Abstract
Tropospheric ozone (O3) is a widespread secondary photochemical air pollutant, which occurs naturally at ground level in low concentration and is regarded the most damaging air toxicant. Current study was conducted to evaluate the potential of ethylenediurea (EDU) on the growth and physiochemical attributes of pea cultivar exposed to ambient O3 stress. Ozone stress reduced growth and biomass production of Pisum sativum. Application of EDU concentration of 450 ppm via soil drenching significantly enhanced growth and biomass of P. sativum. Application of EDU escalated relative water content and ascorbic acid content in P. sativum seedlings ultimately showing a significant increment in air pollution tolerance index up to 38.6% with EDU-450 treatment making the seedling more tolerant to the air pollution. The increasing concentrations of EDU showed significant increase in total soluble sugar, protein and proline contents, while phenolic contents were significantly decreased up to 36.6% with 450 ppm treatment of EDU. Furthermore, EDU treatment enhanced the activity of superoxide dismutase and ascorbate peroxidase. Current research reveals that EDU ameliorates the deleterious O3 effects on P. sativum and it can be used as an effective tool to enhance crop productivity at elevated levels of O3 concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
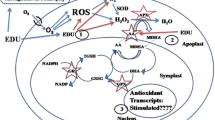
Globally, tropospheric ozone has dramatically suppressed crop productivity on a larger scale (Oksanen et al. 2013). Especially in tropical areas, the higher concentrations of O3 are mainly recognized due to the favorable climatological conditions such as high temperature, atmospheric humidity and high levels of solar radiations (McGrath 2015). South Asia also experiences continuous decline in air quality due to emissions of environmental pollutants such as nitrogen oxides and O3 precursors (Van Dingenen et al. 2009) and reached far more than the threshold level of various plants. When exposed to high concentrations of O3, plants undergo various physiological and phenotypical changes. The amount of response to the ozone stress depends upon the type of crop (Mills et al. 2007) and grown variety (Sarkar and Agrawal 2010). Among the various symptoms and physiological changes in the plants on exposure to high ozone concentrations, some of the most prominent and adverse changes are chlorosis, conductance through stomata, shrinkage of leaves, reduction in the rate of photosynthesis because of lesser activity of RuBisCO, decreased contents of chlorophyll and accelerated senescence (Pandey et al. 2014, 2015). On exposure to the leaves, ozone immediately gains entry into the tissues of the leaf through stomata and get dissolved in the apoplastic solutions (Li et al. 2017), after which get transformed into ROS (reactive oxygen species). Reactive oxygen species then damages the cell membrane, prompts the lipid peroxidation of the membrane, enhances the malondialdehyde levels, reduces the cell membrane stability and eventually causes the programmed death of the cell (Zhang et al. 2017; Dumont et al. 2017). The assimilation process of the plant can also be inhibited along with the changes in the distribution of assimilates, reduction in the growth of plant and reduced productivity of the crops (Singh and Agrawal 2017).
Keeping in view the damages caused by ozone to less resistant crops, a number of various protectants have been employed such as antioxidants (Zhang et al. 2018), anti-senescence agents (Navakoudis et al. 2003), growth regulators (Paoletti et al. 2011), flavonoids and polyamines (Didyk and Blum 2011) and fungicides (Hassan et al. 2007). Ethylenediurea (abbreviated as EDU) is a synthetic compound which is also known as antiozonant is employed to study the crop and plants responses to damages caused by ozone (Manning et al. 2011; Agathokleous et al. 2015). Unusually, this compound is unable to scavenge O3 directly because of the absence of double bond. Du Pont Company had been known for the preparation of the chemicals which can protect tires of cars as well as elastomers from the attack of ozone. In terminology, the chemicals which protect abiotic components from ozone have been known as antiozonants. On the other hand, the chemicals which protect plants from ozone attack are known as ozone protectants.
The first ever reported study on EDU being a protectant against injuries caused by ozone was on beans (Carnahan et al. 1978). Various researches have also approved the efficacy of EDU in decreasing the impacts of ozone damages (Agathokleous 2017; Tiwari 2017). The basic mechanism behind the protective role of EDU in plants is the stimulation of defense antioxidative responses, EDU is proposed to inhibit O3 injuries by upregulating antioxidative defense responses, reduction in the ROS formation and increase in the photosynthetic activity (Pandey et al. 2014, 2015; Yuan et al. 2015) due to which the yield of the plants is also maintained (Pandey et al. 2014, 2015; Yuan et al. 2015).
In the recent studies on EDU experimentations, no side effect on growth and toxicity to plants was reported at the concentrations required for O3 protection (Agathokleous et al. 2016a). For the assessment of ambient ozone effects on vegetation, the EDU has been reported to have great potential being reliable, low-cost and low technology tool (Singh et al. 2015; Agathokleous et al. 2016b, c).
