Abstract
Two cultivars of soybean (Pusa 9814 and Pusa 9712) were investigated to evaluate the impact of ambient and elevated concentrations of ozone (O3) in a suburban site of India with and without application of 400 ppm ethylenediurea (EDU) in open top chambers having filtered air (FCs), non-filtered air (NFCs), and non-filtered plus 20 ppb O3 (NFCs + 20 ppb). Significant reductions were observed in various growth parameters, biomass accumulation, and yield attributes of soybean cultivars due to ambient O3 in NFCs and elevated concentration of O3 in NFCs + 20 ppb. Reductions in all parameters were of lower magnitude in plants treated with EDU as compared to non-EDU treated plants. Yield (weight of seeds plant−1) increased by 29.8% and 33% in Pusa 9712 and by 28.2% and 29.0% in Pusa 9814 due to EDU treatment in plants grown at ambient and elevated levels of O3, respectively. The results clearly showed that (a) EDU can be effectively used to assess phytotoxicity of O3 by providing protection against its deleterious effects, (b) EDU can be used for biomonitoring of O3 in areas experiencing its higher concentrations, and (3) EDU is more effective against higher concentrations of O3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tropospheric O3 levels have increased from pre-industrial period and are predicted to continue to increase in near future (Coyle et al. 2003; Vingarzan 2004; Meehl et al. 2007). It was estimated that air pollution causes reduction in major crop yields and led losses of $4.0 to 7.0 billion alone in USA (Heck et al. 1988). O3 phytotoxicity is due to its oxidative capacity that induces reactive oxygen species in plants exposed to it. Ethylenediurea (EDU) is an antioxidant chemical not available commercially and used only as an experimental tool, which provides protection against O3 injury in a number of plant species (Carnahan et al. 1978; Hassan et al. 2007; Singh and Agrawal 2009; Agrawal et al. 2004, 2005; Wahid et al. 2001; Tiwari and Agrawal 2009; Singh et al. 2010). Extensive use of EDU has been done in the USA and Western Europe to assess the effect of O3 on plant growth and its metabolism (Kuehler and Flagler 1999; Ribas and Penuelas 2000; Manning et al. 2003; Szantoi et al. 2009), whereas limited work has been done in tropical countries including India (Hassan 2006; Agrawal et al. 2005; Al-Qurainy 2008; Singh and Agrawal 2009). EDU is very much effective in protecting plants from O3 and ultimately helps in retaining high yield of various crop plants. However, its effect varies not only between species but also among different cultivars, mode of its application, and also due to variations in prevailing environmental conditions.
Experiments performed in India using EDU that are mainly worked out in ambient atmosphere clearly shown that ambient O3 levels caused significant growth and yield reductions of various crop plants, including vegetables (Varshney and Rout 1998; Agrawal et al. 2004, 2005; Tiwari et al. 2006; Singh and Agrawal 2009; Tiwari and Agrawal 2009; Singh et al. 2010). However, several experiments have been performed open top chambers (OTCs) to determine the effects of O3 specifically apart from other pollutants (Brunschon-Harti et al. 1995; Wahid et al. 2001; Elagoz and Manning 2002; Szantoi et al. 2009; Feng et al. 2010), but no work has been done so far to evaluate the effects of EDU application under ambient and elevated concentrations of O3. OTCs provide near natural field environment and are used to study the response of plants to ambient/without O3 and can also be used to provide elevated levels of O3, CO2, NO2, SO2, etc. to predict effect of these pollutants on plants in the near future. Plants grown in OTCs are treated with charcoal-filtered air (FCs) and non-filtered air (NFCs) under quasi-natural field conditions.
It is a well-known fact that legumes are very sensitive to O3, and huge yield losses (10–65%) are reported in Asian regions due to increasing ambient O3 concentrations. It was observed that Asian grown wheat and rice cultivars are more sensitive to O3 than the North American dose–response relationships, but for legumes, no such equivalent conclusion is set due to scatter in data (Emberson et al. 2009). Under chronic O3 concentrations, legumes exhibited reduced photosynthetic rate, especially at reproductive phase of growth (Morgan et al. 2003; Krupa et al. 1998), clearly showing that unchecked increase of O3 in the future will hamper the productivity of crops sensitive to O3. Thus, in order to compare the sensitivity level of soybean to O3 in Indian environmental conditions, the present study was undertaken to assess how EDU helps to ameliorate O3-induced injury in plants at ambient and elevated levels of O3.
2 Materials and Methods
2.1 Experimental Site
The experiment was carried out under natural field conditions at a suburban area of Varanasi city, situated in the eastern Gangetic plains of Indian subcontinent at 25°14′ N latitude, 82°03′ E longitude, and 76.19 m above sea level in the months of July to October, 2008. Mean monthly minimum temperature varied from 20.3°C to 26.1°C and maximum from 31.8°C to 33.9°C during the experimental period. The mean monthly maximum relative humidity ranges from 83.1% to 90.0% and mean minimum from 71.2% to 82.5%. Total rainfall during the growth period of plant was 785.7 mm, and the maximum was recorded in July (373.5 mm) and August (352.6 mm). The mean monthly sunshine hours varied from 3.3 to 8 h.
2.2 Plant Materials
Seeds of two test cultivars of soybean Glycine max L. cv. Pusa 9712 and Pusa 9814 were obtained from Indian Agricultural Research Institute, New Delhi. Soybean is a nutritious leguminous crop that provides oil seed and vegetable oil. It is also a very good source of high quality protein. It is the fourth most important crop of the world in terms of production (FAO-UN 2003), and India produces half of the world’s average soybean.
