Abstract
A lead resistant fungus was isolated from the Pacific sediment. It was associated with Penicillium according to its partial sequences of 18S and ITS. The fungus could grow in the presence of 24 mM Pb(NO3)2 in a liquid medium, and no growth inhibition was observed at 4 mM and below. When growing in the presence of 4 mM Pb(NO3)2, the fungus accumulated a large amount of lead granules in the cell, as well as adsorbed on the outer layer of cell wall, as observed under a transmission electron microscope. The intracellular lead deposited either in the vicinity of the cytoplasm membrane or in the vacuoles, and also could aggregate into large particles in the cytoplasm. However, lead was not adsorbed on the thick inner wall of the fungus. Energy dispersive X-ray spectroscopy analysis showed that these granules or particles mainly consisted of lead, and other elements could hardly be detected. Selected area electron diffraction analysis showed that there were regular crystalline lattices in the lead precipitates, indicating that they were actually in the form of crystals to some extent. Therefore, both intracellular bioaccumulation and extracellular biosorption had contributed to the high resistance of this fungus to lead. These results suggest that this fungus can be used in biotreatment as a lead trapper.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead is one of the nonessential heavy metals, a group which also include Ag, Cd, Au and Hg. It is detrimental to all organisms even at trivial levels, potentially due to ligand interactions with large biomolecules, such as by inactivation of a series of enzymes. On the other hand, both bacteria and fungi are found to be resistant to lead, although the cases are not as frequently reported as for Cr, Cd and Hg-resistant microorganisms. Ralstonia metallidurans CH34 is the most deeply investigated metal resistant bacterium; its resistance to lead is mediated by a P-type ATPase which can transport lead out of the cell (Diels et al. 1989; Borremans et al. 2001; Mergeay et al. 2003). Other bacteria such as Arthrobacter sp. (Trajanovska, et al., 1997), Pseudomonas marginalis, Bacillus megaterium (Roane, 1999), Staphylococcus aureus and Citrobacter freundii (Levinson, et al, 1996; Levinson and Mahler, 1998) are also reported to be lead resistant, but were not so thoroughly investigated as R. metallidurans CH34.
Compared to bacteria, lead resistant fungi are much less investigated (Bååth 1989; Ngu et al. 1998; Dursun et al. 2003; Zucconi et al. 2003). Fungal biosorption of heavy metals are mostly investigated with dead biomass by far. However, bioaccumulation with living cells is recognized to have irreplaceable advantages in the removal of heavy metals, especially from organic sewage (Malik 2004). Therefore, highly resistant microorganisms and a better understanding of metal-microbe interactions will facilitate the application of this technology.
Deep sea is the least explored area on the earth, where microbial resources are still mostly in the dark. In this report, we isolated a fungus of high resistance to lead (II) from the deep sea sediment of the West Pacific Ocean. Its resistance to lead was attributed to both extracellular adsorption and intracellular accumulation of this metal, which showed a strict preference for certain subcellular structures.
Isolation of lead resistant microorganisms
The lead resistant fungus was isolated from the deep sea sediment of the west Pacific at the site of E147° 8.9206′, N 12° 59.9060′, with water depth of 4,480 m. Lead resistant microorganisms were enriched with 2 mM Pb(NO3)2 as selective pressure in HLB medium. HLB contains 3% NaCl, 0.5% yeast extracts and 1% Tryptone, prepared with distilled water (pH 7.3). Phylogenetic analyses of ITS1-5.8S-ITS2 and 18S of this fungus were performed as described previously (Shao and Sun 2007). The Blastn results of 18S partial sequence (564 bp) showed that it had 99% homology with species of different genera, such as Penicillium, Eupenicillium, Thysanophora, Talaromyces and so on. Certainly, most fungi on the list belonged to Penicillium. The Blastn result of ITS (599 bp) confirmed that this fungus belonged to Penicillium. However, it cannot be affiliated with any specific species because there are several species of Penicillium of 89% identity with the ITS sequence of Psf-2. They are Penicillium commune, P. chrysogenum, P. camemberti, P. aurantiovirens, and so on. Therefore, this fungus is possibly a new species of Penicillium. It was tentatively named Penicillium sp. Psf-2, and deposited in the Marine Culture Collection Center of China with accession number MCCC 3A00002. The sequences of 18S and ITS1-5.8S-ITS2 of this fungus have been deposited in GenBank with accession numbers of EF660440 and EF660439, respectively.