Reiling and Davison (1992) reported the method of screening of cultivars as ozone responses by analyzing the gaseous exchange, fluorescence and contents of chlorophyll. The responses of the ozone in early stages can be easily evaluated by the analysis of these factors (Guidi et al. 2000). In screening, dicots are considered to be less resistant to ozone as compared to the monocots. Legumes are considered as most sensitive crop to the ozone (Singh and Agrawal 2011). In Asia, the loss of productivity of the legumes was 10–65% when the concentration of ozone in ambient environment during the growing season of legumes was varying between 35 and 75 ppb (Emberson et al. 2009).
Pisum sativum, leguminous crop belonging to the Leguminosae family, is highly rich in proteins (Nawab et al. 2008). Pea is a vegetable high in nutrition and one of the most cultivated crop in the whole world. The total amount of trading in pulses is about 40% of the pea vegetable, and it is recognized as the cash crop, highly exported in the world (Ali et al. 2002). It is the most extensively grown crop in the world, ranked the 2nd in terms of cultivation after soybean (Smykal et al. 2012). In the above scenario, the present study was conducted to assess the effects of environmental ozone stress on growth, some biochemical and physiochemical attributes on Meteor cultivar of pea under various EDU applications by two different methods.
Materials and methods
The present experiment was performed in wire fenced area of Botanical Garden situated at Department of Botany, University of the Punjab, Lahore (31.497185°-N, 74.298172°-E), Pakistan.
Procurement of plant material
Seeds of certified pea (Pisum sativum L.) cultivar Meteor were acquired from Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad, Pakistan. Viable and healthy seeds were separated from the desiccated one and used for further experimental work. A mixture of H2O2 and 70% ethanol (1:1 by v/v) was used for the surface sterilization of selected seeds. Afterward, the seeds were washed several times with distilled water. Then, seeds were soaked in a beaker filled with distilled water for 24 h.
Plants raising
Clay pots with a diameter of 36 cm were employed in order to grow the pea plants. Pots were prepared using unsterilized loamy soil along with sand (medium-coarse, mined and sieved through 2 mm mesh size) in 3:1 ratio. The ratio of mixing soil with farmyard manure was 6:1. Four pre-soaked seeds were sown in each pot. Total three replicates were prepared for each treatment. Number of seedling was kept as one per pot specifically in the middle of pot after complete germination. The seedlings were provided treatments of EDU (150, 300, 450 and 600 ppm) solutions after gap of every 10 days for up to 80 days (final harvest) by RCBD (Randomized Complete Block Design). Solutions of EDU were applied using two methods, one as soil drench (100 ml plant−1) another as foliar spray (10 ml plant−1), to the two sets of plants, respectively. The plants were treated early in the morning at 8:30 AM on 10-day interval basis. Control plants were not treated with EDU but received only distilled water as 100 ml per plant. Each and every plant either treated with EDU or not treated with EDU was watered with the tap.
Meteorological parameters
The data of various concerned meteorological parameters including temperature (Tmax and Tmin °C), relative humidity (RHmax and RHmin %), total rainfall (mm) as well as day and night span of the experimental site were obtained from Pakistan Metrological Department, Lahore, for the sake of studying the effects of these parameters on ozone formation in ambient environment.
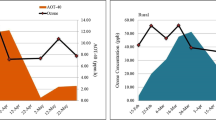
The maximum and minimum values along with means of ambient temperature, light intensity and relative humidity during the crop growing months of pea cultivar are shown in Fig. 1. November was the hottest month with the mean temperature of 20.5 °C. January was the most rainy month with least sunshine hours in growth season.
Ambient ozone monitoring
Concentration of ambient ozone present in the experimental site was recorded on daily basis for 8 h (8:00 AM–16:00 PM) with help of UV absorption photometric O3 analyzer (Horiba, APOA-370, Ozone Monitor, Japan). Monitoring of O3 was done during the entire experimental period.
Plant sampling and crop measurements
For taking up the biomass, three plants from the experiment were sampled randomly at 30 days after sowing from pea plants of each treatment. Plants with intact roots were dug out from the soil substratum, and the adhered soil particles were removed by washing thoroughly under running tap water. Blotting papers were used to remove the excess moisture on plant’s surface. For determination of biomass, the individual plant parts, i.e., leaves, roots and stem, were separated and oven-dried for 12 h at 80 °C for 5 days for the determination of biomass.
Biochemical attributes
Plant pigments
Leaf of treated plant was taken and weighed 500 mg, mixed with 10 ml of 80% acetone and grounded well using pestle and mortar to make homogenous mixture. The homogenate was then centrifuged at 3000 rpm for 15 min. The obtained supernatant was stored for further use. The pellet was exposed to 80% acetone (5 ml) repeatedly until it becomes colorless. All the acquired extracts were collected and used for quantification of pigments. Chlorophyll content was found by the procedure introduced by Arnon (1949) in which absorbance was noted particularly at 645 nm and 663 nm in a spectrophotometer (BMS). The formula used for the calculation of pigment amount is given below;
The amount of Carotenoid was estimated by the method of Kirk and Allen (1965). The same chlorophyll extract was measured at 480 nm in spectrophotometer to estimate the carotenoid content.
