Abstract
A total of 422 bacterial isolates were obtained from the lead (Pb) ore in north-eastern Iran. The Pb tolerances of these strains were studied using microbroth serial dilution approach and 35 strains could grow up to 3250 ppm Pb concentration. Of these strains, 10 of them represented qualitatively high levels of Pb adsorption and were selected for quantitative studies. Strain AS2 which is phylogenetically related to genus Bacillus showed the highest level of Pb remediation. The effects of different factors, including pH, initial Pb concentration, temperature and inoculum size, were studied on the remediation process. Pb remediation capacity was reached at 74.5 mg/g (99.5 % of initial Pb) at pH 4.5, temperature 30 °C, inoculum size 1.0 % (v/v) and an initial Pb concentration of 500 ppm after 24 h. Pb desorption capacity of strain was 66 %. The novel isolate could remove 98 % of Pb from the contaminated industrial wastes after 24 h. Pb uptaking to the cell surface was proven using scanning electron microscopic micrograph and energy-dispersive X-ray spectroscopy analysis. Most Pb removal efficiency was observed in the active cell culture as compared to the inactive cell and extracellular polymeric substances. The novel strain represents a good candidate for removal of environmental anthropogenic Pb pollutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a non-essential heavy metal. It causes serious health hazards, that is, permanent brain damage, learning disabilities, hearing loss, anaemia, insomnia, headache, dizziness, irritability, weakness of muscles, renal damages and heart disease (Naseem and Tahir 2001). Industrial activities, such as production of batteries, pigments and metal smelting, as well as manufacturing of products, such as Pb arsenate insecticides or Pb water pipes, are the main anthropogenic sources of Pb (Jarosławiecka and Piotrowska-Seget 2014; Ismail et al. 2013). Accordingly, large volumes of Pb-containing wastes from these industries are introduced into environments. To protect ecosystems, wastewaters must be treated to reduce Pb to below 0.05–0.10 ppm before discharging into the environment (Saǧ et al. 1995). Different physical and chemical methods have been applied to treat Pb-containing wastes. These methods include adsorption, flotation, ion exchange, membrane filtration and precipitation (Chen et al. 2009; Kabbashi et al. 2009; Landaburu-Aguirre et al. 2009; Zhang et al. 2009). The physico-chemical methods are generally expensive, so the need for hazardous reagents produces other toxic chemicals and requires long treatment time. The biological systems apply a process called bioremediation to protect environments from toxic effects of hazardous components. Since the elemental form of Pb is toxic, it cannot be degraded or transformed (biotransformation/biodegradation) into a less dangerous material. However, the living systems (like bacteria) can precipitate bioavailable soluble and toxic Pb to insoluble forms (biosorption) and reduce drastically its availability and toxicity. The major advantages of the biosorption approach are its high effectiveness in reducing the heavy metal ions and the low cost of the process (Macek and Mackova 2011). Various groups of microorganisms like Bacillus cereus, Arthrobacter species, Corynebacterium species (Roane and Kellogg 1996; Trajanovska et al. 1997; Zanardini et al. 1997), Pseudomonas marginalis, Pseudomonas vesicularis, Enterobacter species (Hasnain et al. 1993; Trajanovska et al. 1997), Saccharomyces cerevisiae and Penicillium species (Chen and Wang 2007; Sun and Shao 2007) were reported for their ability to remediate Pb contaminations using the adsorption method.
Heavy metals like Pb are found in the environments as the natural sources (geogenic) of parent rocks and metallic minerals (metalliferous ores) (Sparks 2005). The long-time contacts of microbial populations with this element in the contaminant regions result in the enrichments of resistant strains by the act of natural selection. Accordingly, these indigenous species are appropriate candidates for bioremediation of anthropogenic (Human made) pollutions. This study describes the isolation of Pb-tolerant and absorbing bacteria from Pb ore, and the influence of various biosorption-related variables, such as pH, inoculums size, temperature and initial Pb concentration, was studied on the selected strain. The ability of novel strain to remediate industrial Pb-containing wastes was also taken into account.
