Abstract
Climate change influences the current tropospheric ozone (O3) budget due to industrialization and urbanization processes. In recent years, the impact of elevated O3 on crop development and yield loss has emerged as one of the most important environmental issues, particularly in rural and suburban areas of the lower Indo-Gangetic Plains of India. The impact of the current tropospheric ozone (O3) on the crop yield, photosynthetic yield, and enzymatic antioxidants of six rice (Oryza sativa L.) cultivars (IR 36, MTU 1010, GB 3, Khitish, IET 4786, and Ganga Kaveri) was investigated with and without the application of ethylenediurea (EDU). The results revealed that O3 stress significantly affected crop yield, photosynthetic yield, and antioxidant enzymes. The findings showed that O3 toxicity induces oxidative stress biomarkers, i.e., malondialdehyde (MDA) content, and was manifested by increasing the enzymatic antioxidants, i.e., superoxidase dismutase (SOD) and catalase (CAT) in four rice cultivars (IR 36, GB 3, IET 4786, and Ganga Kaveri). At the same time, the results also illustrated that the rice cultivars MTU 1010 and Khitish are more tolerant to O3 stress as they had less oxidative damage, greater photosynthetic SPAD value, SOD and CAT activities, and lower MDA activity. The results also elucidated that the application of EDU decreased O3 toxicity in sensitive cultivars of rice by increasing antioxidant defense systems. The current O3 level is likely to show an additional increase in the near future, and the use of tolerant genotypes of rice may reduce the negative impacts of O3 on rice production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ambient ozone (O3), a gaseous pollutant, has assumed a much larger, more intense, and global significance, affecting crop production in many agricultural regions in changing climate scenarios. O3 concentrations depend sensitively upon weather variables, and the primary factors influencing their formation are the intensity of solar radiation and the rise in ambient temperature (Gaur et al. 2014; Tiwari et al. 2008; Xu et al. 2016). Changes in regional O3 levels not only depend upon weather variables but also upon the transportation of O3 from one region to another along with the prevailing winds. Subtropical climatic conditions prevalent in the South Asia region provide favorable conditions for O3 formation due to warm and sunny days in the presence of O3 precursors (Ramanathan and Ramana 2005; Tiwari et al. 2008) due to industrialization and urbanization processes.
High ambient O3 is highly reactive, and a strong oxidant acts as a phytotoxicant upon entering the plant, affecting metabolism, reducing chlorophyll content, modifying leaf senescence, and decreasing biomass accumulation, resulting in crop yield reduction (Ainsworth et al. 2012; Avnery et al. 2011; Mishra et al. 2013; Fahad et al. 2013; Rai and Agarwal 2015). Significant yield reductions of agricultural crops have been well documented due to rising ambient O3 (Cotrozzi et al. 2016; Rai et al. 2010; Yi et al. 2016). In India, the estimated O3-induced yield loss for rice was ~ 6%, and wheat was ~ 21% (Sharma et al. 2019), whereas Lin et al. (2018) estimated rice yield loss of 3.9–15.0%, 8.5–14.0% for winter wheat, and 2.2–5.5% for maize in China. Mills et al. (2018) calculated that O3-induced wheat yield losses ranged from 7.3 to 8.9% in Germany, France, and Italy. In the USA, O3-induced yield losses for wheat and soybean were 4.9% and 6.7%, respectively (Lapina et al. 2016). Predicted and current O3 trends may increase the relative crop production losses in rice, wheat, soybeans, beans, barley, maize, and potatoes in the near future (Emberson et al. 2009; Feng and Kobayashi 2009).
Boro rice is dry-season rice grown in low-lying areas or on medium lands with irrigation after the harvest of wet season rice. The rapid expansion of Boro rice cultivation has taken place in recent years in Eastern India (Uttar Pradesh, Bihar, West Bengal, Assam, Odisha, and Andhra Pradesh) and the river basin deltas of Bangladesh. In 2019–2020, the total production of rice in India was 118 million tonnes, of which Boro rice accounted for ~ 14% (DAC 2021). In the neighboring country of Bangladesh, out of 36.4 million tonnes of rice produced in 2019–2020, the share of Boro rice was about 54% (IFPRI 2020). The crop is known for its high productivity (5–6 t/ha) in areas where rice productivity has traditionally been poor (2–3 t/ha) during the wet season. Low winter temperatures during early crop growth promote photosynthate accumulation, increasing the carbon–nitrogen ratio (Singh 2002). Boro rice is transplanted during December–January and harvested in the hot and humid months of April–May. The total O3 concentration increases during winter and pre-monsoon periods due to the rise in temperature in changing climatic scenarios (Lu et al. 2019; Ramanathan and Ramana 2005; Tiwari et al. 2008). Ozone concentrations below 40 ppb usually do not influence crop yield, though this threshold value (AOT40) often exceeds as high as ~ 67 ppb due to the rise in temperature during the Boro rice cultivation season (Dey et al. 2014; Ganguly 2012; Singh et al. 2021). Elevated O3 levels coincide with the late vegetative and reproductive stages such as flowering and grain filling. Rice has been found moderately sensitive to O3 as other ozone-sensitive crops like wheat and soybean grown during November–May in Southeast Asia, and the O3-affected rice crops have lower yields with poor quality grains (Mills et al. 2007; Feng and Kobayashi 2009; Wang et al. 2012; Liu et al. 2015). It has been predicted that by 2050, large stretches of rice crops will be affected by O3 toxicity, especially in parts of Asia, the Middle East, Africa, and South America (IPCC 2014).