Relative water contents of leaves
Method given by Sivakumaran and Hall (1978) for the estimation of relative water content (RWC) of leaves was used. For that purpose, fresh leaves of treated plants were sampled and weighed, soaked in a beaker in distilled water, dried in oven at 80 °C for 24 h and reweighed. But before drying leaves were blotted and weighed again after 8 h of soaking. The formula used to calculate the RWCs given below.
Leaf pH
Weighed 5 g plant leaf was crushed in 100 ml distilled water until homogenization. The pH of homogenate was measured by an automated pH meter (HANNA).
Ascorbic acid content
The method of Keller and Schwager (1977) was used for the estimation of ascorbic acid. The following formula was used to make the standard curve using various concentrations of ascorbic acid.
where W: weight of the fresh leaf taken, V1: volume of the supernatant taken, V: total volume of the mixture, and value of [Eo − (Es − Et)] is estimated by the standard curve.
Air pollution tolerance index (APTI)
The formula developed by Singh and Rao (1983) was used to determine the air pollution tolerance index (APTI) of pea plants which is given as:
where A: ascorbic acid content of leaf in mg/g dry weight; T: total chlorophyll content of leaf in mg/g dry weight; P: leaf extracts pH; R: percentage relative water content of leaf.
Total soluble protein contents
Method of Lowery et al. (1951) was used to estimate the protein content of plant samples using bovine serum albumin (BSA) as a standard protein.
Proline contents
Method of Bates et al. (1973) was used to measure the proline content.
Total soluble sugar contents
Total soluble sugar was estimated using method of Nelson (1944).
Determination of total phenols
Procedure introduced by Zieslin and Ben-Zaken (1993) was used to determine the total phenolics of plant samples.
Antioxidant enzyme activity
The ascorbate peroxidase (APX) activity of concerned sample was determined with modified method of Nakano and Asada (1987). Assay introduced by Sairam et al. (2002) was used to find out the superoxide dismutase (SOD) activity.
Statistical analysis
The descriptive statistical analysis was performed for calculation of standard deviation, and the expression of data was done in ± S.E of 3 replicates each. The statistical analyses were performed by applying ANOVA and DMRT (Duncan’s Multiple Range Test) by the software of SPSS (Version 20).
Results and discussion
Ozone has been highlighted as the major contributor in air pollution. It has also been found as the most harmful air pollutant due to its damaging effects upon vegetation. With respect to the issues of air quality, its estimated increasing concentration in a year is about 0.1–1 ppb (Coyle et al. 2003). The ozone production is highly promoted during the months of summer and spring by photochemical reactions under the sun. So, in this regard, the higher concentrations of ozone were highly promoted in the presence of long days and higher temperatures. The precursors of ozone have been added up in the environment form the emissions of vehicle and industrial exhausts. The mean values of ozone for the Northern Hemisphere upon mid-latitudes were estimated to be increased as 20–45 ppb which is twice the concentration estimated from the previous century (Vingarzan 2004). The future predictions predict about the concentrations of ozone to be risen globally about 20–25% by the year 2050, and the same predictions were more alarming for India and south Asia confronts to be higher in the year 2020 (Van Dingenen et al. 2009).
Seasonal mean concentration of ambient ozone in the months of November, December and January was 46.4 ppb during the crop growth season 2018–2019 (Fig. 2). The increased ambient levels of ozone in the city of Lahore were estimated to be due to increase in temperature day-by-day, long, shiny, sunny days, less level of winds along with the high emissions of precursors of ozone-promoting substances and mostly highlighted in the backgrounds of the rural areas (Van Dingenen et al. 2009). The published reports were in the support of higher concentrations of ozone at surface for the past few years (Mishra and Agrawal 2015). Monitored air quality data showed up the lower levels of ozone concentrations along with the AOT40 value during the month of January due to lower temperature ranges and less shiny days in contrast to the month of February (Singh et al. 2018b). The maximum value of ozone was recorded as 78 ppb at the harvest of crop during the month of January (Gupta et al. 2018). Oksanen et al. (2013) reviewed that all the concentrations were in accordance with other readings as in India.
