Abstract
Acid mine drainage (AMD) is considered to be one of the major environmental problems faced by the mining industries due to its toxic and lethal impact on the ecosystem. Generation of million tons of mine wastes like tailings, slags, waste rocks/ore bodies, etc. are the major sources of AMD generation in metalliferous/coal mine, due to the biologically mediated oxidation of sulfidic waste. Low pH and high heavy metal and sulfate concentration are the major characteristic features of the AMD environment and considered to be lethal than single-factor pollutants. Owing to its toxic effects, development of both preventive and treatment options remain the main focus of scientific research for decades. Prevention is a better option than complex treatment processes; however, due to several environmental factors preventive measures are limited from its application in several mining locations. In order to minimize the impact of AMD on ecosystem, several remediation technologies have been developed including chemical and biological treatment options. Due to the high capital investment and other reasons, chemical technologies are less preferred over biological treatments. Nowadays, several studies have been conducted to develop promising biological treatment system to attenuate AMD and recover metals from such environment. The present chapter discusses the cause and generation of AMD, available prevention measures, and treatment technologies using metabolic capacities of sulfate-reducing bacteria (SRB), which is the main player of biological treatment system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acid mine drainage
- Bioremediation
- Biostimulation
- Bioaugmentation
- Sulfidogenesis
- Passive and active treatments
- Sulfate-reducing bacteria
8.1 Introduction
Generation of million tons of waste in metalliferous/coal mines containing sulfidic minerals is a major environmental and economic concern worldwide (Nordstrom 2000; Baker and Banfield 2003). These sulfidic minerals upon oxidation generate highly acidic, metal- and sulfate-rich mine waters known as acid mine drainage (AMD) (Nordstrom 2000). AMD is considered to be a multifactor pollutant, detrimental for the receiving ecosystems including aquatic and terrestrial (Gray 1997). Numerous studies have reported the lethal impact of AMD on the aquatic life-forms (Soucek et al. 2000; Schmidt et al. 2002; Gerhardt et al. 2004; Martin and Goldblatt 2007; Van Damme et al. 2008; Gray and Delaney 2008; Jennings et al. 2008; Hogsden and Harding 2011). Owing to its extreme and toxic nature, AMD degrades the quality of river, lakes, and groundwater and also affects the terrestrial ecosystem (Nieto et al. 2007; Olıas et al. 2004; Alhamed and Wohnlich 2014; Gupta and Nikhil 2016; Roychowdhury et al. 2017; Grande et al. 2018). Both active and abandoned mines generate millions of cubic tons of AMD worldwide. In the reports from the US Forest Service 2005, 55700 mines are abandoned in the USA. Almost 15,000–23,000 km AMD-impacted streams are present across the USA (Roychowdhury et al. 2019 and references therein). Near about 600 coal mines in the Zipaquirá Mining District of Colombia generate 70,400 m3 of contaminated mine water each month (Fenalcarbón 2006). Pérez-Ostalé et al. (2013) determined the mining-affected area in the Iberian Pyrite Belt (TPB) region and found that more than 4800 ha areas of 88 sulfidic mines were occupied by mine waste, open pit, tailing dams, etc. Recently, Grande et al. (2018) mapped the impact of AMD on the river network of the IPB. They also demonstrated that 23 water reservoirs near the IPB are also affected by AMD and have excess mining leachates than the established range by Directive 98/83/CE (regulation for the quality of the drinking water in Europe). In the preliminary evaluation of AMD in the Minas Gerias State of Brazil, it was identified that four mining sites can generate about billions of m3 of water at pH 2–3 (de Mello et al. 2006). China has more than 63,433 metal and nonmetal mines, but the quantity of nonmetal mines surpasses 56,000 including 15,000 coal mines (Liu et al. 2018b and references therein). Shu (2003) reported the generation of 25,000 tons of waste rocks and 30,000 tons of tailings annually from the Lechang Pb/Zn mine, China. Saha and Sinha (2018) reported that more than billion tons of waste rocks and tailings are present in the mining area of Canada and have the potential to generate AMD. In India, problem of AMD is also associated with metal and coal mining areas (Tiwary 2001; Pandey et al. 2007; Equeenuddin et al. 2010; Sahoo et al. 2012). Problem of mine drainage is also present in South African mining areas (Manders et al. 2009; Ochieng et al. 2010; McCarthy 2011). Groundwater near the mining area of Johannesburg was contaminated with AMD (Naicker et al. 2003). Chon and Hwang (2000) reported that 41 coal mines in Korea discharge about 141,000 m3/day AMD waste.
In order to reduce the AMD impact on the ecosystem, different prevention measures have been recommended. However, owing to the various environmental factors associated with the mine environment, capital cost, and sustainability factor, application of these preventive methods to combat AMDs is restricted to only few mines/affected areas (Sahoo et al. 2013). Several remediation technologies including chemical and biological options are used to treat AMD and AMD-impacted sites (Johnson and Hallberg 2005a; Neculita et al. 2007; Skousen et al. 2017). In comparison to chemical treatments, biological treatments are preferred as they are eco-friendly, cost-effective, and sustainable in nature (Kieu et al. 2011; Hao et al. 2014). Sulfidogenic activity of the sulfate-reducing bacteria (SRB) is the prime principle of the biological treatments. In order to enhance the growth and activities of SRB, several organic carbon sources were tested, which in turn increase the alkalinity and metal precipitation process during the biological treatment of AMD. Nowadays, acidophilic/neutrophilic SRB consortium/mixture is also applied to enhance the treatment of AMD in sulfidogenic bioreactors (Nancucheo et al. 2017).
The present review chapter provides a broad account of the occurrence of AMD, its ecological impact on ecosystem, and available prevention measures and highlights the remediation options. Bioremediation-based options utilizing the SRB are described in detail including their taxonomic identity, metabolic capabilities, and processes.
8.2 Formation of Acid Mine Drainage
Exposure of sulfidic minerals/ores to air and water during mining activity generates highly acidic, metal- and sulfate-rich acid mine drainage (AMD) in the mining area (Nordstrom 2000). The process of AMD generation starts with the oxidation of sulfidic ores (pyrite) in presence of water and air resulting in the formation of Fe2+, SO42-, and H+ (Eq. 8.1).
The ferrous ions further oxidize to ferric ions (Fe3+), which act as an oxidizing agent for sulfidic ores (Eqs. 8.2 and 8.3) and also form ferric hydroxide as iron precipitates (Eq. 8.4).
The generation of H+ ion increases acidity by reducing the pH of mine drainage which leads to the dissolution of other toxic heavy metals such as cadmium, copper, zinc, cobalt, nickel, lead, chromium, etc. from the host rocks or ores. These toxic metals are highly soluble in acidic environment, hence making the mine drainage hazardous to the environment (Nordstrom and Alpers 1999; Nordstrom 2000). At low pH (3–4), the oxidation of sulfidic ores is very slow (Diaby et al. 2007), but the presence of highly acidophilic chemolithothrophic iron and sulfur oxidizers in mine environment makes this process feasible, as these organisms are capable of oxidizing Fe/S for their energy-generation process (Baker and Banfield 2003). It was observed that the rate of Fe2+ oxidation is enhanced to several folds by these iron- and sulfur-oxidizing bacteria/archaea (Diaby et al. 2007 and references therein). These microorganisms use the sulfur and iron as electron donors for their energy generation (Baker and Banfield 2003; Mendez-Garcia et al. 2015) which leads to further decrease in pH of the mine drainage. Acidithiobacillus, Ferrithrix, Leptospirillum, Ferrovum, Picrophilus, Sulfobacillus, Ferroplasma, etc. are the typical iron and sulfur oxidizers involved in acid mine generation (Mendez-Garcia et al. 2015; Chen et al. 2016; Teng et al. 2017).
8.3 Ecological Impact of Acid Mine Drainage
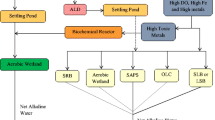
Acid mine drainage is a multifactor system which causes ecological instability due to its hazardous nature. The major factors such as acidity and metal and sulfate concentrations vary and depend upon the prevailing geochemistry of the environment. These factors make the life of both terrestrial and aquatic systems unsustainable (Fig. 8.1). It affects the food chain, increases loss of species, changes the community structure of ecosystem, increases acidity/metal precipitation/soluble metal concentration, and reduces pH (Gray 1997). These deleterious effects of AMD on ecosystem are more pronounced than single-factor pollutants and can be divided into four different categories (chemical, physical, biological, and ecological) (Gray 1997).
High concentration of heavy metals in AMD acts as a metabolic poison for the life in both terrestrial and aquatic systems. These metals when mixed with river streams, ponds, lakes, etc. produce hydroxide precipitate, which in turn reduces the oxygen content for life and also covers the body surface of several organisms and bed of these water bodies, hence making the life of both pelagic and benthic population inhabitable (Soucek et al. 2000; Schmidt et al. 2002; Gerhardt et al. 2004; Jennings et al. 2008; Qiu et al. 2009; Casiot et al. 2009; Hogsden and Harding 2011). Brown and Sadler (1989) reported that the loss of sodium ions from blood and oxygen in the tissue due to the accumulation of precipitates of heavy metals in gills (affect the breathing) was the main cause of fish death in AMD-contaminated water bodies. It has been found that streams that have normal pH levels but high iron concentrations have a small fish population (Koryak et al. 1972). Slaninova et al. (2014) also reported the loss of fish due to the inflow of acidic water which contained higher amount of Al and Fe into the rearing pond. The presence of heavy metals in high amount reduces the reproduction capabilities of organisms (Walton et al. 1982; Marsden and DeWreede 2000). When AMD runoff passes through the field, it reduces pH (increase in acidity) and increases heavy metal and sulfate contents of the soil, which change the nature of the soil (Gray 1997). Heavy metals have negative influences on soil fertility by diminishing the population of soil microbiota and other decomposers of soil organic matter, hence acting as a serious threat to plant productivity (Leita et al. 1995; Giller et al. 1998). Heavy metals as micronutrient support the metabolic precursor for many enzymatic reactions in plant metabolism, but when it exceeds the permissible limit, it starts hampering the growth, reproduction, flowering, etc. (Chibuike and Obiora 2014; Shahid et al. 2015). Plants have several mechanisms to overcome the stress of heavy metals, and one of them is phytoaccumulation, which leads to the bioaccumulation of these metals in the food chain/web and hence alters the ecosystem (Chibuike and Obiora 2014). Near to the mining regions, farmers use AMD-infested water for irrigation purpose, which severely affects the fertility of field (due to the acidity of AMD and accumulation of excessive heavy metal contents) and crop productivity (Garrido et al. 2009; Sun et al. 2015; Liao et al. 2016). Humans also have an indirect effect of AMD on their life. AMD-affected water bodies have low pH and high toxic metal concentration, which are used by the local population for their day-to-day life (He et al. 2018). These toxic metals cause serious diseases related to liver, kidney, brain, reproduction, etc. in humans (Jaishankar et al. 2014). It is of utmost importance to develop proper remediation technologies that can reduce its hazardous impacts on the ecosystem and continue with mining in more sustainable ways.
8.4 Acid Mine Drainage (AMD) Prevention and Control Options
Control and prevention options are considered to be of great importance to eliminate the sulfur oxidation event in waste rock and tailings, thus reducing AMD generation. There are several ways through which this can be achieved, but this is not feasible in many mining sites due to short life span or cost-intensiveness (Sahoo et al. 2013 and references therein). Five different prevention options are available: (i) physical barriers, (ii) bacterial inhibition (bactericides), (iii) chemical passivation, (iv), electrochemical, and (v) desulfurization (Kuyucak 2002) (Fig. 8.1). Physical barriers include dry and wet covers which are used to prevent the oxidation of pyritic minerals. Soil covers, plastic liners, and organic wastes have been used as dry covers to limit the oxygen transfer and oxidation of sulfidic minerals in mining region (Backes et al. 1987; Yanful and Payant 1992; Yanful and Orlandea 2000; Timms and Bennett 2000; Kuyucak 2002; Pandey et al. 2011). Water covers have also been used to prevent the oxidation of sulfidic minerals due to two reasons (i) low solubility of oxygen and (ii) diffusion rate in water as compared to air (Kuyucak 2002; Demers et al. 2008). Prevention of water migration from mining wastes via constructing interceptor structures, slurry walls, and diversion ditches also play an important role in limiting AMD generation (Kuyucak 2002). Vegetative covers are also reported as an option for reducing the acid generation, as well as phytostabilization potential of plants for accumulation of heavy metals (Reid and Naeth 2005; Tamas and Kovacs 2005; Valente et al. 2012; Wang et al. 2017). Bactericidal effect on mine tailings also provides a limited time span prevention option by killing the iron- and sulfur-oxidizing bacteria responsible for AMD generation (Kuyucak 2002; Sahoo et al. 2013 and references therein). Anion surfactants such as sodium dodecyl sulfate (SDS), sodium lauryl sulfate (SLS), and organic acids were found to be effective in reducing the growth and activity of iron- and sulfur-oxidizing bacteria (Kuyucak 2002; Lottermoser 2007). Benzoic acids, fatty acids, amines, etc. were tested at lab scales and were found to be effective in limiting the pyrite oxidation (Kleinmann et al. 1981; Onysko et al. 1984). Chemical passivation is another technique for AMD prevention which deals with the use of chemically inert organic and inorganic materials for coating the surface of sulfidic minerals to limit its oxidation (Zhang and Evangelou 1998). Various organic (humic acid, lipids, fatty acids, polyethylene polyamine, alkoxysilanes, oxalic acid, catechol, 1, 3-benzenediamidoethanthiol, etc.) and inorganic (phosphate, silica, permanganate, alkaline materials, etc.) coating materials are used nowadays to prevent AMD generation from sulfidic minerals (Elsetinow et al. 2003; Zhang et al. 2003a, b; Khummalai and Boonamnuayvitaya 2005; Hao et al. 2009; Ačai et al. 2009; Liu et al. 2013; Shu et al. 2013; Park et al. 2018). Huang and Evangelou (1994) introduced a new microencapsulation technique that uses soluble phosphate salt, hydrogen peroxide, and sodium acetate to form a stable ferric phosphate coating on iron sulfide minerals, which inhibits its oxidation by limiting the oxidant. Electrochemical is a process in which sulfidic minerals act as an electrode as these minerals are known to behave like electronic conductors (Shelp et al. 1995). Several laboratory and field tests were conducted by ENPAR Technology Inc. 2000 and Shelp et al. (1995) where sulfide tailings were converted into electrodes of an electrochemical cell which removed the dissolved oxygen and lowered the redox potential, forming a thermodynamically stable environment and eventually inhibiting the pyrite oxidation. ENPAR Technology Inc. installed a pilot-scale electrochemical system on the pyrrhotite tailings in the Sudbury basin (Ontario, Canada) and Golden Sunlight Mine tailings of Montana, USA, in 2000 and 2002, respectively. Desulfurization is a method based on froth flotation principle which is widely used these days to separate sulfide-rich minerals from the waste, resulting in low-sulfur containing tailings which are non-acid generating (Benzaazoua et al. 2000; Hesketh et al. 2010; Nagase et al. 2011).
