Abstract
In this study, the characteristics of sewage of small community were determined for 6 months to ascertain the type of treatment required in subtropical conditions. The results demarcated sewage of this community as a medium-strength wastewater (chemical oxygen demand: 475 mg/L, biochemical oxygen demand: 240 mg/L and total suspended solids: 434 mg/L). Chemical oxygen demand to sulphate ratio of the sewage (11.6) established that it was amenable to anaerobic digestion. The temperature, strength, biodegradability and components of sewage were suitable for anaerobic digestion, and thus, upflow anaerobic sludge blanket reactor (UASB) was selected for its treatment. These reactors are often shutdown in small communities due to environmental and/or socio-economic factors. The ability of two UASB reactors, seeded with cow dung (UASBCD) and activated sludge of a dairy treatment plant (UASBASDIT) to restart after a long idle period of 12 months, was investigated along with sludge analysis by scanning electron microscope. Biomass in both reactors reactivated rapidly after shutdown period and within 30 days after substrate feeding achieved uniform removal efficiencies for chemical oxygen demand, total suspended solids, total dissolved solids, chloride and oil and grease. Chemical oxygen demand removal efficiency of both reactors became uniform and remained close to 80% after 30 days through reactivation of microbes in sludge bed due to adequate food and temperature conditions. During restart-up, at an average organic loading rate of 0.902 kg COD/m3 per day, methane yields of 0.091 and 0.084 m3/kg COD removed were achieved for UASBCD and UASBASDIT reactors, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pakistan, like many other developing countries, lacks municipal wastewater treatment facilities, and almost total domestic wastewater is indiscriminately discharged untreated in water bodies, especially in the vicinity of metropolitans, where untreated municipal wastewater is also used for irrigation which can cause soil contamination, surface and groundwater contamination and ultimately risk to human beings through food chain. There is a strong need to provide improved water and wastewater facilities to the poor (especially in developing countries) and reduce the environmental impacts of our practices (Gray 2010; Elmitwalli and Otterpohl 2011). This situation also demands to reclaim nutrient-rich municipal wastewater by using simple, efficient and cost-effective sewage treatment techniques compatible with local conditions. Generally, characterization demonstrates the physical, chemical and biological composition of wastewater. However, the characteristics like organic matter, suspended solids and temperature are considered most important in a wastewater treatment plant (van Haandel and Lettinga 1994; Abbasi and Abbasi 2012). The concentration of these pollutants is the main factor that determines the applicability of anaerobic technologies for domestic wastewater treatment. The characteristics of sewage also help determine the wastewater treatment sequence (Levine et al. 1991; Mikosz 2015). Anaerobic digestion is a complex biological process in which the facultative and anaerobic microorganisms digest organic material in the absence of oxygen in order to obtain energy for their survival, and resultantly generate gaseous methane and carbon dioxide (CH4, CO2) emissions. The anaerobic digestion process, in its simplest form, can be explained by following reaction (Reynolds and Richards 1996).
Anaerobic digestion involves various steps, including (1) hydrolysis, (2) acidogenesis, (3) acetogenesis and (4) methanogenesis (van Haandel and Lettinga 1994; Seghezzo 2004). During the hydrolysis, the enzymes excreted by fermentative bacteria decompose the complex and insoluble organic polymers (proteins, carbohydrates, fats) into simple soluble compounds such as fatty acids, amino acids, sugars and alcohols (van Haandel and Lettinga 1994; Shieh et al. 2000). The acidogenesis phase converts the dissolved compounds to ethanol, propionate, butyrate and new cell matter. The acetogenesis process converts the long-chain fatty acids into acetate, hydrogen (H2), CO2 and new cell matter. Finally, methane-producing bacteria complete the decomposition process by producing methane and carbon dioxide through the (1) cleavage of two acetic acid molecules and (2) reduction of carbon dioxide with hydrogen. About 70% of the total methane generated is produced from acetate because limited amount of hydrogen is available to produce methane by the reduction of carbon dioxide. There are two main types of methane-producing microorganisms such as methanothrix and methanosarcina. However, only one species of methanogenic organisms usually dominates in the system depending on the conditions in the reactor (Shieh et al. 2000).
Anaerobic treatment, such as upflow anaerobic sludge blanket (UASB) reactor, is preferred for treatment of municipal wastewater because of its merits over conventional treatment methods (van Lier and Lettinga 1999). These advantages are (1) its ability to treat high organic loads and withstand fluctuation in the influent, (2) biogas formation and (3) effective treatment of wastewater in a short period of time (Chong et al. 2012; Cervantes et al. 2015). Anaerobic reactors reduce pollution load and provide good stabilization of solids. Furthermore, depending on the design of a UASB reactor, a high sludge hold-up time can be obtained so that the excess sludge is discharged only once every 3–4 years (Lettinga 1996; Elmitwalli and Otterpohl 2011).