In order to protect crops from ozone effects, the EDU has been employed as the protecting tool and at the same time the effects of ozone were noted as well under environmental conditions (Yuan et al. 2015; Pandey et al. 2015). In the present research, EDU has been applied both as soil drench and foliar spray upon the pea plants by employing various concentrations including 150, 300, 450 and 600 ppm in order to check the decreased effects of toxic ozone due to EDU application. The foliar method of application has been pointed as the best method for cereal crops and specifically the rice crop due to the simple, reliable and effective behavior of EDU (Feng et al. 2010). The findings of Wahid et al. (2012) are similar to the results of present experimental study that the sesame crop was proved to be more protective under the higher concentrations of EDU including 375 and 500 ppm, while the sesame crop was not safe under the lower concentrations of EDU including 125 ppm and 250 ppm. Among different concentrations of EDU, the most effective concentration in protecting plants from ozone was 450 ppm (Jiang et al. 2018).
A significant increment in total fresh and dry biomass data of shoot, root and leaves by the application of various EDU treatments is shown in Table 1. The pattern of fresh and dry biomass against different EDU concentrations was measured as 450 ppm EDU > 300 ppm > 600 ppm ≥ 150 ppm. The data collected from the experiment indicate that the total fresh biomass of Meteor cultivar was maximum in plants treated with EDU-450 as soil drench, i.e., 13.56 g followed by EDU-450 as foliar spray, i.e., 12.97 g. A statistical significant difference of values of fresh biomass was clearly observed in plants of Meteor cultivar in both methods of application. The dry biomass of leaf, stem and root showed the ameliorative effects of EDU applied as soil drench and foliar spray against the ambient ozone stress on plants of pea cultivar. Ozone effects were suppressed by the application of EDU upon total biomass and dry weights of shoots (Jiang et al. 2018). According to Szantoi et al. (2009), the R. laciniata plants treated with 200, 400 and 600 mg/L EDU concentrations produced percentage increase of 10.5%, 32.9%, 24% for root biomass and 11.9%, 29.6%, 22% for total biomass in contrast to the untreated plants. According to results of Tiwari et al. (2005), the wheat plants produced additive effects in dry weights of root, shoot, leaf and total biomass by treatment with EDU.
In the present experimental study, the EDU applied as soil drench and foliar spray methods caused significant enhancement in the leaf area with more significant results given by EDU-450 treatment. There was an increase of 51.6% in leaf area of Meteor in EDU-450 treated plants of P. sativum. In the experiment done by Wahid et al., the lower EDU treatment of 125 ppm produced similar results as produced by NEDU-treated plants in biomass assessment. The highest EDU treatment of 500 ppm allowed the enhancement of green leaves number by 44–59% in relation with its effects in reducing senescence. By this result, it was deduced that EDU allowed plants to be green for longer period of times and at the same time, greater assimilation effect was recorded in the case of EDU-treated plants as compared to the NEDU-treated plants at less growth indicating the highest levels of leaf senescence.
The relative increase of chlorophyll and carotenoids is the most commonest response produced by plants treated with EDU concentrations (Singh and Agrawal 2011). In the present study, the plant pigments including chlorophyll a, chlorophyll b, total chlorophyll and carotenoids were found to increase significantly in the plants of pea cultivar treated with EDU as soil drench and foliar application. The maximum value of chla, chl b, total chl and carotenoids was recorded in 450 ppm concentration of EDU in soil drench method, i.e., 3.11, 1.01, 7.57 and 0.26 mg/g fresh weight of leaf, respectively (Table 2). The increasing trend of values of all pigments was found as EDU-450 > EDU-300 > EDU-600 ≥ EDU-150 > Control. The same trend was observed in foliar spray method of application with slight reduction in values of all the green pigments being maximum in 450 ppm treatment while minimum in control. Pandey et al. (2014) mentioned in his results about the decrease in chlorophyll degradation content due to the increment in chlorophyll content by the application of EDU. The previous results of Rai et al. 2015 for soybean and of Singh et al. 2009 for wheat supported the results of Ashrafuzzaman et al. (2017) by producing the supportive results for chlorophyll and carotenoids content under the stress of ozone.
Leaf pH showed acidic nature in pea growth season 2018–2019. The leaf pH was found to decrease with the increasing EDU concentrations applied as soil drench. The maximum leaf pH was recorded in control plants, i.e., 6.35 followed by 6.28, 6.25, 6.16 and 6.03 in plants treated with EDU-150, EDU-600, EDU-300 and EDU-450, respectively, when applied as soil drench. A same decreasing trend was followed in foliar spray method of application where the control plants showed maximum value of pH, i.e., 6.32 while the higher concentrations of EDU, i.e., 300 and 450 ppm showed more acidic nature (6.19 and 6.07, respectively).
RWC (relative water content) is defined as the factor to calculate air pollution tolerance of plants (Chaudhary and Rathore 2018). The relative water content of leaves, during the present study, increased by the increase in concentrations of EDU being maximum in 450 ppm EDU, i.e., 79.6% followed by EDU-300 (67.7%) in soil drench method of application. A similar trend of increasing RWC was found in foliar spray application of various EDU concentrations. The study effects of Chaudhary and Rathore (2018) mentioned the negative effects of ozone upon the relative water content of the cultivars of castor. The castor plants showed lesser contents of water in its early stages of growth due to its sensitivity at this stage. Moreover, the reduction in photosynthetic rates is also produced due to the reduction in relative water content (Alinian et al. 2016).
