the development of microbial resistance and environmental toxicity. These disadvantages have stimulated scientists to search for biomolecules derived from microorganisms, plants and animal sources for the development of biological control agents (BCAs), to be used against plant diseases (Carmona-Hernandez et al. 2019; Dukare et al. 2019; Nega 2014; Shuping and Eloff 2017). BCAs have been used as commercial deterrents to pathogens in agricultural applications, including biological pesticides and phytosanitary products (Martinez 2012). These biocontrol agents cause the inhibition of phytopathogens based on the production of secondary metabolites and hydrolytic enzymes, which cause growth inhibition, and nutritional competition by the production of siderophores and catabolism of carbon sources (Taechowisan et al. 2005; Trejo-Estrada et al. 1998).
The potential use of microorganisms in the treatment of plant fungal diseases has been associated with the antagonistic activity of microbes toward fungal pathogens. Several biocontrol strains have been considered as promising alternatives to chemical pesticides and have been commercialized as biofertilizers and BCAs (such as Gallex for Agrobacterium radiobacter, Kodiac for Bacillus subtillis) (Agrios 2005; Carmona-Hernandez et al. 2019; Sharma et al. 2009). In particular, actinobacteria represent abundant sources of antibiotics, bioactive compounds, and industrial enzymes. Actinobacterial strains residing in deserts and arid environments are invaluable and possess noteworthy gene clusters that yield specific metabolites, owing to their ability to survive and thrive under extreme conditions, such as those with limited water and nutrients (Mohammadipanah and Wink 2015; Singh and Dubey 2018). These extremophiles have been described as potent antagonists of phytopathogens owing to their high abundance, their metabolic versatility (hydrolytic enzymes, secondary metabolites), and their antimicrobial and insecticidal properties (Ganesan et al. 2017; Keikha et al. 2015; Shivlata and Tulasi 2015; Singh and Dubey 2018; Wang et al. 2013). They also have the potential to promote plant growth owing to their capacity to reduce iron and to solubilize phosphorus for their assimilation by plants (Francis et al. 2010; Palaniyandi et al. 2013; Valencia-Cantero et al. 2007). For example, an alkoliphilic strain, Kocuria rosea HN01, was demonstrated to reduce Fe3+ into the soluble form Fe2+ and thus make it available for plant growth (Wu et al. 2014).
The use of traditional approaches based on physiological and biochemical assays to identify and to characterize secondary metabolites remains limited and inefficient (Rutledge and Challis 2015). Recently, various effective approaches have been developed such as next generation sequencing technologies and in silico analyses and genome mining techniques. These new technologies have provided an efficient way to rapidly mine the genetic data and discover novel biomolecules. The parallel use of in silico studies to confirm in vitro assays can lead also to a more rational choice of microbes to inhibit diseases (Chen et al. 2007; Shivlata and Tulasi 2015). However, there are a limited number of reports and genomes of endophytic actinobacteria associated with arid plants (Singh and Dubey 2018).
Thus, the genome sequence of an actinobacterium isolated from an arid region, Kocuria rhizophila PT10, was assessed in order to explore its biocontrol and biotechnological potential and to explain the mechanisms used to fight pathogens. This radioresistant strain was previously isolated from irradiated roots of the xerophyte Panicum turgidum (Poaceae family) collected from the oasis of Ksar Ghilane. The antimicrobial activities of strain PT10 against fungi (B. cinerea BC21 and F. graminearum g1) and bacteria (Acinetobacter baumannii PT6, Bacillus subtilis QST713, Escherichia coli DH5α and Staphylococcus pasteuri PT2) were performed using in vitro analyses. In addition, the biocontrol-associated genes/gene clusters were identified based on bioinformatic analyses of its genome (CAZY, antiSMASH and BAGEL) and compared to the genetic potential of others Kocuria strain. This genomic comparison was permitted to underline the presence of one cluster predicted to produce linocin and restricted in few Kocuria species including K. rhizophila.
This work was conducted between 2018 and 2019, in the laboratory UMR5557, Ecologie Microbienne (Villeurbanne, France).
Materials and methods
Bacterial strain isolation, cultural and morphological characteristics
Strain PT10 was isolated from irradiated roots (10 kGy) of the xerophyte P. turgidum as described previously by Guesmi et al. (2021b). The roots were collected from Ksar Ghilane oasis (Tunisia, GPS coordinates N 32° 59.557′, E 9° 36.941′, 221.9 m altitude) in May 2015 (Guesmi et al. 2021a).
Morphological features of strain PT10 were assessed after growth on Tryptic soy agar (TSA) at 30 °C for 48 h using Scanning Electron Microscope (JSM 5400.JEOL, Japan). Briefly, washed bacterial cells were fixed with 2% glutaraldehyde, and dried with a graded ethanol (Sigma Aldrich) series (50, 70, 90 and 100%) (Kaewkla and Franco 2019).
For antimicrobial activity, the strain PT10 was grown in TSA medium at 30 °C for 48 h. The used test organisms were two Gram-positive bacteria: B. subtilis QST713 and S. pasteuri PT2, and two Gram-negative bacteria: A. baumannii PT6 and E. coli DH5α. Both bacterial strains (PT2 and PT6) were obtained from our own culture collections (Guesmi et al. 2021a, b). E. coli DH5α was obtained from the culture collections of laboratory UMR5557, Ecologie Microbienne (Villeurbanne, France) and B. subtilis QST713 was obtained from Serenade Max (Bayer, France) which sells it as a biological control agent for agricultural purposes; it was used here as a positive control. Test and control strains were grown in Lauria Bertani (LB) broth medium for 24 h at 30 °C (PT10 and B. subtilis) or 37 °C (all other strains).
Phytopathogenic fungi and culture conditions
The phytopathogenic fungi used in this study are B. cinerea BC21 and F. graminearum g1, which were obtained from the culture collection of laboratory UMR5557, Ecologie Microbienne (Villeurbanne, France). The fungi were cultivated in potato dextrose agar (PDA) medium composed by 20 g dextrose, 4 g potato infusion, 15 g bacteriological agar, pH 5.6 (European Pharmacopoeia, USP). The plates were incubated at room temperature (22 °C) for 5 to 7 days.
In vitro bacteria–fungus interaction assays
The antifungal activity due to diffusible compounds was detected by performing confrontation assays using a dual culture technique of the pathogen and the test bacteria on TSA. Briefly, the bacterial strains (K. rhizophila PT10 and B. subtilis QST713) were grown overnight on liquid Tryptic soy broth (TSB). After a centrifugation to eliminate the culture medium, they were diluted with sterilized water to an OD600 value with a range from 0.35 to 0.40.