The start-up and restart-up of anaerobic reactors are very important issues for their applicability in the treatment of wastewaters. However, very little information is available about the restart-up and its modelling in the literature (Zhao et al. 2010; Xing et al. 2014). The study of biomass (sludge bed) reactivation is important to determine the ability of UASB reactors to withstand long shutdown period in small community municipal treatment plants which face accidental shutdown due to different environmental (rainy season), economic and social factors (strikes etc.). Shutdown, in seasonally operated industries, occurs repeatedly after every 6 months. The restart potential of anaerobic reactor can be monitored in terms of removal of parameters like volatile fatty acid (VFA), chemical oxygen demand (COD), total suspended solids (TSS), pH fluctuation and methane (CH4) production. Successful restart of anaerobic reactors has been reported in the literature; however, time taken by reactors to restore normal functioning after shutdown varies considerably and is dependent on the history of the reactor, type of packing material/sludge used and type of wastewater treated before and after shutdown (Sanz and Fdz-Polanco 1989; Manariotis and Grigoropoulos 2006, 2008; Dong et al. 2010).
In the present study, the characteristics of raw municipal wastewater of a small community are presented which were monitored for 6 months (January to June, 2005 in Punjab University, Lahore, Pakistan) in terms of (COD), biochemical oxygen demand (BOD), total suspended solids (TSS), volatile suspended solids (VSS), pH, conductivity, turbidity, temperature, alkalinity, sulphide, sulphate, chlorides, ammonia nitrogen, detergent, oil and grease and other elements such as cyanide, arsenic, iron and zinc, to assess the applicability of UASB reactor for its treatment in subtropical conditions. Furthermore, this study evaluates the return of biomass to active state in UASB after a long non-feeding period and the time needed to achieve steady-state under laboratory conditions with specific hydraulic retention time (HRT), substrate and temperature. For this restart-up study, in 2008, in Punjab University Lahore, two UASB reactors seeded with cow dung and activated sludge of a dairy industry wastewater treatment plant termed as UASBCD and UASBASDIT, respectively, previously used to treat sewage were used.
Materials and methods
Sewage sampling
Composite samples of domestic sewage were collected from the Garden Town municipal wastewater pumping station of Metropolitan Lahore. Sampling period was spanned over 6 months (from January to June). Sampling was carried out at an interval of 3 h and 3 days a week during the operation of pumping station. Sewage was collected in 20-L plastic can which was duly labelled, sealed, transported to laboratory and stored at 4 °C for further analysis. Wastewater was then characterized in terms of various parameters (i.e. temperature, pH, turbidity, conductivity, BOD, COD, TSS, VSS, alkalinity, oil and grease, ammonia nitrogen (NH4-N), detergents, chlorides, sulphate and sulphide).
Design of UASB reactor assembly
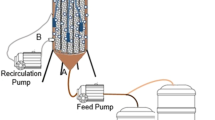
A bench-scale anaerobic UASB reactor was used in this study. The set-up consisted of a pair of UASB reactors, peristaltic pump, influent tank, effluent collection tank and gas trapping system. Schematic diagram of the experimental set-up is shown in Fig. 1, and Fig. 2 shows the photograph of UASBCD and UASBASDIT reactors during restart-up.
The UASB reactor was made of Perspex material and comprised of a tubular section at the bottom and an expanded section termed as gas–liquid–solid separator (GLSS) at the top. Tubular section was a 120-cm-long column with 7 cm internal diameter (ID) and a volume of 4.6 L. The length of the gas–liquid–solid separator was 40 cm and volume was 10.2 L. The GLSS section was further divided into two parts: bottom half was tapered with a slope angle (Ø) of 60° and top half was a 20-cm-long column with an internal diameter of 22 cm. An inverted canopy was also attached with the top lid of GLSS in order to promote coagulation of suspended/colloidal particles, boost the collection of suspended particles and enhance the collection of biogas and to control the washout of particles (Yasar et al. 2007).
Analytical techniques
Methods 5220 C, 4500-Cl C, 4500-NO3 −, 4500-P ascorbic acid method, 4500-SO4 2− turbidimetric method, and 5220 B were used for the determination of COD, chloride, nitrate, phosphate, sulphate, oil and grease contents, respectively.