Materials and methods
Site description, samples collection and analysis
Samples were collected from different sites of Tarik Darreh Pb Ore in north-eastern Iran (35°21′–35°28′N, 60°40′–60°49′E). The samples including soil, water and sediment were collected in sterile plastic containers and kept in the dark at an environmental temperature for a few hours before being analysed in the laboratory. Anions and cations were analysed using titration and atomic absorption methods (atomic absorption spectroscopy, AAS), respectively (Maiti 2004; Saad et al. 1998). Pb concentration was analysed using AAS-7000 (Shimadzu, Japan).
Culture media and growth conditions
Bacteria were isolated under aerobic conditions on Luria–Bertani (LB) agar (Merck). The pH of the medium was adjusted at pH 5.0 by 2 M HNO3. All samples were serially diluted up to 10−6 and plated by spreading method. The plates were incubated at different temperatures of 20, 30 and 40 °C for 2 weeks. Pure isolates were obtained after successive cultivation. The isolates were maintained on the slant LB medium at 4 °C and LB broth medium supplemented with 30 % (v/v) glycerol at −80 °C for short and long preservations, respectively.
Minimum inhibitory concentration (MIC) determination
Since Pb chemically precipitates in alkaline condition (Leung et al. 2001), the pH was adjusted to acidic condition (pH < 5.0) with 2 M HNO3 in all Pb relative experiments. MIC determination was conducted in LB liquid medium by broth microdilution method. It was performed with 96-well, round-bottom microtiter plate. The final volume of wells was 200 μl. Each plate included positive control (bacteria without Pb), negative control (medium only) and serial twofold dilutions of Pb in LB medium. Bacterial inoculum was produced from mid-log culture in Pb-free LB medium. After the addition of a 100 μl inoculum containing 2 × 106 cells/ml to Pb-containing positive control wells, the plates were incubated at 30 °C. Bacterial growth was determined by measuring the turbidity at 600 nm. The lowest concentration of Pb which inhibited the bacterial growth was determined as the MIC of the isolate.
Quantitative and qualitative screening for Pb-absorbing bacteria
In order to evaluate the biosorptive potential of Pb-resistant bacteria, a rapid agar screening method was used, which was introduced by Pümpel et al. (1995). Briefly, bacterial strains were punctually inoculated in Pb-free LB agar and incubated for 48–72 h at 30 °C. The petri dishes were then overlaid by soft agar containing 500 ppm Pb and incubated for 24 h at 30 °C. Afterwards, Pb removal from solid media by microorganisms was visualized by exposing each plate to H2S vapours, which was generated in situ by reacting sodium sulphide with hydrochloric acid in a stoichiometric ratio. Precipitation of lead sulphide (PbS) and halo formation around the colonies were considered for Pb biosorption capacity. For quantitative measurement of Pb adsorption, the liquid LB medium was supplemented with 100 ppm Pb concentration. Cultures were grown in 150 rpm at 30 °C for 24 h. After the incubation, supernatant was collected and Pb absorption was determined using AAS. Cultures containing 100 ppm Pb without inoculum were applied as negative control.
Factors affecting removal process
To understand the effects of various conditions on Pb removal, growth conditions or chemical components were varied one at a time. Organisms were cultured in 25 ml LB medium containing Pb as PbNO3 in 100-ml Erlenmeyer flasks incubated aerobically at 150 rpm in an orbital shaker. These variables consisted of various pH of the medium which was adjusted to pH 3.0, 3.5, 4.0, 4.5 and 5.0; initial Pb concentrations of 50, 100, 200, 300, 400, 500, 600 and 700 ppm; different incubation temperatures of 20, 25, 30, 35 and 40 °C; various inoculum sizes of 0.5, 1.0, 3.0, 5.0 and 7.0 % (v/v); and incubation time up to 36- and 2-h intervals. Residual Pb was measured using AAS.
Biological removal efficiency (BRE) and biological removal capacity (BRC) were calculated as shown in the following formula:
Each experiment was carried out in three independent batches in triplicates (nine replications). The standard error of the mean was calculated and was shown as error bars on the figures. One-way analysis of variance (ANOVA) and Tukey’s honest significant difference (HSD) test were performed using Statistical Package for Social Science (SPSS) ver. 16 software (IBM Co).
