Abstract
Five hundred strains of rhizobacteria were isolated from the rhizosphere of the Central Highlands of Vietnam, where black pepper is cultivated. Of these, seven potent rhizobacteria were evaluated for anti-Phytophthora activity and 16S rRNA gene sequencing and phylogenic analysis classified. Evaluation of their antifungal activity was performed both in vitro and in vivo. The results showed that almost all potent rhizobacteria possessed anti-Phytophthora activity. The rhizobacteria strains displayed over 60% inhibition of Phytophthora during the in vitro test, and six rhizobacteria inhibited Phytophthora by 77.50–98.75% during the in vivo test. Enzymatic activities were measured to determine the antifungal mechanisms; these were identified as protease, chitinase, and β-glucanase. The effects of the rhizobacteria on plant growth and antifungal activity were also investigated. Under greenhouse conditions, black pepper seedlings treated with rhizobacteria were stronger and had lower rates of disease and fatality compared to the control group. The results from the in vitro test also showed that the anti-Phytophthora activity of the rhizobacteria was not dependent on enzyme activity, but rather on their chemical compounds. GC–MS and LC–MS profiles of the culture broth from the promising rhizobacteria strain RBDS.29 revealed seven potent antifungal compounds. The data suggest that Bacillus velezensis RB.DS29 is a promising rhizobacterium that promotes plant growth and the biocontrol of black pepper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vietnam is the biggest producer and exporter of black pepper in the world. The area of cultivation is nearly 100,000 ha, with an annual production of 246,000 t, contributing up to 40% of the world’s black pepper production (http://www.fao.org/faostat/en/#data/QC). Although black pepper production in Vietnam has expanded rapidly, it seems to be unsustainable due to its high use of chemical fertilizers and fungicides to control the disease. Wilt diseases, caused by Phytophthora capsici, Fusarium spp., and nematodes, are a serious problem in black pepper production. The diseases have spread quickly in Vietnam and the rest of the world. In 2016, over 10,000 ha of the black pepper crop was infected and lost. Clearly, chemical pesticides and fungicides are unable to control wilt or root-knot diseases in Vietnam.
Diverse soil microorganisms, especially rhizobacteria and endobacteria, play a very important role in plant growth and protection against disease and pests [1,2,3,4]. Rhizobacteria are rhizosphere-competent bacteria able to multiply and colonize plant roots at all stages of growth. They can enhance nutrition uptake and be used in the biocontrol of crops [2, 5]. Almost all rhizosphere and endophytic bacteria possess nitrogen-fixing abilities, phosphorus solubility, and IAA-producing activity [2, 6, 7]. In recent years, the use of rhizobacteria to promote plant growth has received much attention. Rhizobacteria may not only enhance crop yield through increased nutrient uptake and regulation of plant growth, but also reduce the use of chemical fungicides. In addition, rhizobacteria can help plants adapt to climate change and improve stress tolerance to drought, hot weather or the heavy rainfall common in tropical regions [8,9,10], all of which are ongoing agricultural challenges in the Central Highlands of Vietnam. As such, research into the application of rhizobacteria seems the best method for the sustainable production of black pepper and other crops in the Central Highlands of Vietnam.
The purpose of this study is to evaluate anti-Phytophthora activity in both in vitro and in vivo tests to select the most promising rhizobacteria for further investigation and application.
Experimental procedure
Materials
Rhizobacteria strains were isolated from the rhizosphere of black pepper (Piper nigrum L.) in the Central Highlands of Vietnam, following the methods of White et al. [11]. Among the 500 isolated strains, seven potent strains consistently showed strong anti-Phytophthora effects: RB.CS1, RB.CP15, RB.DS29, RB.EK2, RB.EK4, RB.BH15, and RB.CJ35. These strains were grown on TSA medium at 30 °C for 48 h and then stored in frozen glycerol at − 80 °C. The pathogen Phytophthora was a fungal strain from the collection of the Institute of Biotechnology and Environment at Tay Nguyen University in Vietnam; it was grown on PGA medium at 30 °C.