8.5 Acid Mine Drainage Treatment Options
In order to minimize the impact of AMD on the receiving environments such as rivers, soil, and groundwater, two major types of treatment options are available: (i) abiotic treatment (application of neutralizing agents to neutralize the AMD with precipitation of metals) and (ii) biotic treatment (application of biostimulation and bioaugmentation agents). Both abiotic and biotic remediation strategies are divided into two parts: (i) active and (ii) passive (Fig. 8.1).
8.5.1 Abiotic Remediation Strategies
8.5.1.1 Active Technologies
A wide range of neutralization materials is available that can be used for the treatment of AMD (Johnson and Hallberg 2005a; Skousen et al. 2017). These neutralization agents (limestone, quicklime, hydrated lime, magnesite, dolomite, ammonia, sodium hydroxide, cement kiln dust, barium hydroxide, barium carbonate, fly ash, lime kiln dust, etc.) increase the pH of the AMD which lead to the precipitation of certain heavy metals. This pH-dependent metal precipitation is applicable for most of the heavy metals (Cu, Ni, Zn, Fe, Ag, Se, Pb, Cd, Th, Be, and Sb) but few remain unaffected [Hg. Cr (VI), Mo, and As (III)] or uncertain (Co and Bi) [Taylor et al. 2005]. This neutralization technology is adopted by several mines worldwide to reduce the acidity and recover the metals from the AMD (Skousen et al. 2017 and references therein). The choice of the neutralization agent depends upon the mine type or the metal contents of the AMD (Trumm 2010). Each neutralization agent has specific chemical properties like solubility and pH (Taylor et al. 2005). Calcium- and magnesium-based carbonate materials can be used as neutralization agents for this application but few carbonate minerals such as siderite, rhodochrosite, and ankerite may increase the acidity of Fe and Mn upon treatment of AMD (Taylor et al. 2005). There are other important active treatment options available including reverse osmosis, aeration, ion exchange, electrodialysis, and natural zeolites (Feng et al. 2000; Potgieter-Vermaak et al. 2006; Zhong et al. 2007; Motsi et al. 2009; Buzzi et al. 2013; Rodríguez-Galán et al. 2019 and references therein). Active treatments are useful in minimizing the detrimental effect of AMD, but it has few disadvantages like (i) it requires continuous input of chemicals, (ii) it is expensive, (iii) some of the chemicals are toxic and require safe handling, and (iv) it is labor- intensive.
8.5.1.2 Passive Technology
Abiotic passive treatment of AMD includes anoxic limestone drains (ALD), open limestone channels (OLC), limestone leach bed (LLB), limestone sand treatment (LST), steel slag leach bed (SSLB), limestone diversion well (LDW), and low-pH Fe oxidation channels (Skousen et al. 2017). These strategies are economically more cheap and eco-friendly than active treatment. Anoxic limestone drain (ALD) is the approach where limestone drain is covered to avoid the passage of air into the drain system, and to keep the dissolved iron in the reduced form (Skousen et al. 2017 and references therein). This process is applicable if the dissolved oxygen concentration is below 1mg/L (Taylor et al. 2005). In this system limestone dissolves and increases the pH by adding bicarbonate alkalinity (Taylor et al. 2005). Long residence time is encouraged for higher efficiency as well as low DO is required; otherwise armoring of the limestone takes place with iron minerals, reducing the rate of limestone dissolution. Open limestone channel (OLC) is the drainage system equipped with large coarse limestone over which AMD flows (Skousen et al. 2017). There are several studies in which the efficiency of OLCs has been assessed for the treatment of AMD (Ziemkiewicz et al. 1997; Ziemkiewicz and Brant 1996; Cravotta 2007; Cravotta III 2008a, b; Cravotta and Ward 2008). These studies reported the higher neutralization efficiency of the incoming AMD. It requires a large area for effective operation and gradient of the slope of the drainage such that it neither passes the AMD very quickly nor accumulates the metal precipitates within the void spaces (Skousen et al. 2017). Limestone diversion well (LDW) is consisted of well with crushed limestone. The AMD stream is diverted into these wells through pipelines to increase the alkalinity of the AMD and reduce the acidity with metal precipitation. Armoring of limestone is less due to the adequate mixing of water. Reduction of acidity with increase in pH was observed in a few studies using LDW for treatment of AMD (Arnold 1991; Faulkner and Skousen 1994; Ziemkiewicz and Brant 1996). Limestone leach bed (LLB) is a small basin carrying small-sized limestones to treat AMD or mine discharge. It can be constructed near an AMD seep or underground mine drainage (Skousen et al. 2017 and references therein). A minimum of 30 min time is sufficient to reduce 50% acidic load of moderately acidic AMD (Black et al. 1999). It was suggested that LLB was useful at the upstream of OLC, as it shortened the time and improved the efficiency (Ziemkiewicz et al. 2003). A manually flushed upflow LLB was constructed at Strattanville, PA, to treat water with 400– 650 mg L−1 acidity and pH 4.5 at a flow rate of 380–570 L min−1 (Schueck et al. 2004). Limestone sand treatment (LST) can be used for treatment of AMD stream by the addition of sand-size limestone to the stream (Skousen et al. 2017). It causes precipitation of heavy metals due to neutralization of AMD, and armoring of limestone is less due to agitation caused by the flowing streams. Steel slag leach beds (SSLB) are used to treat AMD due to the alkalinity content (45–78% CaCO3) of basic steel slag. Slags generated from stainless steel are toxic than basic steel slags. So it can be used as a potent neutralization agent for AMD treatment. AMD and metal-free water with acidity were treated by slag (Ziemkiewicz and Skousen 1998; Simmons et al. 2002a, b; Skousen et al. 2017 and references therein). Low-pH Fe oxidation channels can be used to partially treat high Fe discharges (Skousen et al. 2017). In this technique, a shallow channel is built lined with limestone or sandstone to increase the Fe oxidation.
8.5.2 Biological Remediation Technologies
The capacity of iron- and sulfate-reducing microorganisms plays substantial roles in generating alkalinity, heavy metal removal, sulfate reduction, and metal recovery, thereby attenuating the AMD-generation process (Johnson and Hallberg 2005a; Roychowdhury et al. 2015). Macrophytes are also known to play an important role in metal removal in various wetland systems (Roychowdhury et al. 2015). The excessive presence of Fe3+ and SO42- in the AMD system tends to be of major importance as they can generate alkalinity with the help of their respective reducing microbial populations. AMD system is very low in organic carbon content (less than 10 mg/L), as a result of which these indigenous microorganisms cannot promote iron- and sulfate-reducing events naturally (Johnson 2012). In order to promote the growth and activities of these microorganisms, supplementation of organic carbon substrates is required. These organic carbons act as electron donors while the available Fe3+ and SO42- act as electron acceptors for these microbial populations. Sulfate-reducing members on undergoing dissimilatory sulfate reduction process generate H2S which increases the pH of the system. On the contrary, iron reduction by iron-reducing populations generates Fe2+ in the system which on reaction with H2S forms iron sulfide, thereby removing the iron and sulfate contents from the AMD system with raise in pH (Fig. 8.2). Other metals present in the AMD such as Cu, Ni, Zn, and Cd also get precipitated in the form of their metal sulfides upon reaction with H2S. Biologically mediated remediation technologies are presented in this section in details.
Mechanism of sulfate- and iron-reducing bacteria in bioremediation of AMD. SRB and IRB indicate sulfate- and iron-reducing bacteria. Biochemical reactions operated by SRB/IRB during sulfate/iron reduction processes while using organic carbons (lactate, formate, and acetate) and H2. Metal precipitation reaction is represented in red (M: Metal)
8.5.2.1 Passive Treatment Options
Different passive biological treatment options are available which can be used for the development of environment-friendly and sustainable technology for remediation of AMD (Johnson and Hallberg 2005a). Wetland systems (aerobic and anaerobic) have been employed to eliminate metals and sulfate and reduce the acidity of AMD by chemical, physical, microbial, and plant-mediated processes (Pat-Espadas et al. 2018). Aerobic wetlands depend upon the oxidation of metals and precipitation of metals in the form of metal hydroxides (Roychowdhury et al. 2015). Wetland plants remove the metals from the AMD via rhizofiltration (adsorption/adsorption/precipitation of metals in rhizosphere) and phytoextraction (uptake of metals and store them in plant bodies) (Roychowdhury et al. 2015 and references therein). Anaerobic wetland system utilizes the potential of SRB for removal of heavy metal via metal precipitation through sulfate reduction (Pat-Espadas et al. 2018). There are reports where constructed wetland systems decreased the heavy metal contents up to a great extent and reduced the acidity of AMD (Sheoran and Sheoran 2006; Nyquist and Greger 2009; Sheoran 2017; Pat-Espadas et al. 2018). Acidity of AMD, metal contents, and seasonal variations are considered to be important factors of wetland system to enhance the performance of AMD treatment. Permeable reactive barrier (PRB) is the treatment option for AMD where a reactive barrier is installed in the path of contaminated water flow (Benner et al. 1999). Reactive mixture includes limestone and organic matter which promote the activities of SRB, leading to the decrease in metal and sulfate contents and acidity (Benner et al. 1997; Waybrant et al. 1998)). The potential of different organic matters for reactive mixture had been tested for treatment of AMD, and many other studies have been conducted to assess the potential of PRB in remediation (Blowes et al. 2000; Gibert et al. 2004; Golab et al. 2006; Pagnanelli et al. 2009; Gibert et al. 2011; Shabalala et al. 2014 and references therein). Infiltration bed technology passes the surface water contaminated with AMD from the channel comprising a bed of organic substrates that promote and sustain the SRB activities (Vestola 2004). This study reported the removal of Cu, Zn, Fe, and Mn from the AMD-contaminated water up to 99%. Anoxic pond is the open pit which receives AMD and treatment of AMD is performed via addition of organic substrates and SRB (Riekkola-Vanhanen and Mustikkamaki 1997). It was reported that increase in sulfate-reduction activity was observed during treatment of AMD in anoxic pond upon supplementation with liquid manure and press juice from silage. Increase in SRB activities resulted in the decrement of heavy metal and sulfate concentration with slow rise in pH (Riekkola-Vanhanen and Mustikkamaki 1997). Injection of substrates into subsurface is the method to treat the groundwater contaminated by AMD by enhancing the growth and activity of SRB through injecting the substrates into the subsurface by boreholes (Canty 1999). Canty (1999) reported the increase in pH and removal of Al, Cd, and Zn from mine wastewater flowing through the mine shaft containing organic substrates. Unwanted discharge of substrates, leakage, and spill outs are the major concerns of this method (Canty 1999).
8.5.2.2 Active Treatment Options
Active treatment options of biologically mediated remediation technology depend upon the sulfidogenic activity of the sulfate-reducing microorganism. The sulfidogenic activity of the microorganisms radically improves the process of remediation to mitigate the impact of AMD on ecosystem. There are several advantages of active biological treatment systems such as recovery of selective heavy metals, lowering of significant amount of sulfate and heavy metals from the AMD, along with predictable and readily controllable performance of the treatment system (Jonhson and Hallberg 2005a, b; Neculita et al. 2007; Kefeni et al. 2017; Nancucheo et al. 2017; Kiran et al. 2017). Due to these properties, this technology is preferred over the passive treatment options. There are several reports in the literature where sulfidogenic bioreactors were used to treat AMD or wastewater containing heavy metals through sulfate reduction process (Hao et al. 2014; Kefeni et al. 2017; Nancucheo et al. 2017 and references therein). In the current scenario, in order to remediate AMD/acidic mine water, researchers have been employing neutrophilic sulfate-reducing populations in offline bioreactor system, i.e., avoiding the direct exposure of SRB to the acidic water which are lethal to them (Nancucheo et al. 2017). In contrast, few studies have been conducted to treat AMD with the help of acidophilic SRB in sulfidogenic bioreactors (Kimura et al. 2006; Nancucheo and Johnson 2012, Hedrich and Johnson 2014; Santos and Johnson 2017; Johnson and Santos 2018; González et al. 2019).
Several studies have been conducted using the different kinds of sulfidogenic bioreactors/microcosms/mesocosms for the treatment of acid mine drainage which include either of the strategies: (i) biostimulation, (ii) bioaugmentation, and (iii) biostimulation and bioaugmentation (Jong and Parry 2003; Kaksonen et al. 2004; Church et al. 2007; Neculita et al. 2007; Hiibel et al. 2008; Becerra et al. 2009; Bijmans et al. 2009; Hiibel et al. 2011; Neculita et al. 2011; Burns et al. 2012; Nancucheo and Johnson 2012; Xingyu et al. 2013; Zhang and Wang 2016; Nancucheo and Johnson 2014; Lefticariu et al. 2015; Zhang et al. 2016; Kefeni et al. 2017; Vasquez et al. 2018; Johnson and Santos 2018; González et al. 2019). Biostimulation and bioaugmentation are complementing each other as these strategies provide substantial remediation efficiencies than any other systems (Tyagi et al. 2011). Biostimulation denotes the addition of organic carbon as electron donor or nutrient in nutrient-deficient systems to enrich the indigenous population for the remediation purpose. Bioaugmentation describes the addition of extraneous culture of single isolates capable of performing desired functions. Biostimulation and bioaugmentation approaches denote the supplementation of nutrient and biological culture/strain in the treatment systems.