Comparing active–inactive cells and extracellular polymeric substances (EPS) in removal process
Fresh culture media of selected strain were prepared and divided into three parts. Two parts were inactivated by autoclave and treatment by 1 mM sodium azide (Johnson et al. 2007), respectively. All the autoclaved (inactive), sodium azide treatment (inactive) and living cells (active) were harvested by centrifugation at 7500g for 10 min. To determine whether bacterial exopolysaccharides are involved in bioremediation, the EPS of the bacterial culture was extracted as described by Zhang et al. (2002). The ability of active and inactive cells as well as extracted exopolysaccharide to uptake Pb was measured as mentioned in “Quantitative and qualitative screening for Pb-absorbing bacteria” section.
Scanning electron microscopic (SEM) and energy-dispersive X-ray spectroscopy (EDS) analysis
Samples for SEM studies were harvested from LB liquid culture containing 500 ppm concentration of Pb at the optimum condition. Cells pellets were freeze-dried and coated with gold using a sputter coater (EmscopeSc 500). SEM analysis was performed using a LEO.1450.VP/REM (Oberkochen-Zeiss, Germany). EDS elemental analysis was carried out on non-treated bacterial cells as well as bacterial cells interacted with Pb using an ESEM FEI Quanta 200, high-resolution electron microscope.
Measurement of Pb desorption capacity
Bacterial cells were harvested from adsorption experiments and washed with distilled water to remove unbanned Pb(II). The biomass was transferred to desorbent solution containing HNO3, Ca(NO3)2 and ethylenediaminetetraacetic acid (EDTA; 0.10 and 0.01 M, respectively), and the solution was shaken for 18 h. The biomass was collected and analysed to determine the concentration of Pb(II) after desorption (Deng et al. 2007). Desorption capacity was calculated as follows:
Removal of anthropogenic Pb contamination in batch fermentation
Remediation of Pb-contaminated wastes from local batteries and dye industries was carried out in a 1 L batch fermentation system with waste Pb added at 500 ppm concentration to the optimal removal of LB culture. Residual Pb was measured using AAS.
Molecular identification of the strain
The genomic DNA of the strain was extracted using DNA extraction kit (Thermo scientific, Lithuania) according to the manufacturer’s recommended procedure, and the 16S rRNA gene was amplified using the bacterial universal primers 27F and 1492R (Lane et al. 1985). Amplification reactions contained 1.5 μl of each primer, 1 μl of 10 mM deoxynucleotide (dNTP), 5 μl polymerase chain reaction (PCR) buffer, 1.5 μl of 50 mM MgCl2, 4 μl template DNA, 0.25 μl DNA polymerase and 35.25 μl dH2O, in a final volume of 50 μl. The following conditions were used in the amplification of 16S rRNA gene: 94 °C for 2 min, followed by 30 cycles of 94 °C for 60 s, 55 °C for 60 s and 72 °C for 60 s, with a final 7-min extension at 72 °C. PCR products were purified with the DNA purification kit (Bioneer, South Korea), according to the manufacturer’s protocol. The sequencing was conducted on an ABI 3730XL DNA sequencer at Macrogen (Seoul, South Korea).
Results and discussion
Chemical characterization of sample and isolation of bacteria
Several water, soil and sediment samples from Tarik Darreh Ore were obtained and used in this study (pH of the samples was neutral to slightly alkaline (7.3–7.8). The Pb concentration in the soil samples was 400 mg kg−1. Concentration of major cations and anions was as follows (mg/L): Ca2+ (80), Mg2+ (22.4), Na+ (47.5), K+ (5.2), HCO3 − (317.6), SO4 2− (53.4) and Cl− (32.8). The LB agar-isolating medium yielded high numbers of colonies, and the range was 2.5 × 106–4.4 × 106 CFU/ml. A total of 422 pure isolates were obtained by successive cultivation. These strains were characterized as Gram-positive cocci (33 isolates), Gram-negative cocci (10 isolates), Gram-positive bacilli (285 isolates) and Gram-negative bacilli (94 isolates).