Methodology
PCR amplification, sequencing, and phylogenetic analysis of the 16S rRNA gene
Genomic DNA from an overnight culture of each strain was extracted by the Qiagen method [12]. The genomic DNA was used as a template for amplification by PCR. A nearly full-length segment of 16S rRNA gene nucleotides was amplified in a 100-μL reaction tube using the universal primers 27f (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492r (5′-TACGGYTACCTTGTTACGACTT-3′). The 16S rRNA gene was amplified by iCycler thermal cycler (Bio-Rad, USA) using the following schedule: 94 °C for 5 min, repeated by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 2 min. The amplified products were then separated by electrophoresis on agarose gel (1.5%, w/v). The target bands in the agarose gel were cut out and purified using a QIA quick PCR purification (Promega Co., USA). Sequencing reactions were carried out in a CEQ 8000 Genetic Analysis System (Beckman Coulter Inc., USA) using a CEQ Dye Terminator Cycle Sequencing Kit (Beckman Coulter Inc., USA).
The nucleotide sequences (from 1300 to 1440 bps) of the 16S rRNA genes were compared with known sequences in the DDBJ/GenBank/EMBL databases using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the taxonomic positions of the rhizobacteria isolates. A phylogenetic tree was made using MEGA version 6.0 software after multiple alignments of data by CLUSTAL W [13, 14].
Chitinase activity of the rhizobacteria
The rhizobacteria were grown in LB medium supplemented with 0.1% colloidal chitin for 5 days at 30 °C and a shaking speed of 150 rpm. Cells were separated by centrifugation at 6000 × g and 4 °C for 5 min. The supernatant was dialyzed overnight at 4 °C using 20 mM sodium phosphate buffer (pH 6.0). The dialyzed protein solution was used to measure chitinase activity. The chitinase activity assay was conducted in a 600 μL reaction mixture containing 0.1% colloidal chitin as the substrate and an appropriate volume of crude enzyme in 20 mM sodium phosphate buffer (pH 6.0). The reaction mixture was incubated at 37 °C for 15 min. Chitinase activity was determined as per Imoto’s method [15]. One unit of chitinase activity was defined as the amount of enzyme needed to release 1 μmol of reducing sugar per min.
β-Glucanase activity of the rhizobacteria
Rhizobacteria were cultivated in LB medium containing 0.1% β-glucan for 5 days at 30 °C and a shaking speed of 150 rpm. The supernatant was prepared as per the procedure described above for chitinase. The reaction mixture contained 250 µL 1% β-glucan (laminarin) in 50 mM sodium acetate (pH 5) and 125 µL of crude enzyme. The mixture was incubated for 30 min at 37 °C. The amount of reducing sugar released was measured with DNS reagent at 540 nm (UV–Vis, Jasco V630, Japan), as per Miller’s methods [16].
Protease activity of the rhizobacteria
Rhizobacteria were grown in LB medium for 5 days at 30 °C and a shaking speed of 150 rpm. The supernatant was prepared as per the procedures described above for chitinase and glucanase activity. The reaction mixture, containing 5 mL of 1% casein and 1 mL of the crude enzyme, was kept at 35.5 °C for 10 min. The reaction was stopped by the addition of 10 mL of 5% trichloroacetic acid. After filtration, 3 mL Folin–Ciocalteu reagent was added to the solution. It was then kept for 10 min at room temperature before being measured at 660 nm by UV Vis (Jasco V630, Japan) following Anson’s methods [17].
In vitro Phytophthora antagonism by rhizobacteria
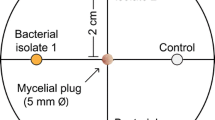
Seven potent rhizobacteria were evaluated for their ability to inhibit Phytophthora on agar plates [12]. A mycelial plug of growing Phytophthora was placed in the center of the PGA medium, and the rhizobacteria were streaked 2 cm on either side of it. The plates were then incubated at 28 °C for 5 days or until the leading edge of Phytophthora in the control reached the edge of the plate. The radial growth of fungal mycelium was measured, and the percentage of growth inhibition was calculated as follows:
where R1 is the diameter of the fungus mycelium grown on the control disk (cm) and R2 is the diameter of the fungus mycelium grown on the treated rhizobacteria disk (cm).