2.3 Experimental Design
Eighteen open top chambers (OTCs) of 1.5-m diameter and 1.8-m height were established at the experimental site following the design of Bell and Ashmore (1986). Six chambers received non-filtered air (NFCs), six chambers received non-filtered air plus 20 ppb elevated O3 (NFCs + 20 ppb) and other six filtered air after passing through activated charcoal filters (FCs). There were three replicate chambers of each treatment. Each OTC contained cultivars of soybean; half of the area was covered by Pusa 9712 and other half with Pusa 9814. Recommended dose of NPK (20:80:40 kg ha−1) was added to the soil using standard agronomic practices. Seeds were inoculated with Rhizobium culture of strain Bradyrhizobium japonicum TSH-501 and sown in rows 15 cm apart inside each chamber. Eighteen OTCs were divided into six treatments as FCs without EDU, FCs with EDU, NFCs without EDU and NFCs with EDU, NFCs + 20 ppb without EDU, and NFCs + 20 ppb with EDU. Each OTC was ventilated with high speed blower for a continuous air supply at three changes per minute. Plants were fumigated with O3 in the respective OTCs with the help of ozone generator (Model Systrocom, India), where oxygen gas was passed across a UV lamp and it breaks into nascent oxygen which reacts with oxygen to form O3. The delivery ends of O3 generator were connected to the distribution system so that air coming from blower became diluted and circulated the O3 gas into the chamber. Plants were exposed to 20 ppb elevated O3 above ambient O3 for 3 h day−1 at the peak O3 period (1100–1400 hours) of local time.
Inside and outside the chambers, microclimatic conditions were measured continuously. The mean temperature within the chamber was 0.1–0.3°C higher as compared to ambient atmosphere. Light intensity was 4–5% less inside the chambers as compared to ambient air. Relative humidity inside the chambers was 2–5% higher than the ambient condition. Above parameters were measured using the portable temperature, and light intensity and humidity were recorded (Model DP223, Omnidata International Inc., USA).
2.4 O3 Monitoring
O3 concentrations were monitored and analyzed using O3 analyzer (Model APOA 370, HORIBA Ltd., Japan) from 900 to 1700 hours during the study period from each chamber. The concentration of O3 was maintained 20 ± 5 ppb above ambient level in NFCs + 20 ppb chambers. Air samples were collected with the help of Teflon tube (0.35-cm diameter) placed above the canopy of plants.
2.5 EDU Application
Nine chambers (FCs, NFCs, and NFCs + 20 ppb) of each cultivar were treated with 400 ppm EDU and others with same amount of deionized water to maintain an equal water regime in both treatments. Fresh solution of EDU was prepared each time using deionized warm water and applied as a soil drench (100 ml plant−1) 10 days after germination (DAG) between 0900 and 1000 hours at an interval of 10 days up to 40 DAG. After that, 200 ml plant−1 EDU was applied till the date of harvest.
2.6 Plant Sampling and Analysis
Random samplings of plants were done at 40, 60, and 80 DAG for various growth analyses. Monoliths of 10 × 10 × 20 cm3 containing intact roots were carefully dug out at random from each chamber. These were thoroughly washed under tap water by placing them on a sieve of 2-mm mesh size. Plant samples were analyzed for root and shoot length, number of leaves, and leaf area. Leaf area was measured using a portable leaf area meter (Model LI-3000, LI-COR, Inc., USA). Plant parts were separated and oven dried (80°C) to constant weight to determine biomass accumulation in each part. Various growth indices were calculated using the standard formulae of Hunt (1982). Different yield parameters, such as number and weight of pod per plant, number of seeds per pod, number and weight of seeds per plant, weight of above ground parts, and test weight (1,000 seed weight), were recorded. Harvest index (HI) was calculated as the ratio of economic yield (weight of seeds per plant) to above ground biomass of the plant.
2.7 Statistical Analysis
Treatment means were statistically compared using the statistical package SPSS software (SPSS Inc., Version 10.0). Data of growth, biomass, and yield were subjected to Student’s t test for assessing the significance of quantitative changes in different parameters due to EDU treatment at different sampling intervals.
Three-way ANOVA was used to determine statistically significant differences in growth, biomass, and yield data due to cultivar and EDU treatments at different O3 levels.
3 Results
3.1 Ozone Concentration
Mean ambient O3 concentration varied from 50.5 to 58.9 ppb during the growth period of soybean. Lowest concentration of O3 was recorded in the month of July 2008 (50.5 ppb), whereas its highest concentration was recorded in October 2008 (Fig. 1). In all the months, mean O3 concentration exceeded 40 ppb, and maximum hourly mean concentration of O3 was 109.1 ppb during the study period. O3 concentration was reduced by 92.6% in FCs as compared to NFCs.
3.2 Visible Injury
Visible injury was observed in both the cultivars of soybean grown in NFCs and NFCs + 20 ppb at both 60 and 80 DAG, but at 40 DAG, injury symptoms were only observed in plants grown in NFCs + 20 ppb. Initially, small chlorotic spots were observed on the adaxial surface of the leaves, but later on, prominent interveinal chlorosis was observed. EDU treatment reduced number of injured leaves by 50.2% and 59.1% in Pusa 9712 and 45.9% and 60.0% in Pusa 9814 grown in ambient and elevated levels of O3, respectively, but the protection was only partial. Percentage of injured leaves at 80 DAG is presented in Fig. 2. No injury symptoms appeared in the plants grown in open top chambers receiving filtered air.
3.3 Growth Characteristics and Biomass Accumulation
Significant increase in shoot length was observed in EDU-treated plants of Pusa 9712 grown in non-filtered air (NFCs) at all the sampling ages and also under elevated level of O3 (NFCs + 20 ppb) at 60 DAG, whereas in Pusa 9814, the increment was only significant in plants grown in NFCs at 80 DAG and in NFCs + 20 ppb at 60 DAG (Fig. 3). Root length did not show significant changes with EDU treatments at most of the sampling ages in Pusa 9712 in both NFCs and NFCs + 20 ppb plants except in NFCs at 40 DAG while the increment was significant in Pusa 9814 grown in NFCs and NFCs + 20 ppb plants at 40 and 60 DAG (Fig. 3). Leaf number represents the leaf production rate of a plant. Results showed a significant increase in number of leaves in both the cultivars treated with EDU as compared to non-EDU-treated ones grown in NFCs and NFCs + 20 ppb at all the ages of observations (Fig. 4). Number of leaves increased by 19.3% and 34.6% in Pusa 9712 and 25.6% and 25.0% in Pusa 9814 in EDU-treated plants grown in NFCs and NFCs + 20 ppb at 80 DAG. Leaf area also showed a similar pattern of increase except in Pusa 9814 grown in NFCs at 60 DAG (Fig. 4). Significant increments of 13.7% and 13.0% in Pusa 9712 and 7.4% and 25.5% in Pusa 9814 were observed (in EDU-treated plants of NFCs and NFCs + 20 ppb) for leaf area at 80 DAG. Increment in number of root nodules were observed in EDU-treated plants of both the cultivars grown in ambient and at elevated levels of O3. Number of nodules reduced by 39.9% and 51.0% in Pusa 9712 and 28.9% and 31.8% in Pusa 9814 in non-EDU-treated plants as compared to EDU-treated ones grown in NFCs and NFCs + 20 ppb.