Fifty microliters of the bacterial suspensions were streaked as a line on TSA in conventional Petri dishes 1 cm from the plate edge and incubated for 48 h at 30 °C. Mycelial disks 8 mm in diameter were cut from the target fungi colonies cultured on PDA plates for seven days and were then placed on the agar at a distance of 3 cm away from the bacterial streak. Control plates with fungi but without bacteria were prepared simultaneously. The plates were incubated at 22 °C for 5 to 7 days and examined for the inhibition of fungal growth (Khan et al. 2018). The fungal growth was measured in the control and assays in order to calculate the inhibition rate (Gasmi et al. 2019). All experiments were done in triplicates.
Antifungal activity of extracellular bacterial metabolites
The antifungal activities of the cell-free supernatant of strain PT10 culture were evaluated using the dual culture method. The antagonistic activity of extracellular bacterial metabolites against the fungi was also examined. Bacterial cultures were grown in TSB at 30 °C for 3 days. Cells were removed by centrifugation (Avanti® J-E) at 13,000g for 30 min and the supernatant filtered through a sterile 0.22 µm membrane (Millipore). Then, 1 mL of the resulting supernatant was supplemented to 14 mL of the PDA medium before pouring in the Petri dish (9 cm). Once the medium had cooled, disks (7 mm in diameter) of the target fungi, taken from the fresh margin of the mycelium, were spaced equally on the Petri dish. The plates were then incubated at 22 °C for 48 h. The inhibitory activity of the filtrate against fungal growth was recorded as the percent reduction in mycelium growth in comparison with that of the control plates (Zhao et al. 2010).
Growth inhibition of bacterial pathogens
Strain PT10 was inoculated into TSB medium and allowed to grow at 30 °C for 72 h until high turbidity was observed. After incubation, the broth culture was centrifuged (Eppendorf, France) twice at 6000g for 30 min and the supernatants were recovered using 0.22 μm Millipore filters. Finally, the supernatants were mixed with the test organisms in liquid LB medium at different volumes (0, 100, 200, 300, 400 and 500 µL) using a microplates system. The microplates were then incubated at 30 °C, and the inhibitory activity of this actinobacterium was continuously observed and recorded over 24 h. The optic density (OD) at 600 nm was measured using microplate reader TECAN (xsInfinite® 200 PRO, Germany).
The antibacterial activity of strain PT10 was assessed using a cross streak assay (Devi et al. 2013). PT10 was inoculated onto TSA plates with a single streak on the margin of Petri dishes. The plates were incubated at 30 °C for 72 h. Bacterial pathogens were streaked perpendicular to the antagonist on the agar medium. The plates were then incubated at 37 °C for 24 h. After further incubation, the antimicrobial interactions were assessed by monitoring bacterial growth (absence or presence).
Screening of fungal cell walldegrading and others beneficial enzyme activities
Fifteen to 20 µL of an overnight bacterial culture of K. rhizophila PT10 was spotted onto TSA plates to test for hydrolytic enzyme activity. After 3–4 days of incubation, bacterial growth was assessed on the plates. Each experiment was repeated three times.
To detect the activity of chitin-degrading enzymes, the colloidal chitin-containing solution was prepared as described by Murthy and collaborators (Murthy and Bleakley 2012) and supplemented into the TSA medium at 10%. After incubation, clear zones around colonies were considered evidence of chitinase activity. The experiment was repeated twice with three replicates per experiment.
Protease activity was determined by spot-inoculation of bacterial cells on skim milk agar (Naik et al. 2008; Smibert and Krieg 1994), which contains, per liter, 5 g pancreatic digest of casein, 2.5 g yeast extract, 1 g dextrose, 7% vol/vol of skim milk solution and 17 g of agar. After 3 days of incubation at 30 °C, the plates were flooded with Coomassie Brilliant Blue (0.25% w/v) dissolved in methanol: acetic acid: water (5:1:4 v/v) for 10 min to enhance the visibility of halos around the bacterial colonies.
To detect cellulase activity, a modified procedure from that of Teather and Wood (1982) was used in which 15 µL of overnight culture was spot plated on carboxymethylcellulose (CMC) agar (0.2% of NaNO3, 0.1% of K2HPO4, 0.05% of MgSO4, 0.05% of KCl, 0.2% of carboxymethylcellulose sodium salt, 0.02% of peptone and 1.7% of agar) (Chantarasiri 2014). Plates incubated at 30 °C for 48 h were flooded with Gram’s iodine (2 g potassium iodide and 1 g iodine were dissolved in 300 mL distilled water) for 3 to 5 min (Kasana et al. 2008).
For pectinase activity, a pectin-containing medium was prepared as described previously by Namasivayam et al. (2011) and plates read after 5 days of incubation. The halos were detected without staining or after adding 0.05% ruthenium red (Sigma-Aldrich) for 20 min followed by several rinses with distilled water.
Amylase activity was qualitatively examined TSA medium containing 0.5% (w/v) of soluble starch and 10% (wt/vol) of NaCl. After 3 days of incubation at 30 °C, strain PT10 was tested for amylase production by flooding bacterial growth with 0.5% of Lugol solution (Sigma-Aldrich). A clear zone around the strain on a purple background was considered to represent a positive test.
For the screening for lipase activity, the agar medium contains 10 g L−1 of peptone, 5 g L−1 of NaCl, 0.1 g L−1 of CaCl2, 2H2O, 15 g L−1 of agar and 1% (v/v) of Tween 80, at pH 7.4 (Haba et al. 2000). Following strain inoculation, the plates were incubated for 3–4 days at 30 °C. Opaque halos around the colonies were taken as the indication of lipase activity.
Genome analyses of K. rhizophila PT10 genetic potentials and comparative genomics
In order to determine the mechanisms implicated in the inhibition of fungi, in silico analyses of the genome of K. rhizophila PT10 were done. The antibiotics and secondary metabolite analysis shell (antiSMASH 6.0.0) (https://antismash.secondarymetabolites.org/#!/start) served as a comprehensive resource for the automatic genomic identification and analysis of biosynthetic gene clusters (Blin et al. 2019). The database BAGEL4 (http://bagel4.molgenrug.nl/) was used to determine and compare biosynthetic gene clusters of bacteriocins and Ribosomally synthesized and Post-translationally modified Peptides (RiPPs). Comparative genomic analysis of biosynthetic gene clusters (BGCs) was made with available genomes of several Kocuria strains including K. rhizophila DC2201, K. flava strain HO-9041, K. palustris strain M14/1 and Bacillus subtilis strain 168. The glycoside hydrolases were determined using the CAZY database (http://www.cazy.org) using several genomes of the closer strain K. rhizophila PT10. BLAST similarity and the presence of other genes were analyzed through the MicroScope pipeline of Genoscope (http://www.genoscope.cns.fr/agc/microscope/mage/). The antifungal activity of K. rhizophila PT10 was also analyzed in silico using BlastP (SwissProt database) and microbial genome annotation MaGe platform (SwissProt EXPASY) (Vallenet et al. 2006).