Resistance of this fungus to lead
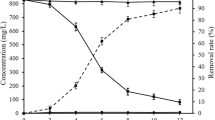
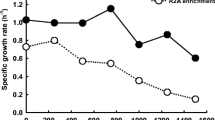
The resistance of Psf-2 against lead was tested in the range from 0 to 24 mM Pb(NO3)2, specifically at 2, 4, 8 and 16 mM. The fungus was inoculated with a suspension of conidiophores and cultivated at 28°C, 150 rpm. The medium is liquid PDA which contains 2% sucrose and 1.2% potato extracts (product of Beijing Shuangxuan Microbial Cultural Medium Factory, China), and supplemented with 3% NaCl and 0.18% MgSO4, and adjusted to pH 4 before autoclaving. Lead was added separately after sterilization of the medium. However, lead precipitation occurred at high concentrations of Pb(NO3)2; the precipitates were visible in the medium above 8 mM. But with the fungus growing, the precipitates finally disappeared from the medium. This may be due to the solubilizing effects of some organic chelators or acids produced by the fungus. Nonetheless, the actual concentration of free lead ions in the medium should be lower than that added to a certain degree.
Although we are not sure about the exact concentration of free Pb ions in the medium, the high resistance of Psf-2 is definite. As a result, Psf-2 can grow even in 24 mM Pb(II), which was the highest Pb concentration tested in this report and it grew normally, i.e., no growth inhibition was observed at 4 mM and below. However, growth was obviously inhibited at 8 mM, which resulted in about 70% biomass reduction of the no Pb control.
It is known that lead is usually toxic to normal fungi even at low concentrations. Such as in baker’s yeast, Saccharomyces cerevisiae, growth was affected at 10 mg/l of lead (II) (about 0.05 mM). Additions of 50 mg/l lead (II) resulted in a 60% reduction in biomass yields of yeast (Donmez and Aksu 1999). The lead tolerance of Pseudomonas vesicularis, Bacillus cereus, Staphylococcus sp. and Streptomyces sp., was reported from approximately 1 to 5 mM (Hasnain et al. 1993; Zanardini et al. 1997). Among the reported lead tolerant fungi, Paecilomyces lilacinus is probably of the highest resistance, and it can grow in medium containing up to 1,434 mg/l Pb (about 7 mM) (Zucconi et al. 2003). A marine fungus Corollospora lacera (ATCC 34603) isolated from sand grains from Bermuda has also been investigated for its lead resistance; about 41% biomass reduction was caused by 250 mg/l lead (about 1.2 mM) after 15 days of incubation in liquid medium (Taboski et al. 2005). However, in this report, even after discounting the insoluble part of Pb in the treatment of 16 or 24 mM, the concentration of Pb (II) would be no less than 8 mM. The resistance of Psf-2 to lead is remarkable. Additionally, its resistance could be further promoted if other conditions were optimized.
But, how does this fungus resist high concentrations of lead? Several kinds of interactions of fungi with toxic metals have been found: extracellular precipitation, binding to the cell wall, intracellular binding to proteins, vacuolar compartmentation and metal transformation (Gadd 1993; Baldrian 2003). In a previous report, we found a fungus from the same deep sea sample, which could grow in medium containing 1,200 mM Mn(II) by accumulating a large quantity of manganese intracellularly (Shao and Sun 2007).
Mechanism of resistance of Psf-2 to lead
To observe the mechanism of this fungus, the hyphae grown in 4 mM Pb(NO3)2 were picked out from the culture and subjected to sample processing for transmission electron microscopy (TEM, model JEM-1230EX). The samples were fixed only with 2.5% glutaraldehyde, dehydrated and embedded in resin as described previously. Fixation with osmic acid was not used to avoid the possible interference of Os. Ultra thin sections of about 600 nm thick were prepared and examined under TEM directly without the regular double staining. However, in order to locate where Pb deposited, part of the ultrathin sections were stained with uranyl acetate.
Results of TEM revealed that lead precipitated both inside and outside of the cell. The precipitation outside the cell seemed to be the first step in a cell’s battle with Pb, as in some cells no Pb accumulated in the cytoplasm, but rather just precipitated on the cell wall which occurred only in young cells. Interestingly, the crust of lead on the cell surface could be peeled off in the process of sample preparation (Fig. 1a). On the whole, nearly all cells had surface deposits, but only some had a deposit layer of Pb as thick as those in Fig. 1a, b; actually, most cells had Pb particles on walls as shown in Fig. 1c–f.