Three-way ANOVA showed that shoot length varied significantly due to all the individual factors and interaction of C × O3 × T (p < 0.05; Table 1). Significant effect was observed on root length due to cultivar (p < 0.05) and EDU treatment (p < 0.05), on number of leaves due to all the individual factors (p < 0.001) and interaction of O3 × T (p < 0.05), and on leaf area due to O3 (p < 0.05), treatment (p < 0.01), and interaction of C × O3 (p < 0.01; Table 1).
Root biomass increased significantly in EDU-treated plants of Pusa 9712 grown in NFCs and NFCs + 20 ppb as compared to non-treated ones at 60 and 80 DAG, whereas in Pusa 9814, increment of root biomass was observed only in plants of NFCs + 20 ppb at both 60 and 80 DAG (Fig. 5). Root biomass varied significantly at 40 DAG only in plants of Pusa 9712 grown in NFCs + 20 ppb (Fig. 5). Root biomass increased by 12.4% and 80.3% in Pusa 9712 and 30.7% and 30.9% in Pusa 9814 in EDU-treated plants grown in NFCs and NFCs + 20 ppb as compared to non-EDU-treated ones at 80 DAG. Significantly, increment in shoot biomass was observed in both the EDU-treated cultivars of NFCs and NFCs + 20 ppb as compared to non-EDU-treated ones at all the sampling ages except in NFCs at 80 DAG (Fig. 6). Shoot biomass increased by 31.2% in Pusa 9712 and 26.7% in Pusa 9814 of NFCs + 20 plants treated with EDU as compared to non-EDU-treated ones at 80 DAG. A similar pattern was observed for leaf biomass in both the cultivars of soybean (Fig. 7). Significant variations in total biomass were observed in all the EDU-treated plants grown in NFCs and NFCs + 20 ppb at all the ages of sampling in both the cultivars (Fig. 8). Increment of total biomass by 19.6% and 16.8% in Pusa 9712 and 22.7% and 22.6% in Pusa 9814 of EDU-treated plants grown in NFCs and NFCs + 20 ppb were observed at 80 DAG.
Statistical analysis showed that shoot biomass and total biomass varied significantly due to all the individual factors (p < 0.001) and interaction of C × O3 (p < 0.001) and O3 × T (p < 0.05, p < 0.01). Significant effect of cultivar on root biomass (p < 0.05) and cultivar and EDU treatment (p < 0.01) on leaf biomass were recorded (Table 1).
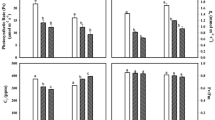
Net assimilation rate (NAR) and leaf area ratio (LAR) showed a similar trend in both the cultivars grown in NFCs and NFCs + 20 ppb (Fig. 9). NAR reduced significantly by 50.8% and 28.6% in EDU-treated plants of NFCs and NFCs + 20 ppb of cultivar Pusa 9712, whereas in Pusa 9814, it decreased by 9.9% and 20.8% in EDU-treated plants of NFCs and NFCs + 20 ppb as compared to the control ones during 40–60 DAG. LAR increased significantly in EDU-treated plants grown only in NFCs + 20 ppb during the age interval of 40–60 DAG in Pusa 9712, but in Pusa 9814, it increased at both the ages of sampling (Fig. 9). Relative growth rate (RGR) decreased significantly in both the EDU-treated cultivars grown in NFCs during the age interval of 40–60 DAG (Fig. 9). Root shoot ratio (RSR) was significantly higher by 20.9% and 30% in EDU-treated plants of Pusa 9712 grown in NFCs + 20 ppb at both 60 and 80 DAG. Leaf weight ratio (LWR) did not vary significantly in both the cultivars treated with EDU (Fig. 10). Specific leaf area (SLA) increased significantly in Pusa 9814 in EDU-treated plants grown in NFCs + 20 ppb at both the ages, while a reduction was observed in NFC-grown plants at 60 DAG (Fig. 10). Specific leaf weight (SLW) showed a similar but reverse trend of SLA (Fig. 10).
ANOVA showed that NAR, RGR, and SLW varied significantly due to cultivar and interaction of C × O3 (p < 0.001, p < 0.01, p < 0.05; Table 1). Significant effect was observed due to O3 on RSR (p < 0.001), due to cultivar (p < 0.001), O3 (p < 0.01), and interaction of C × O3 (p < 0.001) on LWR, and due to cultivar (p < 0.01) and interaction of C × T (p < 0.05) on SLA (Table 1). LAR showed significant variations due to cultivar (p < 0.001) and interaction of C × O3 (p < 0.001), C × T (p < 0.05), O3 × T (p < 0.05), and C × O3 × T (p < 0.05; Table 1).