In vivo Phytophthora antagonism by rhizobacteria
In vivo antagonism tests of the rhizobacteria were conducted as per Dinu’s methods [18]. In brief, black pepper shoots (about 8 cm in length with at least one node) were excised from healthy black pepper vines (Vinh Linh local variety) and washed thoroughly with tap water before surface sterilization with 0.1% sodium hypochlorite for 10 min. The shoots were rinsed five times with sterile distilled water and then dried on sterile paper. The samples were immersed in the rhizobacteria suspension (107 CFU mL−1) for 60 min and then spread on sterile paper to remove excess moisture. The treated shoots were inoculated with Phytophthora and then kept in a plastic tray and incubated at 30 °C for 3 days in the dark. The length of the dark lesions that developed along the inoculated spots on the shoots was measured after 96 h. In the control group, the shoots were not inoculated with Phytophthora.
where D1 is the length of shoots not inoculated with fungus (cm) and D2 is the length of shoots inoculated with fungus (cm).
Evaluation of Phytophthora antagonism in the greenhouse by rhizobacteria
Black pepper seedlings, a Vinh Linh local variety with five leaves, were used for testing in the greenhouse. Rhizobacteria were cultivated in LB (composition L−1: 10 g tryptone, 5 g yeast extract, and 10 g NaCl) for 72 h at 25 °C with a shaking speed of 150 rpm. The bacteria culture was adjusted to 107CFU ml−1 by optical density.
Phytophthora was grown on potato dextrose medium for 3 days at 28 °C and a shaking speed of 150 rpm; spore density was adjusted to 107 spores mL−1. Evaluation of Phytophthora antagonism by the rhizobacteria was conducted in the greenhouse. The test had 9 plots, with 15 seedlings per plot, designed as a random completed block design (RCBD), as per Table 1.
All plots except control groups 1 and 2 were treated with 10 mL of rhizobacteria suspension (107 CFU mL−1). After 15 days, the seedlings were treated with 10 mL of Phytophthora spore suspension (107 spores mL−1). The experiment was conducted for 3 months in the greenhouse. The growth of the seedlings, rate of Phytophthora infection, and rate of fatalities were collected; mean values were calculated from five plants (Fig. 1).
Phylogenetic analysis of the strains based on the 16s rRNA gene sequences. The phylogenetic tree was drawn using the Mega software version 6.0 after multiple alignments of the data by CLUSTAL W. The tree was made using Kimura’s method. The numbers at the branches are bootstrap confidence percentages (%)
GC–MS and LC–MS analysis of fungal antagonist compounds produced by rhizobacteria
Fungal antagonistic compounds produced by strains RB.DS29 and RB.EK2 were analyzed by GC–MS and LC–MS, following the methods described by Lim [19].
The rhizobacteria strain RB.DS29 was cultivated in LB medium supplemented with deactivated Phytophthora spores for 5 days at 30 °C and a shaking speed of 150 rpm. The culture was centrifuged at 13,000 × g and 5 °C for 5 min. The samples were extracted and purified by solid phase extraction using the Quechers method. Ten milliliters of the sample was heated to 50 °C for 30 min, before 500 uL of the solution was analyzed by GC (Agilent 6890 N) and MS5972 (Agilent, USA). GC–MS was equipped with a HP-5 MS capillary column (30 m × 0.25 mm × 0.25 μm). He was the carrier gas (1 mL min−1), and the detector temperature was 250 °C. The column was held for 2 min at 50 °C and then programmed to increase to 300 °C for 20 min at a rate of 15 °C min−1. The source pressure was 7 Pa, the filament voltage was 70 eV, and the scan rate was 1.9 scan s−1. The compounds were identified using data from the Mass Spectra Library (NIST 14.L, “ChemStation Integrater” Agilent Technologies).