8.5.2.2.1 Biostimulation-Based Bioremediation of AMD or Mine Discharge
Different biostimulation agents were used to promote the growth and activities of SRB for the treatment of AMD. Table 8.1 tabulates the amendment use for the biostimulation-based bioremediation of AMD. A detailed list of biostimulation studies performed in last 15 years was prepared and tabulated with their major findings and type of amendments used for achieving the bioremediation goals (Table 8.1). In this section, only those studies are described where microbiological investigation was performed through mesocosms, microcosms, and bioreactors-based treatment of AMD to understand the role of indigenous microbiota in such process (Table 8.2). Kaksonen et al. (2004) reported the use of lactate and ethanol for sulfate reduction to treat AMD and found that microbial diversity of ethanol-fed bioreactors was more diverse than lactate-fed ones and were mostly dominated by members of Proteobacteria (especially Deltaproteobacteria). Kaksonen et al. (2006) reported the use of ethanol in sulfate reduction and higher HRT promoted sulfate removal up to 81%. Members such as Desulfovibrio, Desulfotomaculum, Desulfobulbus, Desulforhabdus, and Desulfobacca were found to be involved in sulfate reduction process in this study. Zagury et al. (2006) attempted to remediate the AMD through addition of six different organic carbon substrates to enhance the sulfate reduction and metal precipitation in batch reactors. The results suggested enhanced metal removal efficiencies (94–99% for Zn, Ni, Cd, Mn, and Fe). This study also suggested that mixture of organic materials was found to better in terms of sulfate reduction followed by ethanol, maple woods, and single substrates. Increase in several orders of SRB cell numbers from initial day to the end of experiment suggested the role of organic carbons in enhancing the activities of SRB. Church et al. (2007) reported the sulfate reduction at low pH and confirmed the involvement of SRB members such as Desulfosporosinus, Bacillus, and Clostridium in sulfate reduction process. Hiibel et al. (2008) compared the microbial community structure of two field-scale pilot sulfate-reducing bioreactors different in configurations used for treating AMD. The results showed that around 60% sulfate were removed from the AMD in one of the reactors, which was almost 40% greater than other bioreactors. The increase in abundance of Desulfovibrio taxa was quantified by qPCR, and clone library suggested that the presence of SRB (Desulfosporosinus, Desulfobulbus, and Desulfovibrio) in the bioreactors confirmed the enhanced reduction of sulfate. The effect of three different organic substrates (ethanol, hay and wood chips, and corn stover and wood chips) on the performance and microbial communities present in pilot-scale biochemical reactors treating mine drainage were observed (Hiibel et al. 2011). Results revealed the differences in microbial diversity across the bioreactors fed with different substrates. Desulfovibrio and Desulfomicrobium were the dominant species detected in the bioreactors. Behum et al. (2011) reported successful treatment of AMD through anaerobic sulfate-reducing bioreactor with increase in pH up to 6.8, 99% removal of Fe and Al, and 42% reduction in sulfate content. Desulfobacca was found to be the dominant SRB in this treatment. Burns et al. 2012 demonstrated the performance of pilot-scale bioreactor and microbial community dynamics treating AMD. The results indicated the successful increase in pH from 3.09 to 6.56 with lowering of total iron and sulfate levels upto 95.9% and 67.4%, respectively. The microbial diversity was assessed through 16S rRNA gene and dsrA gene-based clone library, and the results suggested the involvement of various SRB members. Nancucheo and Johnson (2012) treated synthetic AMD using glycerol and confirmed 98% reduction in sulfate with the help of sulfate-reducing bacteria (Desulfosporosinus). Lefticariu et al. (2015) suggested that organic carbon substrates are promising agents for the enhancement of sulfate reduction. In this study different herbaceous and woody organic substrate along with limestone amended bioreactors were used to treat AMD and the results showed the increase in herbaceous content resulted in more sulfate and metal removal. Microbial diversity analysis also confirmed the increase in cellulolytic and fermentative bacteria along with sulfate reducers. In another study, where different organic reactive mixtures were tested on the treatment of AMD along with role of different hydraulic retention times (HRT) on efficiency, reactive mixture and microbial diversity was also assessed (Vasquez et al. 2016a, b; Vasquez et al. 2018). The results from these studies suggested that mixtures were found to increase the pH and alkalinity, reduced the sulfate (>70%), and removed Fe2+ and Zn2+ (>99%). Different retention times also enhanced the pH and reduced the sulfate content, but the best was observed with 4 days HRT. The microbial diversity assessment of these bioreactors during the treatment suggested the enhancement in abundance of SRB belonging to genera Desulfovibrio, Desulfomicrobium, Desulfobulbus, Syntrophobacter, Desulfococcus, Desulfomona, Desulfobacter, and other fermentative groups. Influence of inorganic ligand availability and organic carbon on the performance of bioreactors and microbial community composition on the treatment of Zn-laden mine water was investigated. The results revealed the removal of zinc in the form of zinc sulfide after few days of treatment and no difference was observed in the microbial community composition of the bioreactors amended with alfalfa and wood chips. Rice bran was used as a carbon source to treat AMD through sulfate-reducing bioreactors (Sato et al. 2018). The results revealed the reduction of sulfate with successful enrichment of SRB member (Desulforhabdus), and decrement of metal concentration was observed. Giachini et al. (2018) used the poultry-derived biochar to treat AMD and was successful in the removal of sulfate and metals. Grubb et al. (2018) reported the successful removal of metals (>90%) from AMD with active involvement of SRB members.
8.5.2.2.2 Bioaugmentation or Biostimulation and Bioaugmentation-Based Bioremediation of AMD or Mine Discharge
In contrast to biostimulation, there are several studies where the potential of acidophilic or neutrophilic sulfate-reducing bacteria was used to treat AMD. These sulfate-reducing microbial consortia were developed either from municipal waste/activated anaerobic sludge (Bai et al. 2013; Zhang et al. 2016; Zhang and Wang 2016) or mine environment (Elliott et al. 1998; Jong and Parry 2003; Nancucheo and Johnson 2012; Jing et al. 2018; Gonzalez et al. 2019). Low-pH sulfidogenic bioreactor was operated by acidophilic SRB as these members were capable to perform sulfidogenesis at low pH (Kimura et al. 2006; Nancucheo and Johnson 2012; Johnson and Santos 2018). A detailed list of bioaugmentation- and biostimulation-based treatment studies were tabulated and represented in Table 8.2. The role of SRB-containing consortium in remediation of acidic metal and sulfate-rich water has been successfully demonstrated (Nancucheo et al. 2017). Thiopaq®, by Paques, the Netherlands, and BioSulphide®, developed by BioteQ Environmental Technologies Inc., Canada, are the two patented bioreactors using SRB which are being implemented in various sites including Budelco zinc refinery, the Netherlands; Gold Mine Pueblo Viejo, Dominican Republic; Kennecott Utah Copper Mine, Utah; and Copper Queen Mine, Bisbee, Arizona, respectively, for AMD remediation and metal recovery (Nancucheo et al. 2017).
8.6 Sulfate-Reducing Bacteria and Their Application in Remediation of Acid Mine Drainage
8.6.1 Taxonomy
Sulfate-reducing prokaryotes are anaerobic and utilize sulfate (most oxidized form of sulfur) as their terminal electron acceptor for oxidation of organic compounds (fatty acids, alcohol, hydrocarbon, etc.) and hydrogen (Plugge et al. 2011; Muyzer and Stams 2008). These bacteria are known to play an important role in global carbon and sulfur cycles (Crowe et al. 2014). Sulfate-reducing prokaryotes are mainly present in Deltaproteobacteria (subdivision of Proteobacteria), while few other members are present in domain of Nitrospira, Thermodesulfobacteria, Clostridia, and Euryarchaeota/Crenarchaeota (Stahl et al. 2002; Rabus et al. 2006; Rabus et al. 2013) (Fig. 8.3). Based on rRNA sequence analysis, these SRB can be classified into four major phylogenetic lineages: Gram-negative mesophilic SRB (e.g., Desulfovibrio, Desulfomonile, Desulfobulbus), Gram-positive spore-forming SRB (e.g., Desulfotomaculum), thermophilic bacterial SRB (e.g., Thermodesulfobacterium and Thermodesulfovibrio), and thermophilic archaeal SRB (e.g., Achaeoglobus) (Castro et al. 2000). Both 16S rRNA and dsrAB (dissimilatory sulfite reductase) genes were used as molecular markers for identification of SRB in any environment. Fingerprinting of dsrAB gene through t-RFLP, DGGE, and gel-retardation analyses were used to determine the SRB populations of any ecosystems (Wagner et al. 2005; Geets et al. 2006). It is important to understand the nutritional requirement of diverse SRB to utilize their potential for bioremediation processes.
Neighbor-joining phylogenetic tree based on 16S rRNA gene sequence of type strain of sulfate-reducing taxa at 1000 bootstrap. Scale bar refers to a phylogenetic distance of 0.05 nucleotide substitutions per site. All the sequences of the type strains were recovered from Ribosomal Database Project except for Caldivirga and Archaeoglobus whose 16S rRNA gene sequences were retrieved from NCBI while n represents the number of type stains used for construction of phylogenetic tr
8.6.2 Metabolic Versatility
Metabolic versatility of SRB is well established in the 1970s (Plugge et al. 2011 and references therein). In the last few decades, several biochemical studies have been conducted to understand their mode of metabolism [autotrophic (grow autotrophically with hydrogen and fixing CO2), litho-autotrophic (reduced mineral and CO2), or heterotrophic (utilize organic carbons)], suitable carbon substrates (electron donor), or terminal electron acceptors for efficient growth and energy generation process (Rabus et al. 2006; Fichtel et al. 2012; Hussain and Qazi 2016; Agostino and Rosenbaum 2018; Qian et al. 2018 and references therein). In addition to sulfate, various sulfur species (sulfite, thiosulfate, and tetrathionate) have been used by SRB as terminal electron acceptors (Rabus et al. 2006; Muyzer and Stams 2008). Various carbon compounds can be used by heterotrophic SRB including sugars, amino acids, alcohols monocarboxylic acids, dicarboxylic acids, and aromatic compounds (Fauque 1995; Fauque and Ollivier 2004; Rabus et al. 2006; Zagury et al. 2007; Muyzer and Stams 2008). Sulfate reducers are known to grow syntrophically with methanogenic populations in both sulfate-rich and sulfate-depleted marine environments for mineralization of organic carbon (Odom and singleton 1993; Raskin et al. 1996; Finke et al. 2007; Xiao et al. 2017). Sulfate-reducing populations coexist with methanogens by competing for common substrates, i.e., hydrogen for their growth and metabolism (Stams and Plugge 2009; Paulo et al. 2015; Ozuolmez et al. 2015). Thus, some SRB showed dual life styles: sulfidogenic and syntrophic metabolism (Plugge et al. 2011). These dual metabolic potentials improve the chance of survival of SRB under sulfate depletion condition.
8.6.3 Dissimilatory Sulfate Reduction Pathway
Sulfate reduction process occurs in three steps: (i) activation of sulfate with two ATP molecules by ATP sulfurylase to generate adenosine phosphosulfate (APS) and pyrophosphate, (ii) reduction of APS into sulfite by APS reductase with release of AMP and consumption of two electrons, and (iii) reduction of sulfite to sulfide. Two mechanisms were proposed for the last step of sulfate reduction: (i) direct pathway, in which sulfite is directly converted to sulfide with the help of bisulfite reductase (Peck and LeGall 1982), and (ii) trithionate pathway, in which sulfite is first converted to trithionate which is further converted into thiosulfate, followed by reduction of thiosulfate to sulfide with the help of sulfite reductase, trithionate reductase, and thiosulfate reductase, respectively (Kobayashi et al. 1969, 1974). Chambers and Trudinger (1975) reported that neither thiosulfate nor trithionate was a normal intermediate in the reduction pathway. Santos et al. (2015) reported that sulfite was first converted to DrsC trisulfide by dsrAB/dsrC genes which was further reduced to DsrCr and sulfide by dsrMKJOP complex. They further reported that DsrC was a co-substrate for sulfite reduction by dsrAB and absence of DsrC or high bisulfite concentration lead to the production of thiosulfate and trithionate as in vitro products of dsrAB. In order to understand the rate of sulfate reduction and sulfate transportation with the formation of different intermediary products intracellularly, isotopic fraction of sulfur was examined to understand the sulfur transfer pathway in dissimilatory sulfate reduction (Sim et al. 2017).
8.6.4 Substrate-Level Phosphorylation
Sulfate-reducing microbes are known to utilize diverse organic carbon for their metabolism. Based on their mode of oxidation, they are categorized into (i) complete oxidizers – which completely oxidize organic carbon to CO2, and (ii) incomplete oxidizers, which form acetate as an end product on oxidation of organic carbon (Castro et al. 2002). Out of the known SRB species, 70% of the species fall into incomplete oxidation category (Qian et al. 2018).
Lactate is considered to be the most common organic carbon substrate/electron donor for the SRB (Plugge et al. 2011). It is oxidized to pyruvate by a D-or L-lactate dehydrogenase which is ultimately oxidized to acetate. Lactate oxidation yields two molecules of ATP through substrate-level phosphorylation, which are used for activation of sulfate during first step of sulfate reduction pathway resulting in no net gain of ATP. Two hypothetical models are proposed for electron transfer from lactate oxidation to sulfate reduction process: (i) H2 cycling pathway in which H2 is produced from cytoplasmic and periplasmic hydrogenases which ultimately transfers electron to terminal reductase via cytochrome and transmembrane complexes (Heidelberg et al. 2004) and (ii) second pathway that transfers electrons from lactate oxidation directly to the membrane-bound menaquinone pool which further transfers the electrons to terminal reductase via transmembrane-bound complexes (Keller and Wall 2011). Citric acid cycle (CAC) and oxidative carbon monoxide dehydrogenase (OCMD) pathways are operated in SRB to completely oxidize the acetate (Goevert and Conrad 2008 and references therein). But most of the organisms utilize OCMD as it operates at more negative redox potential [greater than that of the APS/HSO3- couple (-60 mV) and HSO3- /HS- couple (-116 mV)] than CAC pathway (Thauer et al. 2007). Propionate is another electron donor and carbon source for several incomplete and complete SRB (Widdel and Pfennig 1982; Suzuki et al. 2007; El Houari et al. 2017). Methylmalonyl-CoA pathway was considered to be operated in Desulfobulbus propionicus for its incomplete oxidation (Widdel and Pfenning 1982). This pathway was found to be more thermodynamically/energetically favorable than oxidation of propionyl-CoA via acrylyl-CoA, because redox potential of the acrylyl-CoA/propionyl-CoA couple is more positive (+69 mV) than fumarate/succinate couple (þ33 mV) (Barton and Hamilton 2007). The completely oxidizing sulfate reducers use the classical b-oxidation pathway to degrade fatty acids (Janssen and Schink 1995a, b). The electron donor or carbon source plays an important role in the metabolic pathways of SRB. It was reported that several other important organic carbons were utilized by SRB for sulfate reduction. Complex polysaccharides, amino acid/protein, or alcohol enhance the sulfate-reduction ability of SRB (Neculita et al. 2007; Schmidtova and Baldwin 2011; Ayala-Parra et al. 2016; Hussain et al. 2016).