MIC determination and selection of Pb-absorbing bacteria
A total of 36 strains could not tolerate the minimum amount of Pb and only grew in the absence of Pb. The MIC for other strains was as follows: 100 ppm (40 strains), 500 ppm (34 strains), 1000 ppm (48 strains), 1500 ppm (79 strains), 2000 ppm (97 strains) and 3000 ppm (52 strains). A total of 35 strains showed the highest Pb tolerance and could grow up to 3125 ppm initial Pb concentration. Among the last group, eight strains showed the highest qualitative Pb absorption (Fig. 1) and were selected for further quantitative analysis. One strain was designated as AS2 that uptakes remarkable amount of initial Pb concentration (BRE 87 %; BRC 109 mg/g) and was selected for optimization studies. Geogenic contaminated sites were reported to be good sources for heavy metal tolerance microbes with potential applications in bioremediation. Arsenic-, cadmium-, copper- and zinc-resistant bacteria were isolated from gold, lead–zinc, copper and silver mine soils, respectively (Li et al. 2013; Piotrowska-Seget et al. 2005; Zhu et al. 2012). Heavy metal tolerance of the microbial community in these environments could be acquired by genetically tolerance elements (genes involving in metal resistance are mostly presented in plasmids) and shift in species composition (Hu et al. 2007). More than 90 % of bacterial strains obtained from Pb ore in this study could tolerate at least 100 ppm Pb. There are a wide range of Pb tolerances in scientific reports: Pseudomonas aeruginosa ASU 6a (Gabr et al. 2008) was able to survive under the maximum range of Pb concentration up to 500 ppm, Entrococcus faecum Pb12 tolerates 800 ppm Pb (Bhakta et al. 2012), and Achromobacter species TL-3 isolated from activated sludge samples tolerates 1500 ppm Pb in Luria broth medium (Batta et al. 2013). The novel strain AS2 which was isolated from Pb ore in this study showed the maximum MIC to be higher than previous report and could survive up to 3125 ppm Pb.
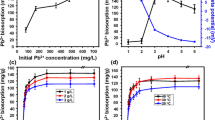
Factors affecting removal process
As shown in Fig. 2, a biological removal efficiency of 100 ppm initial Pb was 87, 90, 95, 90 and 86 % at pH 3.0, 3.5, 4.0, 4.5 and 5.0, respectively. Bacterial growth was increased as the pH of the medium increased. Pb removal capacities for the selected strain were (mg/g): 13.1, 13.6, 14.4, 13.5 and 12.8 at pH 3.0, 3.5, 4.0, 4.5 and 5.0, respectively. Pb adsorption was best at pH 4.0 (p < 0.05) for strain AS2, and this pH was applied in the following experiments. Concentration of hydrogen ion (pH) has significant influence on Pb removal capacity. Under pH condition of above 6, the accumulation of hydroxyl group (OH−) results in the chemical precipitation of Pb as Pb(OH)2 and misunderstanding the bioremoval capacity. In contrast, high acidic condition (pH < 2) decreased the removal capacity as a result of competition for negatively charged binding sites between heavy metal cation and protons (H+). However, the optimal pH condition is also depending on the metal-accepting groups (if any) on the bacterial surface. For example, phosphoryl groups are mainly in unprotonated form already at pH 2 (pKa ≈ 1.5), so bacteria with this uptaking groups can remove Pb at lower pH (Halttunen et al. 2007). Strain AS2 removed maximum Pb at pH 4.5 and postulated that it applied binding groups to higher pKa than phosphoryl groups. Strain AS2 adsorbed 91, 92, 95, 96, 97, 98, 93 and 85 % of Pb at the initial concentrations of 50, 100, 200, 300, 400, 500, 600 and 700 ppm, respectively. However, increase in Pb concentration results in reduction of bacterial growth. The corresponding performances of removal capacity were (mg/g): 7.0, 14.2, 28.8, 43.6, 58.8, 73.5, 84.1 and 80.0 at a mentioned concentration of Pb, respectively (Fig. 2b). For removal efficiency, the highest bioremediation rate was observed