The defensive mechanism of ascorbic acid as an antioxidant has been marked as most important one in defense mechanism (Smirnoff 2000). The results of present investigation showed higher ascorbic acid contents in the leaves of pea plants treated with various concentrations of EDU as compared to control plants and the maximum value was found in EDU-450 treatment in both soil drench and foliar spray methods, i.e., 0.38 and 0.35 mg/g, respectively. While the 300 ppm and 600 ppm concentrations followed it, i.e., 0.31 and 0.27 mg/g, respectively, in soil drench method and 0.28 and 0.24 mg/g in foliar spray method of application (Table 2). Chaudhary and Agrawal (2015) reported that the elevation of ozone levels caused increment in the levels of ascorbic acid in mung bean plants. Plants treated with 150 ppm EDU treatments produced 18.3% increment in the levels of ascorbic acid content for carrot plants (Tiwari and Agrawal 2010).
Air pollution tolerance index (APTI) determination is very simple method which provides a reliable screening of large number of plants with respect to their susceptibility to air pollutants. The APTI of Meteor was observed to increase with the increase in EDU concentrations being maximum in EDU-450-treated plants, i.e., 8.5 in both soil drench and foliar spray methods while with more increase in EDU concentration the APTI tend to decrease (7 and 6.7 in soil drench and foliar spray application of EDU-600, respectively). The minimum APTI value was found in control plants, i.e., 6.1 (Fig. 3). Higher values for APTI showed plants to be more tolerant to air pollution and on the other hand, the lower values for APTI for plants were less tolerant to air pollution (Tiwari and Agrawal 2010). Plants exposed to the industrial sites have more pollution index as compared to the plants located in the non-industrial or non-polluted site. Plant tolerant species in one area may behave as non-tolerant species in another area (Raza et al. 1985).
The total soluble protein, proline and total soluble sugar contents of Meteor cultivar were recorded as being having significant amounts of higher EDU supplemented plants than the control plants, while the phenolic contents were found to decrease with increasing EDU concentrations. Total soluble protein, proline and sugar contents showed maximum values in EDU-450 treatments in both sets of EDU applications while phenolics were found to be maximum in control plants. Increase in total contents of protein has also been reported by Tiwari and Agrawal (2010) and Singh et al. (2009) as soil drench method of application of 500 ppm EDU. An inverse relationship has been developed among proteins and phenolics supporting the protein competition model of phenolic allocation at AO + EDU and EO + EDU producing increased protein content with the decreased phenolic contents (Singh et al. 2018a, b). By the reports of Cotrozzi et al. (2016), there was an increase in plant cell protection from the oxidative stress in the plant of Quercus ilex by the function of proline. Rathore and Chaudhary (2019) mentioned the higher amount of proline, carotenoids and anthocyanin in the cultivar Nidhi-999 by the treatments of EDU resulting in higher levels of protection from oxidative stresses. In an exceptional case of soybean genotype treated with EDU, the content of total phenolics decreased (Singh et al. 2018a, b). The results of Singh et al. (2018b) are contrary to the findings of present study in which they found an increase in phenolic contents in Wardan cultivar of clover by the treatments of EDU resulting an increased protein content at high ambient ozone level causing balance in the pathways of metabolism.
Application of various EDU concentrations altered the activities of antioxidative (APX and SOD) enzymes with varied magnitude in soil drench and foliar spray method of application. Significant maximum increment in APX was found in EDU-450 ppm treatment (0.85 and 0.77 µmol ascorbate/mg protein/2 mint in soil drench and foliar spray, respectively). Same is the case with SOD showing maximum values in EDU-450 ppm followed by EDU-300, EDU-600 and EDU-150 ppm as compared to control. SOD was found in the range of 0.59–0.98 units/mg protein in both methods of application (Fig. 4).
The elevated levels of toxic ROS have been managed by the metalloenzyme SOD. Increase in ascorbate peroxidase (APX), catalase (CAT) and superoxide dismutase (SOD) have been recorded as 16%, 27% and 31%, respectively, by the exposure effects of ozone (Baqasi et al. 2018). Upon application of Kundan variety with EDU, the antioxidants and antioxidative enzymes showed positive response and increased the defensive mechanisms by increasing SOD and CAT levels by protecting them from ozone harmful effects (Rao and Reddy 2008). Contrary results have been reported by Baqasi et al. (2018) with the increasing plant resistance to ozone effects by decreasing antioxidative enzyme activities under EDU treatment. The increase in activities of SOD and APX for Warden have been reported on both of the sites but that SOD increase has been reported only on site R2 by EDU treatment (Singh et al. 2018b). Ozone tolerance effect and increase in the activity of SOD are related (Singh et al. 2015).