8.6.5 Electron Transport Phosphorylation
Electron transport phosphorylation occurs in SRB where H2 acts as an electron donor for dissimilatory sulfate reduction. In this pathway, H2 is oxidized by periplasmic hydrogenase (Hase1) of SRB to generate electron and H+ ion. The generated electron transfers to cytoplasmic heme type cytochrome C3, which transfers it to cytoplasmic dissimilatory sulfite reductase via transmembrane complex (da Silva et al. 2013). The H+ ion generated in this mechanism is pumped across the cytoplasmic membrane with proton motive pump. This results in a H+ gradient between the cytoplasm and periplasm resulting in the generation of ATP through phosphorylation of ADP with the help of membrane-bound ATP synthase (Fitz and Cypionka 1991). Odom and Peck (1981) reported the production of H2 during the growth of Desulfovibrio species on lactate/sulfate media. But genetic evidence confirmed that H2 cycling in Desullfovibrio alaskensis G20 mutant of type 1 cytochrome c3, Desulfovibrio vulgaris Hildenborough, mutants of Tp1-c3, Desulfovibrio gigas mutants of cytoplasmic hydrogenase or periplasmic hydrogenase, was not required for sulfate reduction (Heidelberg et al. 2004; Li et al. 2009; Keller and wall 2011; Sim et al. 2013; Morais-Silva et al. 2013; Keller et al. 2014; Price et al. 2014). Another potential mechanism for electron-transport phosphorylation is formate cycling. Heidelberg et al. (2004) reported that in Desulfovibrio vulgaris Hildenborough, formate is oxidized by formate dehydrogenases present in the periplasm. A putative cytoplasmic formate: hydrogen lyase was reported from the genome of D. alaskensis G20 which could convert cytoplasmic formic acid to H2 and CO2 or vice versa (Pereira et al. 2011). Electrons generated from the oxidation of formate can be transferred to cytoplasmic membrane for sulfate reduction via periplasmic electron carriers and the produced H+ ion creates a gradient across the membrane for ATP generation. Knockout of Desulfovibrio vulgaris Hildenborough for formate dehydrogenase showed reduced growth in lactate/sulfate media indicated the role of formate cycling in energy production (da Silva et al. 2013). In a cycA mutant of Desulfovibrio alaskensis G20, accumulation of formate was observed on lactate/sulfate media which suggested that formate formed during oxidation of lactate oxidation was immediately reoxidized. (Li et al. 2009). There are several transmembrane redox complexes such as Qrc complex, Qmo complex, DsrMKJOP complex, Hmc (high-molecular weight cytochrome) and Tmc (with a periplasmic type II cytochrome c3 subunit), Hdr/flox, nfn, and Rnf identified in SRB which are known to be involved in electron transfer to cytoplasm (Pereira et al. 1998, 2006; Li et al. 2009; Venceslau et al. 2010; Grein et al. 2010; Pereira et al. 2011; Ramos et al. 2012; Quintas et al. 2013; Keller et al. 2014; Meyer et al. 2014).
8.6.6 Extracellular Electron Transfer Between ANME and SRB
Anaerobic methane oxidation by archaeal members in association with sulfate-reducing bacteria offers extracellular electron transfer to SRB for dissimilatory sulfate reduction process (Boetius et al. 2000). Various studies have been conducted to understand the genetic potential of ANME archaea and syntrophic association with SRB members (Skennerton et al. 2017 and references therein). The two possible mechanisms of methane oxidation coupled with sulfate reduction were proposed (Milucka et al. 2012; McGlynn et al. 2015; Wegener et al. 2015; Scheller et al. 2016; Skennerton et al. 2017). Wegener et al. (2015) suggested that under thermophilic anaerobic methane oxidation (TAOM) conditions, both ANME and the HotSeep-1 bacteria overexpressed the extracellular cytochrome production genes and formed cell-to-cell connections by expressing the genes for pili production which is responsible for interspecies electron transfer between syntrophic consortia. In contrast to the previous mechanism, Milucka et al. (2012) confirmed that ANME-2 oxidized methane with a concomitant reduction of sulphate to zero-valent sulfur for extracellular electron transfer. Hence, it can be concluded that AOM is not an obligate syntrophic process. Produced S0 is exported outside the cell where it reacts with sulfide to form disulfide. Disulfide is disproportionated to sulfate and sulfide by sulfate-reducing Deltaproteobacterial members.
8.6.7 SRB in Acidic Condition and Factors Affecting SRB Activities
It was believed that SRB thrive in environmental conditions above pH 5.0 (Hao et al. 1996). In the last decades, several studies confirmed the possibility of sulfate reduction at low pH condition (pH < 5.0) (Sánchez-Andrea et al. 2014). The presence of SRB in acidic environment has been reported in several AMD sites and was suggested that the existence of micro-niches of higher pH in such environments supported the growth and metabolism of SRB (Sánchez-Andrea et al. 2014 and references therein). It is reported that low pH destabilizes the macromolecules (proteins get denatured), changes the conformation of surface protein due to protonation as well as increases energy cost for pH homeostasis (Golyshina and Timmis 2005; Krulwich et al. 2011). Acidophilic organisms have different strategies to maintain the circum-neutral pH in the cytosol by pumping out the H+ ion, changing the permeability of membrane, increasing the positive redox potential of cytoplasm through potassium transporter, and decarboxylation of amino acids (Chen et al. 2015 and references therein). At low pH, H2S and organic acids are also toxic to SRB (Koschorreck 2008). Sulfate reduction process generates H2S as the main product, which remains freely present in undissociated form at pH < 5.0 (Moosa and Harrison 2006). Furthermore, it has a negative impact on cells due its toxic nature as well as high membrane permeability. There are several reports where inhibition of sulfate reduction with increase in sulfiide concentration was observed (Reis et al. 1992; Icgen and Harrison 2006; Koschorreck 2008; Cassidy et al. 2015 and references therein). Similarly, low molecular weight organic compounds such as acetate and lactate are mostly in undissociated state at low pH, which may pass the cell membrane and get dissociated inside the cytoplasm releasing proton which in turn lowers the pH (Sanchez-Andrea et al. 2014 and references therein). The nonionic substrates such as glycerol, hydrogen, and ethanol were more preferred at low pH (Nagpal et al. 2000; Kaksonen et al. 2004; Bijmans et al. 2009). Benner et al. 1999 reported that anaerobic bacteria change their mode of carbohydrate metabolism to avoid the accumulation of organic acid inside the cytoplasm at low pH condition. There are reports where SRB activity decreases with increasing undissociated concentration of lactate or acetate (Reis et al. 1990; Jong and Parry 2006). Other than the abovementioned factors, several other factors are known to be inhibitory for sulfate-reducing taxa. Heavy metals are considered to be toxic to several SRB above certain threshold (Utgikar et al. 2002; Sani et al. 2001). Effect of copper toxicity on sulfate removal efficiency was observed and found that 1.57mM copper concentration reduced the efficiency of SRB for sulfate removal to 50% (Song et al. 1998). Sani et al. 2001 showed the toxic effects of copper, zinc, and lead on Desulfovibrio desulfurican G20. It was found that at copper concentration of 13.3uM, specific growth rate of D. desulfurican G20 got reduced to 50%. In another study 10uM concentration of lead was shown as minimum inhibitory concentration for Desulfovibrio desulfurican G20 at pH 6.0 (Sani et al. 2003). These studies suggest that heavy metals increased the lag phase and lower the specific growth rates of the tested species. Toxic effects of several heavy metals [Cu(II), Zn(II)], Mn(II), Ni(II), and Cr(III)] were evaluated through batch studies on two SRB cultures, Desulfovibrio vulgaris and Desulfovibrio sp (Cabrera et al. 2006 and references therein). The results indicated Cu > Ni > Mn > Cr > Zn showed maximum inhibitory effect on both the SRB cultures. Higher concentration of molybdate may act as an inhibitor for sulfate reduction as it is a structural analog of sulfate (Biswas et al. 2009; de Jesus et al. 2015). Hussain and Qazi (2016) reported that higher concentration (10 and 15 ppm) of Cu, Cr, and Ni inhibited bioprecipitation and sulphate reduction by Desulfotomaculum reducens-HA1 and Desulfotomaculum hydrothermale-HA2. It was reported that sulfate reduction could not be observed at oxic condition or in the presence of nitrate and nitrite (Rubio-Rincón et al. 2017). It was suggested that nitrite formation by nitrate inhibited the SRB activities, because nitrite suppressed the sulfate reduction (Barton and Hamilton 2007; Mohanakrishnan et al. 2008). SRB are known to reduce oxygen as a protective mechanism against its harmful effects (Lamrabet et al. 2011 and reference therein).
8.6.8 Resource Recovery from AMD Treatment Using SRB
Sulfate-reducing bacteria are known to have diverse metabolic properties and have been used in various treatment processes. The ability of SRB to produce biohydrogen, aliphatic hydrocarbon, polyhydroxyalkanoates (PHA), magnetite, and metal sulfides is well documented (Gramp et al. 2007; Gramp et al. 2010; Yu et al. 2011; Peltier et al. 2011; Friedman and Rude 2012; Martins and Pereira 2013; Hai et al. 2004; Hedrich and Johnson 2014; Martins et al. 2015). Sulfate reducers are capable of oxidizing various organic carbons and produce sulfide as the main product of sulfate reduction. Interaction of sulfide with the metals such as Fe, Cu, Ni, Zn, and Cd lead to the formation of metal sulfides. Sulfidogenic treatment of AMD leads to generation of metal sulfide, and also the generated sulfide could be used for the production of elemental sulfur via oxidation (aeration) (Nancucheo et al. 2017). There are two strategies generally used for metal sulfide production during treatment of AMD: (i) direct interaction of sulfide and metals (in-line sulfidogenic reactors) and (ii) sulfide produced during the sulfidogenesis is separated and used as a metal precipitating agent (offline sulfidogenic reactors). Thioteq (Paques) and BioSulfide (BioteQ) are the two offline sulfidogenic systems used for metal recovery from the AMD/wastewater treatment. Biogenic Ni and Zn sulfide precipitation were observed during the growth of SRB in Ni- and Zn-containing medium (Gramp et al. 2007). The Ni-sulfidic phase (Heazelwoodite) was more crystalline than its abiotic control setup. Gramp et al. (2009) reported the formation of iron sulfides (mackinawite and greigite) by the sulfate-reducing bacteria, and with the increase in incubation temperature of the bacteria, crystallinity of the iron sulfides got enhanced. Castillo et al. (2012) observed the zinc precipitation in the two polymorphs of ZnS (sphalerite and wurztite) while investigating the tolerance of SRB against zinc-rich sulfate medium for the application of SRB in AMD remediation. Hedrich and Johnson (2014) used the modular bioreactor for the recovery of metal sulfide (ZnS) during the treatment of metal-containing wastewaster. Zhou et al. (2014) reported the iron sulfide precipitation during the extended incubation of Desulfovibrio vulgaris, and higher sulfide accumulation led to the transformation of mackinawte to gregite. Murray et al. (2017) reported the use of waste H2S gas from AMD remediation to synthesize zinc sulfide quantum dots, which was found to be different in its physical and optical properties from its other chemical counterparts. Picard et al. (2018) reported that Desulfovibrio hydrothermalis AM13 influenced the nucleation and growth of iron sulfidic minerals. Overall, metal sulfides are known to have diverse applications including cancer therapy, antimicrobial agents, textile, optoelectronics, hydro-cracking in fuel refineries, and hydro-processing (Qian et al. 2018 and references therein).
8.7 Conclusion
The toxic and lethal effects of AMD or acidic mine water on environmental health are of major concern. This problem has gained attention from the researchers to find out a promising technology for its treatment or measures to prevent its generation. There are several prevention measures through which its generation can be checked, but several factors limit these options. There are remediation technologies being used including both passive and active treatment strategies. But the choice of remediation technologies depends upon various factors such as (i) mine type, (ii) type of waste generated, (iii) geochemistry of AMD, (iv) cost of the treatment system, and (v) sustainability and long-term treatment options. Due to high capital investment, requirement of large area, continuous input of neutralization agents, sludge accumulation and its disposal, as well as sustainability of chemical treatment options, biological treatments are found to be more attractive nowadays. Biological treatment provides cost-effective, sustainable, and eco-friendly options to treat AMD and recover metals from the AMD environment. Advancement in the molecular approaches allowed the researchers to gain a deeper insight of microbial community and composition, their functions, and interaction in such processes. Sulfidogenic activity and metabolic versatility of SRB play an important role in attenuation of AMD and metal precipitation. In future, research is needed to develop this biological technology for its application in more promising ways to treat AMD or AMD-impacted areas.