LC–MS analysis
The RB.DS29 isolate was cultivated in LB medium supplemented with denatured Phytophthora spores for 5 days at 30 °C and 150 rpm. Five milliliters of the bacterial culture was centrifuged at 13,000 × g and 4 °C for 10 min. Two milliliters of the supernatant was transferred to the tube, 2 ml acetonitrile was added, and the mixture was vortexed for 30 s. The mixture was then centrifuged at 6000 × g for 10 min. Twenty microliters of supernatant was taken for LC–MS analysis. Samples were injected and analyzed by micrOTOF-QII Bruker Daltonic (Germany) with Agilent 1290 UPLC and Dual AJS ESI ion sources. ACE 3 C18 (150 mm × 4.6 mm × 3,5 µm) column and pre-column (Phenomenex Security Guard™) were used to separate the samples. The column temperature was held to 40 °C, and the flow rate was 0.5 ml/min. The mobile phase used deionized water containing 0.1% formic acid and methanol containing 0.1% formic acid at a 90:10 ratio for 0–20 min, and then a ratio of 5:95 for 20–30 min. Acquisition range was from 50 to 1500 m/z, and the scan rate was 1.00 spec s−1. MS was set as follows: capillary voltage 4500 V, nebulizer pressure 1.2 bar, drying gas 8 L/min, gas temperature 200 °C, funnel 1RF 350 V, funnel 2RF 360 V, hexapole RF 350 V, ion energy 8.0 eV, collision RF 300 V, transfer time 120 m/s, and pre-Puls storage 5.0 m/s.
Data analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests in triplicate using SAS 9.1 software. α ≤ 0.05 was considered to be significant.
Results and discussion
Evaluation of Phytophthora antagonist activity by potent rhizobacteria strains
Phytophthora is an oomycete plant pathogen that causes wilt disease in black pepper, as well as blight and fruit rot in peppers and other important crops. Currently, using fungal antagonist bacteria to control Phytophthora is the best choice for green and sustainable agriculture [20,21,22]. Phytophthora has infected and destroyed much of the cultivation area of black pepper in the Central Highlands of Vietnam. Soil and roots were collected in five provinces of the Central Highlands to isolate and screen for bacteria possessing high anti-Phytophthora activity. Five hundred strains of black pepper rhizobacteria were isolated and characterized. After screening, seven potent rhizobacteria were selected, as summarized in Table 2. All potent rhizobacteria were highly antagonistic against Phytophthora, both in vitro and in vivo. The in vitro results showed that rhizobacteria greatly inhibited Phytophthora growth by over 60% (see Table 1). The potent rhizobacteria were evaluated continuously in vivo on black pepper shoots. For five of the rhizobacteria, Phytophthora growth inhibition was greater than 90%. While strain RB.CJ35 did not inhibit Phytophthora growth in vivo, strain RB.DS29 had the highest activity both in vitro and in vivo.
In addition, the results showed that all seven strains were plant-growth-promoting rhizobacteria (PGPR), demonstrating nitrogen-fixing, phosphorus solubilization, and IAA production abilities (unpublished data). The activities of rhizobacteria were also mentioned in previous studies. Toh et al. [22] isolated 129 bacterial endophytic strains from the roots of black pepper. Three strains, KDKS5-49, KRBR-15, and BR(1)6, demonstrated anti-Phytophthora activity of 40.32–48.39% [22]. These results were lower than ours. In another study, 19 rhizobacteria were isolated from black pepper, but their anti-Phytophthora activity ranged widely from 4.7 to 77.4%; 10 of the isolates showed antifungal activity over 50% [18].