Standard method (5210 B) was followed to determine BOD. Dilution water was prepared by adding 1 ml each of phosphate buffer, MgSO4, CaCl2 and FeCl3 solutions per litre of water at 20 °C. Dilution water was then aerated with filtered air, and five dilutions were made for each sample. Wastewater sample and dilution water were filled in airtight 300-ml BOD bottles which were then water sealed and incubated at 20 ± 1 °C for 5 days. Dissolved oxygen (DO) was measured immediately after filling BOD bottles with diluted sample and after 5 days of incubation and blanks. COD was determined according to open reflux method (5220 B) in standard methods. A sample of sewage wastewater was refluxed for 2 h in a highly acidic solution with a known surplus amount of potassium dichromate (K2Cr2O7). After digestion, the remaining potassium dichromate was titrated with ferrous ammonium sulphate using ferroin indicator, in order to determine the amount of K2Cr2O7 consumed. The end point of titration was the change in colour from blue green to reddish brown that remains for 1 min or longer.
Standard method (2540 B) was applied for the determination of total solids (TS). Wastewater sample (100 ml) was evaporated in a pre-weighed dish and dried to constant weight in an oven at 103 °C (APHA et al. 1998). The increase in the weight of dish represented the TS. To determine TDS by standard method (2540 C), wastewater sample was first filtered through a standard pre-weighed glass fibre filter. The filtrate was evaporated to dryness in a pre-weighed dish and dried to constant weight at 180 °C. The increase in dish weight represented the TDS. TSS were measured following standard method (2540 D), wherein the residue on the pre-weighed filter was dried to a constant weight at 103 °C. The increase in weight of the filter represented TSS. VSS content was determined in accordance with standard method (2540 E). The residue of TSS was ignited to a constant weight at 550 °C in a muffle furnace. The ignited residue was transferred to a desiccator to dissipate heat. The process of ignition, cooling and desiccation was repeated until a constant weight was obtained. VSS were calculated from the difference of weight of residue and crucible before and after ignition.
Turbidity was determined by using a portable microprocessor turbidity meter (Hanna, HI 93703) having a resolution of 0.01 and a measuring range of 0.00–1000 NTU. DO was measured by using a DO meter (Hanna, HI 9142) with a measuring range of 0–19.9 mg/L, and resolution of 0.1. pH meter (JENCO, USA 6173) was used to measure pH of water samples.
The heavy metals were determined with Varian atomic absorption spectrophotometer (model-250) using standard methods air–acetylene (3111 B) and nitrous–acetylene (3111 D) flames, and relevant metal element standards.
The methane production was monitored by liquid displacement method. The liquid displaced was alkaline (NaOH) solution. The COD from wastewater that was converted to CH4 was calculated by assuming that 1 ml CH4 displaced 1 ml NaOH solution and that at 25 °C, 1 g COD produces 394 ml moist CH4/382 ml dry CH4 (Ruiz et al. 1998; Board 2004).
Results and discussion
Characteristics of sewage
The composition of sewage is shown in Table 1. Table 2 shows specifically the heavy metal contents of sewage. The average pH value (7.16) of sewage was almost neutral. The BOD and COD contents on average were 240 and 475 mg/L, respectively. The COD, BOD and TSS contents varied over time. BOD and COD contents were 257, and 506 mg/L, respectively, in winter, and 224 and 445 mg/L, respectively, in summer. Lower concentrations of COD, BOD and TSS in summer owe to the dilution of wastewater in rainy season. The TSS content and COD/BOD ratio (1.97–1.99) designate sewage a typical untreated domestic wastewater (Tchobanoglous et al. 2003). VSS/TSS ratio (42.9–45.5) shows high biodegradability of wastewater and renders it amenable to anaerobic treatment. COD to sulphate ratio of the sewage (10.8–12.4) also sufficiently meets the requirement of anaerobic digestion because anaerobic treatment is effective when the COD/sulphate ratio exceeds 10 (Pol et al. 1998). Higher sulphate contents of sewage, however, cause damage to infrastructure due to the production of sulphuric acid (Tchobanoglous et al. 2003). Alkalinity level (146 mg/L) and NH4–N contents (27 mg/L) were also similar to values for domestic wastewater reported in the literature (Seghezzo 2004).
The characteristics of sewage demarcate it a medium-strength municipal wastewater (Mahmoud 2002; Mikosz 2015). Oil and grease contents of sewage (229 mg/L) were, however, higher than medium-strength domestic wastewater, which may owe to the discharge of oily wastewater from a number of restaurants in the area. The average values of arsenic, cyanide, iron and zinc (0.1, 0.009, 0.06 and 0.1 mg/L, respectively) were within the acceptable limits for irrigation water (Ayers and Westcot 1994).