Conclusion
Current research indicates that ambient O3 stress reduced growth and photosynthetic pigments in P. sativum. Ethylenediurea regulated the growth and physiochemical attributes of P. sativum through modulation in antioxidative defense mechanism. EDU can be used as an effective antiozonant in other agronomic crops. Furthermore, molecular mechanisms associated with O3 stress alleviation may be exploited in various crops.
References
Agathokleous E (2017) Perspectives for elucidating the ethylenediurea (EDU) mode of action for protection against O3 phytotoxicity. Ecotoxicol Environ Saf 142:530–537
Agathokleous E, Koike T, Watanabe M, Hoshika Y, Saitanis CJ (2015) Ethylenediurea (EDU), an effective phytoproctectant against O3 deleterious effects and a valuable research tool. J Agric Meteorol 71:185–195
Agathokleous E, Paoletti E, Saitanis CJ, Manning W, Sugai T, Koike T (2016a) Impacts of ethylenediurea (EDU) soil drench and foliar spray in Salix sachalinensisprotection against O3-induced injury. Sci Total Environ 573:1053–1062
Agathokleous E, Paoletti E, Saitanis CJ, Manning WJ, Shi C, Koike T (2016b) High doses of ethylene diurea (EDU) are not toxic to willow and act as nitrogen fertilizer. Sci Total Environ 566:841–850
Agathokleous E, Saitanis CJ, Stamatelopoulos D, Mouzaki-Paxinou AC, Paoletti E, Manning W (2016c) Olive oil for dressing plant leaves so as to avoid O3 injury. Water Air Soil Pollut 227:282
Ali I, Rub A, Hussain SA (2002) Screening of pea germplasm for growth, yield and resistance against powdery mildew under the agro-climatic conditions of Peshawar. Sarhad J Agric 18:177–181
Alinian S, Razmjoo J, Zeinali H (2016) Flavonoids, anthocynins, phenolics and essential oil produced in cumin (Cuminum cyminum L.) accessions under different irrigation regimes. Ind Crop Prod 81:49–55
Arnon DI (1949) Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Ashrafuzzaman MD, Lubna FA, Holtkamp F, Manning WJ, Kraska T, Frei M (2017) Diagnosing ozone stress and differential tolerance in rice (Oryza sativa L.) with ethylenediurea (EDU). Environ Pollut 230:339–350
Baqasi LA, Qari HA, Hassan IA (2018) Physiological and biochemical response of winter wheat (Triticum aestivum L.) to ambient O3 and the antiozonant chemical ethylenediurea (EDU) in Jeddah, Saudi Arabia. Biomed Pharmacol J 11:45–51
Bates LS, Waldran RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208
Carnahan JE, Jenner EL, Wat EKW (1978) Prevention of ozone injury to plants by a new protective chemical. Phytopath 68:1225–1229
Chaudhary N, Agrawal SB (2015) The role of elevated ozone on growth, yield and seed quality amongst six cultivars of mung bean. Ecotoxicol Environ Saf 111:286–294
Chaudhary IJ, Rathore D (2018) Suspended particulate matter deposition and its impact on urban trees. Atmos Pollut Res 9:1072–1082
Cotrozzi L, Remorini D, Pellegrini E, Landi M, Massai R, Nali C, Guidi L, Lorenzini G (2016) Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol Plantarum 157:69–84
Coyle M, Smith R, Fowler D (2003) An ozone budget for the UK: using measurements from the national ozone monitoring network; measured and modelled meteorological data, and a ‘big-leaf’ resistance analogy model of dry deposition. Environ Pollut 123:115–123
Didyk NP, Blum OB (2011) Natural antioxidants of plant origin against ozone damage of sensitive crops. Acta Physiol Plant 33:25–34
Dumont J, Keskisaari S, Keinänen M (2017) Ozone affects ascorbate and glutathione biosynthesis as well as amino acid contents in three Euramerican poplar genotypes. Tree Physiol 34:253–266
Emberson LD, Büker P, Ashmore MR, Mills G, Jackson LS, Agrawal M, Atikuzzaman MD, Cinderby S, Engardt M, Jamir C, Kobayashi K, Oanh NTK, Quadir QF, Wahid A (2009) A comparison of North American and Asian exposure-response data for ozone effects on crop yields. Atmos Environ 43:1945–1953
Feng Z, Wang S, Szantoi Z, Chen S, Wang X (2010) Protection of plants from ambient ozone by applications of ethylenediurea (EDU): a meta-analytic review. Environ Pollut 158:3236–3242
Guidi L, Di Cagno R, Soldatini GF (2000) Screening of bean cultivars for their response to ozone as evaluated by visible symptoms and leaf chlorophyll fluorescence. Environ Pollut 107:349–355
Gupta SK, Sharma M, Majumder B, Maurya VK, Lohani M, Deeba F, Pandey V (2018) Impact of ethylenediurea (EDU) on growth, yield and proteome of two winter wheat varieties under high ambient ozone phytotxicity. Chemosphere 196:161–173