TEM photographs of Psf-2 grown in the presence of 4 mM Pb (II) (no Os fixation and without electron staining, observed at 80 KV). a, b Show the cell wall biosorption of lead; a a loose binding to the outer layer of the cell wall (OW), but no lead had been accumulated in the cell at this point; b a tight and thick binding of lead to the outer layer. c, d Show the intracellular bioaccumulation of lead in the cytoplasm and along the cell membrane, arrow head pointed to the spherical area containing many fine lead granules; e, f show the intracellular bioaccumulation in vacuoles but not on the inner wall (IW). IW was not observed in a and d
Analytically, two layers of the cell wall were observed in most cells. The outer layer grew light, thin and floppy while the inner is dark, thick and dense. Interestingly, lead precipitation mainly occurred on the outer layer. The biosorption occurred to only part of cell surface in some cells (Figs. 1b, 2d) and sometimes around the whole cell (not shown). Therefore, this layer should be the first defense of Psf-2 against lead toxicity.
Ultrastructure of Psf-2 grown in the presence of 4 mM Pb (II) (the same sample as in Fig. 1, observed at 80 KV). a–f Were U-stained sections except e; a, b whole cells to show the interaction of lead with membranes. a Lead deposited in vacuoles and in some vesicles which were enlarged in c and at the corner of a, and the aggregation of fine granules into large particles can be seen (arrow head); b showing lead accumulation in the endoplasmic reticulum (arrow head). d Shows the mitochondria and lead on the cell membrane (arrow head) and cell wall. e, f The controls, which grew in medium without the addition of lead, the section of e is without staining while f is U stained
Apart from cell wall biosorption, intracellular accumulation occurred in Psf-2 as well. Most cells observed by TEM had intracellular deposits of Pb in the form of irregular granules of about 15–100 nm (Fig. 1c, d), and both the protoplasm and vacuoles were the sites of accumulation. The cell membrane seemed to be closely related with Pb enrichment (Fig. 1c–f), as Pb particles dotted on the inner surface of the cell membrane. Conspicuously, in the cytoplasm of some cells, many fine granules of Pb aggregated into a ball (Figs. 1c, d, 2c, arrow head pointed). In vacuoles, the granules were seldom located at the center, but rather near the inner side of the vacuole membrane, and were usually larger than those in the cytoplasm (Figs. 1f, 2b).
In U stained sections, we can see clearly that granules of lead localized mainly in the vicinity of membranes (Fig. 2a), and that they can be aggregated into large particles in vacuoles (Fig. 2b, c). Some localized at the channel of the endoplasmic reticulum as well (Fig. 2b, arrow head), but scarcely in the mitochondria (Fig. 2c, d). Certainly, some lead granules were not membrane bound. It is intriguing to further decide how lead ions are transported from the outside via the membrane and how they are transferred from the cytoplasm to vacuoles. As of now, results in this report are just morphological observations.
To corroborate the results of the above observation, the result of a Pb negative control need to be investigated in which the fungus grew in the same medium without the addition of Pb. No lead particles were observed on sections of the control (Fig. 2e). As a control for U staining, no additional particles of high electron density were produced by U staining to the sections (Fig. 2f).
To reconfirm that the particles of high electron density are the result of Pb accumulation, sections without U staining were observed using a higher power TEM (model: Tecnai F30) equipped with energy dispersive X-ray spectroscopy (EDX). Under 300 kV, the black particles were subjected to EDX analysis and selected area electron diffraction (SAED). EDX analysis confirmed that the black particle formed ring was derived from Pb, and no P element was detected (Fig. 3c); other detected elements, except Cu which is the background signal, were C, N and O, and the atomic ratio of C/N/O/Pb is 3.276/2.333/2.543/91.849. The percentages of C, N and O were very low and might result from the background. This is different than the result of C. cladosporioides which could accumulate manganese as a complex with phosphorus (Shao and Sun 2007). In addition, SAED discovered the crystalline lattice of lead particles (Fig. 3b). These results demonstrated that the particles in Figs. 1 and 2 contained a high percentage of crystalline lead, but whether it is in element form needs to be investigated further.
EDX analysis of lead particles. a Section observed at 300 KV; the black ring is the result of intracellular lead accumulation. b The result of SAEF detection on the ring, showing the crystalline structure of the deposit; arrow head points to the lattice. c EDX result of the black deposit, showing the composition as lead. d EDX result of the center vacuole to show the background of copper derived from the copper grid
In the case of C. lacera (ATCC 34603), more than 90% of lead was trapped on the cell surface, while only 1.7–5.5% of the total Pb was found in the intracellular fraction (Taboski et al. 2005). Our results imply that both biosorption and bioaccumulation of lead contributed to the high tolerance of the fungus Psf-2. We cannot judge which mechanism is more important for metal trapping, but intracellular sequestration and compartmentalization seemed to be the main strategies of metal resistance. The absorption outside cell wall is probably the binding of lead ions to EPS on the cell surface (Bhaskar and Bhosle 2006). This is a quick, non energy consuming process, while intracellular bioaccumulation must be dependent on an active transport system (Bruins et al. 2000), first via the membrane to the cytoplasm and then from the cytoplasm to the vacuoles.