Restart of UASB reactor
The UASBCD and UASBASDIT reactors were restarted after a long shutdown period of 12 months and were kept inside the laboratory at room temperature varying from (15 to 35 °C) during the non-feeding period. The reactors were operated after 12 months at a HRT of 12 h (hrs). The UASB reactor operation parameters during restart-up are given in Table 3. Colour of sludge of both reactors was dark grey at the time of restart, and volume of sludge blanket of UASBCD and UASBASDIT was decreased by 23 and 18% of the initial, respectively, due to compaction. Figures 3 and 4 show the scanning electron microscopic view of sludge mass in UASBCD and UASBASDIT, respectively. The sludge mass morphological configuration in UASBCD was better than UASBASDIT and, therefore, showed better stimulation after substrate feeding as depicted in the scanning electron microscope analysis of sludge of UASBCD and UASBASDIT reactor.
Overall better morphological structure of UASBCDCD reactor’s sludge as compared to UASBASDIT reactor can be explained by the longer time required for sludge development, better granulation, biomass growth and settling characteristics of cow dung sludge during the start-up of these reactors. The sludge in UASBCD reactor was an inhomogeneous suspended mass in the first 3 months during start-up. After that, granulation started and was completed in the fourth month. The sludge bed in UASBASDIT reactor was merely a suspended biomass up to a period of 60 days. After that, granulation of biosolids became noticeable, which indicated successful start-up of the reactor. However, sludge granulation fully appeared after 80 days, and the quality of sludge was comparable with the well-matured sludge of a digester. The cow dung seed sludge was comprised of predominantly organic matter and heavy population of microbes. For UASBCD, VSS/TS ratio gradually increased up to sludge age of 150 days followed by a slight drop in this ratio. In case of UASBASDIT, VSS/TS ratio was lower than the VSS/TS ratio for UASBCD and it gradually increased (0.55–0.68) untill 120 days and decreased afterwards (Rizvi et al. 2015). Difference in time required for the granulation of the sludge can be due to a number of factors including dissimilarities in seed sludge, availability of micro-nutrients, availability of active microbial population in the inoculum, microbial internal storage and pH (Singh and Viraraghavan 2003; Ni et al. 2015). Predominance of active biomass is important for reducing the start-up time and yielding better removal of pollutants. A shortening of start-up time from 50 to 30 days in a UASB reactor has been reported in the literature by substituting seed sludge of domestic wastewater treatment plant with distillery waste treatment plant (Vadlani and Ramachandran, 2008).
Sludge granules are basically developed by self-granulation of microorganisms, and dynamic balance between granule expansion and decomposition results in coexistence of UASB sludges with different sizes in the reactor. Therefore, based on the physicochemical characterization, the sludge having larger granular structure gave better performance in UASB systems (Ahn et al. 2002). Adequate temperature and appropriate alkalinity are required for generation and preservation of granules. The type and strength of substrate along with intra-granular diffusion also play a vital role in determining the microstructure of the granules. The presence of cations such as calcium and iron also enhances granulation by ionic bridging and linking exo-cellular polymers (Tiwari et al. 2006; Abbasi and Abbasi 2012). Similarly, Bae et al. (1995) examined the reactivation characteristics of anaerobic sludges starved for 10 months and observed that microbial activity recovered in the reactor after refeeding. According to the specific biomass activity tests, the methanogenic activity was sustained better in room temperature as compared to refrigerated conditions. The granular sludge was quickly reactivated by refeeding the substrate, and in about 12 days, a 11.2 kg COD/m3 per day loading rate was achieved after refeeding (Bae et al. 1995). Similarly, Jin et al. (2007) demonstrated that pre-granulated seeding sludge could significantly reduce the start-up time. They operated two laboratory-scale and one pilot-scale expanded granular sludge bed (EGSB) reactor to treat wastewater from a treatment plant. The microbiological structure and particle size distribution of three types of sludge (aerobic excess sludge, sanitary landfill sludge digested for 1 year and granular sludge of EGSB reactor after 400 days of operation) were analysed through scanning electron microscopy. The laboratory-scale EGSB reactor seeded with anaerobic sludge (after digestion for 1 year in landfill) showed better COD removal efficiency than one seeded with aerobic excess sludge.