Classification by sequencing and phylogenetic analysis of the 16S rRNA gene
The seven potent rhizobacteria were further classified by sequencing the 16S rRNA gene. The results of the phylogenetic analysis are shown in Table 3 and Fig. 2. All potent rhizobacteria belong to the genus Bacillus. Three strains, RB.EK2, RB.EK4, and RB.CS1, are 99% similar to Bacillus amyloliquefaciens. Strains RB.DS29 and RB.CJ35 are the closest to Bacillus velezensis. RB.BH15 is 100% similar to Bacillus subtilis, and RB.CP15 is similar to Bacillus cereus. All bacteria strains have been reported as antagonistic to plant pathogen fungi and promote plant growth [19,20,21,22,23]. Among these, Bacillus velezensis was recently used in sustainable agriculture. Bacillus velezensis FZB42 was able to form biofilm, in order to increase biocontrol [24, 25]. This strain can also synthesize antifungal compounds such as fengycin, bacillomycin D, difficidin, bacilysin, and amylocyclicin [26,27,28]. Bacillus velezensis has been used in the biocontrol of both wheat powdery mildew disease, caused by the fungus Blumeria graminis, and Wilt disease of strawberries, caused by Fusarium oxysporium [19, 29, 30]. However, no research has reported on the use of Bacillus velezensis in the biocontrol of black pepper. It is interesting that both RB.DS29 and RB.CJ35 belong to Bacillus velezensis, but are isolated from two different regions, and their anti-Phytophthora activity is quite different (Table 1).
GC–MS profiles of the chemical compounds produced by Bacillus velezensis RB.DS29. Samples were analyzed by GC (Agilent 6890 N) and MS5972 (Agilent, USA); HP-5 MS capillary column (30 m × 0.25 mm × 0.25 μm). Operating conditions: He as the carrier gas (1 mL min−1) and a detector temperature of 250 °C. The column was held for 2 min at 50 °C and then increased to 300 °C for 20 min at a rate of 15 °C min−1. Compounds were identified using data from the Mass Spectra Library (NIST 14.L, ChemStation Integrater Agilent Technologies)
Determination of enzymatic activities of rhizobacteria
It is well known that the antifungal activity of bacteria relates to enzymes, including chitinase, protease, and glucanase, since these enzymes can degrade the cell walls of pathogenic fungi [12, 31, 32]. As such, the rhizobacteria were tested for enzymatic activities; the results are shown in Table 2.
The results indicate that all potent rhizobacteria had three enzymatic activities. Protease activity ranged from 0.127 to 0.228 U mL−1, with RB.EK2 showing the highest activity. Compared to other recent studies, protease activity of the rhizobacteria was as high as that of Bacillus licheniformis TKU004 (0.09–0.14 U mL−1) [33], Bacillus mycoides TKU 038 (0.240 U mL−1), Bacillus cereus TKU022 (0.03–0.12 U mL−1) [34], and Serratia marcescen TKU016 (0.370 U mL−1) and TKU011 (0.477 U mL−1) [35], but lower than that of Brevibacillus parabrevis TKU046 (3.2–6.4 U mL−1) [36].
Chitinase activity in the tested rhizobacteria (Table 2) ranged from 2.90 U mL−1 (RB.EK2) to 5.70 U mL−1 (RB.CS1). It was clear that chitinase activity in the seven potent strains was higher than that of other bacteria. For instance, Tran et al. [12] isolated chitinolytic bacteria from Sakata Lake in Niigata, Japan, and found that the chitinase activity of Aeromonas hydrophila was 0.1–0.3 U mL−1. This demonstrated that chitinase activity was related to antifungal activity against Trichoderma reesei. Another study reported that chitinase activity of Bacillus amyloliquefaciens V656 was 0.44 U mL−1 [37]. In our study, chitinase activity was similar to that of Streptomyces thermocarboxydus TKU045 (1–3 U mL−1) [38], but lower than that of Serratia marcescens PRNK-1 (10–15 U mL−1) [31].
It is well known that fungal cell walls consist of two main components: glucan and chitin. Therefore, chitinase and β-glucanase play very important roles in inhibiting the growth of pathogenic fungi. The β-glucanase activity of the rhizobacteria (Table 2) ranged from 0.064 U mL−1 (RB.CJ35) to 0.114 U mL−1 (RB.EK2). These results were higher than that of Delftia tsuruhatensis MV01 (0.035 U mL−1) [39], but a little lower than that of Agrobacterium sp. ZX09 (0.30 U g−1) [40].
