Abstract
In this work, we investigated the lead(II) biosorption mechanism of Bacillus thuringiensis (Bt) 016 through batch and microscopic experiments. We found that the maximum lead(II) biosorption capacity of Bt 016 was 164.77 mg/g (dry weight). The pH value could affect the biosorption of lead(II) in a large extent. Fourier transform infrared analyses and selective passivation experiments suggested that the carboxyl, amide and phosphate functional groups of Bt 016 played an important role in lead(II) biosorption. Scanning electron microscopy observation showed that noticeable lead(II) precipitates were accumulated on bacterial surfaces. Further transmission electron microscopy thin section analysis coupled with energy dispersive X-ray spectroscopy as well as selected area electron diffraction indicated that lead(II) immobilized on the bacteria could be transformated into random-shaped crystalline lead-containing minerals eventually. This work provided a new insight into lead(II) uptake of Bt, highlighting the potential of Bt in the restoration of lead(II) contaminated repositories.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead is one of the most common toxic heavy metals, with a great poisonous impact on nervous, bone marrow hematopoietic, digestive, cardiovascular, reproductive, immune systems and kidney of humans, especially for children (Fowler 1998; Tong et al. 2000; Watt et al. 2000; Gordon et al. 2002). The extensive exploitation and usage of lead compounds has caused serious lead(II) pollution in water and soil environments, even agricultural land currently, threatening the health of animals and humans through food chains.
Traditional physical and chemical strategies for dealing with lead(II) contaminated environments include ion exchange, adsorption, precipitation and leaching. But they are unsuitable for large-scale remediation due to the disadvantages of high cost, recontamination and low efficiency, especially in soil environments or farmland. Microbial remediation strategy for lead(II) contamination is considered as a promising technology with low cost, high effective and environmental-friendly properties in recent years, using bacteria, fungi, algae as well as actinomycetes (Cho and Kim 2003; Tuzun et al. 2005; Akar and Tunali 2006; Sari and Tuzen 2009; Karaduman et al. 2011; Sahin et al. 2013; Subhashini et al. 2013; Edris et al. 2014). Microbes are capable of removal or immobilization lead(II) from water and soil environments through bioaccumulation, precipitation or acceleration the transformation of lead(II) into a very stable mineral mineralization (Aiking et al. 1985; Mire et al. 2004; De et al. 2008; Park et al. 2011; Naik et al. 2013; Naik and Dubey 2013). These would lead to significantly mitigation of lead(II) mobility and bioavailability in environments. However, different microbes exhibit a wide diversity of lead(II)-microbe interaction. More works are still needed to understand the common rules of the lead(II) uptake mechanisms and test the feasibility of the microbial strategy.
Bacillus thuringiensis (Bt) is a ubiquitous gram-positive bacterium with endospore. It is considered as the most widely used microbial biopesticide (Sellami et al. 2013) with insecticidal crystal proteins (ICPs), which are highly specific to target insect and innocuous to humans, plant and animals (Bravo et al. 2007; Soberon et al. 2009). Moreover, many Bt stains also have been reported to possess the potential of removal organic pollutants and heavy metal from water and soil environments (Brar et al. 2009; Dave and Dave 2009; Kebria et al. 2009; Muneer et al. 2009; Zhuang et al. 2011; Huang et al. 2014). These studies suggested a new perspective of entomopathogens in bioremediation of pollutants, highlighting the potential of Bt in the restoration of contaminated agricultural fields. However, works involved in the lead(II) bioremediation with Bt has been scarcely reported in literatures until now.
The purpose of the present article is to investigate the lead(II) biosorption of Bt 016. It is found that Bt 016 had strong ability of lead(II) uptake. Interestingly, lead(II) immobilized on the bacteria could be further transformated into crystalline lead-containing minerals after biosorption. The mechanism of lead(II) biosorption was further investigated through batch and microscopic experiments. The present work provide novel insights into lead(II) uptake mechanism of Bt, and will be beneficial for developing cost-effectively, environmental-friendly bioremediation or long-term management strategies of lead(II) contaminated repositories.