Figures 5, 6, 7, 8 and 9 show the performance of both UASB reactors after restart for the removal of COD, TSS, TDS, chloride and oil and grease content. There was found considerable fluctuation in the removal efficiency of these reactors in the initial 25–30 days for COD, TSS, TDS, chlorides and oil and grease. Figure 5 shows the COD removal of both reactors during restart-up. COD removal efficiency of UASBCD reactor varied between 50 and 81%, whereas COD removal of UASBASDIT reactor was in the range of 33–75% in the initial 28 days after restart. This may owe to the inability of sludge to cope with the COD loading. However, the reactors became stable and restored normal functioning after 30 days and COD removal efficiency of UASBCD and UASBASDIT became uniform and remained close to 80 and 78%, respectively, which may be due to better microbial activity in sludge bed (Kobayashi et al. 2009). Both reactors behaved similarly for the removal of TSS, TDS, chloride and oil/grease. The TSS removal varied between 42 and 75% for both reactors during the first 25 days and became uniform subsequently as depicted in Fig. 6. The TDS removal efficiency of both reactors varied considerably (4–22%) during the first 28–30 days and then became stable up to 65 days (Fig. 7). The removal efficiency for chlorides varied between 32 and 63.5% for both reactors within 25 days as shown in Fig. 8 and remained close to 70% from 30 to 65 days. The removal efficiency for oil and grease of the reactors varied between 58 and 87%, and it took 35 days for them to achieve consistent removal efficiency. The pattern of overall removal efficiency of both reactors with respect to reactivation time was similar. However, the performance of UASBCD in terms of removal percentage was relatively better than UASBASDIT which could be due to better composition, settling, sludge growth, granulation, biomass growth and ionic bridging and linking of exo-cellular polymers of sludge in the former reactor. The time required to achieve steady state in both reactors was considerably short, which could be due to the presence of sufficient methanogenic microbial population retained in the sludge of the reactors (Manariotis and Grigoropoulos 2008; Kobayashi et al. 2009; Dong et al. 2010).
By comparing the results of restart-up with start-up, it is clear that within 35 days both reactors achieved their pre-shutdown removal efficiencies for COD, TSS, chlorides TDS and oil and grease at 12-h HRT. During start-up, at sludge age of 150 days and 30 °C temperature, the UASBCD reactor exhibited COD, TSS and oil and grease removal up to 81, 73 and 93%, respectively. The removal of these parameters by UASBASDIT reactor was 79, 65 and 91%, respectively (Rizvi et al. 2015). The better performance of UASBCD reactor in terms of organics removal was attributed to better granulation and increased growth of biomass, which ultimately resulted into enhanced degradation of organics and removal of suspended solids (Weiland and Rozzi 1991; Yasar et al. 2007; Lew et al. 2011).
These findings are in agreement with the results reported in the literature (Sanz and Fdz Polanco 1989; Manariotis and Grigoropoulos 2002, 2008; Dong et al. 2010). For example, Sanz and Fdz Polanco (1989) reported more than 72% COD removal efficiency after 25 days of restart following a 6-month shutdown period in a UASB reactor treating municipal wastewater. According to Dong and co-workers (Dong et al. 2010), the COD removal efficiency after 4-month shutdown of a UASB reactor treating soybean processing wastewater varied significantly (71.7–89.4%) in the initial 25 days and became uniform after 35 days of operation. Manariotis and Grigoropoulos (2002), however, reported rapid reactivation of anaerobic reactor while treating low-strength wastewater at 26 °C after a 2-year shutdown period, and COD removal efficiency of 83% was achieved in the initial 10-day period after restart. Manariotis and Grigoropoulos (2008) studied the restart performance of four anaerobic reactors after 24-month shutdown and reported that a period of 2.5 months was required for these reactors to achieve the normal treatment efficiency. There is wide variation in COD removal efficiency of UASB (42–80%) reported in the literature. COD removal efficiency (80%) of UASBCD reactor obtained in this study is close to the COD removal reported by Ruiz et al. (1998) who reported COD removal varying from 73 to 80%. Whereas, Alvarez et al. (2006) achieved 58% total COD removal at a temperature of 15ºC and HRT of 11 h. Lower COD removal in their study can be explained by the low-temperature conditions. Kalogo et al. (2001) reported COD removal efficiency of up to 65% in a self-inoculated UASB reactor treating domestic wastewater. In another study, Lew et al. (2011) treated domestic wastewater in an integrated UASB–sludge digester and found COD removal efficiency ranging from 42 to 78% at temperature ranging from 10 to 28 °C. In the same way, Zhang et al. (2012) reported 75.6% COD removal efficiency of modified UASB reactor treating sewage at HRT of 10 h and temperature of 45–50 °C. COD removal in these studies ranges from 42 to 78%.
COD removal efficiency higher than 80% was reported by a few researchers. Khan et al. (2015) reported COD, BOD and TSS removal efficiencies of 65–85% in UASB reactor for domestic wastewater treatment at higher temperature (8–40 °C). Similarly, 83–85% COD removal was obtained with UASB, treating fortified municipal wastewater and high-strength synthetic sewage, respectively (Farajzadehha et al. 2012; Banihani and Field 2013).
The results of this study are comparable and better than results reported in the literature treating medium-strength raw municipal wastewater without fortification in a single-stage UASB reactor at temperature of 30 °C. The higher organics removal reported in some studies mentioned above is due to difference in operating parameters such as temperature (35–40 °C), strength of substrate, fortification of substrate, type of reactor and inoculum used, modification of reactor design and operating parameters such as organic loading rate and hydraulic retention time (Alves et al. 2000; Farajzadehha et al. 2012, Khan et al. 2015; Rizvi et al., 2015).