Psf-2 is the first fungus capable of accumulating a large amount of lead intracellularly. Results in this report together with the manganese-resistant Cladosporium cladosporioides which could sequestrate Mn intracellularly with phosphorus (Shao and Sun 2007) suggested that intracellular accumulation is an important mechanism for fungi from deep sea sediment to resist heavy metals. These fungi are a potential treatment for heavy metal pollution.
References
Bååth E (1989) Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut 47:335–379
Baldrian P (2003) Interactions of heavy metals with white-rot fungi. Enzyme Microb Technol 32:78–91
Bhaskar PV, Bhosle NB (2006) Bacterial extracellular polymeric substance (EPS): a carrier of heavy metals in the marine food-chain. Environ Int 32(2):191–198
Borremans B, Hobman JL, Provoost A, Brown NL, van Der Lelie D (2001) Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J Bacteriol 183:551–568
Bruins MR, Kapil S, Oehme1 FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207
Diels L, Sadouk A, Mergeay M (1989) Large plasmids governing multiple resistance to heavy metals: a genetic approach. Toxicol Environ Chem 23:79–89
Donmez G, Aksu Z (1999) The effect of copper (II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem 35:135–142
Dursun AY, Ulsu G, Cuci Y, Aksu Z (2003) Bioaccumulation of copper(II), lead(II) and chromium(VI) by growing Aspergillus niger. Process Biochem 38(10):1647–1651
Gadd GM (1993) Interactions of fungi with toxic metals. New Phytol 124:25–60
Hasnain S, Yasmin S, Yasmin A (1993) The effects of lead-resistant Pseudomonads on the growth of Triticum aestivum seedlings under lead stress. Environ Pollut 81:179–184
Levinson HS, Mahler I (1998) Phosphatase activity and lead resistance in Citrobacter freundii and Staphylococcus aureus. FEMS Microbiol Lett 161:135–138
Levinson HS, Mahler I, Blackwelder P, Hood T (1996) Lead resistance and sensitivity in Staphylococcus aureus. FEMS Microbiol Lett 145:421–425
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278
Mergeay M, Monchy S, Vallaeys T, Auquier V, Benotmane A, Bertin P, Taghavi S, Dunn J, van der Lelie D, Wattiez R (2003) Ralstonia metallidurans, a bacterium specially adapted to toxic metals: towards a catalogue of metal-responsive genes. FEMS Microbiol Rev 27:385–410
Ngu M, Moya E, Magan N (1998) Tolerance and uptake of cadmium, arsenic and lead by Fusarium pathogens of cereals. Int Biodeterior Biodegradation 42:55–62
Roane TM (1999) Lead resistance in two bacterial isolates from heavy metal-contaminated soils. Microb Ecol 37:218–224
Shao Z, Sun F (2007) Intracellular sequestration of manganese and phosphorus in a metal-resistant fungus Cladosporium cladosporioides from deep sea sediment. Extremophile (published online)
Taboski M, Rand T, Piórko A (2005) Lead and cadmium uptake in the marine fungi Corollospora lacera and Monodictys pelagica. FEMS Microbiol Ecol 53(3):445–453
Trajanovska S, Britz ML, Bhave M (1997) Detection of heavy metal ion resistance genes in gram-positive and gram-negative bacteria isolated from a lead-contaminated site. Biodegradation 8:113–124
Zanardini E, Andreoni V, Borin S, Cappitelli F, Daffonchio D, Talotta P, Sorlini C, Ranallib G, Bruni S, Cariati F (1997) Lead-resistant microorganisms from red stains of marble of the Certosa of Pavia, Italy and use of nucleic acid-based techniques for their detection. Int Biodeterior Biodegradation 40:71–182
Zucconi L, Ripa C, Alianiello F, Benedetti A, Onofri S (2003) Lead resistance, sorption and accumulation in a Paecilomyces lilacinus strain. Biol Fertil Soils 37:17–22
Acknowledgements
This work was supported by the COMRA program (No. DYXM115-02-2), the National Basic Research Program of China (No.2004CB719601) and the National Infrastructure of Natural Resources for Science and Technology Program of China (No. 2005DKA21209).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Driessen.
Rights and permissions
About this article
Cite this article
Sun, F., Shao, Z. Biosorption and bioaccumulation of lead by Penicillium sp. Psf-2 isolated from the deep sea sediment of the Pacific Ocean. Extremophiles 11, 853–858 (2007). https://doi.org/10.1007/s00792-007-0097-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-007-0097-7