Abstract
Acid mine drainage (AMD) is the biggest environmental problem of water related to the mining industry in many countries. Recently, coal mining and mineral mining industries use various AMD treatment systems. The main problem, however, is the very expensive and long retention times for treatment of AMD. For this reason, biosorption technology has been tested recently as a promising method for AMD treatment. The authors review presently available or potential biosorption methods to remove and detoxify toxic heavy metals and organic pollutants from AMD. Biosorption technology is evolving as an attractive option to supplement conventional AMD treatment. However, a literature review indicates that there is a lack of studies examining biosorption of AMD treatment. This paper examines a range of subjects, including laboratory scale experiments and the commercial application of biosorption for treatment of AMD. Our review paper will help direct future studies on the development of biosorption technology for treating AMD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewaters from mining activities are a source of potential environmental problems. In mines, the target ore (gold, silver, copper, etc.) is often rich in sulfide minerals. The wastewater flows over or through sulfur-bearing materials and can generate acid mine drainage (AMD) (Johnson and Hallberg 2005; Nordstrom 2000). A general equation of the AMD production mechanism is:
Equation (1) is a summary of a series of reactions. Subsequent reactions occur biologically and chemically. The first reaction is the oxidation of the sulfide mineral to sulfate solubilizes the ferrous iron. The next step is oxygen-dependent reactions. If the surrounding environment is sufficiently oxidizing, the Fe(II) will oxidize to Fe(III). Iron-oxidizing bacteria, many of which tend to be most active at pH 2.0 to 4.0, can increase the rate of this step (Schippers et al. 2010). Finally, when the pH of AMD is increased, either through contact with fresh water or neutralizing minerals, Fe(III) precipitates as Fe(OH)3 in solution while simultaneously lowering pH. The Fe(OH)3 may be harmful to aquatic systems. The precipitates reduce the amount of light that can penetrate the water, affecting photosynthesis and visibility for animal life. Furthermore, when the precipitate settles, it blankets the stream bed, smothering the bottom-dwellers and their food resources (Moses et al. 1987). Simultaneously, AMD can also dissolve other harmful metals (metalloids) and toxic substances. This phenomenon can contaminate drinking water underground. These environmental pollution problems caused by AMD affect many countries (Chen et al. 2014; Gaikwad and Gupta 2008).
To deal with these problems, a broad range of treatment technologies is available AMD. AMD treatment systems can be broadly categorized as “source control” and “migration control.” Source control is a prevention technique used before the formation of AMD (Johnson and Hallberg 2005). Most source control techniques are to prevent or minimize important sources of reactants such as oxygen, water and sulfur-oxidizing bacteria in the processing of AMD (Lewis 2010; Santos and Johnson 2017). In contrast, migration control techniques are only to minimize the impact of receiving streams and rivers from AMD. In general, these techniques are divided into “active” and “passive” treatment (Akcil and Koldas 2006). More information related to this will be discussed in the next chapter.
One important aspect of environmental pollution caused by AMD is heavy metal contamination into receiving water bodies. If it is released into the environment without removal of hazardous heavy metals, this can cause entry into groundwater or leaching into soil and allow the negative effects to spread even further to harm all that use the stream or environment surrounding it (Hu et al. 2021; Kavehei et al. 2021; Simate and Ndlovu 2014). There are several methods for the removal of heavy metals from acid mine drainage such as chemical precipitation, ion exchange, filtration, oxidation, reverse osmosis, solvent extraction, and adsorption (Gaikwad and Gupta 2008; Hallam et al. 2021). However, these methods of treating AMD have a number of shortcomings including: extensive land utilization, production of large amounts of secondary solid waste, high capital cost and high operating costs. Among these techniques, adsorption is considered to be able to minimize the above disadvantages. Even though sorbents are highly effective in metal removal compared to other removal treatments, the high cost of sorbents is a still limitation. For this reasons, the most recent focus in sorption studies has been to investigate cheap adsorbents capable of replacing the expressive ones. Much research has focused on biosorption in recent years. Unlike bioaccumulation, biosorption technology uses dead biomass, so it is cheaper than other in the manufacture of adsorbents, and it is an economical technology because additional energy is not consumed to adsorption operate (Kratochvil and Volesky 1998; Park et al. 2010).
In the past, there was metal adsorption using biomass, but it is known that the first patent application with biosorption technology was B. Volesky and M. Tsezos in 1982 (Park et al. 2010). Since then, biosorption technology has been used in various fields. The paper in which biosorption technology is used for AMD treatment is known as the uranium adsorption paper using seaweed biosorbent published by Yang J. and Volesky B in 1999 (Yang and Volesky 1999). Since then, biosorption techniques have been used to treat AMD, but have not yet been commercialized. The purpose of this review paper is to discuss recent studies on AMD treatment using biosorption technology for commercializing this technology. No attempt has been made to cover all the available literature on AMD treatment in biosorbents. For this reasons, this paper can contribute greatly to future studies on the development of biosorption technology capable of removing contaminants from a variety of AMDs. First, before dealing with AMD treatment by biosorption, the paper, an overview of the traditional technology on the treatment of AMD is presented. Secondly, the paper investigated cases applied to research in order to find out where biosorption is used. Finally, this paper deals with the case where biosorption was applied single to AMD treatment and the case where it was used in combination with other treatment technologies.
AMD treatment
Source control
Treatment of AMD may be required for many years after mining activities have ended. Results of many experiments indicate that it may be possible to prevent AMD, rather than treating the site after mining. For this reason, appropriate control methods for the site during the early stages of mining would be beneficial (Salomons 1995). Those techniques are called “source control.” The source control methods include: underwater storage of mine tailings, separation and blending of waters with mineral (alkaline) materials, and control of water migration (Johnson and Hallberg 2005).
Various methods exist to prevent or minimize the generation of AMD. First, oxygen methods are commonly used for source control. AMD is almost always begun by an aerobic microbial process in the presence of oxygen. Underwater storage has been used for disposing and storing mine tailings that may prevent contact between the minerals and oxygen. Constructing wetlands represents a modification of the more traditional wet cover method, and a water depth of at least 1.5 m can reduce atmospheric oxygen diffusion into the tailings. Wetlands can be classified as aerobic and anaerobic wetlands. Self-sustaining ecosystems that mimic their natural counterparts are classified as aerobic wetlands (Sheoran and Sheoran 2006). Often these consist of shallow ditches filled with flooded gravel, soil, and organic matter to support wetland plants. An oxygen-consuming organic layer is formed by adding plants, and the water depth can be reduced. Stoltz and Greger demonstrated that the presence of plants also decreased the release of metals by increasing the pH of the substrate (Stoltz and Greger 2006).