Methane generated from UASB reactor can be effectively used to meet partial energy requirements of the system. During restart-up, under ambient temperature conditions (25–30 °C) at hydraulic retention time (HRT) of 12 h, and corresponding average organic loading (OLR) of 0.902 kg COD/m3 per day, average methane production for UASBCD and UASBASDIT reactors was found to be 0.091 and 0.084 m3/kg COD removed, respectively, as shown in Fig. 10. Gas production was nil during the first 7 days and thereafter showed considerable variation on daily basis in both UASB reactors. The methane produced in this study was significantly lower than the theoretical yield of 0.3 m3 methane gas/kg COD removed at 25 °C. The comparatively lower amount of methane generated is attributable to substantial sulphate reduction and methane oversaturation in liquid phase (Souza et al. 2011). However, production of methane indicated that the reactor biomass was attaining active condition and reaching the methane yield of 0.110 and 0.103 m3/kg COD removed achieved in the start-up study for UASBCD and UASBASDIT reactors. Municipal wastewater normally has a low COD concentration and comparatively higher suspended solids’ concentration which leads to small specific methane yield and makes the initial hydrolysis step very important to convert the suspended solids into soluble substrate (Lew et al. 2011). The findings correspond with the results in the literature (Singh et al. 1996; Ruiz et al. 1998; Manariotis and Grigoropoulos 2002, 2008). Singh and co-workers obtained methane yield of 0.141 m3/kg COD removed while treating low-strength domestic wastewater a 20–35 °C at 3-h HRT and OLR of 4 kg COD/m3 per day. Ruiz et al. (1998) reported average methane production of 0.20 L CH4 per litre per day at an OLR of 3 g COD per litre per day, treating domestic wastewater in UASB reactor at 7-h HRT. Similarly, Manariotis and Grigoropoulos (2002) reported a methane yield of 0.102 m3/kg COD removed at 12-h HRT treating domestic wastewater in an anaerobic baffled reactor. Manariotis and Grigoropoulos (2008) reported a COD conversion to biogas ranging from 0.140 to 0.160 m3/kg COD removed at HRT of 0.3 day. The methane generation results show that the digestion process in the UASB is dependent on OLR and operational parameters and that restart-up of reactors treating municipal wastewater is feasible at mesophilic temperature.
Start-up and restart-up are very important phases of UASB reactor with economic implications. Start-up and restart-up are also considered to be the most difficult phases in anaerobic digestion and usually take several months with digested sewage sludge due to slow growth rate of anaerobic microorganisms. In the literature, there is lack of information on selection of sludge and kinetics involved during start-up (Weiland and Rozzi 1991; Show et al. 2004). The results of this study show great potential for restart-up of UASB reactor after 12-month shutdown period by shortening the restart-up time and achieving steady-state removal efficiencies for COD, TDS, TSS, chlorides and oil and grease and methane generation within 30 days by the activation of methanogenic microbial population in the biomass of the reactors.
Conclusion
The COD, BOD and TSS and COD/BOD ratio of sewage designated it a medium-strength wastewater. The VSS/TSS and COD/sulphate ratio reflected higher biodegradability and rendered it amenable to anaerobic treatment. The metal elements contents of sewage were also in the permissible limits for irrigation water. The overall performance of UASBCD reactor was always relatively better than UASBASDIT reactor due to better composition, biomass development, granulation and settling of sludge with time in the former reactor. The restart performance of anaerobic reactors after 12-month shutdown revealed that a period of 2.5 months was enough for these reactors to restore the normal treatment efficiency and use of stored anaerobic granular sludge for restart of reactors was feasible. The UASB technology provides an economical solution for the direct treatment of municipal wastewater and can be applied in small communities where the wastewater flow variation is high due to rainy season or population load is high during the tourist season and where the reactors have to face shutdown due to environmental, technical and socio-economic factors. They can also be used effectively in seasonally operated food industries that have to face shutdown repeatedly.