Results and discussion
K. rhizophila PT10 was isolated from irradiated roots (10 kGy) of P. turgidum using a serial dilution method on TSA plates (Guesmi et al. 2021a). This perennial bunchgrass, belonging to family of Poaceae, was collected from the oasis of Ksar Ghilane. Colonies presented yellow pigmentation after cultivation on TSA for 48 h at 30 °C. They were opaque, smooth and circular with regular edges. Strain PT10 was coccoid in pairs, tetrads and larger aggregates (Guesmi et al. 2021b).
The whole genome of PT10 was previously sequenced, which showed that this novel radioresistant actinobacterium harbored several gene clusters coding for secondary metabolites and hydrolytic enzymes that can be implicated in biocontrol (Guesmi et al. 2021b). Therefore, this strain was evaluated for antimicrobial activities against fungal and bacterial pathogens and the production of hydrolytic enzymes.
Antifungal and antibacterial activities of PT10
The antifungal activity was evaluated using the dual culture method. Strain PT10 was grown for 5 days together with fungi, thus enabling to assess the release and activity of their secondary metabolites. Strain PT10 showed high activity against B. cinerea BC21 and moderate activity against F. graminearum g1 (Show supplementary data Fig. S1).
These results were confirmed by measuring the percent of inhibition of pathogens. Thus, the percent growth inhibition against B. cinerea BC21 was found to be 98.64 ± 0.30% and 98.92 ± 0.12% for K. rhizophila PT10 and B. subtilis QST713, respectively. Strain PT10 possessed also a moderate antifungal activity against F. graminearum g1with an inhibition zone of 42.29 ± 0.14%, compared to B. subtilis QST713, which exhibited 62.67 ± 0.66% of suppression.
As shown in Table 1, strain PT10 exhibited significant antifungal activity against indicator fungal plant diseases in an in vitro bioassay comparable to the positive control B. subtilis QST713, which is used as a biocontrol agent. Moreover, the control efficiency against phytopathogens of the filtrate obtained from strain PT10 supernatant was also assessed (Table 1). The maximum percent of inhibition of the cell-free supernatant was observed toward B. cinerea BC21 (approximately 63.3 ± 0.78%), and the minimum percent inhibition was found for F. graminearum g1 (approximately 39.67 ± 0.28%), underlining the fact that main activity was concentrated in the secretome of PT10.
The results of in vitro bacteria–fungus interaction assays demonstrated that K. rhizophila PT10 has an interesting activity against phytopathogenic fungi. Indeed, PT10 exhibited a considerable activity against F. graminearum g1 and B. cinerea BC2 (Table 1). These results led us to consider K. rhizophila PT0 as a promising source of antimicrobial metabolites, which can be used in various potential biotechnological applications. Previous investigations were had demonstrated that isolates belonging to Kocuria genus have a broad-spectrum of antimicrobial activities against pathogens, such as Kocuria sp. rsk4 (Kumar and Jadeja 2018), K. palustris (Palomo et al. 2013), K. marina (Uzair et al. 2018). The antimicrobial potential exhibited by these strains was attributed to their production of bioactive compounds (Bundale et al. 2019; Singh and Dubey 2018).
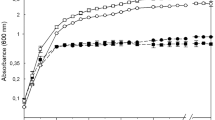
A second screening was done using filtered supernatant of PT10 from a liquid LB broth culture (Fig. 1). The filtrate of strain PT10 exhibited a broad spectrum of antibacterial activity against both Gram-positive and Gram-negative bacteria. Among all the tested organisms, A. baumannii PT6 and S. pasteuri PT2 were found to be the most sensitive to the antibacterial biomolecules of PT10. As shown in Fig. 1, the growth of PT6 and PT2 at OD600 decreased from 2 and 1.81 without PT10 supernatant (as negative control) to 0.45 and 0.57, after adding 300 µL of supernatant, respectively. However, the growth inhibition of B. subtilis QST713 and E. coli DH5α was negligible.
The evaluation of the antibacterial activity demonstrated that strain PT10 has the potential to inhibit the growth of pathogenic bacteria A. baumannii PT6 (Gram-negative) and S. pasteuri PT2 (Gram-positive) (Fig. 1). K. marina CMGS2, a halotolerant marine seaweed endophytic actinobacterium isolated from the brown seaweed Pelvetia canaliculata, was shown to have remarkable antimicrobial activity against two pathogens B. subtilis 168 (ATCC23857) and S. aureus (ATCC 33591–MRSA) (Uzair et al. 2018). However, there are many limits to the use of these biomolecules related to difficulties incurred during their production and identification.
In order to provide insights into their biotechnological potential, we analyzed K. rhizophila PT10 genome for its putative production of antimicrobial metabolites (secondary metabolites and hydrolytic enzymes).
Genomic insights into the antimicrobial activity of Kocuria rhizophila PT10
The secondary metabolites analysis for antimicrobial activities was done using a comprehensive resource for the automatic genomic identification (antiSMASH and BAGEL) that permits rapid genome survey of both bacterial and fungal strains (Medema et al. 2011; Poorinmohammad et al. 2019; Weber et al. 2015).
Secondary metabolites and siderophores putatively involved in the antimicrobial activity of PT10 were studied in our previous work using antiSMASH (Guesmi et al. 2021b). Thereby, genome mining of PT10 strain, using those complementary approaches, refined the prediction to 5 BGCs coding for putative siderophores, bacteriocin, NRPS-like, Type 3 polyketide synthase-betalactone (T3pks) and terpene (Table 2 and Fig. S3). A similar analysis was made in Kocuria genomes available in NCBI database. First, the number of secondary metabolites gene clusters is relatively low compared to other actinobacteria such as Streptomyces or Frankia that harbor up to twenty gene clusters (Choudoir et al. 2018; Udwary et al. 2011). For instance, the analysis of the genome of B. subtilis strain 168 revealed the presence of 14 BGCs (Table S1) compared to the 5 BGCs found in PT10 (data not shown). Concerning the Kocuria species analyzed here, K. flava has the highest number of clusters with 8 biosynthetic clusters, followed by K. rhizophila (5‒6) and then K. palustris (2) (Table 2, Table S1).
Interestingly, all BGCs found in K. rhizophila PT10 were also found in DC2201 strain with at least 82% of similarity using core genes (Table 2) underlining that K. rhizophila strain possesses a conserved predicted secondary metabolome. Among the 5 BGCs, only one cluster (Bacteriocin-RiPP-like) is very conserved in this genus, while the 4 other BGCs have a more scattered distribution in the Kocuria species used in this analysis (K. flava and K. palustris). Due to the few genomes available, a Blast research using same proteins made against different genome projects (Table S2) confirmed that the BGC annotated as Bacteriocin-RiPP-like is less present among Kocuria species. Indeed, in addition to K. rhizophila, this cluster is also present in three new Kocuria species when the core proteins of the other BCGs is widespread in 7 to 19 species. This BGC encodes putatively linocin M18, which is an RiPP produced by Brevibacterium linens as published previously (Valdes-Stauber and Scherer 1994; Motta and Brandelli 2002). The linocin M18, purified from supernatant, was shown to be an inhibitor of Gram-positive bacteria, such as Listeria spp, whereas no activity was found against B. subtilis (Valdes-Stauber and Scherer 1994). This suggests that this compound could be also produced by PT10 in its supernatant and could act negatively against S. aureus growth but its purification is required to confirm this result.