Hassan IA, Bell JNB, Marshall FM (2007) Effects of air filtration on Egyptian clover (Trifoliumalexandrium L. cv. Messkawy) grown in open top chambers in a rural site in Egypt. Res J Biol Sci 2:395–402
Jiang L, Feng Z, Dai L, Shang B, Paoletti E (2018) Large variability in ambient ozone sensitivity across 19 ethylenediurea-treated Chinese cultivars of soybean is driven by total ascorbate. J Environ Sci 64:10–22
Keller T, Schwager H (1977) Air pollution and ascorbic acid. Eur J Pathol 7:338–350
Kirk JTO, Allen RL (1965) Dependence of chloroplast pigments synthesis on protein synthetic effects on actilione. Biochem Biophysics Res J Canada 27:523–530
Li S, Harley PC, Niinemets Ü (2017) Ozone-induced foliar damage and release of stress volatiles is highly dependent on stomatal openness and priming by low-level ozone exposure in Phaseolus vulgaris. Plant, Cell Environ 40:1984–2003
Lowery OH, Rosenbrough MS, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–267
Manning WJ, Paoletti E, Sandermann JH, Ernst D (2011) Ethylenediurea (EDU): a research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ Pollut 159:3283–3293
McGrath JM (2015) An analysis of ozone damage to historical maize and soybean yields in the United States. Proc Natl Acad Sci USA. 112:14390–14395
Mills G, Buse A, Gimeno B, Bermejo V, Holland M, Emberson L, Pleijel H (2007) A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos Environ 41:2630–2643
Mishra AK, Agrawal SB (2015) Biochemical and physiological characteristics of tropical mung bean (Vigna radiata L.) cultivars against chronic ozone stress: an insight to cultivar-specific response. Protoplasma 252:797–811
Nakano Y, Asada K (1987) Purification of ascorbate peroxidase in spinach chloroplast; its inactivation in ascorbate depleted medium and reactivation by monodehydro ascorbate radical. Plant Cell Physiol 28:131–140
Navakoudis E, Lütz C, Langebartels C (2003) Ozone impact on the photosynthetic apparatus and the protective role of polyamines. Bio Chim Biophys Acta 1621:160–169
Nawab NN, Subhani GM, Mahmood K, Shakil Q, Saeed A (2008) Genetic variability, correlation and path analysis studies in garden pea (Pisumsativum L.). J Agric Res 46:333–340
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of sugar. J Biol Chem 153:375
Oksanen E, Pandey V, Pandey AK, Keski-Saari S, Kontunen-Soppela S, Sharma C (2013) Impacts of increasing ozone on Indian plants. Environ Pollut 177:189–200
Pandey AK, Majumder B, Keski-Saari S, Kontunen-Soppela S, Pandey V, Oksanen E (2014) Differences in responses of two mustard cultivars to ethylenediurea (EDU) at high ambient ozone concentrations in India. Agric Ecosyst Environ 196:158–166
Pandey AK, Majumder B, Keski-Saari S, Kontunen-Soppela S, Mishra A, Sahu N, Pandey V, Oksanen E (2015) Searching for common responsive parameters for ozone tolerance in 18 rice cultivars in India: results from ethylenediurea studies. Sci Total Environ 1:230–238
Paoletti E, Manning WJ, Ferrara AM, Tagliaferro F (2011) Soil drench of ethylenediurea (EDU) protects sensitive trees from ozone injury. iForest Biogeosci For 4:66–68
Rai R, Agrawal M, Kumar Choudhary K, Agrawal SB, Emberson L, Büker P (2015) Application of ethylene diurea (EDU) in assessing the response of a tropical soybean cultivar to ambient O3: nitrogen metabolism, antioxidants, reproductive development and yield. Ecotoxicol Environ Saf 112:29–38
Rao CASV, Reddy AR (2008) Glutathione reductase: a putative redox regulatory system in plant cells. In: Khan NA, Singh S, Umar S (eds) Sulfur assimilation and abiotic stresses in plants. Springer, Berlin, pp 111–114
Rathore D, Chaudhary IJ (2019) Ozone risk assessment of castor (Ricinus communis L.) cultivars using open top chamber and ethylenediurea (EDU). Environ Pollut 244:257–269
Raza SN, Vijaykumari N, Murthy MS (1985) Air pollution tolerance index of certain plants of hyderabad. In: Symposium on biomonitoring state of environment. Indian National Science Academy, New Delhi, pp 243–245
Reiling K, Davison AW (1992) Spatial variation in ozone resistance of British populations of Plantago major L. New Phytol 122:699–708