Materials and methods
Bacterium and cultivation
Bt 016 was obtained from Northeast Agricultural University, China. Bacteria were cultured in Luria–Bertani (LB) liquid medium, consisting of NaCl (10 g/L), tryptone (10 g/L) and yeast extract (5 g/L), pH 7.0, at 30 °C with a shaking speed of 160 rpm.
Batch experiments for the lead(II) uptake
The lead(II) stock solution was prepared by dissolving Pb(NO3)2 (AR) in deionized-distilled water. Lead(II) solutions were prepared by diluting the stock solution to desired concentrations. Since further transformation of lead(II) into mineral nanocrystals mediated by Bt 016 might be inhibited when the concentration of lead(II) was over than 400 mg/L as suggested in our preliminary studies, 400 mg/L of lead(II) was chosen as initial concentration in most of our experiments, and lead(II) uptake experiments were carried out in a final biomass of 1 g/L (dry weight) at 30 °C with shaking at 160 rpm unless noted otherwise. Indeed, the cell pellet used in our experiments was live cell of Bt 016. However, for the convenience of evaluating the lead(II) biosorption capacity directly, wet cells were transformed into dry weight in this manuscript, and 10 g wet cells of Bt 016 equal to 1 g dry weight as suggested by our preliminary studies.
The lead(II) uptake capacity of Bt 016 was investigated at different initial lead(II) concentrations ranging from 100 to 700 mg/L. After cultured in LB medium for 24 h, the cell pellet of Bt 016 was harvested and washed twice with 0.01 M NaNO3. And then the cell pellet was transferred into lead(II) solutions supplemented with 0.01 M NaNO3 (pH 4.0). After the equilibrium (24 h) was established, samples were centrifuged, and the residual lead(II) was analyzed using the supernatant.
To evaluate the pH effect on the lead(II) uptake, the cell pellet was re-suspended in lead(II) solution (400 mg/L) supplemented with 0.01 M NaNO3 at different pH values ranging from 1 to 6. After the equilibrium (24 h) was established, the residual lead(II) was analyzed using the supernatant. The pH of solutions was adjusted to desired values using 0.1 M HNO3 or 0.1 M NaOH.
Further lead(II) biosorption experiments were carried out at different bacterial concentrations (1, 2 and 3 g/L, dry weight) and temperatures (20, 30 and 40 °C) respectively with initial lead(II) concentration of 400 mg/L and pH 4.0. Aliquots of samples were withdrawn at predetermined time intervals (10, 20, 30 min, 1, 2, 4, 8 and 24 h). The residual lead(II) was analyzed using the supernatant.
The lead(II) concentration was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES) (Ultima2) in this work.
Zeta potential measurement
The cell pellet was re-suspended in deionized-distilled water at different pH values ranging from 1 to 6. The surface charge of bacteria at different pH was determined using Zeta Potential Analyzer (Brookhaven, USA) within 1 h after the samples were prepared. Then the isoelectric point (IEP) of Bt 016 cells was obtained from the results of zeta potential measurements.
FT-IR analysis
Bacterial samples were obtained by centrifugation at 5000 rpm for 10 min and were washed three times with deionized-distilled water. And then samples were dried at 37 °C and prepared in a KBr pellet with a sample/KBr ratio of 1:100. Infrared spectras were recorded on a Perkin-Elmer Spectrum One FT-IR spectrometer in the range of 2000–500 cm−1.
Modification of the functional groups
In order to determine the effect of functional groups on the lead(II) biosorption, Bt 016 cells were disrupted into cell debris, and selective passivation of different functional groups was carried out respectively by chemical modifications before lead(II) biosorption. Briefly, after cultured and washed in deionized-distilled water for twice, the cell pellet was re-suspended in deionized-distilled water and repeated freeze–thaw cycles using liquid nitrogen for three times. Subsequently, the bacterial suspension was homogenized through sonication at 40 W with 15-s pulses and 30-s intervals under ice cooling treatment. The cell viability was examined by spreading the obtained cell lysate onto agar plate. No colony should be observed on the agar plate after cultivation at 30 °C for 24 h. Cell debris was obtained by centrifugation at 14,000 rpm for 10 min and stored at −20 °C prior to further analysis.