Concerning the other BGCs, the second most conserved is a NRPS-like cluster type found also in K. flava genome. The cluster is A-503083 biosynthesis (antibiotic), which is a capuramycin-type nucleoside antibiotic discovered using a screening aimed at identifying inhibitors of bacterial translocase I, an essential enzyme in peptidoglycan cell wall biosynthesis (Fujita et al. 2008). Capuramycin-type nucleoside and their analogs were shown to have antimycobacterial activity (Biecker et al. 2019).
The next conserved BGC is an arylomycin biosynthetic gene close to T3pks and found also in K. flava (Table 2). Arylomycins have a potential use as antibiotics as type I signal peptidase inhibitors (Smith and Romesberg 2012). They were identified from the culture broth of Streptomyces sp. HCCB10043 and were demonstrated to exhibit antibacterial activities against Staphylococcus epidermidis (Jin et al. 2012) and could be produced by PT10 and play an analogous function against S. aureus in our bioassays.
Concerning terpene BGC, there are many close clusters: isorenieratene, hopene, kanamycin, salinomycin or carotenoids biosynthesis. These biologically active compounds have been isolated from several endophytic actinobacteria, with various interesting biological activities, (including potentially antimicrobial activity (Singh and Dubey 2018) and could also code for a bioactive compound in PT10.
The genome analysis of PT10 indicated also the presence of an interesting cluster encoding a siderophore, largely conserved in all Kocuria species analyzed here (Table 2). This cluster contains four genes (desA, desB, deS and desG), belonging to the desferioxamine group, which were identified in Streptomyces genus (Cruz-Morales et al. 2017). The secretion of these low-molecular-weight metabolites by microorganisms allows taking up iron from their environments (Bakker et al. 2003).
The production of siderophores could also be considered as one of the indirect mechanisms for antimicrobial activity thus permitting to suppress diseases through competition for iron with the pathogens (Whipps 2001). For instance, the study of Medema et al. (2011) demonstrated that acinetobactin-like siderophore produced by Acinetobacter calcoaceticus restricted growth of F. oxysporum. Consequently, the production of siderophores could induce systemic resistance of plants and prevent the growth of pathogenic bacteria and fungi in iron deficient conditions (Carson et al. 1994; Storey et al. 2006) and could be produced in the secretome of PT10 to compete with fungi used in our bioassays. Strains affiliated to Kocuria genus are promising sources of novel antimicrobial compounds against many pathogens due to their potential to produce secondary metabolites. Among metabolites, reports on the characterization of antimicrobial peptides (or AMP) extracted from these actinobacterial genera are available. For instance, a novel antimicrobial peptide produced by Kocuria kristinae (Bundale et al. 2019), a kocurin produced by K. palustris and K. rosea and active against Staphylococcus aureus (Martin et al. 2013; Linares-Otoya et al. 2017) were reported. In the same vein, variacin obtained from K. varians was found to act against a large panel of Gram-positive bacteria (Pridmore et al. 1996) and was tested in different chilled dairy food to control Bacillus cereus development (O’Mahony et al. 2001). The biosynthetic genes encoding the kocurin thiopeptide (accession number: WP_058858334.1) or the variacin (accession number: CAA63706.1) are totally absent in PT10 genome eliminating the hypothesis of their implication in our antimicrobial bioassay.
In addition, the comparison of genes in PT10 linked to the synthesis of secondary metabolites molecules with antifungal and antibacterial activities with those of known biocontrol strains, using the MicroScope pipeline of Genoscope, was done and showed the presence of several genes encoding secondary metabolites or proteins with a potential biocontrol or antimicrobial resistance functions (Table 3). As shown in Table 3, we found two biosynthetic genes encoding proteins (VraR and BceA) that are potentially involved in the resistance of antibiotics. In parallel, other genes are potentially involved in the biosynthesis of metabolites with biocontrol functions (moaC, dcsE, dcsD, bacC). The final metabolites of those biosynthesis pathways can be related the antimicrobial and antifungal molecules such as cyclic pyranopterin, bacilysin or cycloserine. Bacilysin has already been reported to play important roles in the pathogen suppression and the induction of systemic resistance (Chen et al. 2018; Teixeira et al. 2020; Zhang et al. 2015). Indeed, the genomic analysis of Bacillus velezensis revealed the presence of several BGCs, which have an important antifungal potential, especially bacilysin (Teixeira et al. 2020) and could suggested the same potential of K. rhizophila PT10. However, the assignation of these genes as cyclic pyranopterin, bacilysin or cycloserine is very hypothetical due to the small score found (Table 3). Finally, regarding all secondary metabolites putatively (Table 2 and Table 3), the Bacteriocin-RiPP-Like cluster remains a good candidate as antifungal compound putatively secreted by PT10 and could be tested by combining different genomic/metabolomics/proteomic approaches (Kloosterman et al. 2021) and biological bioassays.
In perspective, the genome sequencing of more Kocuria species coupled to metabolome/proteome investigations will permit us to define more precisely their specific genetic potentials. This would lead us to propose potential in vitro and in vivo bioassays before agro-industrial applications.
Hydrolytic enzyme assays and genome assessment
The screening of extracellular hydrolytic enzymes (amylase, cellulose, chitinase, lipase, pectinase and protease) production of K. rhizophila PT10 was assessed by in silico analysis. The identification of bacterial antagonism was also performed by measuring the inhibition zones of mycelial radial growth in plate assays. The results of enzymatic production ability by in silico and in vitro analyses of PT10 are presented in Table 4.
The screening of hydrolytic enzyme activities of strain PT10 showed negative results for cellulose and pectinase production. These results were confirmed by in silico analyses which detected no putative genes associated with both enzymes.
For chitinase production, the absence of activity based on in vitro assay was not confirmed by the genome analysis, which detected the presence of GH23 family (KOCU_v1_10268, KOCU_v1_110006) genes as well as GH18 and GH19 family genes (Oyeleye and Normi 2018). In contrast, a unique bacterial GH23 chitinase gene was identified in Ralstonia sp. (Ueda et al. 2009) and structurally characterized (Arimori et al. 2013). Here, the GH23 gene affiliated to chitinase presents two catalytic domains related to lysozyme (LysZ) and peptidoglycan- binding (LysM), which were described as dormant resuscitation-promoting factors (Rpf) (Mukamolova et al. 1998). The second one (KOCU_v1_110006) is a lytic murein transglycosylase, which would be able to cleave the β-1,4 glycosidic bond between N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) residues of peptidoglycan (PG), suggesting its possible implication in PG biosynthesis and turnover. Thus, the antifungal activity observed in PT10 is most likely based on secondary metabolites production rather than on chitinase secretion.