Plants have a diverse spectrum of metabolic reactions at the biochemical level that help them to cope with oxidative stress caused by O3. Because of the oxidative nature of O3, it penetrates the plant leaf through stomata and dissolves in the aqueous phase of the sub-stomatal cavity and generates reactive oxygen species (ROS) (Tiwari 2018; Grulke and Heath 2019). ROS can harm proteins, lipids, and nucleic acids, as well as plants (Del and Carrasco 2004; Ishida et al. 1999; Mudd 1996). Enzymatic and nonenzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and other substances help plants to reduce the negative effects of ROS (Afzal et al. 2020; Caregnto et al. 2013; Gratão et al. 2005; Mittler et al. 2004; Sharma et al. 2012; Yadav et al. 2014; Fahad et al. 2017). The activities of SOD and CAT are amplified when exposed to elevated O3 levels (Liu et al. 2015; Pandey et al. 2015; Ueda et al. 2013).
The protection of sensitive crops from high ambient O3 could be mitigated in two ways, i.e., either by the application of chemical protectants or by the selection of ozone-tolerant cultivars (Didyk and Blum 2011). A large number of chemicals have been evaluated for the protection of crops from ozone injury, but their application in crop fields is not cost-effective (Archambault et al. 2000). Hence, future agricultural productivity may be dependent on the use of O3-tolerant cultivars or the development of O3-resistant cultivars that can thrive under elevated ambient O3 levels (Frei 2015). Farmers may choose more tolerant varieties in the long run to reduce crop losses. Application of EDU has been recommended as a useful research tool to estimate crop losses, especially in rural and semi-urban areas where research infrastructure is the limiting factor (Feng et al. 2010; Manning et al. 2011; Paoletti et al. 2009; Rai et al. 2015; Singh et al. 2010; Tiwari et al. 2005). EDU has no influence on plant growth (Foster et al. 1983; Szantoi et al. 2007) and can better reveal the differences among the cultivars (Oksanen et al. 2013) when screening tolerant cultivars in areas with high concentrations of O3 (Singh and Agrawal 2017). The present study was conducted with the objective to screen six Boro rice cultivars for their O3 sensitivity or tolerance in terms of growth, biochemical, and yield characteristics. These six varieties of Boro rice have been chosen for the study as they are commonly grown in the eastern Gangetic plains during January–May. EDU treatment was used to protect the rice plant from ozone effects. To our knowledge, no such studies involving Boro rice cultivars have been carried out in the eastern Gangetic plains of India and Bangladesh to reveal the rice production losses from the risk of ozone-induced damage in field conditions.
Materials and methods
The study area and experimental details
During the years 2019 and 2020, field experiments were conducted at two different locations (semi-urban and rural) in West Bengal (India), namely, Nilganj, a semi-urban site (longitude: 88° 26′ E, latitude: 22° 45′ N, altitude: 9 m asl), and Bhabanipur village, a rural site, (longitude: 88° 34′ E, latitude: 22° 55′ N, altitude: 12 m asl). The mean annual rainfall in the study area was in the range of 1100 to 1200 mm with a maximum temperature of 34.0 °C in May and a minimum of 10.0 °C in January. The soil of the study area was moderately alkaline, with a pH ranging from 7.54 to 7.70. The texture is loam and sandy loam having 2.3 to 6.0 g/kg organic carbon, 26 to 117 kg/ha available Bray’s phosphorus (P2O5), and 29 to 62 kg/ha ammonium acetate exchangeable potassium (K).
At each location, field plots of 400 m2 with three replications were established. Six varieties of rice (IR 36, MTU 1010, GB 3, Khitish, IET 4786, and Ganga Kaveri) were collected from ICAR-NRRI (Cuttack, India) and grown during the Boro season (January–April). Rice was transplanted as seedlings following the system of rice intensification (SRI) method. The manure and chemical fertilizer application rates were based on the initial soil test value and percentages of the recommended doses for the crop (N:P:K: 80:40:40 kg/ha). Crops were managed by optimizing plant densities, split N fertilization, timely weeding, and plant protection measures.
Use of ethylenediurea as antiozonant
EDU, an antiozonant chemical [N-(2–2-oxo-1-imidazolidinyl) ethyl-N-phenyl urea], has emerged as an effective research tool for evaluating O3 injury in plants (Agathokleous et al. 2016a; Carnahan et al. 1978; Manning et al. 2011; Singh et al. 2015). EDU does not enter the cell but remains confined to the foliar apoplastic spaces and hence is systemic in nature (Pasqualini et al. 2016; Paoletti et al. 2009). Its retention time in the leaf varies from 8 to 21 days, depending upon the soil fertility conditions (Agathokleous et al. 2016b). As such, repeated application of EDU is recommended for the treatment to be effective.
Both experimental sites were divided into two parts, i.e., one as ambient O3 and the other as EDU treatment plots. Each part had 18 subplots (2 m × 1 m in dimension) for each cultivar. EDU was applied at a concentration of 300 ppm to each plant as a foliar spray until the entire foliage was visibly saturated. The selection of EDU concentration (300 ppm) was based on the earlier study, suggesting that 200–400 ppm of EDU would be the most effective concentration in protecting agricultural plants against O3 stress (Feng et al. 2010; Pandey et al. 2015). In the ambient O3 plots, water was sprayed in place of EDU. To prevent the spread of EDU to control plots, there was 2 m spacing between ambient O3 and EDU-treated plots. The EDU treatment was started 30 days after the transplanting (DAT) of rice seedlings, and 6 numbers of EDU spraying were done at intervals of 15 days until the final harvest (Pandey et al. 2015).
Ozone monitoring and crop yield
Ambient O3 concentrations were monitored using the 2B Tech O3 Monitor (POM) for 6 h per day (10:00 to 16:00 h), regularly at both the experimental sites throughout the growing season (February to May). These experimental locations were not exposed to any direct emissions from air pollution sources.