References
Acai P, Sorrenti E, Polakovič M, Kongolo M, Donato PD (2009) Pyrite passivation by humic acid investigated by inverse liquid chromatography. Colloid Surface Physicochem Eng Asp 337:39–46
Adams R, Ahlfeld D, Sengupta A (2007) Investigating the potential for ongoing pollution from an abandoned pyrite mine. Mine Water Environ 26(1):2–13
Agostino V, Rosenbaum MA (2018) Sulfate-reducing electroautotrophs and their applications in bioelectrochemical systems. Front Energy Res 6:55
Alhamed M, Wohnlich S (2014) Environmental impact of the abandoned coal mines on the surface water and the groundwater quality in the south of Bochum, Germany. Environ Earth Sci 72(9):3251–3267
Arnold DE (1991) Diversion wells–a low-cost approach to treatment of acid mine drainage. In: Proceedings, twelfth west Virginia surface mine drainage task force symposium, pp 3–4
Ayala-Parra P, Sierra-Alvarez R, Field JA (2016) Algae as an electron donor promoting sulfate reduction for the bioremediation of acid rock drainage. J Hazard Mater 317:335–343
Backes CA, Pulford ID, Duncan HJ (1987) Studies on the oxidation of pyrite in colliery spoil. II Inhibition of the oxidation by amendment treatments. Reclamation Revegetation Res 6(1):1–11
Bai H, Kang Y, Quan H, Han Y, Sun J, Feng Y (2013) Treatment of acid mine drainage by sulfate reducing bacteria with iron in bench scale runs. Bioresour Technol 128:818–822
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44(2):139–152
Barton LL, Hamilton WA (eds) (2007) Sulphate-reducing bacteria: environmental and engineered systems. Cambridge University Press, Cambridge
Bayrakdar A, Sahinkaya E, Gungor M, Uyanik S, Atasoy AD (2009) Performance of sulfidogenic anaerobic baffled reactor (ABR) treating acidic and zinc-containing wastewater. Bioresour Technol 100(19):4354–4360
Becerra CA, López-Luna EL, Ergas SJ, Nüsslein K (2009) Microcosm-based study of the attenuation of an acid mine drainage-impacted site through biological sulfate and iron reduction. Geomicrobiol J 26(1):9–20
Behum PT, Lefticariu L, Bender KS, Segid YT, Burns AS, Pugh CW (2011) Remediation of coal-mine drainage by a sulfate-reducing bioreactor: a case study from the Illinois coal basin, USA. Appl Geochem 26:S162–S166
Bekmezci OK, Ucar D, Kaksonen AH, Sahinkaya E (2011) Sulfidogenic biotreatment of synthetic acid mine drainage and sulfide oxidation in anaerobic baffled reactor. J Hazard Mater 189(3):670–676
Benner SG, Blowes DW, Ptacek CJ (1997) A full-scale porous reactive wall for prevention of acid mine drainage. Groundwater Monit Remediation 17(4):99–107
Benner SG, Blowes DW, Gould WD, Herbert RB, Ptacek CJ (1999) Geochemistry of a permeable reactive barrier for metals and acid mine drainage. Environ Sci Technol 33(16):2793–2799
Benzaazoua M, Bussière B, Kongolo M, McLaughlin J, Marion P (2000) Environmental desulphurization of four Canadian mine tailings using froth flotation. Int J Miner Process 60(1):57–74
Bijmans MF, Dopson M, Peeters TW, Lens PN, Buisman CJ (2009) Sulfate reduction at pH 5 in a high-rate membrane bioreactor: reactor performance and microbial community analyses. J Microbiol Biotechnol 19(7):698–708
Biswas KC, Woodards NA, Xu H, Barton LL (2009) Reduction of molybdate by sulfate-reducing bacteria. Biometals 22(1):131–139
Black C, Ziemkiewicz P, Skousen J (1999) Construction of a limestone leach bed and preliminary water quality results in Beaver Creek. In: Proceedings of the 20th West Virginia surface mine drainage task force symposium, Morgantown, WV
Blowes DW, Ptacek CJ, Benner SG, McRae CW, Bennett TA, Puls RW (2000) Treatment of inorganic contaminants using permeable reactive barriers. J Contam Hydrol 45(1–2):123–137
Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, Amann R, Jørgensen BB, Witte U, Pfannkuche O (2000) A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 407(6804):623–626
Brown DJA, Sadler K (1989) Fish survival in acid waters. Acid Toxic Aquat Anim 34:31–44
Burns AS, Pugh CW, Segid YT, Behum PT, Lefticariu L, Bender KS (2012) Performance and microbial community dynamics of a sulfate-reducing bioreactor treating coal generated acid mine drainage. Biodegradation 23(3):415–429
Buzzi DC, Viegas LS, Rodrigues MAS, Bernardes AM, Tenório JAS (2013) Water recovery from acid mine drainage by electrodialysis. Miner Eng 40:82–89
Cabrera G, Pérez R, Gomez JM, Abalos A, Cantero D (2006) Toxic effects of dissolved heavy metals on Desulfovibrio vulgaris and Desulfovibrio sp. strains. J Hazard Mater 135(1–3):40–46
Canty GA (1999) Utilization of coal combustion by-products for in situ treatment of acidic mine waters (Doctoral dissertation)
Casiot C, Egal M, Elbaz-Poulichet F, Bruneel O, Bancon-Montigny C, Cordier MA et al (2009) Hydrological and geochemical control of metals and arsenic in a Mediterranean river contaminated by acid mine drainage (the Amous River, France); preliminary assessment of impacts on fish (Leuciscus cephalus). Appl Geochem 24(5):787–799
Cassidy J, Lubberding HJ, Esposito G, Keesman KJ, Lens PN (2015) Automated biological sulphate reduction: a review on mathematical models, monitoring and bioprocess control. FEMS Microbiol Rev 39(6):823–853
Castillo J, Pérez-López R, Caraballo MA, Nieto JM, Martins M, Costa MC et al (2012) Biologically-induced precipitation of sphalerite–wurtzite nanoparticles by sulfate-reducing bacteria: implications for acid mine drainage treatment. Sci Total Environ 423:176–184
Castro HF, Williams NH, Ogram A (2000) Phylogeny of sulfate-reducing bacteria. FEMS Microbiol Ecol 31(1):1–9
Castro H, Reddy KR, Ogram A (2002) Composition and Function of Sulfate-Reducing Prokaryotes in Eutrophic and Pristine Areas of the Florida Everglades. Appl Environ Microbiol 68(12):6129–6137
Chambers LA, Trudinger PA (1975) Are thiosulfate and trithionate intermediates in dissimilatory sulfate reduction? J Bacteriol 123(1):36–40
Chen LX, Hu M, Huang LN, Hua ZS, Kuang JL, Li SJ, Shu WS (2015) Comparative metagenomic and metatranscriptomic analyses of microbial communities in acid mine drainage. ISME J 9(7):1579
Chen LX, Huang LN, Mendez-Garcia C, Kuang JL, Hua ZS, Liu J, Shu WS (2016) Microbial communities, processes and functions in acid mine drainage ecosystems. Curr Opin Biotechnol 38:150–158
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Appl Environ Soil Sci 2014:708–752
Chon HT, Hwang JH (2000) Geochemical characteristics of the acid mine drainage in the water system in the vicinity of the Dogye coal mine in Korea. Environ Geochem Health 22(2):155–172
Church CD, Wilkin RT, Alpers CN, Rye RO, McCleskey RB (2007) Microbial sulfate reduction and metal attenuation in pH 4 acid mine water. Geochem Trans 8(1):10
Clarke AM, Kirby R, Rose PD (2004) Molecular microbial ecology of lignocellulose mobilisation as a carbon source in mine drainage wastewater treatment. Water SA 30(5):558–661
Clyde EJ, Champagne P, Jamieson HE, Gorman C, Sourial J (2016) The use of a passive treatment system for the mitigation of acid mine drainage at the Williams Brothers Mine (California): pilot-scale study. J Clean Prod 130:116–125
Coggon M, Becerra CA, Nüsslein K, Miller K, Yuretich R, Ergas SJ (2012) Bioavailability of jarosite for stimulating acid mine drainage attenuation. Geochim Cosmochim Acta 78:65–76
Cravotta CA (2007) Passive aerobic treatment of net-alkaline, iron-laden drainage from a flooded underground anthracite mine, Pennsylvania, USA. Mine Water Environ 26(3):128–149
Cravotta CA III (2008a) Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 1: constituent quantities and correlations. Appl Geochem 23(2):166–202
Cravotta CA III (2008b) Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 2: geochemical controls on constituent concentrations. Appl Geochem 23(2):203–226
Cravotta CA, Ward SJ (2008) Downflow limestone beds for treatment of net-acidic, oxic, iron-laden drainage from a flooded anthracite mine, Pennsylvania, USA: 1. Field evaluation. Mine Water Environ 27(2):67–85
Crowe SA, Paris G, Katsev S, Jones C, Kim ST, Zerkle AL, Farquhar J (2014) Sulfate was a trace constituent of Archean seawater. Science 346(6210):735–739
da Silva SM, Voordouw J, Leitao C, Martins M, Voordouw G, Pereira IA (2013) Function of formate dehydrogenases in Desulfovibrio vulgaris Hildenborough energy metabolism. Microbiology 159(8):1760–1769
de Jesus EB, de Andrade Lima LRP, Bernardez LA, Almeida PF (2015) Inhibition of microbial sulfate reduction by molybdate. Braz J Petrol Gas 9(3):95–106
de Mello JWV, Dias LE, Daniel AM, Abrahão WAP, Deschamps E, Schaefer CEGR (2006) Preliminary evaluation of acid mine drainage in Minas Gerais State, Brazil. Rev Bras Ciênc Solo 30(2):365–375
Demers I, Bussière B, Benzaazoua M, Mbonimpa M, Blier A (2008) Column test investigation on the performance of monolayer covers made of desulphurized tailings to prevent acid mine drainage. Miner Eng 21(4):317–329
Deng D, Weidhaas JL, Lin LS (2016) Kinetics and microbial ecology of batch sulfidogenic bioreactors for co-treatment of municipal wastewater and acid mine drainage. J Hazard Mater 305:200–208
Diaby N, Dold B, Pfeifer HR, Holliger C, Johnson DB, Hallberg KB (2007) Microbial communities in a porphyry copper tailings impoundment and their impact on the geochemical dynamics of the mine waste. Environ Microbiol 9(2):298–307
Drennan DM, Almstrand R, Ladderud J, Lee I, Landkamer L, Figueroa L, Sharp JO (2017) Spatial impacts of inorganic ligand availability and localized microbial community structure on mitigation of zinc laden mine water in sulfate-reducing bioreactors. Water Res 115:50–59
El Houari A, Ranchou-Peyruse M, Ranchou-Peyruse A, Dakdaki A, Guignard M, Idouhammou L, Qatibi AI (2017) Desulfobulbus oligotrophicus sp. nov., a sulfate-reducing and propionate-oxidizing bacterium isolated from a municipal anaerobic sewage sludge digester. Int J Syst Evol Microbiol 67(2):275–281
Elliott P, Ragusa S, Catcheside D (1998) Growth of sulfate-reducing bacteria under acidic conditions in an upflow anaerobic bioreactor as a treatment system for acid mine drainage. Water Res 32(12):3724–3730
Elsetinow AR, Borda MJ, Schoonen MA, Strongin DR (2003) Suppression of pyrite oxidation in acidic aqueous environments using lipids having two hydrophobic tails. Adv Environ Res 7(4):969–974
Equeenuddin SM, Tripathy S, Sahoo PK, Panigrahi MK (2010) Hydrogeochemical characteristics of acid mine drainage and water pollution at Makum Coalfield, India. J Geochem Explorat 105(3):75–82
Faulkner BB, Skousen JG (1994) Treatment of acid mine drainage by passive treatment systems. Int Land Reclam Mine Drain Conf 2:250–257
Fauque GD (1995) Ecology of sulfate-reducing bacteria. In: Sulfate-reducing bacteria. Springer, Boston, pp 217–241
Fauque G, Ollivier B (2004) Anaerobes: the sulfate-reducing bacteria as an example of metabolic diversity. In: Microbial diversity and bioprospecting. American Society of Microbiology, Washington, DC, pp 169–176
Fenalcarbón (2006) Acuerdo de concertación para la producción mas limpia de la minería de carbón subterráneo. Federacion Nacional de Carboneros
Feng D, Aldrich C, Tan H (2000) Treatment of acid mine water by use of heavy metal precipitation and ion exchange. Miner Eng 13(6):623–642
Fichtel K, Mathes F, Könneke M, Cypionka H, Engelen B (2012) Isolation of sulfate-reducing bacteria from sediments above the deep-subseafloor aquifer. Front Microbiol 3:65
Finke N, Hoehler TM, Jørgensen BB (2007) Hydrogen ‘leakage’during methanogenesis from methanol and methylamine: implications for anaerobic carbon degradation pathways in aquatic sediments. Environ Microbiol 9(4):1060–1071
Fitz RM, Cypionka H (1991) Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol 155(5):444–448
Friedman L, Rude M (2012) U.S. Patent No. 8,110,093. U.S. Patent and Trademark Office, Washington, DC
Garrido AE, Condori J, Strosnider WH, Nairn RW (2009) Acid mine drainage impacts on irrigation water resources, agricultural soils, and potatoes in Potosi, Bolivia. In: Proceedings America Society of Mining and Reclamation, pp 486–499
Geets J, Borremans B, Diels L, Springael D, Vangronsveld J, Van Der Lelie D, Vanbroekhoven K (2006) DsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria. J Microbiol Methods 66(2):194–205
Gerhardt A, De Bisthoven LJ, Soares AMVM (2004) Macroinvertebrate response to acid mine drainage: community metrics and on-line behavioural toxicity bioassay. Environ Pollut 130(2):263–274
Giachini AJ, Sulzbach TS, Pinto AL, Armas RD, Cortez DH, Silva EP et al (2018) Microbially-enriched poultry litter-derived biochar for the treatment of acid mine drainage. Arch Microbiol 200(8):1227–1237
Gibert O, De Pablo J, Cortina JL, Ayora C (2004) Chemical characterisation of natural organic substrates for biological mitigation of acid mine drainage. Water Res 38(19):4186–4196
Gibert O, de Pablo J, Cortina JL, Ayora C (2005) Municipal compost-based mixture for acid mine drainage bioremediation: metal retention mechanisms. Appl Geochem 20(9):1648–1657
Gibert O, Rötting T, Cortina JL, de Pablo J, Ayora C, Carrera J, Bolzicco J (2011) In-situ remediation of acid mine drainage using a permeable reactive barrier in Aznalcollar (Sw Spain). J Hazard Mater 191(1–3):287–295
Giller KE, Amijee F, Brodrick SJ, Edje OT (1998) Environmental constraints to nodulation and nitrogen fixation of Phaseolus vulgaris L in Tanzania II. Response to N and P fertilizers and inoculation with Rhizobium. Afr Crop Sci J 6(2):171–178
Goevert D, Conrad R (2008) Carbon isotope fractionation by sulfate-reducing bacteria using different pathways for the oxidation of acetate. Environ Sci Technol 42(21):7813–7817
Golab AN, Peterson MA, Indraratna B (2006) Selection of potential reactive materials for a permeable reactive barrier for remediating acidic groundwater in acid sulphate soil terrains. Q J Eng Geol Hydrogeol 39(2):209–223
