Abstract
Trichoderma sp. is a potential cellulase producing mesophilic fungi which grow under mild acidic condition. In this study, growth and nutritional conditions were manipulated for the maximum and cost-effective production of cellulase using lab strain Trichoderma sp. RCK65 and checked for its efficiency in hydrolysis of Prosopis juliflora (a woody substrate). Preliminary studies suggested that when 48 h old secondary fungal culture (20 % v/w) was inoculated in wheat bran moistened with mineral salt solution (pH 4.5 and 1:3 solid to moisture ratio), incubated at 30 °C and after 72 h, it produced maximum cellulase (CMCase 145 U/gds, FPase 38 U/gds and β-glucosidase 105 U/gds). However, using statistical approach a S:L ratio (1:1) was surprisingly found to be optimum that improved cellulase that is CMCase activity by 6.21 %, FPase activity by 23.68 % and β-glucosidase activity by 37.28 %. The estimated cost of crude enzyme (Rs. 5.311/1000 FPase units) seems to be economically feasible which may be due to high enzyme titre, less cultivation time and low media cost. Moreover, when the crude enzyme was used to saccharify pretreated Prosopis juliflora (a woody substrate), it resulted up to 83 % (w/w) saccharification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cellulase market has expanded in recent years with the onset of biorefineries, where fermentable sugars from hydrolysis of cellulosic biomass can be converted to commodities such as bioethanol and other bio-based products [1]. Besides this, cellulases have many other potential applications in textile industry, pulp and paper industry, starch processing, grain alcohol fermentation, malting and brewing, extraction of fruits and vegetable juices [2]. Such wide industrial potential has made cellulases as one of the most useful and in-demand enzyme system. Despite continuous research globally, cellulases production is still in its infancy and needs more precise efforts in this direction. Cost-effective production of cellulases is very critical for the overall process economics for bioconversion of plant materials into value added chemicals and biofuel [3].
Cellulases are a family of three groups of enzymes, endo-(1,4)-β-D-glucanase (EC 3.2.1.4), exo-(1,4)-β-D-glucanase (EC 3.2.1.91), and β-glucosidase (EC 3.2.1.21). The exoglucanase acts on the ends of cellulose chain and releases β-cellobiose as the end product; endoglucanase randomly attacks the internal o-glycosidic bonds, resulting in glucan chains of different lengths; and β-glucosidase act specifically on β-glycosidic bonds of cellobiose and produce glucose [4].
Cellulolytic microorganisms have been identified as members of eubacteria, fungi, some anaerobic protozoa and slime moulds. In general, bacteria degrade cellulose by cell-bound enzymes whereas; fungi secrete most of their enzymes in the surrounding growth medium. Unlike bacterial cellulases which produce membrane bound enzymes in form of cellulase complexes, fungal counterparts secrete cellulases in large amount as independent entities [5]. Therefore, to employ a cellulase producing fungal strain in various industrial applications, a complete cellulase system is required. Among various cellulase producing fungi, genus Trichoderma is the most studied fungus which has been reported to produce fairly good amount of exoglucanase and endoglucanase, but a low titre of β-glucosidase which hampers its efficiency for the conversion of cellulose to sugars. In Trichoderma reesei, 60 % of total protein constitutes of cellobiohydrolase I or exoglucanase and endoglucanase accounts for 20 %, which is thus a major drawback for cellulose hydrolysis [6]. A similar report supporting the preceding statement was reported by Kovacs et al. [7] using Trichoderma reesei Rut C30 producing very low β-glucosidase around 0.07U in comparison to 122 U of endoglucanase and 0.62 U exoglucanase activity in culture filtrate. However, a lot of studies have been done using Trichoderma sp. to produce cellulase, but they all lack in respect of low enzyme titre [8–10] or improper ratio of three enzymes [7, 11]. But still if nutritional and physiological parameters could be optimized, a process can be developed using a fungal strain for efficient cellulase production [3, 12]. Hence, there is still hope to design efficient cellulase production strategy using Trichoderma sp. which would be able to produce a high titre of β-glucosidase along with other two enzymes to remove the limitation in saccharification of cellulosics.