There is no clear correlation between the enzymatic activities (Table 2) and the antagonistic activity to Phytophthora (Table 1). Therefore, further investigation was needed to determine the fungal antagonist mechanism. The results (Table 3) indicate that Phytophthora growth inhibition (%) in mediums supplemented with 1% chitin or 0.1% β-glucan is no different between those with active enzymes and those with enzymes deactivated by boiling. This confirms that chitinase and β-glucanase play little part in the antagonistic activity against Phytophthora. These results are very similar to those shown in Table 2.
Efficacy of rhizobacteria on the growth of black pepper seedlings and antagonism to Phytophthora in the greenhouse
The potent strains of rhizobacteria possessed both antifungal and plant growth-promoting activity. Rhizobacteria were applied to black pepper seedlings in the greenhouse to detect promising strains for further application. After 90 days, as per the results shown in Table 4, all rhizobacteria strongly affected the growth, disease, and fatality rates. Leaf number, plant height, length of roots, and fresh biomass were significantly higher in treated plots than in the control group 1 (no treatment with Phytophthora) or control 2 (treatment with Phytophthora) because all potent rhizobacteria displayed nitrogen-fixing, phosphorus-solubilizing, and IAA-producing abilities. Among the seven strains, RB.DS29 had the greatest impact on growth data, generating results 20–50% higher than the others and twofold that of control group 2. In plots that received strain RB.DS29, the leaf number of the seedlings was 9.66, approximately 50% higher than the others and 300% higher than control 2. Plant height was 42.8 cm, compared to 18.3 cm for the control. The control group that was treated with Phytophthora but not rhizobacteria had the highest rate of root disease and fatalities. After 90 days, the fatality rate in control 2 was 33.3%, double and triple that of plots treated with rhizobacteria.
It is interesting that disease and fatality rates in black pepper treated with strain RB.DS29 were 14.33% and 9.00%, lower than both other strains and the control. The results showed that some strains, such as RB.CJ35, RB.CS1, and RB.BH15, had high enzymatic activities (Table 2), but there were also very high disease and fatality rates in the greenhouse, mostly due to control group 2. These results were similar to previous works. Dastager et al. [7] isolated Serratia nematodiphila NII-0928, a black pepper rhizobacteria. This strain was able to solubilize phosphorus, produce IAA, and enhance nutrient uptake. Nitrogen content was enhanced up to 34.6%, phosphorus by 100%, and potassium by 42.8%. The roots and shoot length also increased by 59–77.7%, respectively, compared to the control [7]. Bacillus amyloliquefaciens Y1 was reported to improve soil fertility, promote black pepper growth, and reduce disease rate [20]. To date, few works have researched the impact of rhizobacteria on the growth and disease resistance of black pepper in vivo, in the greenhouse or in fields. Based on the results of this study, Bacillus velezensis RB.DS29 is a promising strain for use in the biocontrol of black pepper.
The chemical compounds of strain RB.DS20 were identified by GC–MS and LC–MS. The GC–MS profile (Fig. 2) found five chemical compounds: pregn-4-ene-3,20-dione, 17-hydroxy-6-methyl-, bis(O-methyloxime); disulfide, methyl 1-(methylthio)propyl; propanoic acid, 2-methyl-, decyl ester; 1-propanone, 1-(2-benzofuranyl)-3-[(4-methoxyphenyl) amino]; and propanethioic acid, S-pentyl ester. The LC–MS profile (Table 5) also identified two antibiotic compounds: metronidazole-OH and sulfadiazine. These compounds have been reported as possessing potent antibacterial and antifungal activities [41,42,43,44,45]. But no works have been reported that these compounds were produced by Bacillus velezensis.