As described by De Leeuw and Van Zantvoort (1997), AOT40 (accumulated exposure over a threshold of 40 ppb) was used as the exposure index for the O3 concentration. AOT40 is an exposure-plant response index function set by the United Nations Economic Commission for Europe (UNECE) and US-EPA. It is calculated as the sum of differences between the hourly O3 concentration and the threshold value of 40 ppb for each hour when the averaged O3 concentration exceeds 40 ppb. It is expressed mathematically as,
where [O3] = hourly averaged O3 concentration; 40 = threshold value of O3.
An AOT40 value of 10,000 ppb h for daylight hours (radiation > 50 W/m2) over a 6-month period has been established as a critical level for the protection of forests. While for the protection of agricultural crops from a 5% loss in yield, an AOT40 value of 3000 ppb h for daylight hours over a growing season has been established as the critical level (WHO 1996; Beck et al. 1998).
Final harvesting was carried out as per the crop duration of each variety (110–125 days). Harvest parameters such as grain weight (after threshing) were measured for all plants in each plot for both the EDU and the ambient O3-exposed plants within each cultivar and each treatment. Relative yield loss (RYL) calculation of rice based on AOT40 values was done using crop yield data from the EDU-treated plant yield that had resulted without O3-induced damages as per the following equation (Sinha et al. 2015).
Biomass sampling and biochemical measurements
Plant sampling for biochemical analysis of superoxide dismutase (SOD), catalase (CAT), and lipid peroxidation (MDA) was performed at two phases, i.e., the vegetative phase and the maturity phase, as per the crop duration of each cultivar of rice. The samples were taken from the youngest fully mature leaves of five randomly selected plants within each cultivar of each treatment. The leaf samples were immediately frozen in liquid nitrogen and stored at − 20 °C until further analysis.
SOD activity was assayed using the photochemical nitroblue tetrazolium (NBT) method (Dhindsa et al. 1981). Fresh leaves (250 mg) were homogenized in liquid nitrogen and 3 mL of 50 mM sodium phosphate buffer (pH 7.8), including 1.5 mM EDTA and 1.0 mM ascorbic acid. SOD activity was measured in 5 mL reaction mixture containing 50 mM sodium phosphate buffer (pH 7.8), 75 mM NBT, 0.5 mM riboflavin, 13 mM methionine, 0.1 mM EDTA, and enzyme extract. Finally, the sample was measured by using a UV–VIS spectrophotometer at 560 nm. CAT activity was assayed by following the decrease in absorbance at 240 nm as H2O2 was consumed (Aebi 1984). Fresh leaves (250 mg) were homogenized in liquid nitrogen and 3 mL of 50 mM potassium phosphate buffer (pH 7.0). The assay mixture (3.0 mL) was comprised of 75 mM H2O2, 100 mM phosphate buffer, and enzyme extract. The CAT activity was measured at a 240 nm wavelength using a UV–VIS spectrophotometer.
The degree of lipid peroxidation was measured as malondialdehyde (MDA) content by the thiobarbituric (TBA) acid method (Heath and Packer 1968). Briefly, 250 mg of frozen leaves were ground at 4 °C in a mortar with 4 mL of 0.1% trichloroacetic acid (TCA) solution. The homogenate was centrifuged at 15,000 rpm at 4 °C for 10 min. A total of 1 mL of supernatant was mixed with 4 mL of 0.5% TBA. The mixtures were heated at 95 °C for 10–15 min and then quickly cooled in an ice bath. The absorbance of the supernatant was recorded by using a UV–VIS spectrophotometer at wavelengths of 532 and 600 nm.
Chlorophyll content in terms of SPAD value was measured using the Leaf CHL PLUS chlorophyll meter. The SPAD value is related to the amount of chlorophyll present in the leaf between wavelengths of 650 and 940 nm.
Data analysis
Data recorded in the 2019 and 2020 cropping seasons was pooled together on the account of nonsignificant interaction between years, locations, and treatments. The data was then subjected to ANOVA for each year of sampling. The average value of treatments was separated using the least significant difference (LSD) at the 0.05 probability level.
Results
Ozone exposure and yield loss
Ozone formation was high during the months of February to May due to long hours of sunlight and a daily increase in ambient temperatures, which speed up the O3-forming photochemical reactions. The trends in ambient O3 concentrations were higher than the threshold value of 40 ppb (AOT40) in both semi-urban and rural areas during both years (2019 and 2020). Daytime O3 levels often exceeded 40 ppb during the early vegetative phase of rice plants (40 DAT) and attained maximum levels in the reproductive phase (70 DAT). However, accumulated ozone exposure (AOT40) was significantly lower in rural areas as compared to semi-urban areas (Fig. 1). Maximum AOT40 exposure was 12.23 ppm.h in semi-urban areas and 6.83 ppm.h in rural areas.
This line graph depicts the seasonal variation in the concentration of tropospheric ozone in the Boro rice-growing season in semi-urban and rural areas (February–May). The primary x-axis indicates the tropospheric ozone concentration in parts per billion (ppb) at an interval of 10 days. The secondary x-axis indicates the AOT40 value (accumulated exposure over a threshold of 40 ppb per hour during the crop growing period) in parts per billion (ppm.h). (For the interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
The results of the final harvest showed a significant relative yield loss in IR 36, GB 3, IET 4786, and Ganga Kaveri cultivars of Boro rice under tropospheric O3-treated plants (control) at both locations (Fig. 2). There was no significant yield difference in MTU 1010 and Khitish cultivar for EDU and control treatments at both sites of experiments and may be considered well-adapted cultivars for high ozone exposure. All the yield-related parameters showed more decline in the control plots, which might be due to higher tropospheric O3 levels between the vegetative and maturity phases. Rice production losses due to tropospheric O3 exposure were estimated to be 8–11%, using an equation derived from Indian studies. The yield loss was roughly 47% lower in rural areas (37.83 kg/ha), where the AOT40 value was significantly lower than in semi-urban areas (71.72 kg/ha). Ozone exposure-crop yield relationships showed significantly lower relative yields, which may be attributed to the variety of cultivars more sensitive to O3 exposure.