at 500 ppm Pb concentration and was applied for further experiments. It could be explained that at low metal concentration, the biosorption of the biosorbents is not fully utilized. In contrast, the existence of infinite heavy metal concentration reduces the efficiency due to toxicity towards cells. Figure 2c represents the BRE amounts for strain AS2 as 96, 98.2, 98.6 and 98 % at temperature 20, 25, 30 and 35 °C, respectively, while the BRC were (mg/g): 73.0, 73.5, 73.9 and 73.9 at these temperatures, respectively. Pb removal was not different between 25 and 35 °C (p < 0.05). However, environmental temperature (about 30 °C) is more desirable for in situ remediation process (Vijayaraghavan and Yun 2008) and was selected for further studies. Strain AS2 removed 98, 99.5, 93, 86 and 84 % of the initial Pb at inoculum sizes (% v/v) 0.5, 1.0, 3.0, 5.0 and 7.0, respectively. The removal capacities for these variables were (mg/g) 73.8, 74.5, 70.2, 64.5 and 63.3, respectively. The highest bioremediation rate was observed at the 1.0 % (v/v) inoculum size (Fig. 2d). As shown in Fig. 2e, by increasing the incubation time up to 24 h, the amount of removal efficiency and capacity was increased, but incubation time more than 24 h did not affect the rate of remediation. Most remediation was observed in the mid-log cells.
Role of active, inactive and EPS on Pb remediation/SEM observation and EDS analysis
Active cells showed the highest biological removal efficiency (99.5 %). The BRE amounts for autoclaved inactive cells, sodium azide inactive cells and EPS were 67, 63 and 87 %, respectively. A SEM image (Fig. 3) depicts Pb-treated strain AS2 cells, indicating that the bacteria retain their shape and are visually unaffected after the Pb treatment. The image demonstrated exopolysaccharide around the cells adhesion zone to trap the Pb. EDS elemental analysis was carried out on bacterial cells before and after interaction with Pb. Figure 4a depicts the spectrum of pure bacterial cells, and Fig. 4b shows the presence of Pb. Metal ion can be observed in Pb-treated bacteria. Several mechanisms have been proposed to be involved in Pb biosorption: binding to phosphate, hydroxyl carbonyl and amino groups of peptidoglycan (Cabuk et al. 2006); teichoic acid and lipopolysaccharides (Beveridge and Fyfe 1985) of cell envelope; binding of toxic cation to extracellular polymers (like polysaccharides) (Pérez et al. 2008); precipitating Pb(II) inside/outside the cell in the form of an unusual phosphate compound Pb9(PO4)6 (Naik and Dubey 2011); binding of Pb(II) by specific proteins like metallothioneins; and finally extracellular enzymes, such as superoxide dismutase, can also be engaged in Pb(II) biosorption (So et al. 2001). The dependence of pH as well as EDS analysis in our work indicates that ion exchange is probably at least partly responsible for the observed metal binding. However, involvement of EPS was also confirmed by SEM micrograph and the fact that 67 % of the initial Pb could be absorbed in the cell-free polysaccharide extract. It was also hypothesized that Pb removal by strain AS2 is also an enzymatic dependent process, because the remediation rate decreased drastically in the inactive cells.
Remediation of Pb from industrial waste
Remediation of contaminated waste obtained from batteries and dye industries was analysed in a 1 L batch fermentation system. The concentration of Pb in these samples was as high as 1100 ppm. Twofold dilutions of these wastes were added to remediation culture at optimum condition. The bacterial culture was able to reduce 500 ppm initial Pb concentration to less than 10 ppm (98.4 % removal efficiency) in 24 h. High anthropogenic removal efficiency by the culture of this strain as well as more than 66 % adsorption capacity appears to give desirable results for removal of Pb contamination from the environments.