Concerning proteolytic action, strain PT10 presented an efficient activity by inducing clear zones around the cells in skim milk agar medium (Fig. S4). Protease production was confirmed by the presence of 8 putative genes in the genome of PT10, which are CAAX (2), ClpS (1), ClpP (2), Htpx (1), FtsH (1) and HtrA (1). Linked to its capacity to grow under extreme conditions, K. varians was found to produce a thermostable alkaline protease active at high temperature (80 °C) and pH (pH > 11) (Nwagu 2014) or PT10 to resist to γ-radiation (Guesmi et al. 2021b) which are promising properties for various applications in biotechnology.
In addition, strain PT10 showed positive amylase activity by inducing clear zones around the cells in starch agar medium. In silico analysis revealed the presence of 7 putative genes attributed to the production of amylase enzyme, which belong to glycoside hydrolase family GH13. The glycogen debranching enzyme GlgX (KOCU_v1_70005) was detected, which represents a large group of enzymes that hydrolyze the glycosidic bonds between two or more carbohydrates. This bacterial glycogen synthesis and degradation gene cluster comprise amylase-type debranching enzymes with high specificity for hydrolysis of chains consisting of glucose residues (Dauvillee et al. 2005). The mechanism of glycogen breakdown can play an interesting role in the interactions between the host and pathogen (Wilson et al. 2010). This actinobacterial strain was also found to be a potent producer of lipase, which was confirmed by the presence of 4 putative genes attributed to glycoside hydrolase family (Table 4).
Overall, these results revealed that the actinobacterium strain PT10 is a potent producer of hydrolytic enzymes (amylase, chitinase, lipase and protease), which are potentially associated with its ability to inhibit pathogens growth. In addition, the capacity of hydrolytic enzymes production was attributed to the presence of putative genes belonging to the glycoside hydrolase GH family present in the genome of PT10. GH proteins play an important role in the hydrolysis of the prey’s fungal cell wall with a potential to render microorganisms (bacteria or fungi) efficient biological control agents (Tzelepis et al. 2015).
The genome of strain PT10 is in permanent draft stage; it allowed us to detect genes, which code for hydrolytic enzymes as detected using CAZY database and BLAST similarity with hydrolytic enzymes found in other genomes. The screening of hydrolytic enzymes production by in silico and in vitro analyses revealed the PT10 potential to produce different extracellular hydrolytic enzymes, such as protease, amylase, lipase and chitinase (Table 4). The efficient production of hydrolytic enzymes was confirmed by the presence of several putative genes in the genome of PT10. In addition, the antimicrobial activities of PT10 may be associated with its capacity to the production of hydrolytic enzymes belonging to glycoside hydrolase proteins (GH), such as amylase and protease. However, their functions as antimicrobial compounds are few documented, rather, these enzymes represent the largest groups of industrial enzymes (Kirk et al. 2002), which are extensively exploited commercially, in food, pharmaceutical and detergent industries.
Actinobacteria strains have a great capacity for the degradation of different and complex substrates present in their natural habitats (McCarthy and Williams 1992; Tuomela et al. 2000) indicating the variety of their complex metabolites and genomic organization (Bentley et al. 2002; Narayana et al. 2007). These antagonistic strains also showed production of fungal cell wall degrading enzymes, such as proteases, which are known to be involved in antagonistic activity against phytopathogenic fungi and insects (Dunne et al. 1997; Naik et al. 2008). Enzymatic capacities from the identified rare actinobacteria highlight their potential for the production of various hydrolytic enzymes with a promising prospect for various industrial applications.
Actinobacterial strains associated with roots of xerophytes from arid regions represent efficient sources for various putatively products (such as secondary metabolites, siderophores and hydrolytic enzymes) with the opportunities to develop suitable and green applications in agriculture (Guesmi et al. 2021a). These biomolecules have potentialities to promote plant growth; ward off phytopathogens; and help maintain crop productivity under extreme conditions: abiotic stress, salinity, and desiccation (Singh and Dubey 2018).
Conclusion
In the present investigation, the biotechnological potentials of K. rhizophila PT10, was assessed based on in silico and in vitro analyses. This radioresistant actinobacterium isolated from roots of P. turgidum can effectively inhibit pathogens including plant pathogens. The in vitro approach revealed that PT10 played interesting antimicrobial activities against both bacterial (A. baumannii PT6 and S. pasteuri PT2) and fungi pathogens (F. graminearum g1 and B. cinerea BC21). These results were supported by in silico analyses of secondary metabolites and gene clusters identification mainly based on antiSMASH and BAGEL resources. Five biosynthetic gene clusters were detected in the genome of PT10, which were identified as terpene, siderophore, peptide (linocin M18) and PKS antibiotic (arylomycin) coding, respectively. Strain PT10 had also a great potential to the production of hydrolytic enzymes (protease, amylase, chitinase, lipase), which are implicated in the inhibition of growth of pathogens. Several genes attributed to the presence of glycoside hydrolase proteins were detected in its genome. K. rhizophila PT10 has the potential to use several mechanisms to biocontrol pathogens, such as the production of antimicrobial bioactive molecules and hydrolytic enzymes. Even thought, the safety of this species is not documented and requires further investigation; these mechanisms make plausible the applications of this actinobacterium isolated from roots of a xerophyte as plant growth promoting rhizobacteria and biofertilizers in sustainable agriculture ecosystems through hydrolysis and assimilation of carbohydrates. Thus, the advancement in the genomic science and technology through genome sequencing and bioinformatic analysis can be expected to offer rapid means to identify potentially useful biocontrol agents.
Data availability
The whole-genome shotgun sequence of K. rhizophila PT10 is accessible at GenBank/ENA under accession number PRJEB29453. Strain PT10 is available from two collections, the BCCM and the DSMZ under the deposit numbers LMG 31102 and DSM 108617, respectively.