The media components along with the solid substrate play a vital role in obtaining high titers of enzyme under SSF. Optimising media components along with process parameters could lead to reduced energy requirement, use of simpler fermentation medium, absence of rigorous control of fermentation parameters, reduced water requirement, easier control of bacterial contamination, lower cost of downstream processing and finally leading to cost-effective production of enzyme [13, 14]. The methodology employed can be one factor at a time approach (OFAT) or statistical methods like response surface methodology (RSM) which identifies the influential factors and analyse their combinatorial approach [1]. In addition to the various parameter’s optimization, cost analysis for cellulase production is necessary to validate the suitability of the process and its economic feasibility for large scale application [15].
In this study, highly efficient cost-effective cellulase production from fungus Trichoderma sp. RCK65 has been reported by optimising various physiological and nutritional parameters. These parameters have been screened out by OFAT approach followed by further optimization using central composite design (CCD) of RSM to enhance the enzyme titre. The cost analysis of the optimized media has been carried out and a comparative account has been prepared with the cost of production media for cellulase production from various Trichoderma sp. reported in the literature. Further, the crude culture filtrate was tested on different pretreated Prosopis juliflora (a woody biomass) to check its saccharification efficiency on lignocellulosic substrates.
Materials and methods
Raw materials
Lignocellulosic substrates purchased locally were washed thoroughly and dried in hot air oven till constant weight. The lignocellulosic substrate P. juliflora was collected from Aravali forest area of University of Delhi, New Delhi, India, dried and grounded to a particle size of 40–60 mesh (Metrex Scientific Instrumentation, Delhi, India). The processed substrate was pretreated with acid, alkali and sodium chlorite as described earlier [16]. Chemicals such as carboxymethyl cellulose (CMC) and p-nitrophenyl β-d glucopyranoside (PNPG) were purchased from Sigma–Aldrich (St. Louis, USA). All other media components and chemicals of analytical grade were purchased locally.
Microorganism and culture conditions
The fungal isolate Trichoderma sp. RCK65 procured from culture collection of Lignocellulose Biotechnology Laboratory, University of Delhi South Campus, New Delhi, India, was grown and maintained on potato dextrose agar (PDA) (g/L) potato infusion 200; dextrose 20; agar 20 supplemented with carboxymethyl cellulose (CMC) (5.0 g/L) at 30 °C. The fungal cultures were stored at 4 °C.
Inoculum preparation
Fungal inoculum was prepared in 250 mL Erlenmeyer flasks with 100 mL potato dextrose broth (PDB) supplemented with 0.5 % (w/v) CMC. Four fungal discs (8 mm) diameter each were inoculated in the medium and incubated at 150 rpm and 30 °C. After 48 h, secondary inoculum was prepared by adding 5 mL of the primary inoculum into fresh potato dextrose broth (PDB) (100 mL) supplemented with 0.5 % (w/v) CMC and kept at 30 °C, 150 rpm for next 48 h. The secondary inoculum thus developed was then used for inoculating the production medium.
Cellulase production under solid state fermentation
SSF for cellulase production was carried out in a 250 mL Erlenmeyer flasks containing 5.0 g of wheat bran, moistened with solution containing (g/L) KH2PO4 6.0; (NH4)2SO4 3.0; yeast extract 5.0 and soybean meal 24.0 and pH 6.5. A substrate to moisture ratio of 1:3 was set prior to sterilisation. The flasks were sterilised by autoclaving at 121 °C and 15 psi for 15 min, and thereafter cooled to room temperature and inoculated with fungal inoculum 40 % (v/w) corresponds to 0.032 g dry weight. The contents of the flasks were mixed properly with a sterilised glass rod to distribute the inoculum throughout the bran and incubated at 30 °C. The fungal fermented bran after appropriate interval was taken out, suspended in 25 mL citrate phosphate buffer (50 mM, pH 5.0) and mixed thoroughly for 45 min. The wheat bran suspended in buffer solution was squeezed through the muslin cloth to maximize the enzyme extraction and centrifuged at 10,000 rpm and 4 °C for 5 min. The supernatant obtained was used for various assays to check the cellulase production.
Optimization of cellulase production
Various physical and nutritional parameters, such as cultivation time (up to seventh day), temperature (25–40 °C), solid to liquid ratio (1:1–1:5), pH (3.5–7.5), inoculum age (24–144 h), inoculum size (10–50 %, v/w of 48 h old secondary culture), and supplementation of different carbon and nitrogen sources were studied to evaluate their effect on production of cellulase from Trichoderma sp. RCK65. Each factor examined for optimization was incorporated further in the subsequent experiments. All other experiment conditions were kept constant unless otherwise stated.