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Sarkar A, Agrawal SB (2010) Elevated ozone and two modern wheat cultivars: an assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Environ Exp Bot 69:328–337
Singh S, Agrawal SB (2011) Cultivar-specific response of soybean (Glycine max L.) to ambient and elevated concentration of ozone under open top chambers. Water Air Soil Pollut 217:283–302
Singh AA, Agrawal SB (2017) Tropospheric ozone pollution in India: effects on crop yield and product quality. Environ Sci Pollut Res 24:4367–4382
Singh SK, Rao DN (1983) Evaluation of the plants for their tolerance to air pollution. In: Proceedings of the symposium on air pollution control held at IIT, Delhi, pp 218–224
Singh S, Agrawal SB, Agrawal M (2009) Differential protection of ethylenediurea (EDU) against ambient ozone for five cultivars of tropical wheat. Environ Pollut 157:2359–2367
Singh AA, Singh S, Agrawal M, Agrawal SB (2015) Assessment of ethylene diurea-induced protection in plants against ozone phytotoxicity. Rev Environ Contam Toxicol 233:124–189
Singh AA, Chaurasia M, Gupta V, Agrawal M, Agrawal SB (2018a) Responses of Zea mays L. cultivars ‘Buland’ and ‘Prakash’ to an antiozonant ethylene diurea grown under ambient and elevated levels of ozone. Acta Physiol Plant 40:92
Singh S, Singh P, Agrawal SB, Agrawal M (2018b) Use of Ethylenediurea (EDU) in identifying indicator cultivars of Indian clover against ambient ozone. Ecotoxicol Environ Saf 147:1046–1055
Sivakumaran S, Hall MA (1978) Effect of age and water stress in endogenous levels of plants growth regulators in Euphorbia lathyrus. J Exp Bot 29:195–205
Smirnoff N (2000) Ascorbic acid: metabolism and functions of a multifaceted molecule. Curr Opin Plant Biol 3:229–235
Smykal P, Aubert G, Burstin J, Coyne CJ, Ellis NTH, Flavell AJ, Ford R, Hybl M, Macas J, Neumann P, McPhee KE, Redden RJ, Rubiales D, Weller JL, Warkentin TD (2012) Pea (Pisumsativum L.) in the genomic era. Agronomy 2:74–115
Szantoi Z, Chappelka AH, Muntifering RB, Somers GL (2009) Cutleaf coneflower (Rudbeckia laciniata L.) response to ozone and ethylenediurea (EDU). Environ Pollut 157:840–846
Tiwari S (2017) Ethylenediurea as a potential tool in evaluating ozone phytotoxicity: a review study on physiological, biochemical and morphological responses of plants. Environ Sci Pollut Res 24:14019–14039
Tiwari S, Agrawal M (2010) Effectiveness of different EDU concentrations in ameliorating ozone stress in carrot plants. Ecotoxicol Environ Saf 73:1018–1027
Tiwari S, Agrawal M, Manning WJ (2005) Assessing the effects of ambient ozone on growth and productivity of two cultivars of wheat in India using three rates of application of ethylene diurea (EDU). Environ Pollut 138:153–160
Van Dingenen R, Dentener FJ, Raes F, Krol MC, Emberson L, Cofala J (2009) The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos Environ 4:604–618
Vingarzan R (2004) A review of surface ozone background levels and trends. Atmos Environ 38:3431–3442
Wahid A, Ahmad SS, Zhao Y, Bell JNB (2012) Evaluation of ambient air pollution effects on three cultivars of sesame (Sesamum indicum L.) by using ethylenediurea. Pak J Bot 44:99–110
Yuan X, Calatayud V, Jiang L, Manning WJ, Hayes F, Tian Y, Feng Z (2015) Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU). Environ Pollut 205:199–208
Zhang L, Xiao S, Chen YJ (2017) Ozone sensitivity of four Pakchoi cultivars with different leaf colors: physiological and biochemical mechanisms. Photosynthetica 55:478–490
Zhang L, Jia LL, Sui JX (2018) Ameliorating effects of three kinds of antioxidants to ozone-polluted painted nettle (Coleus blumei, benth.). Photosynthetica 56:623–632
Zieslin N, Ben-Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flower. Plant Physiol Biochem 31:333–339
Acknowledgements
We express our sincere thanks to Prof. Dr. Firdaus-e-Bareen, chairperson Department of Botany, University of the Punjab, Lahore, for providing necessary laboratory facilities in Applied Environmental Biology and Biotechnology Research Lab and encouragement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Anna Grobelak.
Rights and permissions
About this article
Cite this article
Jabeen, F., Ahmed, S. Ethylenediurea regulates growth and physiochemical responses of Pisum sativum to ambient O3. Int. J. Environ. Sci. Technol. 18, 3571–3580 (2021). https://doi.org/10.1007/s13762-020-03077-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-03077-1