References
Abbasi T, Abbasi SA (2012) Formation and impact of granules in fostering clean energy production and wastewater treatment in upflow anaerobic sludge blanket (UASB) reactors. Renew Sustain Energy Rev 16:1696–1708
Ahn Y, Song YJ, Lee YJ, Park S (2002) Physicochemical characterization of UASB sludge with different size distributions. Environ Technol 23:889–897
Alvarez JA, Ruiz I, Gómez M, Presas J, Soto M (2006) Start-up alternatives and performance of an UASB pilot plant treating diluted municipal wastewater at low temperature. Bioresour Technol 97(14):1640–1649
Alves M, Cavaleiro AJ, Ferreira EC, Amaral AL, Mota M, da Motta M, Vivier H, Pons MN (2000) Characterization by image analysis of anaerobic sludge under shock conditions. Water Sci Technol 41:207–214
APHA, AWWA, WPCF (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington. pp 536–538
Ayers RS, Westcot DW (1994) Water quality for agriculture FAO irrigation and drainage, FAO Irrigation and Drainage Paper No 29 (1) FAO
Bae BU, Shin HS, Paik BC, Chung JC (1995) Re-activation characteristics of preserved anaerobic granular sludges. Bioresour Technol 53:231–235
Banihani QH, Field JA (2013) Treatment of high-strength synthetic sewage in a laboratory-scale upflow anaerobic sludge bed (UASB) with aerobic activated sludge (AS) post-treatment. J Environ Sci Health A 48(3):338–347
Board NIIR (2004) Handbook on bio gas and its applications. National Institute of Industrial Research, New Delhi, India, p 162
Cervantes FJ, Gomez R, Alvarez LH, Martinez CM, Hernandez-Montoya V (2015) Efficient anaerobic treatment of synthetic textile wastewater in a UASB reactor with granular sludge enriched with humic acids supported on alumina nanoparticles. Biodegradation 26:289–298
Chong S, Sen TK, Kayaalp A, Ang HM (2012) The performance enhancements of upflow anaerobic sludge blanket (UASB) reactors for domestic sludge treatment–a state-of-the-art review. Water Res 46:3434–3470
Dong F, Zhao QB, Zhao JB, Sheng GP, Tang Y, Tong ZH, Yu HQ, Li YY, Harada H (2010) Monitoring the restart-up of an upflow anaerobic sludge blanket (UASB) reactor for the treatment of a soybean processing wastewater. Bioresour Technol 101:1722–1726
Elmitwalli T, Otterpohl R (2011) Grey water treatment in upflow anaerobic sludge blanket (UASB) reactor at different temperatures. Water Sci Technol 64:610–617
Farajzadehha S, Mirbagheri SA, Farajzadehha S, Shayegan J (2012) Lab scale study of HRT and OLR optimization in UASB reactor for pretreating fortified wastewater in various operational temperatures. APCBEE Procedia 1:90–95
Gray NF (2010) Water technology: an introduction for environmental scientists and engineers, edn 3. IWA Publishing, Colchester, UK, pp 600
Jin WA, Zhang ZJ, Zhang ZF, Qaisar M, Zheng P (2007) Production and application of anaerobic granular sludge produced by landfill. J Environ Sci 19(12):1454–1460
Kalogo Y, Seka AB, Verstraete W (2001) Enhancing the start-up of a UASB reactor treating domestic wastewater by adding a water extract of Moringa oleifera seeds. Appl Microbiol Biotechnol 55(5):644–651
Khan AA, Mehrotra I, Kazmi AA (2015) Sludge profiling at varied organic loadings and performance evaluation of UASB reactor treating sewage. Biosyst Eng 131:32–40
Kobayashi T, Yasuda D, Li YY, Kubota K, Harada H, Yu HQ (2009) Characterization of start-up performance and archaeal community shifts during anaerobic self-degradation of waste-activated sludge. Bioresour Technol 100:4981–4988
Lettinga G (1996) Sustainable integrated biological wastewater treatment. Water Sci Technol 33(3):85–98
Levine AD, Tchobanoglous G, Asano T (1991) Size distributions of particulate contaminants in wastewater and their impact on treatability. Water Res 25:911–922
Lew B, Lustig I, Beliavski M, Tarre S, Green M (2011) An integrated UASB-sludge digester system for raw domestic wastewater treatment in temperate climates. Bioresour Technol 102(7):4921–4924
Mahmoud N (2002) Anaerobic pre-treatment of sewage under low temperature (15 °C) conditions. Wageningen University, The Netherlands
Manariotis ID, Grigoropoulos SG (2002) Low-strength wastewater treatment using an anaerobic baffled reactor. Water Environ Res 74:170–176
Manariotis ID, Grigoropoulos SG (2006) Municipal-wastewater treatment using upflow-anaerobic filters. Water Environ Res 78:233–242
Manariotis ID, Grigoropoulos SG (2008) Restart of anaerobic filters treating low-strength wastewater. Bioresour Technol 99:3579–3589