Other control methods aim to eliminate the process of sulfide oxidation. These methods involve adding solid-phase phosphates to pyritic mine waste to precipitate iron(II) as ferric phosphate (Kim et al. 1999). This can reduce the potential of iron to act as an oxidant of sulfide minerals. Application of anionic surfactants can be used to inhibit sulfur-oxidizing bacteria. Iron- and sulfur-oxidizing bacteria play an important role in generating AMD. Anionic surfactants have been used to inhibit sulfur-oxidizing bacteria activity in mineral spoils and tailings (Kleinmann 1990).
Migration control
Source control methods cannot always prevent the formation of AMD, and appropriate treatment methods need to be implemented when AMD generation is unavoidable. In this case, the method used to minimize environmental pollution caused by AMD is called “migration control” (Johnson and Hallberg 2005). Migration control methods of mine sites generally require pH adjustment, oxidizing or reducing (redox) conditions, and/or stabilization of wastes. Biosorption technique is applicable to this method. Migration control methods are commonly classified as either “passive” or “active” and may involve combinations of physical, biological and chemical approaches Passive treatment refers to a method of treating AMD through minimal maintenance without using electricity, chemicals, and manpower, and active treatment is a method of treating AMD by using electricity, chemicals, and manpower (Gazea et al. 1996).
Passive treatment
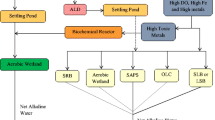
Passive treatment is a method that provides a restrained environment in which natural chemical and biological reactions are used to remediate AMD. Recently, several types of passive treatment systems have been developed without additional chemical reagents. For this reason, passive treatment systems are expected to provide lower costs of construction, operation, and maintenance. The primary passive methods are largely divided into biological systems and non-biological systems with inorganic materials. Table 1 lists the currently used passive treatment methods according to the systems classification.
Anoxic limestone drains (ALDs) and open limestone channels (OLCs) are representative treatment techniques using limestone. ALDs are non-biological systems constructed using buried limestone cells through which anoxic water flows. In this system, limestone supplies additional alkali AMD in the system to avoid ferrous iron oxidation and precipitation (Hedin et al. 1994). OLCs are an open analog to ALDs. These systems are favorably constructed and operated on steep slopes (Alcolea et al. 2012). ALD and OLC have benefits compared to constructed wetlands, because they generate alkalinity at a low cost. However, they only provide short-term decrease in hydroxide precipitation. Another drawback is that, although limestone is an inexpensive and effective way to produce alkalinity, it must be exploited under suitable conditions or its effects are limited.
Constructed wetlands can also be an effective method for treating AMD. Aerobic wetlands, one of the typical passive treatment methods, are a method for precipitating pollutants through aeration with sufficient residence time based on storage space. The best remediation by aerobic wetlands is with mildly acidic or net alkaline waters that are filled with soil or limestone gravel in an open pond that functions as a buffer. Aerobic wetlands can also remove heavy metals. The main drawback to this treatment system is that excessive accumulation of precipitate will severely limit the remediation abilities. In contrast, anaerobic wetlands encourage alkaline water to pass through buried organic substrates, such as a bed of limestone underneath or mixed with an organic substrate that requires exclusion of oxygen and aluminum in the water. These systems neutralize acidity and reduce metals to the sulfide form in organic substrate surface layers. They have the same maintenance and operating cost advantages as other passive treatment systems, but these systems commonly require long retention times and large surface areas to be efficient due to the slow mixing of acidic and alkaline substrate water near the surface (Fabian et al. 2005).
Among other passive treatment methods, permeable reactive barriers (PRBs) have gained recent attention. This method blocks AMD groundwater from underground water flow. PRBs are installed by burying layers of reactive barrier materials, such as limestone, zero valent iron, and organic matter. These materials can promote microbial sulfate reduction reactions and precipitation of iron and other metal sulfide minerals. During this process, these materials remove contaminants from AMD through a variety of reactions, such as adsorption, precipitation, and biological mechanisms. Therefore, the choice of organic matter in barriers is the most important factor in this process (Benner et al. 1999).
Biological reactors, which are referred to as sulfate-reducing bioreactors (SRBs), may be used to reduce sulfate in PRB or wetland systems. SRBs are based on their capacity for reducing sulfate to sulfide as an electron acceptor. As a result, bacteria precipitate as metal sulfides, resulting in increase in pH and alkalinity. For this reason, SRB is an economical treatment system for simultaneously removing metals and sulfides in AMD. However, the removal mechanisms of metals have not been identified, and efficient carbon source selection problems remain (Luptakova and Kusnierova 2005; Zagury et al. 2006).
Alkalinity-producing systems have also been called successive alkalinity-producing systems (SAPS) and reducing and alkalinity-producing systems (RAPS). The systems typically consist of a drainage layer, an organic layer, and a limestone layer. The difference between SAPS and RAPS is that SAPS pass AMD through each layer sequentially, while RAPS pass the material through a combined layer of organic material and limestone. The fundamental concept of these systems is related to the wetland method. These systems have been used to treat high acidity and high concentrations of Fe3+, Al3+, and DO. These systems can also encourage metal precipitation in difficult treatment conditions that include low flow (Barton and Karathanasis 1999).
Active treatment
Passive treatment has many advantages related to maintaining the system. However, most passive treatments require high start-up cost and a large area. Those reasons can result in a move to active treatment methods, which (in its basic concept) is a low technology approach for AMD treatment. Active treatment systems use chemical and physical processes, such as precipitation, biological mediation, sorption, sulfate reduction, electrochemical concentration, and flocculation. Information on the various active treatment systems is summarized in Table 2.
Among active treatment methods that can treat AMD, inorganic alkaline precipitation treatment is the most common and cost-effective method. A wide variety of natural by-product alkaline reagents or manufactured chemical reagents can also be used, determined by availability, cost, and performance. These treatment methods involve addition of chemical neutralizing agents including lime, caustic soda, and calcium carbonate. These reagents increase the pH for precipitation of heavy metals as mainly hydroxide complexion to treat AMD. The reagents vary in cost and effectiveness. Thus, selecting the right reagents for the site is the most important factor for this treatment. For example, sodium hydroxide is less expensive and more effective than limestone. Although active treatment can provide an effective method of treating AMD, it has the disadvantages of sludge disposal problems and continuous operation costs.
Active treatment methods are divided into fixed plant and in-situ methods. Fixed plant treatment of AMD occurs in a fixed location and uses the AMD pump for the plant. The process includes adding and mixing reagents in one or more reactor tanks, collecting treated sludge, and discharging the treated water. Typically, the main advantage of stationary plants is that they can be designed to handle and achieve water quality targets and unexpected contaminants. An important limitation of a fixed plant is that, regardless of AMD source, the affected water must be delivered to the plant. Therefore, there is a significant additional cost when the AMD source is far from the plant. On the other hand, in-situ treatments have a relatively low capital cost regardless of distance, because they use portable and simple systems. All in-situ systems have storage and supply abilities to use similar reagents, such as limestone and calcium hydroxide. However, in-situ systems are limited by reagent, power supply, maintenance parts, and sludge disposal.