To further evaluate the effect of interaction among effective parameters, such as pH, solid to liquid ratio, soybean meal concentration and quantity of wheat bran on cellulase production statistical approach, i.e. RSM was employed. The statistical software package Design Expert 8.0.7.1 (Stat-Ease, USA) was used to develop the experimental design and analyse the results. In experimental design, the minimum (−α) and maximum (+α) value of pH was 3.5 and 5.5, solid to liquid ratio 1:1 and 1:5, soybean meal concentration 1.2 and 3.6 g, respectively, while wheat bran quantity was kept 2 and 8 g.
Cost analysis of cellulase production
In this study, the cost of the cellulase production was estimated by analysing cost of culture medium (\({\text{Cost}}_{\text{cm}}\)), cost of equipment (\({\text{Cost}}_{\text{eq}}\)) and cost of process operation (\({\text{Cost}}_{\text{op}}\)) using the following equations, as described by Osma et al. [15].
where, D max = maximum incubation time, P a and P b = price of autoclave and incubator, LT a and LT b = life time of autoclave and incubator, Cap a and Cap b = capacity of autoclave and incubator.
where, E a and E b = energy consumption by autoclave and incubator.
While the cost of cellulase production (\({\text{Cost}}_{\text{cellulase}}\)) on the basis of maximum FPase activity (\(A_{\text{cellulase}}\)) and volume of enzyme production (\(V_{\text{cellulase}}\)) was calculated as follows:
Saccharification of lignocellulosic substrates
Enzymatic hydrolysis of untreated and pretreated (chlorite treated, alkali treated and acid treated) plant materials, Prosopis juliflora; 10.0 g each was carried out at 5 % (w/v) substrate consistency in 50 mM citrate phosphate buffer (pH 5.0). The substrate with buffer was preincubated at 50 °C on a rotatory shaker (Innova-40, New Brunswick Scientific, Germany) at 150 rpm for 2 h, and thereafter crude culture filtrate containing (20 FPase/g) and Tween 80 (1 % v/v) was also added to the reaction mixture and the reaction continued up to 24 h. Samples of enzymatic hydrolysate were withdrawn at regular intervals and analysed for amount of glucose released.
Analytical methods
The FPase, CMCase and β-glucosidase activities were determined using IUPAC procedures [17]. The release of the reducing sugars was measured as a glucose equivalent using dinitrosalicylic acid method [18]. β-Glucosidase activity was determined by assaying the release of p-nitrophenol (pNP) at 430 nm from a reaction mixture containing 1 mL p-nitrophenyl β-D glucopyranoside (pNPG) (1 mM), 1.8 mL 50 mM acetate buffer (pH 4.8) and 0.2 mL suitably diluted enzyme, incubated at 50 °C for 30 min [19]. One unit of enzyme activity was defined as the amount of enzyme required to liberate 1 µmol of glucose or p-nitrophenol, from their corresponding substrate, per mL of enzyme per min under the assay conditions.
Results and discussion
Time course of cellulase production
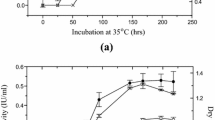
Enzyme production carried out using Trichoderma sp. RCK65 under SSF increased gradually till third day with a subsequent stationary phase on further incubation. The FPase, CMCase and β-glucosidase production reached to a maximum of 19.81 ± 0.06, 53.88 ± 0.38 and 26.73 ± 0.98 U/gds, respectively, on the third day.
Effect of different physiological parameters on cellulase production
Effect of temperature
The optimization of incubation temperature for cellulase production using Trichoderma sp. RCK65 revealed that production of enzyme increased from 25 to 30 °C (Table 1) with maximal enzyme production at 30 °C i.e., CMCase (50.46 ± 2.41 U/gds), FPase (18.73 ± 0.39 U/gds) and β-glucosidase (28.42 ± 0.08 U/gds). Any further increase in temperature decreased the enzyme production, which may be attributed to lower fungal growth at higher temperature. Yang et al. [20] and Das et al. [21]. also reported an optimum temperature ranging 28–30 °C for cellulase production from Trichoderma sp. under SSF condition.