Photosynthetic yield of the plants
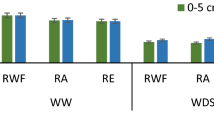
Chlorophyll content plays an important role in determining the photosynthetic yield of the plants. Chlorophyll SPAD values of rice leaves were found significantly higher in EDU-treated plants (Fig. 3). Chlorophyll content was more affected in higher AOT40 areas (semi-urban). Higher AOT40 may prevent chlorophyll synthesis, leading to a decline in SPAD value. At the vegetative phase, the SPAD value was significantly lower as compared to the maturity phase in both EDU and control treatments. SPAD values in MTU 1010 and Khitish cultivars were nonsignificant in both treatments at the vegetative and maturity growth stages (Fig. 3).
Mean chlorophyll SPAD value in rice plants at semi-urban and rural sites of the experiment (mean value of 2 years of data from both study sites. Bars with different letters for each cultivar differ significantly from one other (p < 0.05). All the data represented the average of three replications of each cultivar at each site
Effect on the antioxidant pool
Plants possess a wide range of responses at the biochemical level that assist them in coping with O3-induced oxidative stress. The antioxidative defense was elevated in response to EDU in all cultivars but varied between the developmental phases of rice plants. An increase in CAT activity at the vegetative phase (0.44–0.76 μ mol/ml) suggests increased stress on all rice cultivars (Fig. 4a). Reduced CAT activity at the maturity phase (0.31–0.49 μ mol/ml) may be associated with the exhaustion of the antioxidant defense system after high O3 stress. Increased SOD activity was also recorded at the vegetative phase (4.02–5.44 U mg/pro) with a decline at the maturity phase (3.9–4.39 U mg/pro) (Fig. 4b). The reduction in lipid peroxidation (MDA) content (0.24–0.59 μ mol/ml) in all rice cultivars during the vegetative phase indicates that cell membranes are highly sensitive to ozone damage (Fig. 4c). In MTU 1010 and Khitish cultivars of rice, the values of CAT, SOD, and MDA were found nonsignificant between EDU and control treatments at both the vegetative and maturity phases.
a Response of catalase (CAT) in different cultivars of rice during the vegetative and maturity phases (mean value of 2-year data from both study sites). Bars with different letters for each cultivar differ significantly from one other (p < 0.05). All the data represented the average of three replications of each cultivar at each site. b Response of superoxide dismutase (SOD) in different cultivars of rice during the vegetative and maturity phases (mean value of 2-year data from both study sites). Bars with different letters for each cultivar differ significantly from one other (p < 0.05). All the data represented the average of three replications of each cultivar at each site. c Response of reduced lipid peroxidation (MDA) in different cultivars of rice during the vegetative and maturity phases (mean value of 2 year data from both study sites). Bars with different letters for each cultivar differ significantly from one other (p < 0.05). All the data represented the average of three replications of each cultivar at each site
Discussion
The O3 concentration at a rural and semi-urban site clearly showed sharp monthly variations. The rise in O3 concentrations during March and May can be attributed to its linear relationship with the increase in solar radiation (Han et al. 2011). Low O3 levels were observed during the month of April due to cloudy days and pre-monsoon rain that reduced the availability of precursors for the production of O3 (Tiwari and Agrawal 2018). O3 precursors such as carbon monoxide (CO), volatile organic compounds (VOCs), and nitrogen oxides (NOx) are major contributors to O3 production. It was observed that total O3 concentration was much higher in semi-urban areas than in rural areas due to increases in industrial activity as well as a large increase in the number of vehicles (Kurokawa et al. 2013). Several studies suggest that reductions in the emissions of O3 precursors are the main reason for the decreasing trend of O3 (Butler et al. 2011; Monks et al. 2015; Hogrefe et al. 2011).
Boro rice plants were exposed to very high O3 concentrations (> 50 ppb), particularly during the vegetative and reproductive phases (February–March). These O3 concentrations are in line with several other measurements in the IGP region of India (Deb Roy et al. 2009; Oksanen et al. 2013; Singh et al. 2010). Atmospheric O3 above the threshold value (> 40 ppb) has the potential to damage plant tissues, accelerate leaf senescence, and alter nitrogen cycling in plant ecosystems, which thereby influences plant growth and biomass production (Pandey et al. 2015; Singh and Agrawal 2017). The AOT40 indices at both sites exceeded the critical limit of 3 ppm.h for cereal plants (Mills et al. 2007; Rai et al. 2010). RYL values based on the AOT40 index were in the range of 41 to 147 kg/ha. The maximum area of Boro rice in India is in the eastern region (~ 2.32 million ha) followed by the southern (~ 1.42 million ha) and northern regions (~ 0.10 million ha). Due to the high concentration of tropospheric O3 in the eastern region of India, the total Boro rice crop loss per annum may be in the range of 0.56 to 0.78 million metric tonnes (8–11%) if O3-resistant cultivars of rice are not grown. This loss in rice production could be about 83.75 million USD. Again, it is very explicit that the loss estimated using the AOT40 metrics is much higher in the neighboring country Bangladesh (~ 2.1 million metric tonnes) from the 4.77 million hectare area of Boro rice. Similar results on rice yield loss caused by O3 from all over the world have been reported (Cao et al. 2020; Dingenen et al. 2009; Danh et al. 2016; Mills et al. 2007; Pandey et al. 2015; Singh and Agrawal 2017; Zhao et al. 2020). The yield loss for rice is significantly higher in India (Fig. 5), while in the other regions, it is less than 5% (Lal et al. 2017; Sinha et al. 2015; Sharma et al. 2019). Two highly responsive cultivars of rice, i.e., MTU 1010 and Khitish, had a grain yield per plot higher than the mean grain yield in both rural and semi-urban areas and have the potential to sustain the attainable yield in unfavorable tropospheric O3 concentration. The varietal details of the six rice cultivars used in this study are given in Table 1. The difference in crop duration of all the rice varieties was only 10–15 days at the harvesting stage.