Biosorption
Definition of biosorption
Biosorption is a type of sorption technology and can be defined as passive uptake of ionic pollutants (heavy metals, dyes, and precious metals) by dead or inactive materials derived from biological sources (Volesky 2007). Figure 1 shows the overall process of biosorption. The mechanisms of biosorption are due to a number of metabolism-independent processes in the cell wall. The cell wall of the biomass is mainly composed of polysaccharides, proteins and lipids. The biomass cell wall has many functional groups, which are able to engage in physico-chemical interactions with pollutants ions. Functional groups most commonly involved in such interactions include carboxylate, hydroxyl, amine and phosphoryl groups present within the cell wall components (e.g., polysaccharides, lipids and proteins) (Naja and Volesky 2011). Therefore, the pollutant uptake will differ according to the biomass type.
A number of biological sources, such as algae, bacteria, fungi, industrial wastes, natural residues, bio-industrial wastes, and other biological compounds, have been investigated as cheap adsorbents capable of replacing well known but more expensive commercial adsorbents. When selecting a biomass for biosorption, one primarily considers cost effectiveness and the efficiency of ionic pollutants of the biomass. Many researchers have studied new biomass types with low cost and high efficiency. Furthermore, development of methods for pretreatment of biomass and physical or chemical surface-modification has been of interest for enhancing biosorptive capacity in recent years (Vijayaraghavan et al. 2008). In general, biomass pretreatment basically aims to clean up biomass to enhance biosorption capacity. Common chemical pretreatments involve acidic and basic pretreatments. Other chemical pretreatments include alkaline, ethanol and acetone treatments of the biomass (Khosravihaftkhany et al. 2015; Puranik and Paknikar 1999; Vijayaraghavan et al. 2008). Several researchers also studied enhancing/modifying a particular functional group of the biomass (Deng and Ting 2005). Carboxyl, amine, phosphonate, sulfonate and hydroxyl functional groups present in the biomass were specifically studied. The various methods for enhancing/modifying biosorbents include the removal of interfering sites, replacing interfering sites with binding sites and the addition of ionic polymer coatings and/or grafts.
Application of biosorption
The advantages of biosorption are its eco-friendly products, absence of secondary pollution, and low cost. Thus, it has achieved significant attention for remediating industrial wastewater. In addition, biosorbents allow removal of contaminants by dead organisms. Thus, the biosorption process is simpler and less expensive than using living biomass that requires supply of nutrients and energy. Since the first study on biosorption that focused on copper adsorption by fungi, various types of biomass have been reported to remove heavy metals (metalloids) and radionuclides. In addition, biosorption studies and applications have broadened to include removal of organic pollutants and recovery of precious metals including gold, platinum, palladium, and silver (Park et al. 2010).
Dye removal from dye wastewater
Wastewater containing dyes may be a serious hazard and can be toxic, carcinogenic, and even mutagenic to aquatic systems. Efficient dye removal from textile industry wastewater is one of the most urgent environmental challenges. Dyes are often difficult to remove from wastewater because they generally have a synthetic origin and a complex aromatic molecular structure (Aksu 2005). Biosorption has been studied as an effective method for treatment of dye wastewater. Various types of biomass have been used for biosorption of a wide range of dyes and are sorted as anionic (direct, acid, and reactive dyes), cationic (basic dyes), and nonionic (disperse dyes) (Fu and Viraraghavan 2001). Table 3 shows the biosorption of dyes by various biomass materials under various conditions. The main mechanism of dye biosorption is physico-chemical interactions, such as adsorption, deposition, and ion-exchange. Earlier studies that focused on removal of dye via biosorption reported many biosorbents available on a laboratory scale. However, many dye biosorption studies use synthetic dye wastewater, which does not consider behavior of these sorbents with different competitors or with physico-chemical parameters that can differ considerably in real conditions. For this reason, future research should be conducted using actual dye wastewater more actively.
Removal of heavy metals from industrial wastewater
Pollution of water by heavy metals occurs by various industrial and human activities and is one of the most important environmental problems of recent times. Conventional methods, such as precipitation, coagulation, oxidation–reduction, ion exchange, membrane, and adsorption, are expensive for removal of toxic heavy metal ions from wastewater due to the non-regenerable materials used and the sludge treatment costs (Fu and Wang 2011). The use of biosorption to remove heavy metals from aqueous solutions is one of the most recent developments in environmental technology. The main advantage of this technology is that the production and operating costs are less than those of other technologies. In addition, it has high efficiency, minimal chemical sludge, allows regeneration of biosorbent, and has a possibility of metal recovery. For these reasons, many researchers have studied the biosorptive capacities of various biomasses for removal of heavy metals.
Heavy metal biosorption occurs due to specific functional groups on the cell wall. Carboxyl groups and amine groups of the cell wall are the major functional groups used for removing metal ions. Carboxyl groups actively participate in the binding of cationic metals, and amine groups remove both cationic metals and anionic metals via electrostatic interactions or hydrogen bonding. The use of inexpensive and efficient materials for heavy metal biosorption is summarized in Table 4. Furthermore, biosorption requires a structural biosorbent, adsorption of multiple metals, desorption, mechanistic modeling, enhancement of biosorption capacity through modification of biosorbents, and continuous reactor studies. However, information on these studies is insufficient for process scale-up and for design of real plants.
Recovery of precious metals
Precious metals are broadly used in various industries due to their unique physical and chemical properties. However, the limited availability of precious metals has led to excessive increases in their prices. Therefore, recovery of precious metals from aqueous solutions is economically attractive.
Various methods have been employed to recover precious metals. The main technologies used for recovering precious metals from wastewater include solvent extraction, ion exchange, precipitation, electrolytic recovery, and the oxidation–reduction method. Different processes have demonstrated various efficacies for different metals. Some traditional methods for recovering precious metal are not only inefficient, but they also damage the environment. Therefore, advanced recovery technologies, such as electrochemical and bio-metallurgical, have recently been used to overcome the drawbacks of these traditional methods. Currently, biosorption is a rising technology for recovering precious metals from aqueous solutions. For this reason, research has focused on biosorption to replace conventional methods to recover precious metals. Table 5 summarizes the various biosorbents used for precious metal biosorption.
As a result of analyzing a number of papers, the current precious metal recovery research using biosorption technology is focusing on improving the performance of the biosorbent by using various surface modification methods. Research is also being conducted to apply the developed high-performance biosorbent to pilot-scale continuous processes.
Treatment of AMD using biosorption
Single system
As mentioned in the previous chapter, biosorption technology is increasingly being applied in various fields. However, there are very few cases of biosorbents that have succeeded in industrialization due to the lack of research on actual wastewater treatment (Beni and Esmaeili 2020). Thus, much work in this area tries to demonstrate its possibilities on actual wastewater treatment systems. One of the targets of the study to the actual waste water is AMD. As mentioned in the previous chapter, acid mine drainage is different from general wastewater pollution as it is represented by low pH and high content of heavy metals and since the pollution generation scale is very large, it requires a lot of cost to treat it. For this reasons, research is being conducted to treat AMD using biosorption technology directly, which has an advantage in terms of cost and is known as an eco-friendly technology.