Effect of substrate to moisture ratio
At microscopic level, the influence of cell water flux occur for the maintenance of a constant cell turgor pressure, constant branching orientation and hyphal growth rate but at macroscopic level, influence of the hydration occurs for the maintenance of radial growth rate, evolution of germination, sporulation and metabolic activity of a fungal colony [22]. Moreover, solute diffusion as well as maintenance of osmotic balance in a cell also occurs in aqueous medium. The results in this study showed that enzyme production enhanced with increase in initial substrate to moisture ratio of the medium from 1:1 to 1:3. At substrate to moisture ratio of 1:3, the maximal enzyme production was CMCase (52.30 ± 0.59 U/gds), FPase (19.57 ± 0.42 U/gds) and β-glucosidase (30.78 ± 0.95 U/gds) (Table 1). However, increasing the moisture content beyond 1:3 ratio decreased the enzyme production, which may be due to the presence of excess free water which creates an additional diffusion barrier leading to decrease in growth and enzyme production [13]. Similar observation was also reported in case of cellulase production using Fomitopsis sp. RCK2010 by Deswal et al. [3] and Thermoascus aurantiacus RCKK by Jain et al. [12].
Effect of inoculum concentration
An optimal inoculum level is necessary for maintaining balance between the proliferating biomass and available nutrients to produce maximum enzyme [23]. In this study, the effect of inoculum concentration ranging from 10 to 50 % (v/w) revealed that 20 % (v/w) is the optimal inoculum concentration for maximum cellulase production, i.e. CMCase (56.26 ± 0.89 U/gds), FPase (19.55 ± 1.86 U/gds) and β-glucosidase (67.61 ± 1.08 U/gds), which decreased on further increase of inoculum concentration (Table 1). Hu et al. [10] also reported 20 % (v/w) as optimal concentration for cellulase production from Trichoderma viride. While the decrease in enzyme activity by increasing the inoculum concentration beyond optimal limit could be due to faster nutrient depletion [13].
Effect of initial pH
The cellulase production by Trichoderma sp. RCK65 under SSF condition was also checked at different pH ranging from 3.5 to 7.5. The fungus produced maximum CMCase (146.53 ± 0.29 U/gds), FPase (39.07 ± 1.21 U/gds) and β-glucosidase (105.02 ± 0.55 U/gds) at initial medium pH 4.5 (Table 1). An increase in initial pH of the medium beyond 4.5 reduced the cellulase production drastically. Yang et al. [20] and Das et al. [21] also observed the optimum cellulase production at pH 4.5, respectively, when studying the cellulase production using Trichoderma sp.
Effect of inoculum age
An inoculum age of 48 h was found optimum for the production of CMCase (146.48 ± 1.44 U/gds), FPase (41.49 ± 0.58 U/gds) and β-glucosidase (106.41 ± 0.28 U/gds), when inocula of age ranging 24–144 h were evaluated (Table 1). Further use of older inoculum led to substantial decrease in the production of all the enzymes which may be attributed to the lesser number of fungal cells in growing phase. Our results are also in good accordance with Jain et al. [12]. who reported a rapid decrease in cellulase production when more than 72 h old culture was used as inoculum.
Effect of nutritional parameters on cellulase production
Effect of substrate and its concentration
The influence of various substrates on cellulase production is shown in Table 2. Among tested carbon sources, wheat bran led to maximum production of CMCase (146.55 ± 1.04 U/gds), FPase (41.09 ± 0.68 U/gds) and β-glucosidase (104.64 ± 0.73 U/gds). This may be attributed to the fact that wheat bran is a nutrient base comprising blends of complex carbohydrates as celluloses, hemicelluloses and also a source of substituted insoluble xylan, in addition to digestible nitrogen [1]. Moreover, it also acts as a complete nutritious feed containing various soluble sugars, which are helpful for the initiation of growth and replication of microorganisms and remains loose even under moist conditions providing a large surface area [24]. On the other hand, Prosopis juliflora, Lantana camara and corn cob when used as supplement for cellulase production by Trichoderma sp. RCK65, the production level was substantially lower, which might be due to inappropriate physical properties of these substrates like particle size, geometry and compactness of the substrate which creates problem in heat-mass transfer and aeration required for growth of the fungus [13]. Amongst various concentration of wheat bran tested 5 g gave maximum CMCase (146.7 ± 1.05 U/gds), FPase (41.18 ± 1.96 U/gds) and β-glucosidase (104.89 ± 0.78 U/gds) (Fig. 1).