Golyshina OV, Timmis KN (2005) Ferroplasma and relatives, recently discovered cell wall-lacking archaea making a living in extremely acid, heavy metal-rich environments. Environ Microbiol 7(9):1277–1288
González D, Liu Y, Gomez DV, Southam G, Hedrich S, Galleguillos P et al (2019) Performance of a sulfidogenic bioreactor inoculated with indigenous acidic communities for treating an extremely acidic mine water. Miner Eng 131:370–375
Gramp JP, Bigham JM, Sasaki K, Tuovinen OH (2007) Formation of Ni-and Zn-sulfides in cultures of sulfate-reducing bacteria. Geomicrobiol J 24(7–8):609–614
Gramp JP, Wang H, Bigham JM, Jones FS, Tuovinen OH (2009) Biogenic synthesis and reduction of Fe (III)-hydroxysulfates. Geomicrobiol J 26(4):275–280
Gramp JP, Bigham JM, Jones FS, Tuovinen OH (2010) Formation of Fe-sulfides in cultures of sulfate-reducing bacteria. J Hazard Mater 175(1–3):1062–1067
Grande JA, Santisteban M, de la Torre ML, Dávila JM, Pérez-Ostalé E (2018) Map of impact by acid mine drainage in the river network of The Iberian Pyrite Belt (Sw Spain). Chemosphere 199:269–277
Gray NF (1997) Environmental impact and remediation of acid mine drainage: a management problem. Environ Geol 30(1–2):62–71
Gray NF, Delaney E (2008) Comparison of benthic macroinvertebrate indices for the assessment of the impact of acid mine drainage on an Irish river below an abandoned Cu–S mine. Environ Pollut 155(1):31–40
Grein F, Venceslau SS, Schneider L, Hildebrandt P, Todorovic S, Pereira IA, Dahl C (2010) DsrJ, an essential part of the DsrMKJOP transmembrane complex in the purple sulfur bacterium Allochromatium vinosum, is an unusual triheme cytochrome c. Biochemistry 49(38):8290–8299
Grubb DG, Landers DG, Guerra PA, Miller B, Bilgin A, Hernandez MT (2018) Sugarcane bagasse as a microbial host media for the passive treatment of acid mine drainage. J Environ Eng 144(10):04018108
Gupta SK, Nikhil K (2016) Ground water contamination in coal mining areas: a critical review. Int J Eng Appl Sci 3(2):295–304
Gupta A, Dutta A, Sarkar J, Panigrahi MK, Sar P (2018) Low-abundance members of the firmicutes facilitate bioremediation of soil impacted by highly acidic mine drainage from the Malanjkhand copper project, India. Front Microbiol 9:2882
Hai T, Lange D, Rabus R, Steinbüchel A (2004) Polyhydroxyalkanoate (PHA) accumulation in sulfate-reducing bacteria and identification of a class III PHA synthase (PhaEC) in Desulfococcus multivorans. Appl Environ Microbiol 70(8):4440–4448
Hao OJ, Chen JM, Huang L, Buglass RL (1996) Sulfate-reducing bacteria. Crit Rev Environ Sci Technol 26(2):155–187
Hao J, Murphy R, Lim E, Schoonen MA, Strongin DR (2009) Effects of phospholipid on pyrite oxidation in the presence of autotrophic and heterotrophic bacteria. Geochim Cosmochim Acta 73(14):4111–4123
Hao TW, Xiang PY, Mackey HR, Chi K, Lu H, Chui HK, Chen GH (2014) A review of biological sulfate conversions in wastewater treatment. Water Res 65:1–21
Harrison JM (2005) Ferric iron and sulfate reduction in the attenuation of acid mine drainage: a microcosm study (Doctoral dissertation, MS Thesis, Department of Civil and Environmental Engineering, University of Massachusetts-Amherst, 144p)
He L, Gao B, Luo X, Jiao J, Qin H, Zhang C, Dong Y (2018) Health risk assessment of heavy metals in surface water near a uranium tailing pond in Jiangxi Province, south China. Sustainability 10(4):1113
Hedrich S, Johnson DB (2014) Remediation and selective recovery of metals from acidic mine waters using novel modular bioreactors. Environ Sci Technol 48(20):12206–12212
Heidelberg JF, Seshadri R, Haveman SA, Hemme CL, Paulsen IT, Kolonay JF et al (2004) The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat Biotechnol 22(5):554
Hesketh AH, Broadhurst JL, Harrison STL (2010) Mitigating the generation of acid mine drainage from copper sulfide tailings impoundments in perpetuity: a case study for an integrated management strategy. Miner Eng 23(3):225–229
Hiibel SR, Pereyra LP, Inman LY, Tischer A, Reisman DJ, Reardon KF, Pruden A (2008) Microbial community analysis of two field-scale sulfate-reducing bioreactors treating mine drainage. Environ Microbiol 10(8):2087–2097
Hiibel SR, Pereyra LP, Breazeal MVR, Reisman DJ, Reardon KF, Pruden A (2011) Effect of organic substrate on the microbial community structure in pilot-scale sulfate-reducing biochemical reactors treating mine drainage. Environ Eng Sci 28(8):563–572
Hogsden KL, Harding JS (2011) Consequences of acid mine drainage for the structure and function of benthic stream communities: a review. Freshwat Sci 31(1):108–120
Huang X, Evangelou VP (1994) Suppression of pyrite oxidation rate by phosphate addition. In: Alper CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. American Chemical Society, Washington, DC
Hussain A, Qazi JI (2016) Metals-induced functional stress in sulphate-reducing thermophiles. 3 Biotech 6(1):17
Hussain A, Hasan A, Javid A, Qazi JI, (2016) Exploited application of sulfate-reducing bacteria for concomitant treatment of metallic and non-metallic wastes: a mini review. 3 Biotech 6(2)
Hwang SK, Jho EH (2018) Heavy metal and sulfate removal from sulfate-rich synthetic mine drainages using sulfate reducing bacteria. Sci Total Environ 635:1308–1316
Icgen B, Harrison S (2006) Exposure to sulfide causes populations shifts in sulfate-reducing consortia. Res Microbiol 157(8):784–791
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Jameson E, Rowe OF, Hallberg KB, Johnson DB (2010) Sulfidogenesis and selective precipitation of metals at low pH mediated by Acidithiobacillus spp. and acidophilic sulfate-reducing bacteria. Hydrometallurgy 104(3–4):488–493
Janssen PH, Schink B (1995a) Catabolic and anabolic enzyme activities and energetics of acetone metabolism of the sulfatereducing bacterium Desulfococcus biacutus. J Bacteriol 177:277–282
Janssen PH, Schink B (1995b) Pathway of butyrate catabolism by Desulfobacterium cetonicum. J Bacteriol 177:3870–3872
Jennings SR, Blicker PS, Neuman DR (2008) Acid mine drainage and effects on fish health and ecology: a review. Reclamation Research Group, Bozeman
Jin S, Fallgren PH, Morris JM, Gossard RB (2008) Biological source treatment of acid mine drainage using microbial and substrate amendments: microcosm studies. Mine Water Environ 27(1):20–30
Jing Q, Zhang M, Liu X, Li Y, Wang Z, Wen J (2018) Bench-scale microbial remediation of the model acid mine drainage: effects of nutrients and microbes on the source bioremediation. Int Biodeterior Biodegradation 128:117–121
Johnson DB (2012) Geomicrobiology of extremely acidic subsurface environments. FEMS Microbiol Ecol 81(1):2–12
Johnson DB, Hallberg KB (2005a) Acid mine drainage remediation options: a review. Sci Total Environ 338(1–2):3–14
Johnson DB, Hallberg KB (2005b) Biogeochemistry of the compost bioreactor components of a composite acid mine drainage passive remediation system. Sci Total Environ 338(1–2):81–93
Johnson DB, Santos AL (2018) Design and application of a low pH upflow biofilm sulfidogenic bioreactor for recovering transition metals from synthetic waste water at a Brazilian copper mine. Front Microbiol 9:2051
Jong T, Parry DL (2003) Removal of sulfate and heavy metals by sulfate reducing bacteria in short-term bench scale upflow anaerobic packed bed reactor runs. Water Res 37(14):3379–3389
Jong T, Parry DL (2006) Microbial sulfate reduction under sequentially acidic conditions in an upflow anaerobic packed bed bioreactor. Water Res 40(13):2561–2571
Kaksonen AH, Franzmann PD, Puhakka JA (2003) Performance and ethanol oxidation kinetics of a sulfate-reducing fluidized-bed reactor treating acidic metal-containing wastewater. Biodegradation 14(3):207–217
Kaksonen AH, Plumb JJ, Franzmann PD, Puhakka JA (2004) Simple organic electron donors support diverse sulfate-reducing communities in fluidized-bed reactors treating acidic metal-and sulfate-containing wastewater. FEMS Microbiol Ecol 47(3):279–289
Kaksonen AH, Plumb JJ, Robertson WJ, Riekkola-Vanhanen M, Franzmann PD, Puhakka JA (2006) The performance, kinetics and microbiology of sulfidogenic fluidized-bed treatment of acidic metal-and sulfate-containing wastewater. Hydrometallurgy 83(1–4):204–213
Kefeni KK, Msagati TA, Mamba BB (2017) Acid mine drainage: prevention, treatment options, and resource recovery: a review. J Clean Prod 151:475–493
Keller KL, Wall JD (2011) Genetics and molecular biology of the electron flow for sulfate respiration in Desulfovibrio. Front Microbiol 2:135
Keller KL, Rapp-Giles BJ, Semkiw ES, Porat I, Brown SD, Wall JD (2014) New model for electron flow for sulfate reduction in Desulfovibrio alaskensis G20. Appl Environ Microbiol 80(3):855–868
Khummalai N, Boonamnuayvitaya V (2005) Suppression of arsenopyrite surface oxidation by sol-gel coatings. J Biosci Bioeng 99(3):277–284
Kieu HT, Müller E, Horn H (2011) Heavy metal removal in anaerobic semi-continuous stirred tank reactors by a consortium of sulfate-reducing bacteria. Water Res 45(13):3863–3870
Kimura S, Hallberg KB, Johnson DB (2006) Sulfidogenesis in low pH (3.8–4.2) media by a mixed population of acidophilic bacteria. Biodegradation 17(2):57–65
Kiran MG, Pakshirajan K, Das G (2017) An overview of sulfidogenic biological reactors for the simultaneous treatment of sulfate and heavy metal rich wastewater. Chem Eng Sci 158:606–620
Kleinmann RL, Crerar DA, Pacelli RR (1981) Biogeochemistry of acid mine drainage and a method to control acid formation. Min Eng (NY);(United States) 33(3)
Kobayashi K, Tachibana S, Ishimoto M (1969) Intermediary formation of trithionate in sulfite reduction by a sulfate-reducing bacterium. J Biochem 65:155–157
Kobayashi K, Seki Y, Ishimoto M (1974) Biochemical studies on sulfate-reducing bacteria. XIII. Sulfite reductase from Desulfovibrio vulgaris-mechanism of trithionate, thiosulfate, and sulfite formation and enzymatic properties. J Biochem 75:519–529
Kolmert Å, Johnson DB (2001) Remediation of acidic waste waters using immobilised, acidophilic sulfate-reducing bacteria. J Chem Technol Biotechnol: Int Res Process, Environ Clean Technol 76(8):836–843
Koryak M, Shapiro MA, Sykora JL (1972) Riffle zoobenthos in streams receiving acid mine drainage. Water Res 6(10):1239–1247
Koschorreck M (2008) Microbial sulphate reduction at a low pH. FEMS Microbiol Ecol 64(3):329–342
Krulwich TA, Sachs G, Padan E (2011) Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol 9(5):330
Kuyucak N (2002) Acid mine drainage prevention and control options. CIM Bull 95:96–102
Lamrabet O, Pieulle L, Aubert C, Mouhamar F, Stocker P, Dolla A, Brasseur G (2011) Oxygen reduction in the strict anaerobe Desulfovibrio vulgaris Hildenborough: characterization of two membrane-bound oxygen reductases. Microbiology 157(9):2720–2732
Le Pape P, Battaglia-Brunet F, Parmentier M, Joulian C, Gassaud C, Fernandez-Rojo L et al (2017) Complete removal of arsenic and zinc from a heavily contaminated acid mine drainage via an indigenous SRB consortium. J Hazard Mater 321:764–772
Lefticariu L, Walters ER, Pugh CW, Bender KS (2015) Sulfate reducing bioreactor dependence on organic substrates for remediation of coal-generated acid mine drainage: field experiments. Appl Geochem 63:70–82
Leita L, De Nobili M, Muhlbachova G, Mondini C, Marchiol L, Zerbi G (1995) Bioavailability and effects of heavy metals on soil microbial biomass survival during laboratory incubation. Biol Fertil Soils 19(2–3):103–108
Li X, Luo Q, Wofford NQ, Keller KL, McInerney MJ, Wall JD, Krumholz LR (2009) A molybdopterin oxidoreductase is involved in H2 oxidation in Desulfovibrio desulfuricans G20. J Bacteriol 191(8):2675–2682
Liao J, Wen Z, Ru X, Chen J, Wu H, Wei C (2016) Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: public health implications in Guangdong Province, China. Ecotoxicol Environ Saf 124:460–469
Liu Y, Dang Z, Xu Y, Xu T (2013) Pyrite passivation by triethylenetetramine: an electrochemical study. J Anal Methods Chem 2013:387124
Liu Z, Li L, Li Z, Tian X (2018a) Removal of sulfate and heavy metals by sulfate-reducing bacteria in an expanded granular sludge bed reactor. Environ Technol 39(14):1814–1822
Liu F, Qiao X, Zhou L, Zhang J (2018b) Migration and fate of acid mine drainage pollutants in calcareous soil. Int J Environ Res Public Health 15(8):1759
Logan MV, Reardon KF, Figueroa LA, McLain JE, Ahmann DM (2005) Microbial community activities during establishment, performance, and decline of bench-scale passive treatment systems for mine drainage. Water Res 39(18):4537–4551
Lopez-Luna EL (2008) Contributions of iron (III) and sulfate-reducing bacteria to attenuation of an Acid Mine Drainage site: linking microcosm studies and geochemistry. University of Massachusetts Amherst, Amherst
Lottermoser B (2007) Mine wastes. Springer-Verlag, Berlin Heidelberg, pp 43–118
Lu J, Chen T, Wu J, Wilson PC, Hao X, Qian J (2011) Acid tolerance of an acid mine drainage bioremediation system based on biological sulfate reduction. Bioresour Technol 102(22):10401–10406
Manders P, Godfrey L, Hobbs P (2009) Briefing note: acid mine drainage in South Africa. CSIR, Pretoria. http://www.csir.co.za. Accessed 1 Mar 2013
Marsden AD, DeWreede RE (2000) Marine macroalgal community structure, metal content and reproductive function near an acid mine drainage outflow. Environ Pollut 110(3):431–440
Martin AJ, Goldblatt R (2007) Speciation, behavior, and bioavailability of copper downstream of a mine-impacted lake. Environ Toxicol Chem, Int J 26(12):2594–2603
Martins M, Pereira IA (2013) Sulfate-reducing bacteria as new microorganisms for biological hydrogen production. Int J Hydrog Energy 38(28):12294–12301
Martins M, Santos ES, Faleiro ML, Chaves S, Tenreiro R, Barros RJ, Costa MC (2011) Performance and bacterial community shifts during bioremediation of acid mine drainage from two Portuguese mines. Int Biodeterior Biodegradation 65(7):972–981