Identification of Pb-adsorbing bacteria
The 16S rRNA gene sequences of strain AS2 were obtained (GenBank/EMBL/DDBJ accession number KT943756). Gene sequence analysis revealed strain AS2 to be a member of the genus Bacillus. The closest relative of this strain was Bacillus sonorensis NBCR101234T, with a 16S rRNA gene sequence similarity of 99.5 %.
Conclusion
A novel indigenous bacterium from geogenic contaminated site with very high resistance to Pb (3125 ppm) and high biosorption activity (99.5 % of initial Pb) was isolated. This strain probably uptakes Pb via different pathways. Pb concentration, pH and inoculum size are the most effective variables on the remediation process. Anthropogenic Pb removal by the culture of this strain resulted in more than 98.4 % remediation of the initial Pb. Application of this strain with more than 66 % adsorption capacity appears to give desirable results for removal of Pb contamination from the environments.
References
Batta N, Subudhi S, Lal B, Devi A (2013) Isolation of a lead tolerant novel bacterial species, Achromobacter sp. TL-3: assessment of bioflocculant activity. Indian J Exp Biol 51:1004–1011
Beveridge TJ, Fyfe WS (1985) Metal fixation by bacterial cell walls. Can J Earth Sci 22:1892–1898
Bhakta JN, Munekage Y, Ohnishi K, Jana BB (2012) Isolation and identification of cadmium- and lead-resistant lactic acid bacteria for application as metal removing probiotic. Int J Environ Sci Technol 9:433–440
Cabuk A, Akar T, Tunali S, Tabak O (2006) Biosorption characteristics of Bacillus sp. ATS-2 immobilized in silica gel for removal of Pb(II). J Hazard Mater 136:317–323
Chen C, Wang J (2007) Response of Saccharomyces cerevisiae to lead ion stress. Appl Microbiol Biotechnol 74:683–687
Chen QY, Luo Z, Hills C, Xue G, Tyrer M (2009) Precipitation of heavy metals from wastewater using simulated flue gas: sequent additions of fly ash, lime and carbon dioxide. Water Res 43:2605–2614
Deng LP, Su YY, Su H, Wang XT, Zhu XB (2007) Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J Hazard Mater 143:220–225
Gabr RM, Hassan SHA, Shoreit AAM (2008) Biosorption of lead and nickel by living and non-living cells of Pseudomonas aeruginosa ASU 6a. Int Biodeterior Biodegrad 62:195–203
Halttunen T, Salminen S, Tahvonen R (2007) Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int J Food Microbiol 114:30–35
Hasnain S, Yasmin S, Yasmin A (1993) The effects of lead resistant Pseudomonads on the growth of Triticum aestivum seedlings under lead stress. Environ Pollut 81:179–184
Hu Q, Qi HY, Zeng JH, Zhang HX (2007) Bacterial diversity in soils around a lead and zinc mine. J Environ Sci 19:74–79
Ismail Z, Salim K, Othman SZ, Ramli AH, Shirazi SM, Karim R, Khoo SY (2013) Determining and comparing the levels of heavy metal concentrations in two selected urban river water. Measurement 46:4135–4144
Jarosławiecka A, Piotrowska-Seget Z (2014) Lead resistance in micro-organisms. Microbiology 160:12–25
Johnson KJ, Ams DA, Wedel AN, Szymanowski JES, Weber DL, Schneegurt MA, Fein JB (2007) The impact of metabolic state on Cd adsorption onto bacterial cells. Geobiology 5:211–218
Kabbashi NA, Atieh MA, Al-Mamun A, Mirghami MES, Alam MDZ, Yahya N (2009) Kinetic adsorption of application of carbon nanotubes for Pb(II) removal from aqueous solution. J Environ Sci 21:539–544
Landaburu-Aguirre J, García V, Pongrácz E, Keiski RL (2009) The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: statistical design of experiments. Desalination 240:262–269
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA 82:6955–6959
Leung WC, Chua H, Lo W (2001) Biosorption of heavy metals by bacteria isolated from activated sludge. Appl Biochem Biotechnol 91:171–184