Effect of nitrogen source and its concentration
Among different nitrogen sources tested, the fungus Trichoderma sp. RCK65 produces maximum cellulases, viz. CMCase (149.64 ± 1.20 U/gds), FPase (41.09 ± 1.73 U/gds) and β-glucosidase (106.27 ± 0.62 U/gds) in the presence of 2.4 % (w/v) soybean meal as organic nitrogen source (Table 2). The enhanced production of cellulase in presence of soybean meal may be attributed to organic nitrogen source mediated regulation. Gomes et al. [25] also reported presence of organic nitrogen in the medium lead to increase in production of hydrolysing enzymes by T. aurantiacus. Moreover, it has also been reported that nitrogen can significantly affect the pH of the medium during the course of fermentation as reported by Haapala et al. [26], thereby influences the microbial metabolism. Similar findings were reported by Deswal et al. [3] and Jain et al. [12]. Further the concentrations of soybean meal were checked and a maximum of CMCase (137.98 ± 2.74 U/gds) and β-glucosidase (103.52 ± 3.51 U/gds) with 2 % (w/v) soybean meal whereas maximum FPase (45.35 ± 1.67 U/gds) with 3 % (w/v) (Fig. 2).
Statistical optimization of cellulase production using RSM
The experimental results obtained by CCD of RSM experiment are presented along with the mean observed responses in Table 3. The maximum cellulase activity was observed in run 19 where pH 4.5, solid to liquid ratio 1:1, soybean meal 2.4 % (w/v) and wheat bran 5 g was used in the production medium, while the minimum activity was obtained in run 16 where the fungus was grown on medium of pH 5.0, solid to liquid ratio 1:4, soybean meal 3.0 % (w/v), wheat bran 6.5 g. The analysis of experimental design provided following quadratic equations by which the predicted values could be estimated at varied level of factors:
where A (pH), B (S:L), C is the soybean meal concentration (%) and D is the wheat bran quantity (g) for the three responses CMCase, FPase and β-glucosidase, respectively, which can be used for predicting the responses at any combination of four predicted variables in and around their experimental range. The model on analysis was found significant, as the p < F value to be less than 0.001 for all the three responses that is CMCase, FPase and β-glucosidase enzyme activity and was predicted by the software itself. The analysis of variance showed that for all the enzymes the R 2 value was closer to one that is 0.9789 (CMCase), 0.9507 (FPase) and 0.9544 (β-glucosidase) which are in close agreement with the adjusted and predicted R 2 values (Table 4). The closer the R 2 (adj R 2) value to one, the stronger the model is and better it predicts the response. Besides the adequate precision values of all the enzyme were observed to be higher than 4.0, which is desirable. The parameter coefficient and the P value suggested that all the variables selected for RSM have significant effect on cellulase production.
The 3D response surfaces plots were employed to determine the interaction of the media components and the optimum levels that have the most significant effect on enzyme activity. The effect of four factors on each of the cellulase enzyme was depicted by keeping two variables constant or at zero level and varying the other two, revealing a centre point for best possible interaction for maximum enzyme production. Figure 3a showed that increasing the quantity of wheat bran up to a certain level with respect to pH increased the CMCase activity, but when increased more than 5 g, it decreased the production as increasing the carbon source after a certain concentration decreases the cellulase production. Soybean meal at centre point of 2.4 g gave the maximum CMCase production with respect to wheat bran Fig. 3b. Whereas Fig. 3c, d showed the effect of pH and S:L ratio with respect to soybean meal and wheat bran giving optimum value of 4.5 and 1:1 for maximum CMCase production.
Optimum FPase activity with respect to the four factors was also checked. Figure 4a, b showed that soybean meal and wheat bran at centre point of 5 and 2.4 g exhibited higher enzyme production, which revealed that increasing or decreasing the carbon and nitrogen content effects the cellulase level [27]. Whereas Fig. 4c, d revealed that deceasing the S:L ratio and pH to 1:1 and 4.5, respectively, increased the FPase production. It was due to the fact that water holding capacity depends on the substrate used, and as it is known that wheat bran has higher water holding capacity, this may be attributed to lower moisture content for optimum production [28].