Conclusions
Based on the obtained results, rhizobacteria significantly affect the growth of black pepper and are strongly antagonistic against the Phytophthora fungus that causes wilt disease. After screening both in vitro and in vivo under greenhouse conditions, a promising strain for biocontrol and plant growth promotion is Bacillus velezensis RB.DS29. Not only does this strain demonstrate nitrogen fixing, phosphorus solubilizing, and IAA production, but also it possesses several chemical compounds that have great potential as biofungicides.
References
S. Nakkeeran, K. Kavitha, G. Chandrasekar, P. Renukadevi, W.G.D. Fernando, Biocontrol Sci. Technol. 16, 403 (2006)
F. Ahmad, I. Ahmad, M.S. Khan, Microbiol. Res. 163, 173 (2008)
P.C. Trivedi, in Bacteria in Agrobiology: Disease Management, ed. by D.K. Maheshwari (Springer, Berlin, 2013), p. 349
K.N. Anith, K.M. Faseela, P.A. Archana, K.D. Prathapan, Symbiosis 55, 11 (2011)
B. Lugtenberg, F. Kamilova, Annu. Rev. Microbiol. 63, 541 (2009)
K.N. Anith, N.V. Radhakrishnan, T.P. Manomohandas, Microbiol. Res. 158, 91 (2003)
S. Dastager, D. Kumaran, A. Pandey, Biology 6, 801 (2011)
S.S.K.P. Vurukonda, S. Vardharajula, M. Shrivastava, A. Skz, Microbiol. Res. 184, 13 (2016)
E. Ngumbi, J. Kloepper, Appl. Soil. Ecol. 105, 109 (2016)
F.F. Da Mota, E.A. Gomes, L. Seldin, J. Microbiol. 56, 75 (2008)
L.J. White, V.S. Brözel, S. Subramanian, Bio-Protocol 5, 16 (2015)
M.D. Tran, H. Sugimoto, D.A. Nguyen, T. Watanabe, K. Suzuki, Biosci. Biotechnol. Biochem. 82, 1 (2018)
K. Tamura, G. Stecher, D. Peterson, A. Fillipski, S. Kumar, Mol. Biol. Evol. 30, 2725 (2013)
M.A. Larkin, G. Blackshields, N.P. Brown, Bioinformatics 23, 2947 (2007)
T. Imoto, K. Yagishita, Agric. Biol. Chem. 35, 1154 (1971)
G.L. Miller, Anal. Chem. 31, 426 (1959)
M.L. Anson, J. Gen. Physiol. 22, 79 (1938)
A. Dinu, A. Kumar, R. Aravind, S.J. Eapen, J. Spices Aromat. Crops 16, 1 (2007)
S.M. Lim, M.Y. Yoon, G.J. Choi, Y.H. Choi, K.S. Jang, T.S. Shin, H.W. Park, N.H. Yu, Y.H. Kim, J.C. Kim, Plant Pathol. J. 33, 488 (2017)
R. Aravind, A. Kumar, S.J. Eapen, K.V. Ramana, Lett. Appl. Microbiol. 48, 58 (2009)
N. Sheoran, A.V. Nadakkakath, V. Munjal, A. Kundu, K. Subaharan, A. Venugopal, S. Rajamma, S.J. Eapen, A. Kumar, Microbiol. Res. 173, 66 (2015)
S.C. Toh, S. Lihan, A.S.A.H. Awang, Int. Food Res. J. 23, 2616 (2016)
Q. Jamal, Y.S. Lee, H.D. Jeon, K.Y. Kim, Plant Protect. Sci. 54, 129 (2018)
M. Krober, B. Verwaaijen, D. Wibberg, A. Winkler, A. Pühler, A. Schlüter, J. Biotechnol. 231, 212 (2016)
B. Fan, J. Blom, H.P. Klenk, R. Borris, Front. Microbiol. 8, 22 (2017)
R. Scholz, J. Vater, A. Budiharjo, Z. Wang, Y. He, K. Dietel, T. Schwecke, S. Herfort, P. Lasch, R. Borriss, J. Bacteriol. 196, 1842 (2014)