The carboxyl group on biomass was esterified using acidic methanol method (Kapoor and Viraraghavan 1997; Cheng et al. 2010). Briefly, 1 g of biomass was suspended in 10 mL of anhydrous methanol and 5.4 mL of concentrated HCl. The reaction mixture was shaken at 160 rpm for 24 h. The esterification of phosphate groups was performed by suspending 1 g of biomass in 14 mL triethyl phosphate/nitromethane solution (4:3, v/v) with shaking at 160 rpm for 3 h (Kapoor and Viraraghavan 1997). The acetylation (A) of amino groups on the biomass was carried out by suspending 1 g of biomass in 10 mL acetic anhydride/alcohol solution (1:10, v/v) with shaking at 160 rpm for 2 h (Cheng et al. 2010). After chemical modification, modified samples were obtained by centrifugation at 14,000 rpm for 10 min and washed in deionized-distilled water for three times.
Since the lead(II) biosorption capacity of Bt 016 cell debris was significantly lower than that of Bt 016 cells as suggested in our preliminary studies, the modified samples and non-modified samples (control) were further interacted with 100 mg/L lead(II) for 24 h in this study. The remaining lead(II) concentration in the suspensions was determined by ICP-OES.
Microscopic investigation
Samples for SEM observation were centrifuged at 5, 000 rpm, then the cell pellets were first fixed by 2.0 % glutaraldehyde for 24 h, and washed three times with 0.1 M sodium dimethylarsenate buffer for 3 min, then post-fixed with 1 % osmic acid for 2 h. After washed three times with 0.1 M sodium dimethylarsenate buffer for 3 min again, samples were dehydrated step by step with 35, 50, 70, 80, 95 and 100 % acetone for 3 min respectively. SEM investigations were performed using a JEOL JSM-6700F scanning electron microscope coupled with an EDS (Oxford).
The preparation of TEM sample was the same as for SEM from the harvest to dehydration steps. Then the bacterial cells were embedded in resin. The ultramicrotome (Leica EM UC6) was used to obtain ultrathin sections, then stained with uranyl acetate and lead citrate. Finally the samples were observed using a JEM-2010 transmission electron microscope coupled with an EDS (Oxford) system at 200 kV.
Results
Lead(II) uptake by Bt 016
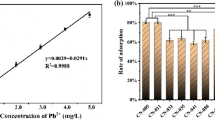
The lead(II) biosorption capacity of Bt 016 was investigated at different initial lead(II) concentrations in this work. As shown in Fig. 1a, the amount of lead(II) biosorption on Bt 016 increased with the rise of initial lead(II) concentration from 100 to 600 mg/L, while it was slightly decreased when the lead(II) concentration was 700 mg/L. The biosorption isotherm analysis suggested that the biosorption data were better fitted to Langmuir model, and the maximum lead(II) biosorption of Bt 016 was 164.77 mg/g (dry weight) under our experimental conditions (as shown in Supplementary Information, Fig. S1 and Table S1). Figure 1b shows the effect of pH on lead(II) biosorption as well as the zeta potential of the cell surface. Only a little amount of lead(II) could be adsorbed by when pH < 3, while the biosorption of lead(II) was significantly increased when pH ≥ 3. The maximum lead(II) biosorption was obtained at pH of 3 and 4. The results indicated that the lead(II) biosorption of Bt 016 depended on the pH in a large extent. Zeta potential analyses demonstrated that the surface charge of Bt 016 cells was positive at pH values below 3 and negative at pH values above 3. The results suggested that electrostatic interaction played an important role in the lead(II) biosorption of Bt 016. Besides, it was also found that the lead(II) biosorption was gradually declined when the pH value was increased from 4 to 6.