Abbreviations
- AMP:
-
Antimicrobial peptides
- antiSMASH:
-
Antibiotics and secondary metabolite analysis shell
- BCAs:
-
Biological control agents
- BGCs:
-
Biosynthetic gene clusters
- OD:
-
Optic density
- TSA:
-
Tryptic soy agar
- TSB:
-
Tryptic soy broth
- PDA:
-
Potato dextrose agar
References
AFDB (2019) L'agriculture africaine. In: A. D. B. Group (ed). https://am.afdb.org/fr. 1 pp
Agrios GN (2005) Plant pathology, 5th edn. Academic Press, New York
Agrios GN (2009) Plant pathogens and disease: general introduction. Elsevier, pp 613–646
Arimori T, Kawamoto N, Shinya S, Okazaki N, Nakazawa M, Miyatake K, Fukamizo T, Ueda M, Tamada T (2013) Crystal structures of the catalytic domain of a novel glycohydrolase family 23 chitinase from Ralstonia sp A-471 reveals a unique arrangement of the catalytic residues for inverting chitin hydrolysis. J Biol Chem 288(26):18696–18706. https://doi.org/10.1074/jbc.M113.462135
Bakker PAHM, Ran LX, Pieterse CMJ, van Loon LC (2003) Understanding the involvement of rhizobacteria-mediated induction of systemic resistance in biocontrol of plant diseases. Can J Plant Path 25(1):5–9. https://doi.org/10.1080/07060660309507043
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor a3(2). Nature 417(6885):141–147. https://doi.org/10.1038/417141a
Biecker AL, Liu X, Thorson JS, Yang Z, Van Lanen SG (2019) Biosynthetic and Synthetic Strategies for Assembling Capuramycin-Type Antituberculosis Antibiotics. Molecules. 24:433. https://doi.org/10.3390/molecules24030433
Blin K, Shaw S, Steinke K, Villebro R, Ziemert N, Lee SY, Medema MH, Weber T (2019) Antismash 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. https://doi.org/10.1093/nar/gkz310
Bundale S, Singh J, Begde D, Nashikkar N, Upadhyay A (2019) Rare actinobacteria: a potential source of bioactive polyketides and peptides. World J Microbiol Biotechnol 35(6):92. https://doi.org/10.1007/s11274-019-2668-z
Carmona-Hernandez S, Reyes-Pérez J, Chiquito-Contreras R, Rincon-Enriquez G, Cerdán CR, Hernandez-Montiel LG (2019) Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: a review. Agronomy 9(3):121. https://doi.org/10.3390/agronomy9030121
Carson K, Glenn AR, Dilworth MJ (1994) Specificity of siderophore-mediated transport of iron in rhizobia. Arch Microbiol 161(4):333–339
Chantarasiri A (2014) Novel halotolerant cellulolytic Bacillus methylotrophicus ryc01101 isolated from ruminant feces in thailand and its application for bioethanol production. KMUTNB Int J Appl Sci Technol 7:63–68. https://doi.org/10.14416/j.ijast.2014.07.001
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Sussmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens fzb42. Nat Biotechnol 25(9):1007–1014. https://doi.org/10.1038/nbt1325
Chen L, Heng J, Qin S, Bian K (2018) A comprehensive understanding of the biocontrol potential of Bacillus velezensis lm2303 against Fusarium head blight. PLoS ONE 13(6):e0198560. https://doi.org/10.1371/journal.pone.0198560
Choudoir MJ, Pepe-Ranney C, Buckley DH (2018) Diversification of secondary metabolite biosynthetic gene clusters coincides with lineage divergence in Streptomyces. Antibiotics (basel, Switzerland) 7(1):15. https://doi.org/10.3390/antibiotics7010012
Cruz-Morales P, Ramos-Aboites HE, Licona-Cassani C, Selem-Mojica N, Mejia-Ponce PM, Souza-Saldivar V, Barona-Gomez F (2017) Actinobacteria phylogenomics, selective isolation from an iron oligotrophic environment and siderophore functional characterization, unveil new desferrioxamine traits. FEMS Microbiol Ecol 93(9):12. https://doi.org/10.1093/femsec/fix086
Dauvillee D, Kinderf IS, Li Z, Kosar-Hashemi B, Samuel MS, Rampling L, Ball S, Morell MK (2005) Role of the Escherichia coli glgx gene in glycogen metabolism. J Bacteriol 187(4):1465–1473. https://doi.org/10.1128/jb.187.4.1465-1473.2005
Devi SC, Kumari A, Nitin J, Jemimah N, Mohanasrinivasan V (2013) Screening of actinomycetes isolated from soil samples for antibacterial and antioxidant activity. Int J Pharm Pharm Sci 5(4):483–489
Dukare AS, Paul S, Nambi VE, Gupta RK, Singh R, Sharma K, Vishwakarma RK (2019) Exploitation of microbial antagonists for the control of postharvest diseases of fruits: a review. Crit Rev Food Sci Nutr 59(9):1498–1513. https://doi.org/10.1080/10408398.2017.1417235
Dunne C, Crowley JJ, Moënne-Loccoz Y, Dowling DN, de Bruijn FJ, O’Gara F (1997) Biological control of pythium ultimum by Stenotrophomonas maltophilia w81 is mediated by an extracellular proteolytic activity. Microbiology (reading, England) 143(12):3921–3931
Francis I, Holsters M, Vereecke D (2010) The gram-positive side of plant-microbe interactions. Environ Microbiol 12(1):1–12. https://doi.org/10.1111/j.1462-2920.2009.01989.x
Fujita Y, Murakami R, Muramatsu Y, Miyakoshi S, Takatsu T (2008) A-94964, novel inhibitor of bacterial translocase i, produced by Streptomyces sp. Sank 60404. J Antibiot 61(9):545–549. https://doi.org/10.1038/ja.2008.72
Ganesan P, Reegan AD, David RHA, Gandhi MR, Paulraj MG, Al-Dhabi NA, Ignacimuthu S (2017) Antimicrobial activity of some actinomycetes from western ghats of Tamil Nadu, India. Alex J Med 53(2):101–110. https://doi.org/10.1016/j.ajme.2016.03.004
Gasmi M, Kitouni M, Carro L, Pujic P, Normand P, Boubakri H (2019) Chitinolytic actinobacteria isolated from an algerian semi-arid soil: development of an antifungal chitinase-dependent assay and gh18 chitinase gene identification. Ann Microbiol 69(4):395–405. https://doi.org/10.1007/s13213-018-1426-z
Guesmi S, Najjari A, Pujic P, Ghedira K, Ouertani R, Jabberi M, Cherif A, Armengaud J, Normand P, Sghaier H (2021a) Roots of the xerophyte Panicum turgidum host a cohort of ionizing-radiation-resistant biotechnologically-valuable bacteria. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2021.09.020
Guesmi S, Pujic P, Nouioui I, Dubost A, Najjari A, Ghedira K, Igual JM, Miotello G, Cherif A, Armengaud J, Klenk H, Normand P, Sghaier H (2021b) Ionizing-radiation-resistant Kocuria rhizophila PT10 isolated from the tunisian sahara xerophyte Panicum turgidum: polyphasic characterization and proteogenomic arsenal. Genomics 113(1, Part 1):317–330. https://doi.org/10.1016/j.ygeno.2020.11.029
Haba E, Bresco O, Ferrer C, Marques A, Busquets M, Manresa A (2000) Isolation of lipase-secreting bacteria by deploying used frying oil as selective substrate. Enzyme Microb Technol 26(1):40–44. https://doi.org/10.1016/S0141-0229(99)00125-8