Martins M, Mourato C, Pereira IA (2015) Desulfovibrio vulgaris growth coupled to formate-driven H2 production. Environ Sci Technol 49(24):14655–14662
McCarthy TS (2011) The impact of acid mine drainage in South Africa. S Afr J Sci 107(5–6):01–07
McCullough CD, Lund MA (2011) Bioremediation of Acidic and Metalliferous Drainage (AMD) through organic carbon amendment by municipal sewage and green waste. J Environ Manag 92(10):2419–2426
McGlynn SE, Chadwick GL, Kempes CP, Orphan VJ (2015) Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 526(7574):531
Méndez-García C, Peláez AI, Mesa V, Sánchez J, Golyshina OV, Ferrer M (2015) Microbial diversity and metabolic networks in acid mine drainage habitats. Front Microbiol 6:475
Meyer B, Kuehl JV, Price MN, Ray J, Deutschbauer AM, Arkin AP, Stahl DA (2014) The energy-conserving electron transfer system used by D esulfovibrio alaskensis strain G 20 during pyruvate fermentation involves reduction of endogenously formed fumarate and cytoplasmic and membrane-bound complexes, Hdr-Flox and Rnf. Environ Microbiol 16(11):3463–3486
Milucka J, Ferdelman TG, Polerecky L, Franzke D, Wegener G, Schmid M et al (2012) Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 491(7425):541
Mohanakrishnan J, Gutierrez O, Meyer RL, Yuan Z (2008) Nitrite effectively inhibits sulfide and methane production in a laboratory scale sewer reactor. Water Res 42(14):3961–3971
Monserrate M (2004) Field and column studies of sulfate reducing bacteria in acid mine drainage (Doctoral dissertation, MS Thesis. Department of Civil and Environmental Engineering, University of Massachusetts-Amherst, 100p)
Moosa S, Harrison STL (2006) Product inhibition by sulphide species on biological sulphate reduction for the treatment of acid mine drainage. Hydrometallurgy 83(1–4):214–222
Morais-Silva FO, Santos CI, Rodrigues R, Pereira IA, Rodrigues-Pousada C (2013) Roles of HynAB and Ech, the only two hydrogenases found in the model sulfate reducer Desulfovibrio gigas. J Bacteriol 195(20):4753–4760
Morales TA, Dopson M, Athar R, Herbert RB Jr (2005) Analysis of bacterial diversity in acidic pond water and compost after treatment of artificial acid mine drainage for metal removal. Biotechnol Bioeng 90(5):543–551
Motsi T, Rowson NA, Simmons MJH (2009) Adsorption of heavy metals from acid mine drainage by natural zeolite. Int J Miner Process 92(1–2):42–48
Muhammad SN, Kusin FM, Madzin Z (2018) Coupled physicochemical and bacterial reduction mechanisms for passive remediation of sulfate-and metal-rich acid mine drainage. Int J Environ Sci Technol 15(11):2325–2336
Murray AJ, Roussel J, Rolley J, Woodhall F, Mikheenko IP, Johnson DB et al (2017) Biosynthesis of zinc sulfide quantum dots using waste off-gas from a metal bioremediation process. RSC Adv 7(35):21484–21491
Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6(6):441
Nagase N, Asano S, Takano M, Takeda K, Heguri S, Idegami A (2011) U.S. Patent No. 8,052,774. U.S. Patent and Trademark Office, Washington, DC
Nagpal S, Chuichulcherm S, Peeva L, Livingston A (2000) Microbial sulfate reduction in a liquid–solid fluidized bed reactor. Biotechnol Bioeng 70(4):370–380
Naicker K, Cukrowska E, McCarthy TS (2003) Acid mine drainage arising from gold mining activity in Johannesburg, South Africa and environs. Environ Pollut 122(1):29–40
Ňancucheo I, Johnson DB (2012) Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb Biotechnol 5(1):34–44
Ňancucheo I, Johnson DB (2014) Removal of sulfate from extremely acidic mine waters using low pH sulfidogenic bioreactors. Hydrometallurgy 150:222–226
Nancucheo I, Bitencourt JA, Sahoo PK, Alves JO, Siqueira JO, Oliveira G (2017) Recent developments for remediating acidic mine waters using sulfidogenic bacteria. Biomed Res Int 2017:1–17
Neculita CM, Zagury GJ, Bussière B (2007) Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria. J Environ Qual 36(1):1–16
Neculita CM, Zagury GJ, Bussière B (2008) Effectiveness of sulfate-reducing passive bioreactors for treating highly contaminated acid mine drainage: I. Effect of hydraulic retention time. Appl Geochem 23(12):3442–3451
Neculita CM, Zagury GJ, Kulnieks V (2010) Short-term and long-term bioreactors for acid mine drainage treatment. Proc Annu Int Conf Soils, Sedim, Water Energy 12(1):2
Neculita CM, Yim GJ, Lee G, Ji SW, Jung JW, Park HS, Song H (2011) Comparative effectiveness of mixed organic substrates to mushroom compost for treatment of mine drainage in passive bioreactors. Chemosphere 83(1):76–82
Nielsen G, Janin A, Coudert L, Blais JF, Mercier G (2018) Performance of sulfate-reducing passive bioreactors for the removal of cd and Zn from mine drainage in a cold climate. Mine Water Environ 37(1):42–55
Nieto JM, Sarmiento AM, Olías M, Canovas CR, Riba I, Kalman J, Delvalls TA (2007) Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ Int 33(4):445–455
Nordstrom DK (2000) Advances in the hydrogeochemistry and microbiology of acid mine waters. Int Geol Rev 42(6):499–515
Nordstrom DK, Alpers CN (1999) Negative pH, efflorescent mineralogy, and consequences for environmental restoration at the Iron Mountain Superfund site, California. Proc Natl Acad Sci 96(7):3455–3462
Nyquist J, Greger M (2009) A field study of constructed wetlands for preventing and treating acid mine drainage. Ecol Eng 35(5):630–642
Ochieng GM, Seanego ES, Nkwonta OI (2010) Impacts of mining on water resources in South Africa: a review. Sci Res Essays 5(22):3351–3357
Odom JM, Peck HD Jr (1981) Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol Lett 12(1):47–50
Odom JM, Singleton R (1993) The sulfate-reducing bacteria: contemporary perspectives. Springer-Verlag, New York, pp 189–210
Olıas M, Nieto JM, Sarmiento AM, Cerón JC, Cánovas CR (2004) Seasonal water quality variations in a river affected by acid mine drainage: the Odiel River (South West Spain). Sci Total Environ 333(1–3):267–281
Onysko SJ, Kleinmann RL, Erickson PM (1984) Ferrous iron oxidation by thiobacillus ferrooxidans: inhibition with benzoic acid, sorbic acid, and sodium lauryl sulfate. Appl Environ Microbiol 48(1):229–231
Ozuolmez D, Na H, Lever MA, Kjeldsen KU, Jørgensen BB, Plugge CM (2015) Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: teamwork or coexistence? Front Microbiol 6:492
Pagnanelli F, Viggi CC, Mainelli S, Toro L (2009) Assessment of solid reactive mixtures for the development of biological permeable reactive barriers. J Hazard Mater 170(2–3):998–1005
Pandey PK, Sharma R, Roy M, Pandey M (2007) Toxic mine drainage from Asia’s biggest copper mine at Malanjkhand, India. Environ Geochem Health 29(3):237–248
Pandey S, Yacob TW, Silverstein J, Rajaram H, Minchow K, Basta J (2011) Prevention of acid mine drainage through complexation of ferric iron by soluble microbial growth products. In: AGU Fall Meeting Abstracts
Park I, Tabelin CB, Jeon S, Li X, Seno K, Ito M, Hiroyoshi N (2018) A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 219:588–606
Pat-Espadas A, Loredo Portales R, Amabilis-Sosa L, Gómez G, Vidal G (2018) Review of constructed wetlands for acid mine drainage treatment. Water 10(11):1685
Paulo LM, Stams AJ, Sousa DZ (2015) Methanogens, sulphate and heavy metals: a complex system. Rev Environ Sci Biotechnol 14(4):537–553
Peck HD, LeGall J (1982) Biochemistry of dissimilatory sulphate reduction. Philos Trans R Soc Lond B Biol Sci 298(1093):443–466
Peltier E, Ilipilla P, Fowle D (2011) Structure and reactivity of zinc sulfide precipitates formed in the presence of sulfate-reducing bacteria. Appl Geochem 26(9–10):1673–1680
Pereira IA, Romão CV, Xavier AV, LeGall J, Teixeira M (1998) Electron transfer between hydrogenases and mono-and multiheme cytochromes in Desulfovibrio ssp. JBIC J Biol Inorganic Chem 3(5):494–498
Pereira PM, Teixeira M, Xavier AV, Louro RO, Pereira IA (2006) The Tmc complex from Desulfovibrio vulgaris Hildenborough is involved in transmembrane electron transfer from periplasmic hydrogen oxidation. Biochemistry 45(34):10359–10367
Pereira IA, Ramos AR, Grein F, Marques MC, Da Silva SM, Venceslau SS (2011) A comparative genomic analysis of energy metabolism in sulfate reducing bacteria and archaea. Front Microbiol 2:69
Pereyra LP, Hiibel SR, Perrault EM, Reardon KF, Pruden A (2012) Effect of bioaugmentation and biostimulation on sulfate-reducing column startup captured by functional gene profiling. FEMS Microbiol Ecol 82(1):135–147
Pérez N, Schwarz AO, Barahona E, Sanhueza P, Diaz I, Urrutia H (2018) Performance of two differently designed permeable reactive barriers with sulfate and zinc solutions. Sci Total Environ 642:894–903
Pérez-Ostalé E, Grande JA, de la Torre ML, Valente T, Cerón JC, Santisteban M (2013) Inventory of mining and quantification of affected areas in the iberian pyrite belt (SW Spain). A methodological contribution to environmental management. Int Multidisciplin Sci GeoConf: SGEM: Surv Geol Mining Ecol Manag 1:613
Picard A, Gartman A, Clarke DR, Girguis PR (2018) Sulfate-reducing bacteria influence the nucleation and growth of mackinawite and greigite. Geochim Cosmochim Acta 220:367–384
Plugge CM, Zhang W, Scholten J, Stams AJ (2011) Metabolic flexibility of sulfate-reducing bacteria. Front Microbiol 2:81
Potgieter-Vermaak SS, Potgieter JH, Monama P, Van Grieken R (2006) Comparison of limestone, dolomite and fly ash as pre-treatment agents for acid mine drainage. Miner Eng 19(5):454–462
Price MN, Ray J, Wetmore KM, Kuehl JV, Bauer S, Deutschbauer AM, Arkin AP (2014) The genetic basis of energy conservation in the sulfate-reducing bacterium Desulfovibrio alaskensis G20. Front Microbiol 5:577
Pruden A, Messner N, Pereyra L, Hanson RE, Hiibel SR, Reardon KF (2007) The effect of inoculum on the performance of sulfate-reducing columns treating heavy metal contaminated water. Water Res 41(4):904–914
Qian Z, Tianwei H, Mackey HR, van Loosdrecht MC, Guanghao C (2018) Recent advances in dissimilatory sulfate reduction: from metabolic study to application. Water Res 150:162–181
Qiu G, Feng X, Wang S, Fu X, Shang L (2009) Mercury distribution and speciation in water and fish from abandoned Hg mines in Wanshan, Guizhou province, China. Sci Total Environ 407(18):5162–5168
Quintas PO, Oliveira MS, Catarino T, Turner DL (2013) Electron transfer between multihaem cytochromes c3 from Desulfovibrio africanus. Biochim Biophys Acta (BBA)-Bioenerg 1827(4):502–506
Rabus R, Hansen TA, Widdel F (2006) Dissimilatory sulfate- and sulfur-reducing prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes, Ecophysiology and biochemistry, vol 2. Springer, Berlin, pp 659–768
Rabus R, Hansen TA, Widdel F (2013) Dissimilatory sulfate-and sulfur-reducing prokaryotes. In: The prokaryotes: prokaryotic physiology and biochemistry. Springer-Verlag, Berlin Heidelberg, pp 309–404
Ramos AR, Keller KL, Wall JD, Pereira IAC (2012) The membrane QmoABC complex interacts directly with the dissimilatory adenosine 5-phosphosulfate reductase in sulfate reducing bacteria. Front Microbiol 3:137
Raskin L, Rittmann BE, Stahl DA (1996) Competition and coexistence of sulfate-reducing and methanogenic populations in anaerobic biofilms. Appl Environ Microbiol 62(10):3847–3857
Reid NB, Naeth MA (2005) Establishment of a vegetation cover on tundra kimberlite mine tailings: 2. A field study. Restorat Ecol 13(4):602–608
Reis MAM, Lemos PC, Almeida JS, Carrondo MJT (1990) Influence of produced acetic acid on growth of sulfate reducing bacteria. Biotechnol Lett 12(2):145–148
Reis MAM, Almeida JS, Lemos PC, Carrondo MJT (1992) Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol Bioeng 40(5):593–600
Riekkola-Vanhanen M, Mustikkamäki UP (1997) In situ treatment of acid mine drainage by sulphate reducing bacteria in an open pit mine. In: Proceedings of biomine international conference, Sydney, Australia, pp E-ROM3.1-E-ROM3.8
Rodríguez-Galán M, Baena-Moreno FM, Vázquez S, Arroyo-Torralvo F, Vilches LF, Zhang Z (2019) Remediation of acid mine drainage. Environ Chem Lett 2019:1–10
RoyChowdhury A, Sarkar D, Datta R (2015) Remediation of acid mine drainage-impacted water. Curr Pollut Rep 1(3):131–141
RoyChowdhury A, Sarkar D, Deng Y, Datta R (2017) Assessment of soil and water contamination at the tab-simco coal mine: a case study. Mine Water Environ 36(2):248–254
RoyChowdhury A, Sarkar D, Datta R (2019) Removal of acidity and metals from acid mine drainage-impacted water using industrial byproducts. Environ Manag 63(1):148–158
Rubio-Rincón F, Lopez-Vazquez C, Welles L, van den Brand T, Abbas B, van Loosdrecht M, Brdjanovic D (2017) Effects of electron acceptors on sulphate reduction activity in activated sludge processes. Appl Microbiol Biotechnol 101(15):6229–6240
Saha S, Sinha A (2018) A review on treatment of acid mine drainage with waste materials: a novel approach. Global NEST J 20(3):512–528
Sahinkaya E, Yurtsever A, Toker Y, Elcik H, Cakmaci M, Kaksonen AH (2015) Biotreatment of As-containing simulated acid mine drainage using laboratory scale sulfate reducing upflow anaerobic sludge blanket reactor. Miner Eng 75:133–139
Sahoo PK, Tripathy S, Equeenuddin SM, Panigrahi MK (2012) Geochemical characteristics of coal mine discharge vis-à-vis behavior of rare earth elements at Jaintia Hills coalfield, northeastern India. J Geochem Explor 112:235–243
Sahoo PK, Kim K, Equeenuddin SM, Powell MA (2013) Current approaches for mitigating acid mine drainage. In: Reviews of environmental contamination and toxicology, vol 226. Springer, New York, pp 1–32
Sánchez-Andrea I, Triana D, Sanz JL (2012) Bioremediation of acid mine drainage coupled with domestic wastewater treatment. Water Sci Technol 66(11):2425–2431
Sánchez-Andrea I, Sanz JL, Bijmans MF, Stams AJ (2014) Sulfate reduction at low pH to remediate acid mine drainage. J Hazard Mater 269:98–109