3.4 Yield and Yield Attributes
The results of the present study showed ameliorating effects of EDU on yield losses of both the cultivars of soybean. Both the cultivars (Pusa 9712 and 9814) behaved in a similar pattern of protection with respect to application of 400 ppm EDU. Increased yield reduction was observed in plants grown under elevated levels of O3 than plants grown under ambient conditions (Fig. 11). Number of pods per plant, number of seeds per plant, and weight of seeds per plant showed significant increases after EDU application in both the cultivars as compared to non-EDU-treated plants grown in NFCs and in NFCs + 20 ppb (Fig. 11). Number of seeds increased significantly by 37.4% and 31.6% in Pusa 9712 and 25.6% and 25.9% in Pusa 9814 in EDU-treated plants grown in NFCs and NFCs + 20 ppb as compared to the control ones. Weight of seeds per plant were significantly higher by 29.8% and 33% in Pusa 9712 and 28.2% and 29.0% in Pusa 9814 in EDU-treated plants grown in NFCs and NFCs + 20 ppb. Above ground biomass was significantly higher in plants grown only under elevated levels of O3 in both the cultivars (Fig. 12). Test weight showed a significant increment of 12.6% in EDU-treated plants of Pusa 9712 grown in NFCs + 20 ppb whereas it increased by 16.6% and 14.9% in Pusa 9814 grown in NFCs and NFCs + 20 ppb (Fig. 12). HI did not change significantly due to EDU in cultivar Pusa 9712; however, it decreased significantly in Pusa 9814 under NFCs + 20 ppb (Fig. 12).
Three-way ANOVA showed that variations in yield (weight of seeds per plant), number of seeds per plant, above ground biomass, and number of pods per plant were significant due to cultivar (p < 0.001, p < 0.01), O3 (p < 0.001), and EDU treatment (p < 0.001) and their interactions except C × T and C × O3 × T (Table 1). Variation in weight of pods per plant was significant due to all the individual factors (p < 0.001) and interaction of C × O3, O3 × T (p < 0.001), and C × O3 × T (p < 0.05) except C × T (Table 1). Test weight and HI showed significant variations due to all the individual factors (p < 0.001) and their interactions except C × O3 for test weight and C × T and O3 × T for HI (Table 1).
Responses of both the cultivars of soybean did not vary significantly between EDU-treated and non-treated plants grown in FCs in most of the parameters assessed at different sampling ages. However, EDU treatment caused significant increments in leaf area, leaf biomass, total biomass, and other growth indices of Pusa 9712 during the initial phase of plant growth and HI of Pusa 9712.
4 Discussion
A relatively higher amount of surface O3 was observed during the entire period of experiment. Increase in anthropogenic O3 precursor emissions lead to increase of O3 in troposphere. In many parts of the northern hemisphere, O3 concentrations are found to be increasing in the free troposphere as well as near surface (Naja and Lal 2002; Lal et al. 2000; Varotsos et al. 1994; Nair et al. 2002; Debaje and Kakade 2009), and it is known that prevailing warm climatic conditions and long sunshine hours may be responsible for higher O3 formation in northern regions (Tiwari et al. 2008). A study conducted in a peri-urban area of Malaysia reported a mean O3 concentration of 32.5 ppb with an hourly maximum level of 90.8 ppb during September 2000 to January 2001 (Ishii et al. 2004). The present study also showed the similar findings as reported by Rai and Agrawal (2008) with lowest O3 concentration in July (30.5 ppb) and highest in October 2006 (45.4 ppb). Soybean plants are grown from July to October and then harvested in November. Rainy season starts from mid June to September. Low O3 concentration of July and August can be correlated with the heavy rainfall, responsible for high relative humidity, and diffused solar radiation due to high cloud cover. Rainfall washes out the active radicals involved in O3 formation process. Higher O3 concentrations during October can be directly explained due to less rainfall and increased sunshine hours, which is conducive for O3 formation. Low O3 concentration in early August (13.5 ppb) which increased to 52.9 ppb in September was observed during the experiment with rice in OTCs in an experiment in Pakistan (Wahid et al. 1995). Sarkar and Agrawal (2009) also observed low O3 concentration (12 h) during July and August (41.3–44.7 ppb) which increased in October (59.9) during the experiment of rice in OTCs in the year 2007. Debaje and Kakade (2009) also observed low O3 concentrations (10–15 ppb) during the month of June–September as compared to summer/winter months at five different sites of western Maharashtra for the period between 2001 and 2005. AOT40 is the critical level of accumulated O3 concentration above threshold of 40 ppb, and if it exceeds above 3 ppm h during the growing season of agricultural crops, it may cause significant yield loss. Rai et al. (2010) reported an AOT40 value of 2.1 ppm h for the period of July to October 2005 in OTCs receiving ambient concentrations of O3. Recently, Sarkar and Agrawal (2010) reported ambient AOT40 value of 7.9 and 8.7 ppm h, and at elevated level, it was 13.1 and 14.4 ppm h for the year 2007–2008 and 2008–2009, respectively, during December to March. Both these studies were conducted at the same site where the present study has been performed.
Results are well in agreement with earlier studies made on Phaseolus vulgaris, G. max, Raphanus sativus, Brassica rapa, and Solanum tuberosum (Carnahan et al. 1978; Ribas and Penuelas 2000; Wahid et al. 2001; Hassan et al. 1995; Hassan 2006), showing lower O3 injuries due to EDU treatment indicating that it provided partial protection against O3 injuries. Higher foliar injury symptoms in NFCs + 20 ppb-grown plants reflected the higher levels of O3 in open top chambers. Protective effect of EDU against foliar injury was also reported in different cultivars of potato and soybean (Clarke et al. 1990; Brennan et al. 1990). Hassan (2006) too has found higher foliar injury at site where higher ambient O3 was recorded and reported partial protection due to EDU application in potato. In a laboratory experiment, it was found that 400 ppm EDU application was sufficient to give complete protection to soybean plants against visible injury from fumigation with O3 at 100 ppb (Wahid et al. 2001).