ROS produced due to high O3 concentration affected the chloroplasts formation, leading to the destruction of photosynthetic pigments (Rai and Agrawal 2014), and thus a reduction in the chlorophyll SPAD value of O3 susceptible cultivars of rice plants during the vegetative phase. Similar results were observed in O3 susceptible plants in several studies (Kollner and Krause 2003; Pellegrini 2014; Singh et al. 2009), which may be considered indicative of O3 stress on chlorophyll-binding proteins. The destruction of plant cells was higher during early vegetative stages when the defense mechanisms were insufficient to scavenge the produced ROS. During the later stages, plants were acclimatized to O3 stress conditions and loss of chlorophyll was minimized (Singh et al. 2009).
Most of the responses in biochemical parameters related to oxidative stress and antioxidative defense varied between the rice cultivars. The response of the plant defense system to O3 stress varies with the time and duration of high O3 contact and plant developmental stage. ROS operates as an indicator molecule for initiating protective responses at a specific threshold, with H2O2 acting as a secondary messenger. As a result, antioxidant enzymes such as CAT and SOD work together to detoxify ROS (Gill and Tuteja 2010; Suzuki et al. 2012). Antioxidant enzymes in the tolerant cultivar alleviated the stressful condition by inhibiting stress impact by protecting ROS formation. In this study, we observed that O3 stress-induced CAT and SOD activity were significantly higher in tolerant rice cultivars (MTU 1010 and Khitish), compared to sensitive cultivars, implying a function for CAT and SOD in O3 tolerance by detoxification of H2O2. There was an upregulation of MDA content in EDU-treated plants, thereby suggesting low exposure of O3. EDU protects the membranes against lipid peroxidation through increased levels of ROS scavenging enzymes. Plants having high antioxidant levels, either naturally occurring or induced, were found more resistant to oxidative damage (Wang et al. 2013; Singh et al. 2010). The efficacy of the ROS scavenging system to maintain the cellular redox steady state of the leaf tissue was responsible for genotypic differences among all rice cultivars (Castagna and Ranieri 2009; Giacomo et al. 2010).
Conclusions
Ozone in the troposphere is a serious problem of concern due to its phytotoxicity, limiting crop productivity worldwide. Rice plants show a wide range of responses to higher concentrations of O3, which are mostly manifested by a variety of alterations in the chlorophyll content, biochemical attributes, antioxidant enzyme activities, and economic yield. Although high O3 concentrations may cause negative effects on the overall growth and development of the rice plants, the vegetative and reproductive phases are the most affected. Other noticeable effects of O3 stresses were damaged photosynthetic machinery and oxidative damage in sensitive cultivars of rice. Recent advances have been made in reducing the detrimental consequences of ozone stress, either through genetic methods or stress resistance induction. In this study, two O3-tolerant rice cultivars had less oxidative damage and showed higher levels of photosynthetic SPAD value, SOD and CAT activities, and a lower level of MDA activity as compared to O3-sensitive cultivars. In view of the rising tropospheric O3 problem, emphasis needs to be given to developing O3-resistant cultivars either through conventional breeding methods or through the utilization of biotechnological tools to reduce the impact of O3 on staple food crops like rice to ensure food security under changing climatic scenarios. Cultivation of O3-tolerant rice cultivars may help in reducing the detrimental environmental effects associated with increased levels of O3. The findings also demonstrated the utility of EDU as a technique for monitoring cultivar-specific sensitivity to ambient O3 and suggested that it may be used more regularly, particularly in rural and semi-urban locations where research infrastructure is limited.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aebi H (1984) Catalase in vitro. Methods in enzymology, vol 105. Academic Press, San Diego, pp 121–126
Afzal J, Saleem MH, Batool F, Elyamine AM, Rana MS, Shaheen A, El-Esawi MA, Tariq Javed M, Ali Q, Arslan Ashraf M, Hussain GS, Hu C (2020) Role of ferrous sulfate (FeSO4) in resistance to cadmium stress in two rice (Oryza sativa L.) genotypes. Biomolecules 10(12):1693. https://doi.org/10.3390/biom10121693
Agathokleous E, Mouzaki-Paxinou A, Saitanis CJ, Paoletti E, Manning WJ (2016a) The first toxicological study of the antiozonant and research tool ethylenediurea (EDU) using Lemna minor L. bioassay: hints to its mode of action. Environ Pollut 213:996–1006
Agathokleous E, Paoletti E, Saitanis CJ, Manning WJ, Sugai T, Koike T (2016b) Impacts of ethylenediurea (EDU) soil drench and foliar spray in Salix sachalinensis protection against O3 induced injury. Sci Total Environ 573:1053–1062
Ainsworth AE, Yendrek Craig R, Stephen S, Collins William J, Emberson Lisa D (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu Rev Plant Biol 63:637–661
Archambault D, Slaski DJ, Li JJ (2000) Ozone protection in plants. The potential use of chemical protectants to measure oxidant damage in Alberta crops. Report prepared for the Air Research Users Group, Alberta Environment, Edmonton