Sani RK, Peyton BM, Brown LT (2001) Copper-induced inhibition of growth of Desulfovibrio desulfuricans G20: assessment of its toxicity and correlation with those of zinc and lead. Appl Environ Microbiol 67(10):4765–4772
Sani RK, Peyton BM, Jandhyala M (2003) Toxicity of lead in aqueous medium to Desulfovibrio desulfuricans G20. Environ Toxicol Chem 22(2):252–260
Santos AL, Johnson DB (2017) The effects of temperature and pH on the kinetics of an acidophilic sulfidogenic bioreactor and indigenous microbial communities. Hydrometallurgy 168:116–120
Santos AA, Venceslau SS, Grein F, Leavitt WD, Dahl C, Johnston DT, Pereira IA (2015) A protein trisulfide couples dissimilatory sulfate reduction to energy conservation. Science 350(6267):1541–1545
Sato Y, Hamai T, Hori T, Habe H, Kobayashi M, Sakata T (2018) Year-round performance of a passive sulfate-reducing bioreactor that uses rice bran as an organic carbon source to treat acid mine drainage. Mine Water Environ 37(3):586–594
Scheller S, Yu H, Chadwick GL, McGlynn SE, Orphan VJ (2016) Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science 351(6274):703–707
Schmidt TS, Soucek DJ, Cherry DS (2002) Modification of an ecotoxicological rating to bioassess small acid mine drainage-impacted watersheds exclusive of benthic macroinvertebrate analysis. Environ Toxicol Chem Int J 21(5):1091–1097
Schmidtova J, Baldwin SA (2011) Correlation of bacterial communities supported by different organic materials with sulfate reduction in metal-rich landfill leachate. Water Res 45(3):1115–1128
Schueck JH, Helfrich DR, Fromell DJ (2004) Limestone upflow pond with siphon discharge design considerations: a simple solution to high volume, high metals AMD discharges. In: Proceedings of the 6th statewide conference on abandoned mine reclamation, Indiana, PA298
Shabalala AN, Ekolu SO, Diop S (2014) Permeable reactive barriers for acid mine drainage treatment: a review. https://doi.org/10.3233/978-1-61499-466-4-1416
Shahid M, Khalid S, Abbas G, Shahid N, Nadeem M, Sabir M et al (2015) Heavy metal stress and crop productivity. In: Crop production and global environmental issues. Springer, Cham, pp 1–25
Shelp G, Chesworth W, Spiers G, Liu L (1995) Cathodic protection of a weathering orebody. In: Proceedings of Sudbury ‘95—mining and the environment, pp 1035–1042
Sheoran AS (2017) Management of acidic mine waste water by constructed wetland treatment systems: a bench scale study. Eur J Sustain Dev 6(2):245–255
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19(2):105–116
Shu WS (2003) Exploring the potential utilization of vetiver in treating acid mine drainage (AMD). In: Proceedings of the third international vetiver conference, Guangzhou, China
Shu X, Dang Z, Zhang Q, Yi X, Lu G, Guo C, Yang C (2013) Passivation of metal-sulfide tailings by covalent coating. Miner Eng 42:36–42
Sim MS, Wang DT, Zane GM, Wall JD, Bosak T, Ono S (2013) Fractionation of sulfur isotopes by Desulfovibrio vulgaris mutants lacking hydrogenases or type I tetraheme cytochrome c3. Front Microbiol 4:171
Sim MS, Paris G, Adkins JF, Orphan VJ, Sessions AL (2017) Quantification and isotopic analysis of intracellular sulfur metabolites in the dissimilatory sulfate reduction pathway. Geochim Cosmochim Acta 206:57–72
Simmons J, Ziemkiewicz P, Black D (2002a) Use of steel slag leach beds for the treatment of acid mine drainage: the McCarty Highwall Project. In: Proceedings of the 19th ASMR Conference, Lexington, KY, pp 527–529
Simmons J, Ziemkiewicz P, Black DC (2002b) Use of steel slag leach beds for the treatment of acid mine drainage. Mine Water Environ 21(2):91–99
Skennerton CT, Chourey K, Iyer R, Hettich RL, Tyson GW, Orphan VJ (2017) Methane-fueled syntrophy through extracellular electron transfer: uncovering the genomic traits conserved within diverse bacterial partners of anaerobic methanotrophic archaea. MBio 8(4):e00530–e00517
Skousen J, Zipper CE, Rose A, Ziemkiewicz PF, Nairn R, McDonald LM, Kleinmann RL (2017) Review of passive systems for acid mine drainage treatment. Mine Water Environ 36(1):133–153
Slaninova A, Machova J, Svobodova Z (2014) Fish kill caused by aluminium and iron contamination in a natural pond used for fish rearing: a case report. Veterinarni Med 59(11):573–581
Song YC, Piak BC, Shin HS, La SJ (1998) Influence of electron donor and toxic materials on the activity of sulfate-reducing bacteria for the treatment of electroplating wastewater. Water Sci Tech 38:187–194
Song H, Yim GJ, Ji SW, Neculita CM, Hwang T (2012) Pilot-scale passive bioreactors for the treatment of acid mine drainage: efficiency of mushroom compost vs. mixed substrates for metal removal. J Environ Manag 111:150–158
Soucek DJ, Cherry DS, Trent GC (2000) Relative acute toxicity of acid mine drainage water column and sediments to Daphnia magna in the Puckett’s creek watershed, Virginia, USA. Arch Environ Contam Toxicol 38(3):305–310
Stahl DA, Fishbain S, Klein M, Baker BJ, Wagner M (2002) Origins and diversification of sulfate-respiring microorganisms. Antonie Van Leeuwenhoek 81(1–4):189
Stams AJ, Plugge CM (2009) Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7(8):568
Sun M, Xiao T, Ning Z, Xiao E, Sun W (2015) Microbial community analysis in rice paddy soils irrigated by acid mine drainage contaminated water. Appl Microbiol Biotechnol 99(6):2911–2922
Suzuki D, Ueki A, Amaishi A, Ueki K (2007) Desulfobulbus japonicus sp. nov., a novel Gram-negative propionate-oxidizing, sulfate-reducing bacterium isolated from an estuarine sediment in Japan. Int J Syst Evol Microbiol 57(4):849–855
Tamás J, Kovács E (2005) Vegetation pattern and heavy metal accumulation at a mine tailing at Gyöngyösoroszi, Hungary. Zeitschrift für Naturforschung C 60(3–4):362–368
Taylor J, Pape S, Murphy N (2005) A summary of passive and active treatment technologies for acid and metalliferous drainage (AMD). In: Proceedings of the in fifth Australian workshop on acid mine drainage
Teng W, Kuang J, Luo Z, Shu W (2017) Microbial diversity and community assembly across environmental gradients in acid mine drainage. Fortschr Mineral 7(6):106
Thauer RK, Stackebrandt E, Hamilton WA (2007) Energy metabolism and phylogenetic diversity of sulphate-reducing bacteria. Sulphate-reducing bacteria: environmental and engineered systems. Cambridge University Press, Cambridge, pp 1–37
Timms GP, Bennett JW (2000) The effectiveness of covers at Rum Jungle after fifteen years. In: Proceedings fifth international conference on acid rock drainage, pp 813–818
Tiwary RK (2001) Environmental impact of coal mining on water regime and its management. Water Air Soil Pollut 132(1–2):185–199
Trumm D (2010) Selection of active and passive treatment systems for AMD—flow charts for New Zealand conditions. N Z J Geol Geophys 53(2–3):195–210
Tyagi M, da Fonseca MMR, de Carvalho CCCR (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22(2):231–241
Utgikar VP, Harmon SM, Chaudhary N, Tabak HH, Govind R, Haines JR (2002) Inhibition of sulfate-reducing bacteria by metal sulfide formation in bioremediation of acid mine drainage. Environ Toxicol Int J 17(1):40–48
Valente T, Gomes P, Pamplona J, de la Torre ML (2012) Natural stabilization of mine waste-dumps—evolution of the vegetation cover in distinctive geochemical and mineralogical environments. J Geochem Explor 123:152–161
Van Damme PA, Hamel C, Ayala A, Bervoets L (2008) Macroinvertebrate community response to acid mine drainage in rivers of the High Andes (Bolivia). Environ Pollut 156(3):1061–1068
Vasquez Y, Escobar MC, Neculita CM, Arbeli Z, Roldan F (2016a) Biochemical passive reactors for treatment of acid mine drainage: effect of hydraulic retention time on changes in efficiency, composition of reactive mixture, and microbial activity. Chemosphere 153:244–253
Vasquez Y, Escobar MC, Neculita CM, Arbeli Z, Roldan F (2016b) Selection of reactive mixture for biochemical passive treatment of acid mine drainage. Environ Earth Sci 75(7):576
Vasquez Y, Escobar MC, Saenz JS, Quiceno-Vallejo MF, Neculita CM, Arbeli Z, Roldan F (2018) Effect of hydraulic retention time on microbial community in biochemical passive reactors during treatment of acid mine drainage. Bioresour Technol 247:624–632
Venceslau SS, Lino RR, Pereira IA (2010) The Qrc membrane complex, related to the alternative complex III, is a menaquinone reductase involved in sulfate respiration. J Biol Chem 285(30):22774–22783
Vestola E (2004) Treatment of acid mine drainage by sulphate reducing bacteria. Master’s Thesis, Helsinki University of Technology, Helsinki, Finland [in Finnish]
Wagner M, Loy A, Klein M, Lee N, Ramsing NB, Stahl DA, Friedrich MW (2005) Functional marker genes for identification of sulfate-reducing prokaryotes. Methods Enzymol 397:469–489
Walton WE, Compton SM, Allan JD, Daniels RE (1982) The effect of acid stress on survivorship and reproduction of Daphnia pulex (Crustacea: Cladocera). Can J Zool 60(4):573–579
Wang L, Ji B, Hu Y, Liu R, Sun W (2017) A review on in situ phytoremediation of mine tailings. Chemosphere 184:594–600
Waybrant KR, Blowes DW, Ptacek CJ (1998) Selection of reactive mixtures for use in permeable reactive walls for treatment of mine drainage. Environ Sci Technol 32(13):1972–1979
Wegener G, Krukenberg V, Riedel D, Tegetmeyer HE, Boetius A (2015) Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature 526(7574):587
Wendt-Potthoff K, Bozau E, Frömmichen R, Meier J, Koschorreck M (2010) Microbial iron reduction during passive in situ remediation of an acidic mine pit lake mesocosm. Limnologica-Ecol Manag Inland Waters 40(2):175–181
Widdel F, Pfennig N (1982) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids II. Incomplete oxidation of propionate by Desulfobulbus propionicus gen. nov., sp. nov. Arch Microbiol 131(4):360–365
Xiao KQ, Beulig F, Kjeldsen KU, Jørgensen BB, Risgaard-Petersen N (2017) Concurrent methane production and oxidation in surface sediment from Aarhus Bay, Denmark. Front Microbiol 8:1198
Xingyu L, Zou G, Wang X, Zou L, Wen J, Ruan R, Wang D (2013) A novel low pH sulfidogenic bioreactor using activated sludge as carbon source to treat acid mine drainage (AMD) and recovery metal sulfides: pilot scale study. Miner Eng 48:51–55
Yanful EK, Orlandea MP (2000) Controlling acid drainage in a pyritic mine waste rock. Part II: geochemistry of drainage. Water Air Soil Pollut 124(3–4):259–283
Yanful EK, Payant SC (1992) Evaluation of techniques for preventing acidic rock drainage. Milestone research report, MEND program, December
Yu L, Duan J, Zhao W, Huang Y, Hou B (2011) Characteristics of hydrogen evolution and oxidation catalyzed by Desulfovibrio caledoniensis biofilm on pyrolytic graphite electrode. Electrochim Acta 56(25):9041–9047
Zagury GJ, Kulnieks VI, Neculita CM (2006) Characterization and reactivity assessment of organic substrates for sulphate-reducing bacteria in acid mine drainage treatment. Chemosphere 64(6):944–954
Zagury GJ, Neculita C, Bussiere B (2007) Passive treatment of acid mine drainage in bioreactors: short review, applications, and research needs. In: Proceedings of the 60th Canadian geotechnical conference and 8th joint CGS/IAH-CNC specialty groundwater conference, Ottawa, Canada, pp 1439–1446
Zhang YL, Evangelou VP (1998) Formation of ferric hydroxide-silica coatings on pyrite and its oxidation behavior. Soil Sci 163:53–62
Zhang M, Wang H (2016) Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Miner Eng 92:63–71
Zhang X, Borda MJ, Schoonen MA, Strongin DR (2003a) Adsorption of phospholipids on pyrite and their effect on surface oxidation. Langmuir 19(21):8787–8792
Zhang X, Borda MJ, Schoonen MA, Strongin DR (2003b) Pyrite oxidation inhibition by a cross-linked lipid coating. Geochem Trans 4(1):8
Zhang M, Wang H, Han X (2016) Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere 154:215–223
Zhang M, Liu X, Li Y, Wang G, Wang Z, Wen J (2017) Microbial community and metabolic pathway succession driven by changed nutrient inputs in tailings: effects of different nutrients on tailing remediation. Sci Rep 7(1):474
Zhong CM, Xu ZL, Fang XH, Cheng L (2007) Treatment of acid mine drainage (AMD) by ultra-low-pressure reverse osmosis and nanofiltration. Environ Eng Sci 24(9):1297–1306
Zhou C, Vannela R, Hayes KF, Rittmann BE (2014) Effect of growth conditions on microbial activity and iron-sulfide production by Desulfovibrio vulgaris. J Hazard Mater 272:28–35
Ziemkiewicz PF, Brant DL (1996) The Casselman river restoration project. In: Proceedings, eighteenth west Virginia surface mine drainage task force symposium, pp 15–16
Ziemkiewicz P, Skousen J (1998) The use of steel slag in acid mine drainage treatment and control. Green Lands 28(1):46–56
Ziemkiewicz PF, Skousen JG, Brant DL, Sterner PL, Lovett RJ (1997) Acid mine drainage treatment with armored limestone in open limestone channels. J Environ Qual 26(4):1017–1024
Ziemkiewicz PF, Skousen JG, Simmons J (2003) Long-term performance of passive acid mine drainage treatment systems. Mine Water Environ 22(3):118–129
Acknowledgments
Authors are grateful to the Department of Biotechnology, Government of India, for funding the AMD-related project (BT/PR 7533/BCE/8/959/2013, Dated 10/12/2013). AG thanks the Department of Biotechnology, Government of India, for providing fellowship under DBT-JRF category (DBT/2014/IITKH/113).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, A., Sar, P. (2020). Treatment Options for Acid Mine Drainage: Remedial Achievements Through Microbial-Mediated Processes. In: Shah, M., Banerjee, A. (eds) Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant. Springer, Singapore. https://doi.org/10.1007/978-981-15-0497-6_8
Download citation
DOI: https://doi.org/10.1007/978-981-15-0497-6_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-0496-9
Online ISBN: 978-981-15-0497-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)