In the present study, EDU treatment resulted in an increment of shoot and root lengths of both the cultivars grown under ambient and elevated concentrations of O3. Significant increment in shoot length was observed in both the EDU-treated cultivars grown in non-filtered air but not in plants of elevated O3 at 80 DAG with more favorable effect in Pusa 9712. Significant increment in root length with both the levels of O3 in EDU-treated plants of Pusa 9814 both at 40 and 60 DAG showed that EDU was more effective in improving shoot length of Pusa 9712 and root length of Pusa 9814. Significant reduction was observed in root tip formation, root length, and root elongation rate of Trifolium subterraneum exposed to 75 ppb O3 for 8 h during daytime for five consecutive days (Vollsnes et al. 2010).Using 500 ppm EDU as soil drench, Agrawal et al. (2005) reported an increase of 12.3% in shoot length of Vigna radiata plants. Tiwari et al. (2005) also observed a significantly higher shoot length at 300 ppm and root length at 450 ppm EDU concentration in Triticum aestivum L. cv M533. Disturbances in various metabolic activities, impairment of photosynthetic capacity, and early senescence due to high levels of O3 lead to reductions in plant growth (Agrawal et al. 2005; Hassan 2006; Singh et al. 2009, 2010). An increment of 20.7% in shoot length of EDU-treated (400 ppm) soybean (G. max L.) plants grown at a roadside rural area having mean O3 concentration of 48 ppb was observed (Wahid et al. 2001). Morgan et al. (2003) reported 7% shorter plant exposed to elevated O3 in soybean. Shoot length increased significantly by 19.7% in palak (Beta vulgaris L. var. All Green) plants treated with 300 ppm EDU at O3 concentrations varying from 52 to 73 ppb at a suburban site of Varanasi, but root length did not vary significantly (Tiwari and Agrawal 2009). Significant increment of 22.8% in shoot length of mungbean was recorded upon 400 ppm EDU treatment in an area experiencing O3 concentration between 52.9 and 64.5 ppb (Singh et al. 2010). They also reported significantly higher root length in EDU-treated plants at 30 DAG. EDU-treated plants of both the cultivars showed significant increment in number of leaves and leaf area, and EDU was found more effective for plants grown at elevated levels of O3 than grown at ambient levels. Increments in various growth characteristics showed protective nature of EDU. Number of leaves and leaf area were significantly higher by 28.0% and 27.6% in palak plants (B. vulgaris L. var. All Green) due to EDU treatment (Tiwari and Agrawal 2009). A similar trend was also observed in five wheat cultivars treated with 400 ppm EDU at a suburban area experiencing an O3 concentration between 34.2 and 54.2 ppb (Singh and Agrawal 2009).
The variability in the response of EDU at two different O3 levels could be explained due to enhancement of leaf senescence under elevated level of O3. Phenylurea is an important component of EDU and thus helps in cell proliferation and induction of these parameters. Earlier studies also suggested cytokinin-like activities of EDU, such as prevention of chlorophyll degradation, protein and RNA synthesis, cell proliferation, etc. (Lee and Chen 1982). EDU brings about specific metabolic changes through sustaining RNA and protein synthesis in cells. EDU-induced leaf production has been reported in G. max L. (Wahid et al. 2001), T. aestivum L. (Agrawal et al. 2004), V. radiata L. (Agrawal et al. 2005), Lycopersicon esculentum L. (Varshney and Rout 1998). Nodulation is an important process in leguminous plants, and it has been found that O3 stress can modify its formation. Tingey and Blum (1973) observed decreased nodulation in soybean plants when exposed to O3. In this study, more reduction in number of nodules was observed in Pusa 9712 as compared to Pusa 9814, and it further decreased under elevated level of O3. EDU treatment positively affected this parameter, but it was more effective in Pusa 9712 which showed more sensitivity toward O3. Agrawal et al. (2005) also noticed an increment of 71.4% in number of root nodules of mungbean after EDU treatment. EDU application at the rate of 1,000 ppm increased 84% root nodules per plant in peanut (Arachis hypogea L. cv. USDA PI 268661; Ensing et al. 1985).
Components of plants significantly increased with EDU treatment grown in non-filtered air and elevated levels of O3 in both the cultivars. Increase in biomass was observed in Pusa 9814 as compared to Pusa 9712 due to EDU application. This trend showed that EDU was more effective in cultivar Pusa 9814 in terms of biomass accumulation. Biswas et al. (2008) observed reduction in total mass, shoot mass, root mass, and root/shoot ratio in 20 O3-exposed wheat cultivars. Ball et al. (1998) analyzed the results of exposure of EDU and non-EDU treated Trifolium repens L. cv. Menna at 12 sites; nine countries in 1994, 1995, and 1996 showed that there was a decrease in the biomass ratio of non-EDU to EDU-treated plants with increasing AOT 40. Shoot biomass in R. sativus L. (cv. Cherry Belle) and P. vulgaris L. (cv. Lit) was higher in EDU-treated plants than in non-EDU-treated plants in Sweden in 1990 and Catalonia (Spain) in 1994–1996 (Pleijel et al. 1999; Ribas and Penuelas 2000). Significant increments were reported in root, shoot, and total dry weight of turnip and radish plants after EDU treatment of 500 ppm in an area having high mean O3 concentration (66.9 ppb; Hassan et al. 1995). Agrawal et al. (2005) and Singh and Agrawal (2009) reported that EDU treatment increased root, shoot, and total dry weight of mungbean and different cultivars of wheat. Higher root and shoot biomass were also observed in EDU-treated plants of tomato at higher level of ambient O3 in an experiment conducted in New Delhi, India (Varshney and Rout 1998). However, Szantoi et al. (2009) reported significant reductions in root biomass by 10.5% and 32.9% and in total biomass by 24% and 11.9%, 29.6% and 22% at 200, 400, and 600 ppm EDU-treated Rudbeckia laciniata plants, respectively, as compared to non-EDU-treated ones.
LAR of EDU-treated plants increased when compared with non-treated ones which represent a leaf growth in terms of increased leaf area. Cell elongation is more sensitive to pollution stress than cell division. Reduction of RGR in EDU-treated plants was mainly due to more reduction in NAR as RGR depends on both NAR and LAR. In contrast, Tiwari and Agrawal (2009) reported significant increment in RGR and reduced NAR in EDU-treated palak plants. Increased SLA in EDU-treated plants of Pusa 9814 indicates that use of photosynthates is more toward leaf elongation. RSR increased significantly only in Pusa 9712 grown in elevated O3 suggesting its important positive role on more allocation of photosynthates to roots. Ozone is known to modify the biomass allocation pattern more to source than sinks such as in case of roots and reproductive parts (Pandey and Agrawal 1994). Significant increment in LAR and RSR of mungbean due to EDU application was observed in urd plants (Singh et al. 2010). Agrawal et al. (2005) also reported increased RSR in mungbean due to EDU treatment. However, Singh and Agrawal (2009) found reduction in RSR of different wheat cultivars after EDU treatment.