Jin X, Rao M, Wei W, Ge M, Liu J, Chen D, Liang Y (2012) Biosynthesis of new lipopentapeptides by an engineered strain of Streptomyces sp. Biotechnol Lett 34(12):2283–2289. https://doi.org/10.1007/s10529-012-1032-2
Kaewkla O, Franco CMM (2019) Actinomycetospora callitridis sp. Nov., an endophytic actinobacterium isolated from the surface-sterilised root of an australian native pine tree. Antonie Van Leeuwenhoek 112(3):331–337. https://doi.org/10.1007/s10482-018-1162-1
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr Microbiol 57(5):503–507. https://doi.org/10.1007/s00284-008-9276-8
Keikha N, Ayatollahi Mousavi SA, Nakhaei AR, Yadegari MH, Shahidi Bonjar GH, Amiri S (2015) In vitro evaluation of enzymatic and antifungal activities of soil-actinomycetes isolates and their molecular identification by pcr. Jundishapur J Microbiol 8(5):e14874. https://doi.org/10.5812/jjm.8(5)2015.14874
Khan N, Martínez-Hidalgo P, Ice TA, Maymon M, Humm EA, Nejat N, Sanders ER, Kaplan D, Hirsch AM (2018) Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol 9:2363. https://doi.org/10.3389/fmicb.2018.02363
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13(4):345–351
Kloosterman AM, Medema MH, van Wezel GP (2021) Omics-based strategies to discover novel classes of RiPP natural products. Curr Opin Biotechnol 69:60–67
Kumar RR, Jadeja VJ (2018) Characterization and partial purification of an antibacterial agent from halophilic actinomycetes Kocuria sp. Strain rsk4. Bioimpacts 8(4):253–261. https://doi.org/10.15171/bi.2018.28
Linares-Otoya L, Linares-Otoya V, Armas-Mantilla L, Blanco-Olano C, Crüsemann M, Ganoza-Yupanqui ML, Campos-Florian J, König GM, Schäberle TF (2017) Identification and heterologous expression of the kocurin biosynthetic gene cluster. Microbiology (reading) 163(10):1409–1414. https://doi.org/10.1099/mic.0.000538
Martin J, da Sousa ST, Crespo G, Palomo S, Gonzalez I, Tormo JR, de la Cruz M, Anderson M, Hill RT, Vicente F, Genilloud O, Reyes F (2013) Kocurin, the true structure of pm181104, an anti-methicillin-resistant Staphylococcus aureus (mrsa) thiazolyl peptide from the marine-derived bacterium Kocuria palustris. Mar Drugs 11(2):387–398. https://doi.org/10.3390/md11020387
Martinez AJ (2012) Natural fungicides obtained from plants. In: Thajuddin N, Panneerselvam A, Dhanasekaran D (eds) Fungicides for plant and animal diseases. IntechOpen
McCarthy AJ, Williams ST (1992) Actinomycetes as agents of biodegradation in the environment. Gene 115(1–2):189–192
Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R (2011) Antismash: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39(Web Server issue):W339-346. https://doi.org/10.1093/nar/gkr466
Mohammadipanah F, Wink J (2015) Actinobacteria from arid and desert habitats: diversity and biological activity. Front Microbiol 6:1541. https://doi.org/10.3389/fmicb.2015.01541
Motta AS, Brandelli A (2002) Characterization of an antibacterial peptide produced by Brevibacterium linens. J Appl Microbiol 92(1):63–70. https://doi.org/10.1046/j.1365-2672.2002.01490.x
Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB (1998) A bacterial cytokine. Proc Natl Acad Sci USA 95(15):8916–8921. https://doi.org/10.1073/pnas.95.15.8916
Murthy N, Bleakley B (2012) Simplified method of preparing colloidal chitin used for screening of chitinase- producing microorganisms. Internet J Microbiol. https://doi.org/10.5580/2e93
Naik PR, Raman G, Narayanan KB, Sakthivel N (2008) Assessment of genetic and functional diversity of phosphate solubilizing fluorescent Pseudomonads isolated from rhizospheric soil. BMC Microbiol 8(1):230. https://doi.org/10.1186/1471-2180-8-230
Namasivayam E, JohnRavindar D, Mariappan K, Jiji AC, Kumar MA, Jayaraj RL (2011) Production of extracellular pectinase by Bacillus cereus isolated from market solid waste. J Bioanal Biomed 3:070–075
Narayana KJ, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y, Krishna PS (2007) Biological activity of phenylpropionic acid isolated from a terrestrial streptomycetes. Pol J Microbiol 56(3):191–197
Nega A (2014) Review on concepts in biological control of plant pathogens. J Biol Agric Healthc 4(27):33–54
Nwagu TN (2014) Production of a thermostable alkaline protease from alkalophilic Kocuria varians grown on various agricultural wastes. Acta Aliment 44(3):317–325. https://doi.org/10.1556/AAlim.2014.0008
O’Mahony T, Rekhif N, Cavadini C, Fitzgerald GF (2001) The application of a fermented food ingredient containing ‘variacin’, a novel antimicrobial produced by Kocuria varians, to control the growth of Bacillus cereus in chilled dairy products. J Appl Microbiol 90(1):106–111
Oyeleye A, Normi YM (2018) Chitinase: diversity, limitations, and trends in engineering for suitable applications. Biosci Rep 38(4):21. https://doi.org/10.1042/BSR20180323
Palaniyandi SA, Yang SH, Zhang L, Suh JW (2013) Effects of actinobacteria on plant disease suppression and growth promotion. Appl Microbiol Biotechnol 97(22):9621–9636. https://doi.org/10.1007/s00253-013-5206-1
Palomo S, González I, de la Cruz M, Martín J, Tormo JR, Anderson M, Hill RT, Vicente F, Reyes F, Genilloud O (2013) Sponge-derived Kocuria and Micrococcus spp. As sources of the new thiazolyl peptide antibiotic kocurin. Mar Drugs 11(4):1071–1086. https://doi.org/10.3390/md11041071
Poorinmohammad N, Bagheban-Shemirani R, Hamedi J (2019) Genome mining for ribosomally synthesised and post-translationally modified peptides (ripps) reveals undiscovered bioactive potentials of actinobacteria. Antonie Van Leeuwenhoek. https://doi.org/10.1007/s10482-019-01276-6
Pridmore D, Rekhif N, Pittet AC, Suri B, Mollet B (1996) Variacin, a new lanthionine-containing bacteriocin produced by Micrococcus varians: Comparison to lacticin 481 of Lactococcus lactis. Appl Environ Microbiol 62(5):1799–1802
Rutledge PJ, Challis GL (2015) Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat Rev Microbiol 13(8):509–523. https://doi.org/10.1038/nrmicro3496
Sharma R, Singh D, Singh R (2009) Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: a review. Biol Control 50(3):205–221. https://doi.org/10.1016/j.biocontrol.2009.05.001
Shivlata L, Tulasi S (2015) Thermophilic and alkaliphilic actinobacteria: biology and potential applications. Front Microbiol 6(1014):29. https://doi.org/10.3389/fmicb.2015.01014