Avnery S, Mauzerall DL, Liu J, Horowitz LW (2011) Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmos Environ 45:2284–2296
Beck JP, Krzyzanowski M, Koffi B (1998) Tropospheric ozone in the European Union – the consolidated report. Topic report no. 8/1998
Butler TJ, Vermeylen FM, Rury M, Likens GE, Lee B, Lee B, Bowker GE, McCluney L (2011) Response of ozone and nitrate to stationary source NOx emission reductions in the eastern USA. Atmos Environ 45:1084–1094. https://doi.org/10.1016/j.atmosenv.2010.11.040.2011
Cao J, Wang X, Zhao H, Ma M, Chang M (2020) Evaluating the effects of ground-level O3 on rice yield and economic losses in Southern China. Environ Pollut 267:115694
Caregnato FF, Bortolin RF, Divan Junior AM, Moreira JCF (2013) Exposure to elevated ozone levels differentially affects the antioxidant capacity and the redox homeostasis of two subtropical Phaseolus vulgaris L. varieties. Chemosphere 93(2):320–330
Carnahan J, Jenner E, Wat E (1978) Prevention of ozone injury to plants by a new protectant chemical. Phytopathology 68:1229
Castagna A, Ranieri A (2009) Detoxification and repair process of ozone injury: from O3 uptake to gene expression adjustment. Environ Pollut 157:1461–1469
Cotrozzi L, Remorini D, Pellegrini E, Landi M, Massai R, Nali C, Guidi L, Lorenzini G (2016) Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol Plant 157:69–84
DAC (2021) First Advance Estimates of Production of Foodgrains for 2020–21. Ministry of Agriculture and Farmers Welfare Department of Agriculture, Cooperation and Farmers Welfare, New Delhi
Danh NT, Huy LN, Oanh NTK (2016) Assessment of rice yield loss due to exposure to ozone pollution in Southern Vietnam. Sci Total Environ 566–567:1069–1079
De Leeuw F, Van Zantvoort E (1997) Mapping of exceedances of ozone critical levels for crops and forest trees in the Netherlands: Preliminary results. Environ Pollut 96:89–98
Deb Roy S, Beig G, Ghude SD (2009) Exposure-plant response of ambient ozone over the tropical Indian region. Atmos Chem Phy 9:5253–5260
Del Valle-Tascon S, Carrasco-Rodriguez J (2004) Impact of ozone on crops. Production Practices and Quality Assessment of Food Crops, vol 1. Springer, Berlin, pp 189–208
Dey S, Pati C, Gupta S (2014) Measurement and analysis of surface ozone and its precursors at three different sites in an urban region in eastern India. Environ Forensics 2014:112–120
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(1):93–101
Didyk NP, Blum OB (2011) Natural antioxidants of plant origin against ozone damage of sensitive crops. Acta Physiol Plant 33:25–34. https://doi.org/10.1007/s11738-010-0527-5
Dingenen RV, Dentener FJ, Raes F, Krol MC, Emberson L, Cofala J (2009) The global impact of O3 on agricultural crop yields under current and future air quality legislation. Atmos Environ 43:604–618
Emberson LD, Buker P, Ashmore M, Mills G, Jackson L, Agrawal M, Atikuzzaman M, Cinderby S, Engardt M, Jamir C, Kobayashi K, Oanh N, Quadir Q, Wahid A (2009) A comparison of North American and Asian exposure-response data for ozone effects on crop yields. Atmos Environ 43:1945–1953
Fahad S, Chen Y, Saud S, Wang K, Xiong D, Chen C, Wu C, Shah F, Nie L, Huang J (2013) Ultraviolet radiation effect on photosynthetic pigments, biochemical attributes, antioxidant enzyme activity and hormonal contents of wheat. J Food, Agri Environ 11(3&4):1635–1641
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, NasimW AS, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and Management Options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Feng Z, Kobayashi K (2009) Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos Environ 43:1510–1519
Feng Z, Wang S, Szantoi Z, Chen S, Wang X (2010) Protection of plants from ambient ozone by applications of ethylenediurea (EDU): a meta-analytic review. Environ Pollut 158:3236–3242
Foster K, Guerard J, Oshima R, Bishop J, Timm H (1983) Differential ozone susceptibility of centennial russet and white rose potato as demonstrated by fumigation and antioxidant treatments. Am Potato J 60:127–139
Frei M (2015) Breeding of ozone resistant rice: relevance approaches and challenges. Environ Pollut 197:144–155
Ganguly ND (2012) Influence of stratospheric intrusion on the surface ozone levels in India. Int J Meteorol 1:7
Gaur A, Tripathi SN, Kanawade VP, Tare V, Shukla SP (2014) Four-year measurements of trace gases (SO2, NOx, CO, and O3) at an urban location, Kanpur, in Northern India. J Atmos Chem 71:283–301
Giacomo B, Forino LMC, Tagliasacchi AM, Bernardi R, Durante M (2010) Ozone damage and tolerance in leaves of two poplar genotypes. Caryologia 63:422–434
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Grulke NE, Heath RL (2019) Ozone effects on plants in natural ecosystems. Plant Biol 22(S1):12–27. https://doi.org/10.1111/plb.12971
Han S, Bian H, Feng Y, Liu A, Li X, Zeng F, Zhang X (2011) Analysis of the relationship between O3, NO and NO2 in Tianjin, China. Aerosol Air Qual Res 11:128–139
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Hogrefe C, Hao W, Zalewsky EE, Ku JY, Lynn B, Rosenzweig C, Schultz MG, Rast S, Newchurch MJ, Wang L, Kinney PL, Sistla G (2011) An analysis of long-term regional-scale ozone simulations over the Northeastern United States: variability and trends. Atmos Chem Phys 11:567–582. https://doi.org/10.5194/acp-11-567-2011