Yield responses observed in the present study suggest that the increase in O3 concentration leads to more yield losses in both the cultivars of soybean. The results are in agreement with the previous studies showing beneficial effects of EDU on yield components of soybean plants (Wahid et al. 2001; Ribas and Penuelas 2000; Elagoz and Manning 2002; Agrawal et al. 2005). Weight of seeds per plant increased more in Pusa 9712 as compared to Pusa 9814 at both the O3 levels due to EDU which could be related to increases number of seeds per plant and also due to more translocation of assimilates to seeds. On the other hand, more energy of photoassimilates might be diverted in the repair processes of plants due to O3 in Pusa 9814. Significant increase in above ground biomass of both the cultivars in NFCs + 20 ppb due to EDU treatment suggests that EDU was more effective at higher O3 concentration. Ozone is well known to cause yield reductions in many major crop plants by altering the normal functioning of plants (Rai et al. 2007; Rai and Agrawal 2008; Wahid et al. 1995; Pang et al. 2009). Gelang et al. (2000) observed that accelerated senescence of flag leaves shortened the grain-filling duration and thereby reduced grain yield under elevated O3. Another possible reason for O3-induced yield loss could be due to toxic effects of O3 on pollen tube growth and fertilization in wheat (Black et al. 2000; Mckee et al. 1997; Mulholland et al. 1998) or high sensitivity of certain snap bean and potato cultivars to O3 (Heagle et al. 2003; Heagle et al. 2002). Wang et al. (2007) compared EDU-treated with untreated wheat plants and concluded that local ambient levels of O3 with an annual mean concentrations of 74 ppb mainly affected wheat but not rice. Grain weight per plant was significantly higher by 18.8%, 19.1%, and 20.5% in M 533 and 24.8%, 66.9%, and 66.8% in M 234 of wheat plants treated with 150, 300, and 450 ppm EDU as compared to their control ones (Tiwari et al. 2005). Rai et al. (2007) found an increment of 20.7% in weight of grains per plant in wheat grown in filtered chambers (FCs) as compared to non-filtered chambers (NFCs). Agrawal et al. (2005) found an increment in test weight of mungbean by 25.3% when treated with 500 ppm EDU. Elagoz and Manning (2002) reported a significant increase in above ground biomass, pod, and seed weight of O3-sensitive bean cultivar (S156) when treated with 300 ppm EDU as foliar spray as compared with control whereas an opposite trend was noticed in O3-tolerant cultivar R123. The study conducted by Hassan (2006) in an Egyptian field also found a significant increase in tuber weight of potato after treatment with 300 ppm EDU and exposure to 95.5 ppb O3.
5 Conclusion
The results of the present study clearly support the hypothesis that negative impact of O3 on the yield of soybean increased with the increase of its concentration and response is cultivar specific. The findings further imply that EDU may be used as an antiozonant in ameliorating the O3 stress and helped in minimizing the yield losses in both the test cultivars. EDU treatment showed that cultivar Pusa 9712 was more sensitive toward O3 in terms of economic yield as compared to Pusa 9814, and EDU protection was higher at elevated O3 concentration. In spite of more biomass accumulation in Pusa 9814, higher yield was found in Pusa 9712 due to EDU treatment which could be due to more investment of photoassimilates toward reproductive parts in this cultivar and more utilization of assimilates for repair processes in Pusa 9814. Findings will be useful for plant breeders to select the O3 resistant cultivars of soybean especially for the rural areas having higher concentrations of O3.
References
Agrawal, S. B., Singh, A., & Rathore, D. (2004). Assessing the effects of ambient air pollution on growth, biochemical and yield characteristics of three cultivars of wheat (Triticum aestivum L.) with ethylenediurea and ascorbic acid. Journal of Plant Biology, 31(3), 165–172.
Agrawal, S. B., Singh, A., & Rathore, D. (2005). Role of ethylenediurea (EDU) in assessing impact of ozone on Vigna radiata L. plants in a suburban area of Allahabad (India). Chemosphere, 61, 218–228.
Al-Qurainy, F. H. (2008). Effect of air pollution and ethylenediurea on broad bean plants grown at two localities in KSA. International Journal of Botany, 4(1), 117–122.
Ball, G. R., Benton, J., Palmer-Brown, D., Fuhrer, J., Skarby, L., Gimeno, B. S., et al. (1998). Identifying factors which modify the effects of ambient ozone on white clover (Trifolium repens) in Europe. Environmental Pollution, 103, 7–16.
Bell, J. N. B., & Ashmore, M. R. (1986). Design and construction of open top chambers and methods of filtration (equipments and cost). In Proceedings of II European Open Top Chambers Workshop, September 1986, Freiburg. CEC, Brussels.
Biswas, D. K., Xu, H., Li, Y. G., Sun, J. Z., Wang, X. Z., Han, X. G., et al. (2008). Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biology, 14, 46–59.
Black, V. J., Black, C. R., Roberts, J. A., & Stewart, C. A. (2000). Impact of ozone on the reproductive development of plants. New Phytologist, 147, 421–447.
Brennan, E. G., Clarke, B. B., Greenhalgh-weidman, B., & Smith, G. (1990). An assessment of the impact of ambient ozone on field grown crops in New Jersey using the EDU method: Part 2—soybean (Glycine max L.) Merr.). Environmental Pollution, 66, 361–373.
Brunschon-Harti, S., Fangmeire, A., & Jager, H. J. (1995). Influence of ozone and ethylenediurea (EDU) on growth and yield of bean (Phaseolus vulgaris L.) in open-top chambers. Environmental Pollution, 90, 89–94.
Carnahan, J. E., Jenner, E. L., & Wat, E. K. W. (1978). Prevention of ozone injury in plants by a new protective chemical. Phytopathology, 68, 1225–1229.
Clarke, B. B., Greenhalgh-Weidman, B., & Brennan, E. G. (1990). An assessment of the impact of ambient ozone on field grown crops in New Jersey using the EDU (ethylene diurea) method: Part 1—white potato. Environmental Pollution, 66, 351–360.