Influence of the factors on β-glucosidase activity as shown in Fig. 5a–c was observed optimum for maximum β-glucosidase production at wheat bran 5 g, pH 4.5 and S:L of 1:1. While Fig. 5d showed that increasing soybean meal increased the β-glucosidase production up to a certain concentration (2.4 g) and remained almost constant thereafter. Our results are in well accordance with the work of Lee et al. [29] where they used organic nitrogen source soy peptone 2.3 g giving maximum β-glucosidase activity. The predicted values were in good agreement with the values measured in these experiments, thus justifying the validity of the response model and the necessity for optimization.
The statistical optimisation surprisingly revealed very low moisture content of 1:1 ratio suitable for all three cellulase production using Trichoderma sp. RCK65 within a short duration of 72 h. A solid to moisture ratio of 1:1 for cellulase production was also reported by Shu et al. 2013 [30] using Trichoderma reesei HY07, but with incubation time of 5 days. Thus, the optimisation resulted in 2.39, 2.86 and 5.4 fold increase in FPase, CMCase and β-glucosidase, respectively. The enzyme activities reported in this study was found to be highest in respect to all the three enzyme activities reported from Trichoderma sp. when compared with the available literature (Table 5).
Cost analysis
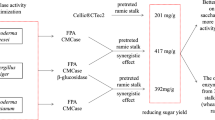
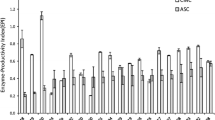
Cost evaluation of the crude culture filtrate of Trichoderma sp. RCK65 was calculated according to Osma et al. [15] and coworkers. Using the Eqs. (1, 2, 3, 4), the total cost of culture medium, equipment and operation was estimated as Rs. 0.911, Rs. 0.150 and Rs. 0.196, respectively. Using the following equities, the net cost of crude enzyme obtained was estimated as Rs. 5.311/1000 units of FPase. Further we have attempted to compare the production cost of enzymes reported in the literature using the methodology reported by Osma et al. 2011 [15]. The media cost, cost of equipment and processing cost was considered in rupees and the enzyme production cost was calculated with respect to FPase activities. It was seen that the cost of our crude enzyme was lowest when compared with the calculated cost based on FPase activities from other reports on Trichoderma cellulase (Fig. 6), which may be attributed by the high enzyme titer and lower incubation time in our case.
Enzymatic saccharification
The crude enzyme of Trichoderma sp. RCK65 when applied for enzymatic saccharification of the varied pretreated lignocellulosic substrate (P. juliflora) showed a perpetual rise in saccharification efficiency starting from 29.88 % in untreated substrate to 83.25 % in chlorite treated substrate with a sugar release of 12.21 and 46.61 g/L, respectively (Table 6). The table depicts that the enhancement in saccharification efficiency was directly proportional to the holocellulose content of the substrate, which may be due to enhanced availability of cellulosic moieties to the enzyme due to partial removal of lignin and/or hemicellulose. Our results were also in accordance with our earlier reports on saccharification of delignified substrates (P. Juliflora, Lantana camara, newspaper waste and corncob) using commercial enzymes yielding 82.8 % [31], 80.0 % [32], 59.8 % [33] and 78 % enzymatic saccharification [34]. It also confirms that the enzyme obtained from Trichoderma sp. RCK65 is comparable to the commercial enzymes used in these studies.
Conclusions
The results presented here demonstrate the enhanced production of cellulase enzyme with high enzyme productivity, which makes the enzyme of immense industrial potential. Moreover, lower incubation time and high enzyme titer help in reducing the cost of cellulase. The enzyme showed wide applicability in hydrolysis of varied pretreated biomass substrate revealing its potential application in the production of second generation bioethanol.
Acknowledgments
SC and RG would like to acknowledge University Grants Commission (40-133/2011 (SR)), New Delhi, India and Department of Science and Technology (SERB/FT/LS-229/2012) for providing the financial support. Moreover, the support from University of Delhi, New Delhi, India, and Central University of Haryana is also highly acknowledged.