IFPRI (2020) Boro rice procurement in Bangladesh: implications for policy. International Food Policy Research Institute Bangladesh, Dhaka
IPCC (2014) Climate Change 2014-Mitigation of Climate Change. IPSS 5th Assessment Report, Bonn
Ishida H, Makino A, Mae T (1999) Fragmentation of the large subunit of ribulose-1,5-bisphosphate carboxylase by reactive oxygen species occurs near gly-329. J Biol Chem 274:5222–5522
Kollner B, Krause GHM (2003) Effects of two different ozone exposure regimes on chlorophyll and sucrose content of leaves and yield parameters of sugar beet (Beta vulgaris L.) and rape (Brassica napus L.). Water Air Soil Pollut 144:317–332
Kurokawa J, Ohara T, Morikawa T, Hanayama S, Janssens-Maenhout G, Fukui T, Kawashima K, Akimoto H (2013) Emissions of air pollutants and greenhouse gases over Asian regions during 2000–2008: regional emission inventory in Asia (REAS) version 2. Atmos Chem Phys 13:11019–11058. https://doi.org/10.5194/acp-13-11019-2013,2013
Lal S, Venkataramani S, Naja M, Kuniyal JC, Kumar MKS (2017) Loss of crop yields in India due to surface ozone: an estimation based on a network of observations. Environ Sci Pollut Res Int 24:1–10
Lapina K, Henze DK, Milford JB, Travis K (2016) Impacts of foreign, domestic, and state-level emissions on ozone-induced vegetation loss in the United States. Environ Sci Technol 50:806–813
Lin Y, Jiang F, Zhao J, Zhu G, He X, Ma X, Li S, Sabel CE, Wang H (2018) Impacts of O3 on premature mortality and crop yield loss across China. Atmos Environ 194:41–47. https://doi.org/10.1016/j.atmosenv.2018.09.024
Liu X, Sui L, Huang Y, Geng C, Yin B (2015) Physiological and visible injury responses in different growth stages of winter wheat to ozone stress and the protection of spermidine. Atmos Pollut Res 6:596–604
Lu X, Zhang L, Shen L (2019) Meteorology and climate influences on tropospheric ozone: a review of natural sources, chemistry and transport pattern. Curr Pollut Rep 5:238–260
Manning WJ, Paoletti E, Sandermann H Jr, Ernst D (2011) Ethylenediurea (EDU): a research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ Pollut 159:3283–3293
Mills G, Buse A, Gimeno B, Bermejo V, Holland M, Emberson L, Pleijel H (2007) A synthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos Environ 41:2630–2643
Mills G, Sharps K, Simpson D, Pleijel H, Frei M, Burkey K, Emberson L, Uddling J, Broberg M, Feng Z, Kobayashi K, Agrawal M (2018) Closing the global ozone yield gap: quantification and co-benefits for multi-stress tolerance. Global Change Biol 24:4869–4893
Mishra AK, Rai R, Agarwal SB (2013) Differential response of dwarf and tall tropical wheat cultivars to elevated ozone with and without carbon dioxide enrichment: growth, yield and grain quality. Field Crop Res 145:21–32. https://doi.org/10.1016/j.fcr.2013.02.007
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Monks PS, Archibald AT, Colette A, Cooper O, Coyle M, Derwent R, Fowler D, Granier C, Law KS, Mills GE, Stevenson DS, Tarasova O, Thouret V, von Schneidemesser E, Sommariva R, Wild O, Williams ML (2015) Tropospheric ozone and its precursors from the urban to the global scale from air quality to short lived climate forcer. Atmos Chem Phys 15:8889–8973
Mudd J (1996) Biochemical basis for the toxicity of ozone. Plant Response to Air Pollution. John Wiley and Sons Ltd, Hoboken
Oksanen E, Pandey V, Pandey AK, Keski-Saari S, Kontunen-Soppela S, Sharma C (2013) Impacts of increasing ozone on Indian plants. Environ Pollut 177:189–200
Pandey AK, Majumder B, Keski-Saari S, Soppela SK, Mishra A, Sahu N, Pandey V, Oksanen E (2015) Searching for common responsive parameters for ozone tolerance in 18 rice cultivars in India: results from ethylenediurea studies. Sci Total Environ 532:230–238
Paoletti E, Contran N, Manning WJ, Ferrara AM (2009) Use of the antiozonant ethylenediurea (EDU) in Italy: verification of the effects of ambient ozone on crop plants and trees and investigation of EDU’s mode of action. Environ Pollut 157:1453–1460
Pasqualini S, Paoletti E, Cruciani G, Pellegrino R, Ederli L (2016) Effects of different routes of application on ethylenediurea persistence in tobacco leaves. Environ Pollut 212:559–564
Pellegrini E (2014) PSII photochemistry is the primary target of oxidative stress imposed by ozone in Tilia americana. Urban Urban Green 13:94–102
Rai R, Agrawal M (2014) Assessment of competitive ability of two Indian wheat cultivars under ambient O3 at different developmental stages. Environ Sci Pollut Res 21:1039–1053
Rai R, Agrawal M, Agrawal S (2010) Threat to food security under current levels of ground level ozone: a case study for Indian cultivars of rice. Atmos Environ 44:4272–4282
Rai R, Agrawal M, Choudhary KK, Agrawal S, Emberson L, Büker P (2015) Application of ethylene diurea (EDU) in assessing the response of a tropical soybean cultivar to ambient O3: nitrogen metabolism, antioxidants, reproductive development and yield. Ecotoxicol Environ Saf 112:29–38
Ramanathan V, Ramana MV (2005) Persistent, widespread, and strongly absorbing haze over the Himalayan foothills and the Indo-Gangetic Plains. Pure Appl Geophys 162:1609–1626. https://doi.org/10.1007/s00024-005-2685-8
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26
Sharma A, Ojha N, Pozzer A, Beig G, Gunthe SS (2019) Revisiting the crop yield loss in India attributable to ozone. Atmos Environ 1:100008