The technology that treated AMD using biosorption technology can be divided into a technology that uses only biosorbents and combines technology with other treatment technologies. Treatment of AMD using biosorption single systems is summarized in Table 6. Normally, a series of batch treatment studies and lab-scale continuous column studies is conducted to optimize the design parameters and conditions for real-scale treatment processes. Batch experiments generally tested the factors influencing biosorption, which are important in the assessment of the biosorption potential of any biomaterial. Batch experiments must be used to evaluate the required fundamental information including optimum experimental conditions, biosorbent efficiency, biosorption rate, and the potentiality of biomass regeneration. Various batch studies were conducted to treat AMD by biosorbents. The biosorption of AMD using chitosan (or chitin) is one of the more frequently reported approaches for removal of pollutants in batch experiments First, chitosan is one of the most plentiful and inexpensive types of biomass. Second, it presents a significant number of functional groups in the biomass cell wall, such as amino/acetamido and hydroxyl groups. Functional groups in the chitosan have helped to remove various metallic ions from effluents. Furthermore, natural chitosan has been modified by many techniques to not only improve its sorption capacity, but also its application potential in support materials for immobilization. Due to its high adsorption capacity, biosorbent research has increased in recent years (Sarode et al. 2019). Most recently, magnetic chitosan and bentonite composites were synthesized and used to treat AMD for heavy metal removal. The sorbents containing chitosan showed excellent performance for actual remediation of AMD containing heavy metals (Cd, Cr, Cu, Fe Zn, Ni, and Pb). The percentage biosorbent removal of heavy metals from actual AMD was greater than 84% (Feng et al. 2019). Robinson-Lora and Brennan (2009) also studied chitin as a biosorbent for AMD treatment in a continuous system and indicated that chitin can play a major role in neutralization of AMD and removal of metal contaminants, such as sulfate (Robinson-Lora and Brennan 2009). Moreover, chitosan is naturally associated with CaCO3 and proteins, allowing gradual release of alkaline species and nitrogen into the aqueous system. As a result, the previous neutralization treatment is not required to control the pH of the wastewater, thus reducing costs and treatment time. Laus et al. (2007) noted that chitosan could remove Fe(II), Al(III), and Cu(II) from AMD with less than 7 g/L of biosorbent, and the pH increased from 2.58 to 6.20 (Laus et al. 2007). Some researchers have studied the performance of crab shell products in removing pollutants from actual AMD in batch-scale study(Daubert and Brennan 2007; Pinto et al. 2011).
According to the literature, there have been many studies on the use of industrial or agricultural biowaste as biosorbents for removal of heavy metals from AMD. The availability and low cost of these types of biowaste make them attractive as biosorbents (Park et al. 2010). Rice husks are an example of biowaste used for biosorption of Cu, Fe, and Zn from AMD (Chockalingam and Subramanian 2006). Bark was also assessed as a biosorbent in the treatment of AMD. The bark was able to remove 96% of iron, 92% of copper, 75% of zinc and 41% of sulfate from AMD (Chockalingam and Subramanian 2009).
A majority of the available data on treatment of AMD by biosorbents has been produced by batch experiments. However, batch-scale results are challenging to apply to real AMD, because the volume of mine effluents requiring treatment is on a huge scale. It is more advantageous to use a continuous reactor type system that is easier to maintain and can continuously treat pollutants than a batch reactor type for the industrialization of AMD treatment system. Therefore, a continuous system is needed for real AMD treatment applications. Despite the ample research on biosorption with batch testing, there are a few biosorbents employed in continuous systems. Yang et al. (2014) studied iron-rich sludge as a biosorbent for treating arsenic in AMD in a continuous system through a column reactor. In that study, the breakthrough point of arsenic was 200 bed volumes, and the sorption capacity was 9.3 mg/g. The main mechanism of As removal in this sludge is adsorption onto iron oxides. This paper showed that not only the surface of the sludge, but also the substances constituting the sludge can be involved in the adsorption of heavy metals in AMD. (Yang et al. 2014). Non-viable activated sludge (non-living sludge) was evaluated in the presence of a synthetic solution of copper and zinc (which closely mimicked AMD composition) using packed-bed flow columns (Utgikar et al. 2000).
Combined systems
Each treatment processes for AMD has unique advantages. Often, treatment requires a combined strategy to minimize the effect of variability on treatment performance. For example, SAPS combines treatment concepts from both wetlands and ALDs. Biosorption for treating AMD can also be performed with existing treatment systems such as precipitation (Santos et al. 2004), bioreactors (Choi and Lee 2015; Hurtado et al. 2018) and various passive treatment systems (Groudev et al. 2008; Jeen and Mattson 2016). These combined systems compensate for the defects in the existing treatments of AMD. Table 7 lists treatment facilities that combine biosorption with various AMD processing systems.
Precipitation using alkaline reagents is the most commonly used active treatment method for removing metals from AMD. It also has found its wide application for metals removal. Research has been conducted to combine precipitation and biosorption to more effectively remove heavy metals present in AMD (Holub et al. 2018). Although the process is cost effective, this method generates large volumes of hazardous, concentrated, mixed-water sludge. It can also be extremely difficult to filter and requires large and expensive solid–liquid separation units. To solve this problem, biosorption treatment was used to produce an easily filterable pulp and a solution containing metals. The study concluded that addition of biomass (grape stalks or cork powder) to synthetic AMD removed iron from the solution and improved the rate of sedimentation and filtration operations (Santos et al. 2004).
Choi and Lee (2015) studied the effects of a combined biosorbent and bioreactor system for treatment of AMD. The reactor consisted of two parts, calcined eggshell powder as a biosorbent and supernatant of organized microalgae. In this reactor, the first part of the process removes heavy metals using biosorption. In addition, the calcined eggshell neutralized the acidity from the AMD in this combined system. The combined reactors using the eggshell and microalgae hybrid system showed total heavy metal removal values from AMD effluents of 99.66% for Fe, 99.47% for Cu, 99.90% for Zn, 99.81% for Mn, 100% for As, and 100% for Cd (Choi and Lee 2015).
Heavy metals are a problem, but high sulfate concentration is also a problem in AMD. Thus, SRB, a biological reactor, has recently been used for efficient removal of metals and sulfide. A study using biosorption technology to increase SRB efficiency has also been reported (Hurtado et al. 2018). According to that study, it was possible to remove metal and sulfate from AMD through the synergistic combination of a biosorption treatment system with an SRB to eliminate copper.