Abbreviations
- \({\text{Cost}}_{\text{cm}}\) :
-
Cost of culture medium
- \({\text{Cost}}_{\text{eq}}\) :
-
Cost of equipment
- \({\text{Cost}}_{\text{op}}\) :
-
Operating cost
- \(D_{\hbox{max} }\) :
-
Maximum incubation time
- \(P_{a}\) :
-
Price of autoclave
- \({\text{LT}}_{a}\) :
-
Life time of autoclave
- \({\text{Cap}}_{a}\) :
-
Capacity of autoclave
- \(P_{b}\) :
-
Price of incubator
- \({\text{LT}}_{b}\) :
-
Life time of incubator
- \({\text{Cap}}_{b}\) :
-
Capacity of incubator
- \(E_{a}\) :
-
Energy consumption by autoclave
- \(E_{b}\) :
-
Energy consumption by incubator
- gds:
-
Gram dry substrate
References
Dave BR, Sudhir AP, Parmar P et al (2013) Enhancement of cellulase activity by a new strain of Thermoascus aurantiacus: optimisation by statistical design response surface methodology. Biocatal Agric Biotechnol 2:108–115. doi:10.1016/j.bcab.2013.02.003
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res 2011:1–10. doi:10.4061/2011/280696
Deswal D, Khasa YP, Kuhad RC (2011) Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour Technol 102:6065–6072. doi:10.1016/j.biortech.2011.03.032
Kuhad RC, Singh A, Eriksson K-EL (1997) Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Adv Biochem Eng Biotechnol 281:1647–1650. doi:10.1007/BFb0102072
Malherbe S, Cloete TE (2002) Lignocellulose biodegradation: fundamentals and applications. Rev Environ Sci Biotechnol 1:105–114. doi:10.1023/A:1020858910646
Tiwari P, Misra BN, Sangwan NS (2013) β-glucosidases from the fungus trichoderma: an efficient cellulase machinery in biotechnological applications. BioMed Res Int 2013:1–10. doi:10.1155/2013/203735
Kovács K, Megyeri L, Szakacs G et al (2008) Trichoderma atroviride mutants with enhanced production of cellulase and β-glucosidase on pretreated willow. Enzyme Microb Technol 43:48–55. doi:10.1016/j.enzmictec.2008.02.006
Vyas A, Vyas D (2005) Production of fungal cellulases by solid state bioprocessing of groundnut shell wastes. J Sci Ind Res 64:767–770
Rocky-Salimi K, Hamidi-Esfahani Z (2010) Evaluation of the effect of particle size, aeration rate and harvest time on the production of cellulase by Trichoderma reesei QM9414 using response surface methodology. Food Bioprod Process 88:61–66. doi:10.1016/j.fbp.2009.06.006
Hu T, Zhou Y, Dai L et al (2011) Enhanced cellulase production by solid state fermentation with polyurethane foam as inert supports. Procedia Eng 18:335–340. doi:10.1016/j.proeng.2011.11.053
Raghuwanshi S, Deswal D, Karp M, Kuhad RC (2014) Bioprocessing of enhanced cellulase production from a mutant of Trichoderma asperellum RCK2011 and its application in hydrolysis of cellulose. Fuel 124:183–189. doi:10.1016/j.fuel.2014.01.107
Jain KK, Bhanja Dey T, Kumar S, Kuhad RC (2015) Production of thermostable hydrolases (cellulases and xylanase) from Thermoascus aurantiacus RCKK: a potential fungus. Bioprocess Biosyst Eng 38:787–796. doi:10.1007/s00449-014-1320-4
Krishna C (2005) Solid-state fermentation systems—an overview. Crit Rev Biotechnol 25:1–30. doi:10.1080/07388550590925383
Singhania RR, Sukumaran RK, Pandey A (2007) Improved cellulase production by Trichoderma reesei RUT C30 under SSF through process optimization. Appl Biochem Biotechnol 142:60–70. doi:10.1007/s12010-007-0019-2
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2011) Cost analysis in laccase production. J Environ Manage 92:2907–2912. doi:10.1016/j.jenvman.2011.06.052
Gupta R, Khasa YP, Kuhad RC (2011) Evaluation of pretreatment methods in improving the enzymatic saccharification of cellulosic materials. Carbohydr Polym 84:1103–1109. doi:10.1016/j.carbpol.2010.12.074
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. doi:10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Wood TM, Bhat MK (1988) Measuring cellulase activities. Methods Enzymol 160C:87–112. doi:10.1016/0076-6879(88)60109-1
Yang YH, Wang BC, Wang QH et al (2004) Research on solid-state fermentation on rice chaff with a microbial consortium. Colloids Surf B Biointerfaces 34:1–6. doi:10.1016/j.colsurfb.2003.10.009