Singh AA, Agrawal SB (2017) Tropospheric ozone pollution in India: effects on crop yield and product quality. Environ Sci Pollut Res 24(5):4367–4382. https://doi.org/10.1007/s11356-016-8178-8
Singh E, Tiwari S, Agrawal M (2009) Effects of elevated ozone on photosynthesis and stomatal conductance of two soybean varieties: a case study to assess impacts of one component of predicted global climate change. Plant Biol 11(Suppl. 1):101–108
Singh S, Kaur D, Agrawal S, Agrawal M (2010) Responses of two cultivars of Trifolium repens L. to ethylenediurea in relation to ambient ozone. J Environ Sci 22:1096–1103
Singh AA, Singh S, Agrawal M, Agrawal SB (2015) Assessment of ethylenediurea-induced protection in plants against ozone phytotoxicity. Rev Environ Contam 233:129–184
Singh AK, Ghorai AK, Majumdar B, Behera MS, Mitra S, Roy S, Saha R, Kar G (2021) Impact of tropospheric ozone on crop production under jute-rice cropping system. Research Bulletin 01/2021 ICAR-Central Research Institute for Jute and Allied Fibres, Barrackpore, Kolkata
Singh UP (2002) Boro rice in eastern India. Rice-Wheat Consortium for the Indo-Gangetic Plains, New Delhi, India
Sinha B, Singh SK, Maurya Y, Kumar V, Sarkar C, Chandra BP, Sinha V (2015) Assessment of crop yield losses in Punjab and Haryana using 2 years of continuous in situ ozone measurements. Atmos Chem Phy 15:9555–9576
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Pl Cell Environ 35:259–270
Szantoi Z, Chappelka AH, Muntifering RB, Somers GL (2007) Use of ethylenediurea (EDU) to ameliorate ozone effects on purple coneflower (Echinacea purpurea). Environ Pollut 150:200–208
Tiwari S, Agrawal M (2018) Tropospheric ozone and its impacts on crop plants. Springer International Publishing AG, Berlin. https://doi.org/10.1007/978-3-319-71873-6_3
Tiwari S, Agrawal M, Manning WJ (2005) Assessing the impact of ambient ozone on growth and productivity of two cultivars of wheat in India using three rates of application of ethylenediurea (EDU). Environ Pollut 138:153–160
Tiwari S, Rai R, Agrawal M (2008) Annual and seasonal variations in tropospheric ozone concentrations around Varanasi. Int J Remote Sens 9(15):4499–4514
Ueda Y, Uehara N, Sasaki H, Kobayashi K, Yamakawa T (2013) Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol Biochem 70:396–402
Wang X, Zhang Q, Zheng F, Zheng Q, Yao F, Chen Z, Zhang W, Hou P, Feng Z, Song W, Feng Z, Fe Lu (2012) Effects of elevated O3 concentration on winter wheat and rice yields in the Yangtze River Delta. China Environ Pollut 171:118–125
Wang J, Zeng Q, Zhu J, Tang H (2013) Dissimilarity of ascorbate glutathione (AsA-GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free air ozone exposure. Agric Ecosyst Environ 165:39–49
WHO (1996) Update and revision of the WHO air quality guidelines for Europe Ecotoxic Effects, Ozone Effects on Vegetation. European Center for Environment and Health, Bilthoven
Xu W, Lin W, Xu X, Tang J, Huang J, Wu H, Zhang X (2016) Long-term trends of surface ozone and its influencing factors at the Mt Waliguan GAW station, China-Part 1: overall trends and characteristic. Atmos Chem Phy 16:6191–6205
Yadav DK, Prasad A, Kruk J, Pospíšil P (2014) Evidence for the involvement of loosely bound plastosemiquinones in superoxide anion radical production in photosystem II. PLoS ONE 9:115466. https://doi.org/10.1371/journal.pone.0115466
Yi F, Jiang F, Zhong F, Zhou X, Ding A (2016) The impacts of surface ozone pollution on winter wheat productivity in China- an econometric approach. Environ Pollut 208:326–335
Zhao H, Zheng Y, Zhang Y, Li T (2020) Evaluating the effects of surface O3 on three main food crops across China during 2015–2018. Environ Pollut 258:113794
Acknowledgements
We are thankful to the National Innovations in Climate Resilient Agriculture Programme of ICAR-Central Research Institute for Dryland Agriculture, Hyderabad, for providing financial assistance for this study under the NICRA Project. The authors gratefully acknowledge the Director, ICAR-Central Research Institute for Jute and Allied Fibres, Barrackpore (India), for providing research farm and laboratory facilities to carry out this work.
Funding
The authors disclosed the receipt of the following financial support for conducting the research: National Innovations in Climate Resilient Agriculture Programme of ICAR-Central Research Institute for Dryland Agriculture, Hyderabad, under NICRA Project. No funding for publication as open access.
Author information
Authors and Affiliations
Contributions
Arvind Kumar Singh: conceptualization, investigation, methodology, data curation, formal analysis, validation, project management, writing-original draft; Sabyasachi Mitra: methodology, writing-review, and editing; Gouranga Kar: project administration; writing-review, and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So, it is not applicable.
Consent for publication
Our manuscript does not contain any data from any individual person, so it is “not applicable.”
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, A., Mitra, S. & Kar, G. Assessing the impact of current tropospheric ozone on yield loss and antioxidant defense of six cultivars of rice using ethylenediurea in the lower Gangetic Plains of India. Environ Sci Pollut Res 29, 40146–40156 (2022). https://doi.org/10.1007/s11356-022-18938-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-18938-0