Jeen and Mattson (2016) studied column experiments for passive treatment systems of AMD from a waste rock storage area. They evaluated biomaterials such as chicken manure, mushroom compost, and straw in comparison to limestone for use in the column reactor. All the column reactors had the following results: sulfate was reduced to sulfide, pH was increased, alkalinity was generated, and heavy metals were removed from the pollutants. These results revealed that the reaction mechanism for heavy metal removal is sulfate reduction followed by precipitation of sulfides, secondary carbonates, and hydroxides. As a result, precipitation and biosorption onto a biomaterial contributed to removal of metals from this system (Jeen and Mattson 2016).
PRB can also be used in a combined system with biosorption for treatment of AMD. Groudev et al. (2008) treated AMD during a 10-year period using different passive systems including ALD, wetlands, a rock filter, and permeable reactive multi-barriers. These methods were used separately or in different combinations. In their study, biosorption technology was combined with permeable reactive multi-barriers. A section of the multi-barriers was filled with a mixture of biodegradable solid organic substrates, such as cow manure, plant compost, and straw. In that study, much of the As, U, and Ra and most of the heavy metals were removed by increase in pH and biosorption (Groudev et al. 2008).
Conclusion
In this review, we presented several biosorption technologies that have been employed for AMD treatment. Relative to biosorption literature, the treatment of AMD via this process is still in its infancy. As discussed in this review, various biosorbents have been used for AMD treatment, which can occur through many mechanisms and can target various contaminants. Furthermore, it may be possible to reduce the cost of biosorbent use and improve the design of the process. Treatment of AMD is not an easy task due to the large scale. Therefore, economics can be the most important factor in the choice of technology. In most cases, expensive technology cannot be applied in a low-income country. Biosorption represents a technique for using economical, biological materials for treatment of AMD. This review paper can help in guiding future studies on the development of biologically-based sorbent materials capable of removing contaminants from a variety of AMDs.
References
Akcil A, Koldas S (2006) Acid mine drainage (AMD): causes, treatment and case studies. J Clean Prod. 14:1139–1145. https://doi.org/10.1016/j.jclepro.2004.09.006
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: A review. Process Biochem 40:997–1026. https://doi.org/10.1016/j.procbio.2004.04.008
Alcolea A, Vázquez M, Caparrós A, Ibarra I, García C, Linares R, Rodríguez R (2012) Heavy metal removal of intermittent acid mine drainage with an open limestone channel. Miner Eng 26:86–98
Barrera H, Urena-Nunez F, Bilyeu B, Barrera-Diaz C (2006) Removal of chromium and toxic ions present in mine drainage by Ectodermis of Opuntia. J Hazard Mater 136:846–853. https://doi.org/10.1016/j.jhazmat.2006.01.021
Barton CD, Karathanasis AD (1999) Renovation of a failed constructed wetland treating acid mine drainage. Environ Geol 39:39–50
Beni AA, Esmaeili A (2020) Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ Technol Innov. https://doi.org/10.1016/j.eti.2019.100503
Benner SG, Blowes DW, Gould WD, Herbert RB, Ptacek CJ (1999) Geochemistry of a permeable reactive barrier for metals and acid mine drainage. Environ Sci Technol 33:2793–2799. https://doi.org/10.1021/es981040u
Cantuaria ML, de Almeida Neto AF, Nascimento ES, Vieira MGA (2016) Adsorption of silver from aqueous solution onto pre-treated bentonite clay: complete batch system evaluation. J Clean Prod 112(1):1112–1121. https://doi.org/10.1016/j.jclepro.2015.07.021
Champagne P, Van Geel P, Parker W (2008) Impact of temperature and loading on the mitigation of AMD in peat biofilter columns. Mine Water Environ 27:225. https://doi.org/10.1007/s10230-008-0053-5
Chen T, Yan B, Lei C, Xiao X (2014) Pollution control and metal resource recovery for acid mine drainage. Hydrometallurgy 147–148:112–119. https://doi.org/10.1016/j.hydromet.2014.04.024
Chockalingam E, Subramanian S (2009) Utility of Eucalyptus tereticornis (Smith) bark and Desulfotomaculum nigrificans for the remediation of acid mine drainage. Bioresource Technol. 100:615–621. https://doi.org/10.1016/j.biortech.2008.07.004
Chockalingam E, Subramanian S (2006) Studies on removal of metal ions and sulphate reduction using rice husk and Desulfotomaculum nigrificans with reference to remediation of acid mine drainage. Chemosphere 62:699–708. https://doi.org/10.1016/j.chemosphere.2005.05.013
Choi HJ, Lee SM (2015) Heavy metal removal from acid mine drainage by calcined eggshell and microalgae hybrid system. Environ Sci Pollut R 22:13404–13411. https://doi.org/10.1007/s11356-015-4623-3
Christoforidis AK, Orfanidis S, Papageorgiou SK, Lazaridou AN, Favvas EP, Mitropoulos AC (2015) Study of Cu(II) removal by Cystoseira crinitophylla biomass in batch and continuous flow biosorption. Chem Eng J. 277:334–340. https://doi.org/10.1016/j.cej.2015.04.138
Côrtes LN, Tanabe EH, Bertuol DA, Dotto GL (2015) Biosorption of gold from computer microprocessor leachate solutions using chitin. Waste Manage 45:272–279. https://doi.org/10.1016/j.wasman.2015.07.016
Das D, Vimala R, Das N (2015) Removal of Ag(I) and Zn(II) ions from single and binary solution using sulfonated form of gum arabic-powdered mushroom composite hollow semispheres: Equilibrium, kinetic, thermodynamic and ex-situ studies. Ecol Eng. 75:116–122. https://doi.org/10.1016/j.ecoleng.2014.11.037
Daubert LN, Brennan RA (2007) Passive remediation of acid mine drainage using crab shell chitin Environ. Eng Sci 24:1475–1480. https://doi.org/10.1089/ees.2006.0199
Deng S, Ting YP (2005) Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res. 39:2167–2177. https://doi.org/10.1016/j.watres.2005.03.033
Donia AM, Atia AA, Elwakeel KZ (2007) Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy. 87:197–206. https://doi.org/10.1016/j.hydromet.2007.03.007