L.M. Wu, H.J. Wu, L. Chen, X. Yu, R. Borriss, X. Gao, Sci. Rep. 5, 12975 (2015)
Q. Gu, Y. Yang, Q. Yuan, G. Shi, L. Wu, Z. Lou, R. Huo, H. Wu, R. Borriss, X. Gao, Appl. Environ. Microbiol. 83, e01075 (2017)
J.M. Palazzini, C.A. Dunlap, M.J. Bowman, S.N. Chulze, Microbiol. Res. 192, 30 (2016)
X.C. Cai, C.H. Liu, B.T. Wang, Y.R. Xue, Microbiol. Res. 196, 89 (2017)
C. Moon, D.J. Seo, Y.S. Song, S.H. Hong, S.H. Choi, W.J. Jung, Microb. Pathog. 113, 218 (2017)
S.H. Hong, Y.S. Song, D.J. Seo, W.J. Jung, Microb. Pathog. 110, 159 (2017)
C.T. Doan, N.T. Tran, M.T. Nguyen, V.B. Nguyen, A.D. Nguyen, S.L. Wang, Molecules 24, 691 (2017)
C.L. Wang, J.W. Su, T.W. Liang, A.D. Nguyen, S.L. Wang, Res. Chem. Intermed. 40, 2237 (2014)
C.L. Wang, C.J. Chen, A.D. Nguyen, T.W. Liang, Y.K. Twu, S.Y. Huang, S.L. Wang, Res. Chem. Intermed. 40, 2363 (2014)
C.T. Doan, T.N. Tran, V.B. Nguyen, T.P.K. Vo, A.D. Nguyen, S.L. Wang, Int. J. Biol. Macromol. 131, 706 (2019)
S.L. Wang, I.L. Shih, T.W. Liang, C.H. Wang, J. Agric. Food Chem. 50, 2241 (2002)
T.N. Tran, C.T. Doan, V.B. Nguyen, A.D. Nguyen, S.L. Wang, Res. Chem. Intermed. 45, 727 (2018)
V. Blattel, M. Larisika, P. Pfeiffer, C. Nowak, A. Eich, J. Eckelt, H. König, Appl. Environ. Microbiol. 77, 983 (2011)
Y. Chen, H. Xu, M. Zhou, Y. Wang, S. Wang, J. Zhang, PLoS ONE 10, e0134799 (2015)
G.L. Backes, D.M. Neumann, B.S. Jursic, Bioorg. Med. Chem. 22, 4629 (2014)
Ł. Popiołek, A. Biernasiuk, A. Malm, J. Heterocycl. Chem. 53, 1589 (2015)
A.S.O. Mohareb, I.E.A. Kherallah, M.E.I. Badawy, M.Z.M. Salem, H.A. Yousef, J. Appl. Biotechnol. Bioeng. 3, 331 (2017)
C.S.C. Kumar, L.Y. Then, T.S. Chia, S. Chandraju, Y.F. Win, S.F. Sulaiman, N.S. Hashim, K.L. Ooi, C.K. Quah, H.K. Fun, Molecules 20, 16566 (2015)
T. Mouri, T. Yano, S. Kochi, T. Ando, M. Hori, J. Pestic. Sci. 30, 209 (2005)
Acknowledgements
Authors are expressed to thank Ministry of Education and Training, Vietnam granted the Science and Technology program: Application of Biotechnology for sustainable black pepper production in the Central highland. B 2017–2019. This work was also supported in part by a grant from the Ministry of Science and Technology, Taiwan (MOST 106-2320-B-032-001-MY3).
Author information
Authors and Affiliations
Contributions
ADN conceived the study. ADN, HTT, and VBN designed and performed the study. AND and S-LW contributed the reagents/materials/analysis tools. QVH, CTD, VBN, and PKV analyzed the data. AND and S-LW wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Trinh, T.H.T., Wang, SL., Nguyen, V.B. et al. A potent antifungal rhizobacteria Bacillus velezensis RB.DS29 isolated from black pepper (Piper nigrum L.). Res Chem Intermed 45, 5309–5323 (2019). https://doi.org/10.1007/s11164-019-03971-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03971-5