Coyle, M., Fowler, D., & Ashmore, M. (2003). New directions: implications of increasing Tropospheric background ozone concentrations for vegetation. Atmospheric Environment, 37, 153–154.
Debaje, S. B., & Kakade, A. D. (2009). Surface ozone variability over western Maharashtra, India. Journal of Hazardous Materials, 161, 686–700.
Elagoz, V., & Manning, W. J. (2002). Ozone and bean plants: morphology matters. Environmental Pollution, 120, 521–524.
Emberson, L. D., Buker, P., Ashmore, M. R., Mills, G., Jackson, L. S., Agrawal, M., et al. (2009). A comparison of North America and Asian exposure-response data for ozone effects on crop yields. Atmospheric Environment, 43, 1945–1953.
Ensing, J., Hofstra, G., & Roy, R. C. (1985). The impact of ozone on peanut exposed in the laboratory and field. Phytopathology, 75(4), 429–432.
Feng, Z., Pang, J., Kobayashi, K., Zhu, J., & Ort, D. R. (2010). Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Global Change Biology. doi:10.1111/j.1365-2486.2010.02184.X.
FAO-UN, (2003). FAO Trade Yearbook, vol. 165. Publising Management Service, Information Division, FAO.
Gelang, J., Pleijel, H., Sild, E., Danielsson, H., Younis, S., & Selldén, G. (2000). Rate and duration of grain filling in relation to flag leaf senescence and grain yield in spring wheat (Triticum aestivum) exposed to different concentrations of ozone. Physiologia Plantarum, 110, 366–375.
Hassan, I. A. (2006). Physiology and biochemical response of potato (Solanum tuberosum L. cv. Kara) to O3 and antioxidant chemicals: possible roles of antioxidant enzymes. Annals of Applied Biology, 148, 197–206.
Hassan, I. A., Ashmore, M. R., & Bell, J. N. B. (1995). Effect of ozone on radish and turnip under Egyptian field conditions. Environmental Pollution, 89, 107–114.
Hassan, I. A., Bell, J. N. B., & Marshall, F. M. (2007). Effects of air filtration on Egyptian clover (Trifolium alexandrinum L. cv. Messkawy) grown in open-top chambers in a rural site in Egypt. Research Journal of Biological Science, 2(4), 395–402.
Heagle, A. S., Miller, J. E., Burkey, K. O., Eason, G., & Pursley, W. A. (2002). Growth and yield responses of snap bean to mixtures of carbon dioxide and ozone. Journal of Environmental Quality, 31, 2008–2014.
Heagle, A. S., Miller, J. E., & Pursley, W. A. (2003). Atmospheric pollutants and trace gases: growth and yield responses of potato to mixtures of carbon dioxide and ozone. Journal of Environmental Quality, 32, 1603–1610.
Heck, W. W., Taylor, O. C., & Tingey, D. T. (1988). Assessment of crop loss from air pollutants. New York: Elsevier.
Hunt, R. (1982). Growth curves. London: Edward Arnold Publishers.
Ishii, S., Marshall, F. M., Bell, J. N. B., & Abdullah, A. M. (2004). Impact of ambient air pollution on locally grown rice cultivars (Oryza sativa L.) in Malaysia. Water Air and Soil Pollution, 154, 187–201.
Krupa, S. V., Nosal, M., & Legge, A. H. (1998). A numerical analysis of the combined open top chamber data from the USA and Europe on ambient ozone and negative crop responses. Environmental Pollution, 101, 157–160.
Kuehler, E. A., & Flagler, R. B. (1999). The effects of sodium erythorbate and ethylenediurea on Photosynthetic function of ozone-exposed loblolly pine seedlings. Environmental Pollution, 105, 25–35.
Lal, S., Naja, M., & Subbaraya, B. H. (2000). Seasonal variations in surface ozone and its precursors over an urban site in India. Atmospheric Environment, 34, 2713–2724.
Lee, E. H., & Chen, C. M. (1982). Studies on the mechanisms of ozone tolerance. Cytokinin like activity of N [2-(2-oxo-1-imidazolidinyl) ethyl]-N-phenylurea, a compound protecting against O3 injury. Physiologia Plantarum, 56, 486–491.
Manning, W. J., Flagler, R. B., & Frenkel, M. A. (2003). Assessing plant response to ambient ozone: growth of ozone-sensitive loblolly pine seedlings treated with ethylene diurea or sodium erythorbate. Environmental Pollution, 126, 73–81.
McKee, I. F., Eiblmeier, M., & Polle, A. (1997). Enhanced ozone-tolerance in wheat grown at an elevated CO2 concentration: ozone exclusion and detoxification. New Phytologist, 135, 275–284.
Meehl, G. A., Stocker, T. F., Collins, W. D., Friedlingstein, P., Gaye, A. T., Gregory, J. M., et al. (2007). Global climate projections. In S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, & H. L. Miller (Eds.), Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Morgan, P. B., Ainsworth, E. A., & Long, S. P. (2003). How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell and Environment, 26, 1317–1328.
Mulholland, J. A., Butler, A. J., Wilkinson, J. G., & Russell, A. G. (1998). Temporal and spatial distributions of ozone in Atlanta: Regulatory and epidemiologic implications. Journal of Air Waste Management Association, 48, 418–426.
Nair, P. R., Chand, D., Lal, S., Modh, K. S., Naja, M., Parameswaran, K., et al. (2002). Temporal variations in surface ozone at Thumba (8.6°N, 77°E): A tropical coastal site in India. Atmospheric Environment, 36, 603–610.
Naja, M., & Lal, S. (2002). Surface ozone and precursor gases at Gadanki (13.5°N, 79.2°E): A tropical rural site in India. Journal of Geophysical Research, 107(14), 417–418.
Pandey, J., & Agrawal, M. (1994). Evaluation of air pollution phytotoxicity in a seasonally dry tropical urban environment using three woody perennials. New Phytologist, 126, 53–61.
Pang, J., Kobayashi, K., & Zhu, J. (2009). Yield and photosynthetic characteristics of flag leaves in Chinese rice (Oryza sativa L.) varities subjected to free-air release of ozone. Agriculture, Ecosystems and Environment, 132, 203–211.