Fabian D, Younger PL, Aplin AC (2005) Constructed wetlands for the passive treatment of acid mine drainage allow a quantitative appraisal of the biogeochemical removal of iron, sulphur, and other pollutants. Abstr Pap Am Chem S. 230:U1785–U1786
Fathima A, Aravindhan R, Rao JR, Nair BU (2015) Biomass of Termitomyces clypeatus for chromium (III) removal from chrome tanning wastewater. Clean Technol Environ Policy. 17:541–547
Feng G, Ma J, Zhang X, Zhang Q, Xiao Y, Ma Q, Wang S (2019) Magnetic natural composite Fe3O4-chitosan@bentonite for removal of heavy metals from acid mine drainage. J Colloid Interface Sci. 538:132–141
Ferraz AI, Amorim C, Tavares T, Teixeira JA (2015) Chromium(III) biosorption onto spent grains residual from brewing industry: equilibrium, kinetics and column studies. Int J Environ Sci Technol. 12:1591–1602. https://doi.org/10.1007/s13762-014-0539-6
Fu Y, Viraraghavan T (2001) Fungal decolorization of dye wastewaters: a review. Bioresource Technol 79:251–262. https://doi.org/10.1016/S0960-8524(01)00028-1
Fu Y, Viraraghavan T (2002) Dye biosorption sites in Aspergillus niger. Bioresource Technol 82:139–145. https://doi.org/10.1016/S0960-8524(01)00172-9
Fu FL, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Gaikwad RW, Gupta DV (2008) Review on removal of heavy metals from acid mine drainage. Appl Ecol Environ Res 6:79–96. https://doi.org/10.15666/aeer/0603_081098
Gazea B, Adam K, Kontopoulos A (1996) A review of passive systems for the treatment of acid mine drainage. Miner Eng 9:23–42. https://doi.org/10.1016/0892-6875(95)00129-8
Gong QQ, Guo XY, Liang S, Wang C, Tian QH (2016) Study on the adsorption behavior of modified persimmon powder biosorbent on Pt(IV). Int J Environ Sci Technol. 13:47–54. https://doi.org/10.1007/s13762-015-0809-y
Groudev S, Georgiev P, Spasova I, Nicolova M (2008) Bioremediation of acid mine drainage in a uranium deposit. Hydrometallurgy 94:93–99. https://doi.org/10.1016/j.hydromet.2008.05.023
Gutiérrez C, Hansen HK, Hernández P, Pinilla C (2015) Biosorption of cadmium with brown macroalgae. Chemosphere 138:164–169. https://doi.org/10.1016/j.chemosphere.2015.06.002
Hallam L, Papasergio AE, Lessio M, Veliscek-Carolan J (2021) Phosphate functionalised titania for heavy metal removal from acidic sulfate solutions. J Colloid Interface Sci 600:719–728. https://doi.org/10.1016/j.jcis.2021.05.047
Hedin RS, Watzlaf GR, Nairn RW (1994) Passive treatment of acid-mine drainage with limestone. J Environ Qual 23:1338–1345
Holub M, Balintova M, Pavlikova P (2018) Proposal and testing of multi-step process for acid mine drainage treatment. Inz Miner. https://doi.org/10.29227/Im-2018-02-25
Hong H-J, Yu H, Hong S, Hwang JY, Kim SM, Park MS, Jeong HS (2020) Modified tunicate nanocellulose liquid crystalline fiber as closed loop for recycling platinum-group metals. Carbohyd Polym 228:115424. https://doi.org/10.1016/j.carbpol.2019.115424
Hosseinzadeh H, Mohammadi S (2016) Biosorption of anionic dyes from aqueous solutions using a novel magnetic nanocomposite adsorbent based on rice husk ash. Sep Sci Technol (philadelphia) 51:939–953. https://doi.org/10.1080/01496395.2016.1142564
Hu J, Liu J, Li J, Lv X, Yu L, Wu K, Yang Y (2021) Metal contamination, bioaccumulation, ROS generation, and epigenotoxicity influences on zebrafish exposed to river water polluted by mining activities. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124150
Hurtado C, Viedma P, Cotoras D (2018) Design of a bioprocess for metal and sulfate removal from acid mine drainage. Hydrometallurgy 180:72–77. https://doi.org/10.1016/j.hydromet.2018.07.006
Jeen SW, Mattson B (2016) Evaluation of layered and mixed passive treatment systems for acid mine drainage. Environ Technol 37:2835–2851. https://doi.org/10.1080/09593330.2016.1167249
Johnson DB, Hallberg KB (2005) Acid mine drainage remediation options: a review. Sci Total Environ 338:3–14. https://doi.org/10.1016/j.scitotenv.2004.09.002
Kavehei A, Hose GC, Gore DB (2021) History of environmental contamination at Sunny Corner Ag–Pb–Zn mine, eastern Australia: A meta-analysis approach. Environ Pollution. https://doi.org/10.1016/j.envpol.2020.115742
Khosravihaftkhany S, Morad N, Abdullah AZ, Teng TT, Ismail N (2015) Biosorption of Pb(II) and Fe(III) from aqueous co-solutions using chemically pretreated oil palm fronds. RSC Advances. 5:106498–106508. https://doi.org/10.1039/c5ra15325e
Kim SD, Kilbane Ii JJ, Cha DK (1999) Prevention of acid mine drainage by sulfate reducing bacteria: organic substrate addition to mine waste piles Environ. Eng Sci 16:139–145
Kim N, Park M, Park D (2015a) A new efficient forest biowaste as biosorbent for removal of cationic heavy metals. Bioresource Technol 175:629–632. https://doi.org/10.1016/j.biortech.2014.10.092
Kim MS, Kim JG (2020) Adsorption characteristics of spent coffee grounds as an alternative adsorbent for cadmium in solution. Environments. https://doi.org/10.3390/environments7040024
Kim S, Song M-H, Wei W, Yun Y-S (2015) Selective biosorption behavior of Escherichia coli biomass toward Pd(II) in Pt(IV)–Pd(II) binary solution. J Hazard Mater. 283:657–662. https://doi.org/10.1016/j.jhazmat.2014.10.008
Kleinmann RLP (1990) At-source control of acid mine drainage. Int J Mine Water 9:85–96. https://doi.org/10.1007/BF02503685
Kratochvil D, Volesky B (1998) Advances in the biosorption of heavy metals. Trends Biotechnol 16:291–300. https://doi.org/10.1016/S0167-7799(98)01218-9
Kwak IS, Yun YS (2010) Recovery of zero-valent gold from cyanide solution by a combined method of biosorption and incineration. Bioresource Technol 101:8587–8592. https://doi.org/10.1016/j.biortech.2010.06.080
Laus R, Geremias R, Vasconcelos HL, Laranjeira MCM, Favere VT (2007) Reduction of acidity and removal of metal ions from coal mining effluents using chitosan microspheres. J Hazard Mater 149:471–474. https://doi.org/10.1016/j.jhazmat.2007.04.012
Lewis AE (2010) Review of metal sulphide precipitation. Hydrometallurgy 104:222–234. https://doi.org/10.1016/j.hydromet.2010.06.010