The relationship between lead(II) biosorption and initial lead(II) concentration, pH, bacterial concentration as well as temperature. a The effect of initial lead(II) concentration on lead(II) biosorption; b the effect of pH on lead(II) biosorption as well as the zeta potential of the cell surface; c the effect of bacterial concentration on lead(II) biosorption; d the effect of temperature on lead(II) biosorption
Figure 1c shows the effects of biomass on lead(II) biosorption. The amount of lead(II) biosorption was decreased as biomass increasing from 1 to 3 g/L at pH 4.0 (Fig. 1c). Besides, it was found that lead(II) could be rapidly adsorbed within the first 2 h (Fig. 1c). The equilibrium was established at about 4 h and lead(II) biosorption remained nearly constant within 4–24 h (Fig. 1c). The result indicated that lead(II) uptake of Bt 016 was a quick biosorption process (Fig. 1c). Further lead(II) biosorption experiments were carried out at different temperatures. As shown in Fig. 1d, the lead(II) biosorption capacity of Bt 016 increased with an increase in the temperature, although the amount of lead(II) biosorption was slightly reduced at 40 than that at 30 °C. The maximum lead(II) uptake could be observed at 30 °C in this work.
The effect of functional groups on lead(II) biosorption
FT-IR analysis was carried out to investigate the active functional groups of biomass that could possibly participate lead(II) biosorption. As shown in Fig. 2a, it could be observed that the FT-IR spectra of raw bacteria display a number of adsorption peaks. However, the peak around 1727.89 cm−1 disappeared after lead(II) biosorption, while the peak around 1238.84 cm−1 appeared, which could be assigned as the C=O stretching vibration of carboxylic groups and antisymmetric –P=O stretching (Pan et al. 2006; Al-Qadiri et al. 2008; Shamala et al. 2009). Moreover, the peak at 1657.10 cm−1 moved to lower frequency, which contributed to acid amide C=O stretching (Murugesan et al. 2011). Whereas the peak at about 1383.72 and 1056.42 cm−1 transferred to higher frequency, which might be attributed to typical amide III, C(=O)O− symmetric stretching vibration of carboxylate, C-N stretching vibration, P-O-C links of the organic phosphated groups and P-O vibration of the (C-PO −23 ) moiety (Park et al. 2005; Pan et al. 2006). The results suggested that carboxyl, amide and phosphate functional groups might be involved in the biosorption of lead(II).
The impact of functional groups on the lead(II) biosorption. a FT-IR spectra of intact bacteria and Pb(II)-loaded bacteria; b comparison of lead(II) biosorption of the cell debris with and without functional groups passivation. Control represents the sample without chemical modification; PO-E represents esterification of phosphate groups; NH-A represents acetylation of amino; CH-E represents esterification of carboxyl groups
In order to confirm the hypothesis that carboxyl, amide and phosphate groups were responsible for the lead(II) biosorption, cell debris with and without functional groups modification were used for lead(II) biosorption respectively. Figure 2b shows that the lead(II) biosorption capacity of cell debris without chemical modification (control) was 71 mg/g. However, when the carboxyl group was esterificated, the content of lead(II) biosorption of cell debris was only about 4 mg/g. The lead(II) biosorption of Bt 016 cell debris was also significantly decreased to about 9 mg/g and 14 mg/g respectively when amide and phosphate functional groups were passivated by individual modifications. The results confirmed that the chelating activity of the carboxyl, amide and phosphate functional groups was responsible for the lead(II) biosorption of Bt 016.
Microscopic investigation of lead(II) uptake
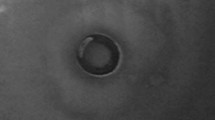
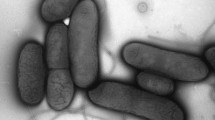
Microscopic investigation was carried out to elucidate the morphologic changes and detailed distribution situation of lead(II) on Bt 016. As shown in Fig. 3, SEM observation showed that the cells of Bt 016 exhibited rod shaped with plump and smooth surfaces (Fig. 3a). After treated with 400 mg/L lead(II), bacterial surfaces became rough with a certain amount of precipitates (Fig. 3c). EDS spectra indicated that the accumulation of precipitates on cell surfaces contained lead.