Shuping DSS, Eloff JN (2017) The use of plants to protect plants and food against fungal pathogens: a review. Afr J Tradit Complement Altern Med 14(4):120–127. https://doi.org/10.21010/ajtcam.v14i4.14
Singh R, Dubey AK (2018) Diversity and applications of endophytic actinobacteria of plants in special and other ecological niches. Front Microbiol 9:1767. https://doi.org/10.3389/fmicb.2018.01767
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: GerhardtR P, MurrayW GE, Wood A, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, pp 607–654
Smith PA, Romesberg FE (2012) Mechanism of action of the arylomycin antibiotics and effects of signal peptidase I inhibition. Antimicrob Agents Chemother 56(10):5054–5060. https://doi.org/10.1128/aac.00785-12
Storey EP, Boghozian R, Little JL, Lowman DW, Chakraborty R (2006) Characterization of “schizokinen”; a dihydroxamate-type siderophore produced by Rhizobium leguminosarum iari 917. Biometals 19(6):637–649. https://doi.org/10.1007/s10534-006-9001-7
Taechowisan T, Lu C, Shen Y, Lumyong S (2005) Secondary metabolites from endophytic Streptomyces aureofaciens cmuac130 and their antifungal activity. Microbiology (reading, England) 151(Pt 5):1691–1695. https://doi.org/10.1099/mic.0.27758-0
Teather RM, Wood PJ (1982) Use of congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43(4):777–780
Teixeira GM, Mosela M, Nicoletto MLA, Ribeiro RA, Hungria M, Youssef K, Higashi AY, Mian S, Ferreira AS, Gonçalves LSA, Pereira UP, de Oliveira AG (2020) Genomic insights into the antifungal activity and plant growth-promoting ability in Bacillus velezensis cmrp 4490. Front Microbiol 11:618415. https://doi.org/10.3389/fmicb.2020.618415
Trejo-Estrada R, Paszczynski A, Crawford DL (1998) Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger yced-9. J Ind Microbiol Biotechnol 21(1–2):81–90. https://doi.org/10.1038/sj.jim.2900549
Tuomela M, Vikman M, Hatakka A, Itävaara M (2000) Biodegradation of lignin in a compost environment: a review. Biores Technol 72(2):169–183. https://doi.org/10.1016/S0960-8524(99)00104-2
Tzelepis G, Dubey M, Jensen DF, Karlsson M (2015) Identifying glycoside hydrolase family 18 genes in the mycoparasitic fungal species Clonostachys rosea. Microbiology (reading, England) 161(7):1407–1419. https://doi.org/10.1099/mic.0.000096
Udwary DW, Gontang EA, Jones AC, Jones CS, Schultz AW, Winter JM, Yang JY, Beauchemin N, Capson TL, Clark BR, Esquenazi E, Eustaquio AS, Freel K, Gerwick L, Gerwick WH, Gonzalez D, Liu WT, Malloy KL, Maloney KN, Nett M, Nunnery JK, Penn K, Prieto-Davo A, Simmons TL, Weitz S, Wilson MC, Tisa LS, Dorrestein PC, Moore BS (2011) Significant natural product biosynthetic potential of actinorhizal symbionts of the genus Frankia, as revealed by comparative genomic and proteomic analyses. Appl Environ Microbiol 77(11):3617–3625. https://doi.org/10.1128/aem.00038-11
Ueda M, Ohata K, Konishi T, Sutrisno A, Okada H, Nakazawa M, Miyatake K (2009) A novel goose-type lysozyme gene with chitinolytic activity from the moderately thermophilic bacterium Ralstonia sp A-471: cloning, sequencing, and expression. Appl Microbiol Biotechnol 81(6):1077–1085. https://doi.org/10.1007/s00253-008-1676-y
Uzair B, Menaa F, Khan BA, Mohammad FV, Ahmad VU, Djeribi R, Menaa B (2018) Isolation, purification, structural elucidation and antimicrobial activities of kocumarin, a novel antibiotic isolated from actinobacterium Kocuria marina cmg s2 associated with the brown seaweed Pelvetia canaliculata. Microbiol Res 206:186–197. https://doi.org/10.1016/j.micres.2017.10.007
Valdes-Stauber N, Scherer S (1994) Isolation and characterization of linocin m18, a bacteriocin produced by Brevibacterium linens. Appl Environ Microbiol 60(10):3809–3814
Valencia-Cantero E, Hernàndez-Calderón E, Velázquez-Becerra C, López-Meza J, Alfaro-Cuevas R, López-Bucio J (2007) Role of dissimilatory fermentative iron-reducing bacteria in Fe uptake by common bean (Phaseolus vulgaris L.) plants grown in alkaline soil. Plant Soil 291(1–2):263–273. https://doi.org/10.1007/s11104-007-9191-y
Vallenet D, Labarre L, Rouy Z, Barbe V, Bocs S, Cruveiller S, Lajus A, Pascal G, Scarpelli C, Médigue C (2006) Mage: a microbial genome annotation system supported by synteny results. Nucleic Acids Res 34(1):53–65. https://doi.org/10.1093/nar/gkj406
Wang C, Wang Z, Qiao X, Li Z, Li F, Chen M, Wang Y, Huang Y, Cui H (2013) Antifungal activity of volatile organic compounds from Streptomyces alboflavus td-1. FEMS Microbiol Lett 341(1):45–51. https://doi.org/10.1111/1574-6968.12088
Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, Lee SY, Fischbach MA, Muller R, Wohlleben W, Breitling R, Takano E, Medema MH (2015) Antismash 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res 43(W1):W237-243. https://doi.org/10.1093/nar/gkv437
Wilson WA, Roach PJ, Montero M, Baroja-Fernández E, Muñoz FJ, Eydallin G, Viale AM, Pozueta-Romero J (2010) Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol Rev 34(6):952–985. https://doi.org/10.1111/j.1574-6976.2010.00220.x
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52(suppl_1):487–511. https://doi.org/10.1093/jexbot/52.suppl_1.487
Wu C-Y, Chen N, Li H, Li Q-F (2014) Kocuria rosea hn01, a newly alkaliphilic humus-reducing bacterium isolated from cassava dreg compost. J Soils Sediments 14(2):423–431. https://doi.org/10.1007/s11368-013-0679-1
Zhang N, Yang D, Wang D, Miao Y, Shao J, Zhou X, Xu Z, Li Q, Feng H, Li S, Shen Q, Zhang R (2015) Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens sqr9 during enhanced biofilm formation regulated by maize root exudates. BMC Genomics 16(1):685. https://doi.org/10.1186/s12864-015-1825-5
Zhao Z, Wang Q, Wang K, Brian K, Liu C, Gu Y (2010) Study of the antifungal activity of Bacillus vallismortis zz185 in vitro and identification of its antifungal components. Bioresour Technol 101(1):292–297. https://doi.org/10.1016/j.biortech.2009.07.071
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no confict of interest regarding the publication of this article.
Additional information
Editorial responsibility: Chenxi Li.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guesmi, S., Mahjoubi, M., Pujic, P. et al. Biotechnological potential of Kocuria rhizophila PT10 isolated from roots of Panicum turgidum. Int. J. Environ. Sci. Technol. 19, 10105–10118 (2022). https://doi.org/10.1007/s13762-021-03824-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03824-y