Li Z, Ge Y, Wan L (2015b) Fabrication of a green porous lignin-based sphere for the removal of lead ions from aqueous media. J Hazard Mater 285:77–83. https://doi.org/10.1016/j.jhazmat.2014.11.033
Li WC, Law FY, Chan YHM (2015) Biosorption studies on copper (II) and cadmium (II) using pretreated rice straw and rice husk. Environ Sci Pollut R. https://doi.org/10.1007/s11356-015-5081-7
Lin S, Wei W, Wu X, Zhou T, Mao J, Yun Y-S (2015) Selective recovery of Pd(II) from extremely acidic solution using ion-imprinted chitosan fiber: adsorption performance and mechanisms. J Hazard Mater. 299:10–17. https://doi.org/10.1016/j.jhazmat.2015.05.050
Liu X, Gao C, Liang C, Nie S, Wang S (2016) Copper (II) removed from aqueous solutions by aminated bagasse pith. Jo Residuals Sci Technol. https://doi.org/10.12783/issn.1544-8053/13/3/6
Luptakova A, Kusnierova M (2005) Bioremediation of acid mine drainage contaminated by SRB. Hydrometallurgy 77:97–102. https://doi.org/10.1016/j.hydromet.2004.10.019
Mao J, Lee SY, Won SW, Yun Y-S (2010) Surface modified bacterial biosorbent with poly(allylamine hydrochloride): Development using response surface methodology and use for recovery of hexachloroplatinate(IV) from aqueous solution. Water Res. 44:5919–5928. https://doi.org/10.1016/j.watres.2010.07.034
Massocatto CL et al (2015) Biosorption of Pb2+, Cr3+, and Cu2+ by peach palm sheath modified colonized by Agaricus Blazei. Desalination Water Treatment. https://doi.org/10.1080/19443994.2015.1107503
Masukume M, Onyango MS, Maree JP (2014) Sea shell derived adsorbent and its potential for treating acid mine drainage. Int J Miner Process 133:52–59. https://doi.org/10.1016/j.minpro.2014.09.005
Meneghel AP, Gonçalves AC, Rubio F, Dragunski DC, Lindino CA, Strey L (2013) Biosorption of cadmium from water using moringa (Moringa oleifera Lam.) seeds water. Air Soil Pollution 224:1–13. https://doi.org/10.1007/s11270-012-1383-2
Mirmohseni A, Seyed Dorraji MS, Figoli A, Tasselli F (2012) Chitosan hollow fibers as effective biosorbent toward dye: Preparation and modeling. Bioresource Technol 121:212–220. https://doi.org/10.1016/j.biortech.2012.06.067
Moghaddam MR, Fatemi S, Keshtkar A (2013) Adsorption of lead (Pb2+) and uranium cations by brown algae; experimental and thermodynamic modeling. Chem Eng J. 231:294–303. https://doi.org/10.1016/j.cej.2013.07.037
Mohd Bahari Z, Ali Hamood Altowayti W, Ibrahim Z, Jaafar J, Shahir S (2013) Biosorption of As (III) by non-living biomass of an arsenic-hypertolerant Bacillus cereus strain SZ2 isolated from a gold mining environment: equilibrium and kinetic study. Appl Biochem Biotechnol. 171:2247–2261. https://doi.org/10.1007/s12010-013-0490-x
Monier M, Abdel-Latif DA, Abou El-Reash YG (2016) Ion-imprinted modified chitosan resin for selective removal of Pd(II) ions. J Colloi Interface Sci. 469:344–354. https://doi.org/10.1016/j.jcis.2016.01.074
Moses CO, Nordstrom DK, Herman JS, Mills AL (1987) Aqueous pyrite oxidation by dissolved-oxygen and by ferric iron. Geochim Cosmochim Ac 51:1561–1571. https://doi.org/10.1016/0016-7037(87)90337-1
Moyo M, Chirinda A, Nharingo T (2016) Removal of Copper from Aqueous Solution Using Chemically Treated Potato (Solanum tuberosum) Leaf Powder CLEAN–Soil, Air, Water
Naja G, Volesky B (2011) The mechanism of metal cation and anion biosorption. Microbial Biosorption of Metals. https://doi.org/10.1007/978-94-007-0443-5_3
Nascimento MRL, Fukuma HT, Da Costa WC, Quinelato AL, Garcia O Jr, Gomes HA (2006) Removal of radionuclides from acid mine waters by retention on adsorbing materials. J Radioanal Nucl Chem 269:755–759. https://doi.org/10.1007/s10967-006-0296-1
Nordstrom DK (2000) Advances in the hydrogeochemistry and microbiology of acid mine waters. Int Geol Rev 42:499–515
Noreen S, Bhatti HN, Nausheen S, Sadaf S, Ashfaq M (2013) Batch and fixed bed adsorption study for the removal of Drimarine Black CL-B dye from aqueous solution using a lignocellulosic waste: a cost affective adsorbent. Ind Crops Prod 50:568–579. https://doi.org/10.1016/j.indcrop.2013.07.065
Park D, Yun YS, Park JM (2010) The past present, and future trends of biosorption. Biotechnol Bioproc E 15:86–102. https://doi.org/10.1007/s12257-009-0199-4
Park SI et al (2012) Recovery of gold as a type of porous fiber by using biosorption followed by incineration. Bioresource Technol 104:208–214. https://doi.org/10.1016/j.biortech.2011.11.018
Parshetti GK, Chowdhury S, Balasubramanian R (2014) Hydrothermal conversion of urban food waste to chars for removal of textile dyes from contaminated waters. Bioresource Technol 161:310–319. https://doi.org/10.1016/j.biortech.2014.03.087
Pat-Espadas AM, Field JA, Otero-Gonzalez L, Razo-Flores E, Cervantes FJ, Sierra-Alvarez R (2016) Recovery of palladium(II) by methanogenic granular sludge. Chemosphere. 144:745–75. https://doi.org/10.1016/j.chemosphere.2015.09.035
Pinto PX, Al-Abed SR, Reisman DJ (2011) Biosorption of heavy metals from mining influenced water onto chitin products. Chem Eng J 166:1002–1009. https://doi.org/10.1016/j.cej.2010.11.091
Podstawczyk D, Witek-Krowiak A, Chojnacka K, Sadowski Z (2014) Biosorption of malachite green by eggshells: mechanism identification and process optimization. Bioresource Technol 160:161–165. https://doi.org/10.1016/j.biortech.2014.01.015
Puranik PR, Paknikar KM (1999) Biosorption of lead, cadmium, and zinc by Citrobacter strain MCM B-181: characterization studies. Biotechnol Progress. 15:228–237. https://doi.org/10.1021/bp990002r
Rahman MS, Sathasivam KV (2016) Heavy metal biosorption potential of a Malaysian Rhodophyte (Eucheuma denticulatum) from aqueous solutions. Int J Environ Sci Technol. 13:1973–1988. https://doi.org/10.1007/s13762-016-1022-3
Ramakul P, Yanachawakul Y, Leepipatpiboon N, Sunsandee N (2012) Biosorption of palladium(II) and platinum(IV) from aqueous solution using tannin from Indian almond (Terminalia catappa L.) leaf biomass: Kinetic and equilibrium studies. Chem Eng J. 193–194:102–111. https://doi.org/10.1016/j.cej.2012.04.035