Das M, Banerjee R, Bal S (2008) Multivariable parameter optimization for the endoglucanase production by Trichoderma reesei Rut C30 from Ocimum gratissimum seed. Braz Arch Biol Technol 51:35–41. doi:10.1590/S1516-89132008000100005
Gervais P, Molin P (2003) The role of water in solid-state fermentation. Biochem Eng J 13:85–101. doi:10.1016/S1369-703X(02)00122-5
Matkar K, Chapla D, Divecha J et al (2013) Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int Biodeterior Biodegrad 78:24–33. doi:10.1016/j.ibiod.2012.12.002
Kuhad RC, Singh A (1993) Lignocellulose biotechnology: current and future prospects. Crit Rev Biotechnol 13:151–172. doi:10.3109/07388559309040630
Gomes DJ, Gomes J, Steiner W (1994) Factors influencing the induction of endo-xylanase by Thermoascus aurantiacus. J Biotechnol 33:87–94. doi:10.1016/0168-1656(94)90101-5
Haapala R, Linko S, Parkkinen E, Suominen P (1994) Production of endo-1,4-β-glucanase and xylanase by Trichoderma reesei immobilized on polyurethane foam. Biotechnol Tech 8:401–406. doi:10.1007/BF00154311
Hao X-C, Yu X-B, Yan Z-L (2006) Optimization of the medium for the production of cellulase by the mutant Trichoderma reesei WX-112 using response surface methodology. Food Technol Biotechnol 44:89–94
Deswal D, Gupta R, Kuhad RC (2012) Enhanced exoglucanase production by brown rot fungus Fomitopsis sp. RCK2010 and its application for cellulose saccharification. Appl Biochem Biotechnol 168:2004–2016. doi:10.1007/s12010-012-9913-3
Lee YM, Lee H, Kim JS et al (2014) Optimization of medium components for β-glucosidase production in Schizophyllum commune KUC9397 and enzymatic hydrolysis of lignocellulosic biomass. BioResources 9:4358–4368. doi:10.15376/biores.9.3.4358-4368
Shu G, Yang H, Chen H, Wang S (2013) Optimization of cellulase production by Trichoderma reesei HY07 using response surface methodology. Res J App Sci Eng Tech 5(23):5438–5442
Gupta R, Sharma KK, Kuhad RC (2009) Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, a woody substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis-NCIM 3498. Bioresour Technol 100:1214–1220. doi:10.1016/j.biortech.2008.08.033
Kuhad RC, Gupta R, Khasa YP, Singh A (2010) Bioethanol production from Lantana camara (red sage): pretreatment, saccharification and fermentation. Bioresour Technol 101:8348–8354. doi:10.1016/j.biortech.2010.06.043
Kuhad RC, Mehta G, Gupta R, Sharma KK (2010) Fed batch enzymatic saccharification of newspaper cellulosics improves the sugar content in the hydrolysates and eventually the ethanol fermentation by Saccharomyces cerevisiae. Biomass Bioenergy 34:1189–1194. doi:10.1016/j.biombioe.2010.03.009
Gupta R, Mehta G, Chander Kuhad R (2012) Fermentation of pentose and hexose sugars from corncob, a low cost feedstock into ethanol. Biomass Bioenergy 47:334–341. doi:10.1016/j.biombioe.2012.09.027
Latifian M, Hamidiesfahani Z, Barzegar M (2007) Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermentation conditions. Bioresour Technol 98:3634–3637. doi:10.1016/j.biortech.2006.11.019
Mekala NK, Singhania RR, Sukumaran RK, Pandey A (2008) Cellulase production under solid-state fermentation by Trichoderma reesei RUT C30: statistical optimization of process parameters. Appl Biochem Biotechnol 151:122–131. doi:10.1007/s12010-008-8156-9
Alam MZ, Mamun AA, Qudsieh IY et al (2009) Solid state bioconversion of oil palm empty fruit bunches for cellulase enzyme production using a rotary drum bioreactor. Biochem Eng J 46:61–64. doi:10.1016/j.bej.2009.03.010
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakraborty, S., Gupta, R., Jain, K.K. et al. Cost-effective production of cellulose hydrolysing enzymes from Trichoderma sp. RCK65 under SSF and its evaluation in saccharification of cellulosic substrates. Bioprocess Biosyst Eng 39, 1659–1670 (2016). https://doi.org/10.1007/s00449-016-1641-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1641-6