Pleijel, H., Mortensen, L., Fuhrer, J., Ojanpera, K., & Danielsson, H. (1999). Grain protein accumulation in relation to grain yield of spring wheat (Triticum aestivum L.) grown in open-top chambers with different concentrations of ozone, carbon dioxide and water availability. Agriculture, Ecosystems and Environment, 72, 262–270.
Rai, R., & Agrawal, M. (2008). Evaluation of physiological and biochemical responses of two rice (Oryza sativa L.) cultivars to ambient air pollution using open top chambers at a rural site in India. Science of the Total Environment, 407, 679–691.
Rai, R., Agrawal, M., & Agrawal, S. B. (2007). Assessment of yield losses in tropical wheat using open top chambers. Atmospheric Environment, 41, 9543–9554.
Rai, R., Agrawal, M., & Agrawal, S. B. (2010). Threat to food security under current levels of ground level ozone: a case study for Indian cultivars of rice. Atmospheric Environment. doi:10/1016/j.atmosenv.2010.06.022.
Ribas, A., & Penuelas, J. (2000). Effects of ethylenediurea as a protective antiozonant on beans (Phaseolus vulgaris cv Lit) exposed to different tropospheric ozone doses in Catalonia (NE Spain). Water Air and Soil Pollution, 117, 263–271.
Sarkar, A., & Agrawal, S. B. (2009). Identication of ozone stress in Indian rice through foliar injury and differential protein profile. Environmental Monitoring and Assessment, 161, 205–215.
Sarkar, A., & Agrawal, S. B. (2010). Elevated ozone and two modern wheat cultivars: An assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Environmental and Experimental Botany, 69, 328–337.
Singh, S., & Agrawal, S. B. (2009). Use of ethylenediurea (EDU) in assessing the impact of ozone on growth and productivity of five cultivars of Indian wheat (Triticum aestivum L.). Environmental Monitoring and Assessment, 159(1), 125–141.
Singh, S., Agrawal, S. B., & Agrawal, M. (2009). Differential protection of ethylenediurea against ambient ozone for five cultivars of tropical wheat. Environmental Pollution, 157, 2359–2367.
Singh, S., Agrawal, M., Agrawal, S. B., Emberson, L., & Büker, P. (2010). Use of ethylenediurea for assessing the impact of ozone on mung bean plants at a rural site in a dry tropical region of India. International Journal of Environmental Waste Management, 5(1/2), 125–139.
Szantoi, Z., Chappelka, A. H., Muntifering, R. B., & Somers, G. L. (2009). Cutleaf coneflower (Rudbeckia laciniata L.) response to ozone and ethylenediurea (EDU). Environmental Pollution, 157, 840–846.
Tingey, D. T., & Blum, U. (1973). Effects of ozone on soybean nodules. Journal of Environmental Quality, 2, 314–342.
Tiwari, S., & Agrawal, M. (2009). Protection of palak (Beta vulgaris L. var Allgreen) plants from ozone injury ethylenediurea (EDU): roles of biochemical and physiological variations in alleviating the adverse impacts. Chemosphere, 75, 1492–1499.
Tiwari, S., Agrawal, M., & Manning, W. J. (2005). Assessing the effects of ambient ozone on growth and productivity of two cultivars of wheat in India using three rates of application of ethylenediurea (EDU. Environmental Pollution, 138, 153–160.
Tiwari, S., Agrawal, M., & Marshall, F. (2006). Evaluation of ambient air pollution impact on carrot plants at a suburban site using open top chamber. Environmental Monitoring and Assessment, 266, 15–30.
Tiwari, S., Rai, R., & Agrawal, M. (2008). Annual and seasonal variations in tropospheric ozone concentrations around Varanasi. International Journal of Remote Sensing, 29, 4499–4514.
Varotsos, C., Chronopoulos, G., & Kalabokas, P. (1994). Seasonal variation and cross tropopause ozone exchange at Athens, Greece. In Proceedings of the 3rd EUROTRAC symposium-photooxidants: Precursors and products, Garmish-Partenkirchen, Germany, pp 305–309.
Varshney, C. K., & Rout, C. (1998). Ethylenediurea (EDU) protection against ozone injury in Tomato plants in Delhi. Bulletin of Environmental Contamination and Toxicology, 61, 188–193.
Vingarzan, R. (2004). A review of surface ozone background levels and trends. Atmospheric Environment, 38, 3431–3442.
Vollsnes, A. V., Krusea, O. M. O., Eriksenb, A. B., Oxaala, U., & Futsaethera, C. M. (2010). In vivo root growth dynamics of ozone exposed Trifolium subterraneum. Environmental and Experimental Botany. doi:10.1016/j.envexpbot.2010.03.007.
Wahid, A., Maggs, R., Shamshi, S. R. A., Bell, J. N. B., & Ashmore, M. R. (1995). Air pollution and its impact on rice yield in Pakistan Punjab. Environment Pollution, 90, 323–329.
Wahid, A., Milne, E., Shamshi, S. R., Ashmore, M. R., & Marshall, F. M. (2001). Effects of oxidants on soybean growth and yield in the Pakistan Punjab. Environmental Pollution, 113, 271–280.
Wang, X. K., Zheng, Q. W., Yao, F. F., Chen, Z., Feng, Z. Z., & Manning, W. J. (2007). Assessing the impact of ambient ozone on growth and yield of a rice (Oryza sativa L.) and a wheat (Triticum aestivum L.) cultivar grown in the Yangtze Delta, China, using three rates of application of ethylenediurea (EDU). Environmental Pollution, 148, 390–395.
Acknowledgments
The authors are thankful to the Head, Department of Botany, Banaras Hindu University for laboratory facilities and to the Ministry of Environment and Forest, New Delhi for providing financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S., Agrawal, S.B. Cultivar-Specific Response of Soybean (Glycine max L.) to Ambient and Elevated Concentrations of Ozone Under Open Top Chambers. Water Air Soil Pollut 217, 283–302 (2011). https://doi.org/10.1007/s11270-010-0586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-010-0586-7