Robinson-Lora MA, Brennan RA (2009) Efficient metal removal and neutralization of acid mine drainage by crab-shell chitin under batch and continuous-flow conditions. Biores Technol 100:5063–5071. https://doi.org/10.1016/j.biortech.2008.11.063
Rubio F, Gonçalves AC Jr, Meneghel AP, Teixeira Tarley CR, Schwantes D, Coelho GF (2013) Removal of cadmium from water using by-product Crambe abyssinica Hochst seeds as biosorbent material. Water Sci Technol 68:227–233. https://doi.org/10.2166/wst.2013.233
Salomons W (1995) Environmental-impact of metals derived from mining activities - processes predictions, prevention. J Geochem Explor 52:5–23. https://doi.org/10.1016/0375-6742(94)00039-E
Santos AL, Johnson DB (2017) The effects of temperature and pH on the kinetics of an acidophilic sulfidogenic bioreactor and indigenous microbial communities. Hydrometallurgy 168:116–120
Santos S, Machado R, Correia MJN, Carvalho JR (2004) Treatment of acid mining waters. Minerals Eng 17:225–232. https://doi.org/10.1016/j.mineng.2003.09.015
Sarode S, Upadhyay P, Khosa MA, Mak T, Shakir A, Song S, Ullah A (2019) Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int J Biol Macromol 121:1086–1100
Schippers A, Breuker A, Blazejak A, Bosecker K, Kock D, Wright TL (2010) The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy. 104:342–350. https://doi.org/10.1016/j.hydromet.2010.01.012
Seo JH, Kim N, Park M, Lee S, Yeon S, Park D (2020) Evaluation of metal removal performance of rod-type biosorbent prepared from sewage-sludge. Environ Eng Res 25:700–706. https://doi.org/10.4491/eer.2019.201
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116. https://doi.org/10.1016/j.mineng.2005.08.006
Simate GS, Ndlovu S (2014) Acid mine drainage: challenges and opportunities Journal of Environmental. Chem Eng 2:1785–1803. https://doi.org/10.1016/j.jece.2014.07.021
Song D, Park SJ, Kang HW, Park SB, Han JI (2013) Recovery of lithium(I), strontium(II), and lanthanum(III) using Ca-alginate beads. J Chem Eng Data. 58:2455–2464. https://doi.org/10.1021/je400317v
Stoltz E, Greger M (2006) Influences of wetland plants on weathered acidic mine tailings. Environ Pollut 144:689–694. https://doi.org/10.1016/j.envpol.2005.12.038
Su W, Yang Y, Dai H, Jiang L (2015) Biosorption of heavy metal ions from aqueous solution on chinese fir bark modified by sodium hypochlorite. BioResources 10:6993–7008
Sulaymon AH, Mohammed AA, Al-Musawi TJ (2014) Comparative study of removal of cadmium (II) and chromium (III) ions from aqueous solution using low-cost biosorbent. Int J Chem Reactor Eng. 12:477–486
Sun L, Chen D, Wan S, Yu Z (2015) Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresource Technol. 198:300–308. https://doi.org/10.1016/j.biortech.2015.09.026
Tan WS, Ting ASY (2014) Alginate-immobilized bentonite clay: Adsorption efficacy and reusability for Cu(II) removal from aqueous solution. Bioresource Technol. 160:115–118. https://doi.org/10.1016/j.biortech.2013.12.056
Utgikar V, Chen BY, Tabak HH, Bishop DF, Govind R (2000) Treatment of acid mine drainage: I equilibrium biosorption of zinc and copper on non-viable activated sludge. Int Biodeter Biodegr 46:19–28. https://doi.org/10.1016/S0964-8305(00)00053-6
Varia J, Zegeye A, Roy S, Yahaya S, Bull S (2014) Shewanella putrefaciens for the remediation of Au3+, Co2+ and Fe3+ metal ions from aqueous systems. Biochem Eng J. 85:101–109. https://doi.org/10.1016/j.bej.2014.02.002
Vijayaraghavan K, Won SW, Mao J, Yun YS (2008) Chemical modification of corynebacterium glutamicum to improve methylene blue biosorption. Chem Eng J 145:1–6. https://doi.org/10.1016/j.cej.2008.02.011
Vijayaraghavan K, Joshi UM (2014) Application of Ulva sp. biomass for single and binary biosorption of chromium (III) and manganese (II) ions: equilibrium modeling. Environ Progress Sustainable Energy. 33:147–153
Volesky B (2007) Biosorption and me. Water Res 41:4017–4029. https://doi.org/10.1016/j.watres.2007.05.062
Wang T, Sun H (2013) Biosorption of heavy metals from aqueous solution by UV-mutant bacillus subtilis. Environ Sci Pollut R 20:7450–7463. https://doi.org/10.1007/s11356-013-1767-x
Wang M-X, Zhang Q-L, Yao S-J (2015) A novel biosorbent formed of marine-derived penicillium janthinellum mycelial pellets for removing dyes from dye-containing wastewater. Chem Eng J 259:837–844. https://doi.org/10.1016/j.cej.2014.08.003
Won SW, Mao J, Kwak I-S, Sathishkumar M, Yun Y-S (2010) Platinum recovery from ICP wastewater by a combined method of biosorption and incineration. Bioresource Technol 101:1135–1140. https://doi.org/10.1016/j.biortech.2009.09.056
Yang J, Volesky B (1999) Removal and concentration of uranium by seaweed biosorbent. doi:https://doi.org/10.1016/S1572-4409(99)80137-0
Yang JS, Kim YS, Park SM, Baek K (2014) Removal of As(III) and As(V) using iron-rich sludge produced from coal mine drainage treatment plant. Environ Sci Pollution Res. 21:10878–10889. https://doi.org/10.1007/s11356-014-3023-4
Zagury GJ, Kulnieks VI, Neculita CM (2006) Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 64:944–954. https://doi.org/10.1016/j.chemosphere.2006.01.001
Zhang M, Helleur R, Zhang Y (2015) Ion-imprinted chitosan gel beads for selective adsorption of Ag+ from aqueous solutions. Carbohydrate Polym. 130:206–212. https://doi.org/10.1016/j.carbpol.2015.05.038
Zhao Y, Wang D, Xie H, Won SW, Cui L, Wu G (2015) Adsorption of Ag (I) from aqueous solution by waste yeast: kinetic, equilibrium and mechanism studies. Bioprocess Biosystems Eng. 38:69–77. https://doi.org/10.1007/s00449-014-1244-z
Zhao S, Zhou T (2016) Biosorption of methylene blue from wastewater by an extraction residue of Salvia miltiorrhiza Bge. Bioresource Technol 219:330–337. https://doi.org/10.1016/j.biortech.2016.07.121
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (2019R1A6A3A01096685).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Maryam Shabani.
Rights and permissions
About this article
Cite this article
Kim, N., Park, D. Biosorptive treatment of acid mine drainage: a review. Int. J. Environ. Sci. Technol. 19, 9115–9128 (2022). https://doi.org/10.1007/s13762-021-03631-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03631-5