Mikosz J (2015) Determination of permissible industrial pollution load at a municipal wastewater treatment plant. Int J Environ Sci Technol 12:827–836
Ni BJ, Batstone D, Zhao BH, Yu HQ (2015) Microbial internal storage alters the carbon transformation in dynamic anaerobic fermentation. Environ Sci Technol 15:9159–9167
Pol LWH, Lens PN, Stams AJ, Lettinga G (1998) Anaerobic treatment of sulphate-rich wastewaters. Biodegradation 9:213–224
Reynolds TD, Richards PA (1996) Unit operations and processes in environmental engineering, 2nd edn. PWS Publishing Co., Boston
Rizvi H, Ahmad N, Abbas F, Bukhari IH, Yasar A, Ali S, Yasmeen T, Riaz M (2015) Start-up of UASB reactors treating municipal wastewater and effect of temperature/sludge age and hydraulic retention time (HRT) on its performance. Arab J Chem 8:780–786
Ruiz I, Soto M, Veiga MC, Ligero P, Vega A, Blàzquez R (1998) Performance of and biomass characterisation in a UASB reactor treating domestic waste water at ambient temperature. Water SA 24(3):215–222
Sanz I, Fdz Polanco F (1989) Anaerobic treatment of municipal sewage in UASB and AFBR reactors. Environ Technol 10(5):453–462
Seghezzo L (2004) Anaerobic treatment of domestic wastewater in subtropical regions. Ph. D. thesis, Wageningen University, Wageningen, The Netherlands
Shieh WY, Chen AL, Chiu HH (2000) Vibrio aerogenes sp a facultatively anaerobic marine bacterium that ferments glucose with gas production. Int J Syst Evol Microbiol 50:321–329
Show KY, Wang Y, Foong SF, Tay JH (2004) Accelerated start-up and enhanced granulation in upflow anaerobic sludge blanket reactors. Water Res 38(9):2293–2304
Singh KS, Viraraghavan T (2003) Impact of temperature on performance, microbiological, and hydrodynamic aspects of UASB reactors treating municipal wastewater. Water Sci Technol 48(6):211–217
Singh KS, Harada H, Viraraghavan T (1996) Low-strength wastewater treatment by a UASB reactor. Bioresour Technol 55(3):187–194
Souza CL, Chernicharo CAL, Aquino S (2011) Quantification of dissolved methane in UASB reactors treating domestic wastewater under different operating conditions. Water Sci Technol 64(11):2259–2264
Tchobangolous G, Burton FL, Stensel HD (eds) (2003) Constituents in wastewater. In: Wastewater engineering: treatment and reuse, 4th edn. McGraw-Hill, New York, pp 27–137
Tiwari MK, Guha S, Harendranath CS, Tripathi S (2006) Influence of extrinsic factors on granulation in UASB reactor. Appl Microbiol Biotechnol 71:145–154
Vadlani PV, Ramachandran KB (2008) Evaluation of UASB reactor performance during start-up operation using synthetic mixed-acid waste. Bioresour Technol 99(17):8231–8236
Van Haandel AC, Lettinga G (1994) Anaerobic sewage treatment: a practical guide for regions with a hot climate. Wiley, New York
Van Lier JB, Lettinga G (1999) Appropriate technologies for effective management of industrial and domestic waste waters: the decentralised approach. Water Sci Technol 40:171–183
Weiland P, Rozzi A (1991) The start-up, operation and monitoring of high-rate anaerobic treatment systems: discusser’s report. Water Sci Technol 24(8):257–277
Xing BS, Guo Q, Zhang ZZ, Zhang J, Wang HZ, Jin RC (2014) Optimization of process performance in a granule-based anaerobic ammonium oxidation (anammox) upflow anaerobic sludge blanket (UASB) reactor. Bioresour Technol 170:404–412
Yasar A, Ahmad N, Chaudhry MN, Khan AA (2007) Sludge granulation and efficiency of phase separator in UASB reactor treating combined industrial effluent. J Environ Sci China 19:553–558
Zhang SJ, Liu NR, Zhang CX (2012) Study on the performance of modified UASB process treating sewage. Adv Mater Res 610:2174–2178
Zhao BH, Mu Y, Dong F, Ni BJ, Zhao JB, Sheng GP, Yu HQ, Li YY, Harada H (2010) Dynamic modeling the anaerobic reactor startup process. Ind Eng Chem Res 49:7193–7200
Acknowledgements
The authors are highly thankful to the Higher Education Commission (HEC), Islamabad, Pakistan, for the financial support under the indigenous Ph. D. Fellowship Program (Grant No: 042-120874Ls2-344).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: V.K. Gupta.
Rights and permissions
About this article
Cite this article
Rizvi, H., Ali, S., Yasar, A. et al. Applicability of upflow anaerobic sludge blanket (UASB) reactor for typical sewage of a small community: its biomass reactivation after shutdown. Int. J. Environ. Sci. Technol. 15, 1745–1756 (2018). https://doi.org/10.1007/s13762-017-1537-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1537-2