Li L, Liu H, Shi Z, Wang G (2013) Sphingobium cupriresistens sp. nov., a copper-resistant bacterium isolated from copper mine soil, and emended description of the genus Sphingobium. Int J Syst Evol Microbiol 63:604–609
Macek T, Mackova M (2011) Potential of biosorption technology. In: Kotrba P, Mackova M, Macek T (eds) Microbial biosorption of metals. Springer, London, pp 1–17
Maiti SK (2004) Handbook of Methods in Environmental Studies Vol. 1: Water and Wastewater Analysis, 2nd edn. ABD Publishers
Naik MM, Dubey SK (2011) Lead-enhanced siderophore production and alteration in cell morphology in a Pb-resistant Pseudomonas aeruginosa strain 4EA. Curr Microbiol 62:409–414
Naseem R, Tahir SS (2001) Removal of Pb (ii) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res 16:3982–3986
Pérez MPJA, García-Ribera R, Quesada T, Aguilera M, Ramos-Cormenzana A, Monteoliva-Sánchez M (2008) Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J Microbiol Biotechnol 24:2699–2704
Piotrowska-Seget Z, Cycoń M, Kozdrój J (2005) Metal-tolerant bacteria occurring in heavily polluted soil and mine spoil. Appl Soil Ecol 28:237–246
Pümpel T, Pernfub B, Pigher B, Diels L, Schinner F (1995) A rapid screening method for the isolation of metal-accumulating microorganisms. J Ind Microbiol 14:213–217
Roane TM, Kellogg ST (1996) Characterization of bacterial communities in heavy metal contaminated soils. Can J Microbiol 42:593–603
Saad B, Pok FW, Sujari ANA, Saleh MI (1998) Analysis of anions and cations in drinking water samples by Capillary Ion Analysis. Food Chem 61:249–254
Saǧ Y, Özer D, Kutsal T (1995) A comparative study of the biosorption of lead (II) ions to Z. ramigera and R. arrhizus. Process Biochem 30:169–174
So NW, Rho JY, Lee SY, Hancock IC, Kim JH (2001) A lead-absorbing protein with superoxide dismutase activity from Streptomyces subrutilus. FEMS Microbiol Lett 194:93–98
Sparks DL (2005) Toxic metals in the environment: the role of surfaces. Elements 1:193–197
Sun F, Shao Z (2007) Biosorption and bioaccumulation of lead by Penicillium sp. Psf-2 isolated from the deep sea sediment of the Pacific Ocean. Extremophiles 11:853–858
Trajanovska S, Britz ML, Bhave M (1997) Detection of heavy metal ion resistance genes in gram-positive and gram-negative bacteria isolated from a lead-contaminated site. Biodegradation 8:113–124
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Zanardini E, Andreoni V, Borina S, Cappitellia F, Daffonchio D, Talottaa P, Sorlinia C, Ranallib G, Brunic S, Cariatic F (1997) Lead-resistant microorganisms from red stains of marble of the Certosa of Pavia, Italy and use of nucleic acid based techniques for their detection. Int Biodeter Biodegrad 40:171–182
Zhang J, Wang R, Jiang P, Liu Z (2002) Production of an exopolysaccharide bioflocculant by Sorangium cellulosum. Lett Appl Microbiol 34:178–181
Zhang Q, Pan B, Zhang W, Pan B, Lv L, Wang X, Wu J, Tao X (2009) Selective removal of Pb(II), Cd(II), and Zn(II) ions from waters by an inorganic exchanger Zr(HPO3S)2. J Hazard Mater 170:824–828
Zhu H, Guo J, Chen M, Feng G, Yao Q (2012) Burkholderia dabaoshanensis sp. nov., a heavy-metal-tolerant bacteria isolated from Dabaoshan mining area soil in China. PLoS One 7:1–6
Acknowledgments
This work was supported by grant from Ferdowsi University of Mashhad (23889/3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cephidian, A., Makhdoumi, A., Mashreghi, M. et al. Removal of anthropogenic lead pollutions by a potent Bacillus species AS2 isolated from geogenic contaminated site. Int. J. Environ. Sci. Technol. 13, 2135–2142 (2016). https://doi.org/10.1007/s13762-016-1023-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1023-2