The ultrathin sections of bacteria were further investigated by TEM combined with EDS and selected area electron diffraction (SAED). Figure 4a shows that the ultrathin sections of bacterial cells were rod or oval by different section angles. After the lead(II) uptake for 4 days, a certain amount of precipitates was accumulated along the outside of cell wall, while some random-shaped minerals could be found inside of the bacterial cell (Fig. 4b). EDS spectra confirmed that these rod shape minerals contained lead. SAED analysis showed a series of diffraction spots, suggesting that the lead(II) could be further transformed into random-shaped mineral nanocrystals by Bt 016 (Fig. 4b).
Discussion
The application of microbes in heavy metal remediation has been the focus of much research. Bacteria are capable of immoblization lead(II) in the natural environment through diverse strategies as reported in previous studies (Naik and Dubey 2013). In this work, it was found that Bt 016 possessed the capacity of lead(II) biosorption with the maximum uptake of 164.77 mg/g. The lead(II) biosorption could be significantly affect by the pH value. Zeta potential analyses suggested that the pH would greatly change the electric charge of bacterial surface, and then varied the biosorption of lead(II) indirectly. Meanwhile, the pH value could also alter the species of lead hydroxide complexes, leading to reduce of lead(II) biosorption when the pH was increased from 4 to 6 in this work. The results was consistent with previous studies on Bacillus gibsonii S-2, Rhodococcus opacus as well as Symphoricarpus albus (Bueno et al. 2008; Akar et al. 2009; Zhang et al. 2013), suggesting that electrostatic attraction played an important role in lead(II) biosorption of bacteria. Time-course profiles for biosorption indicated that lead(II) biosorption rapid increased initially and slows down to approach equilibrium within 2–4 h. The quick lead(II) biosorption in the first 2 h also supports the hypothesis that electrostatic attraction might be the main driving force in the initial process of lead(II) uptake. The capacity of lead(II) biosorption could increase with a rise of initial lead(II) concentration due to a larger driving force for promoting mass transfer between solid and aqueous phase with higher ion concentration. However, the decrease of lead(II) biosorption would occur when initial lead(II) concentrations was over 600 mg/L as suggested in this work. It might be caused by cell death and membrane leakage accompanied with death at high concentration of lead(II). On the contrary, increase in biomass dosage would lead to decrease the capacity of lead(II) biosorption. It was supposed that biomass granulates would be more likely to adhere together or agglomerate at high concentration of biomass, resulting in the reduction of surface area as well as binding sites. Besides, the lead(II) biosorption capacity increased with increasing temperature in this work might suggest that lead (II) biosorption of Bt 016 is an endothermic process.
Further studies on the effect of functional groups on lead(II) biosorption through FT-IR analyses and selective passivation experiments suggested that the carboxyl, amide as well as phosphate functional groups of Bt 016 involved in binding of lead(II). Similar results have been reported in previous works (Bueno et al. 2008; Naik and Dubey 2013; Zhang et al. 2013). Microscopic investigation demonstrated that the immobilization lead(II) could be accumulated outside of cell in the form of lead-containing precipitates. Interestingly, after 4 days, a portion of lead(II) could be further transformed into random-shaped mineral nanocrystals inside of the cell as indicated by TEM investigation. To our knowledge, this is the first report of Bt mediating the transformation of lead(II) into mineral nanocrystals. This mineral nanocrystals might be lead phosphate [Pb9(PO4)6], pyromorphite [Pb5(PO4)3Cl] or galena [PbS] as previously described in study of Klebsiella aerogenes, Bacillus iodinium GP13, Vibrio harveyi, Providencia alcalifaciens strain 2EA and fungi (Mire et al. 2004; Park et al. 2011; Naik and Dubey 2013; Rhee et al. 2014). These minerals have stronger stability and lower solubility, and will drastically reduce the migration, bioavailability and toxicity of lead(II) in the environment.
Bacterial remediation strategy is a promising alternative to the traditional treatment technologies for lead(II)-contaminated environments through biosorption and biomineralization. As commercially available biopesticides, Bt is more feasible in practical application of bioremediation compared with other bacteria due to the following advantages: (1) Bt is non-pathogens of humans, plant and animals, and it is safety for large-scale application; (2) Bt can be cheaply and readily obtained from industrial fermentation processes in large scale; (3) Bt is widespread in various natural habitats, and it can adapt to the climate, pH, temperature of nature environments or agricultural fields; (4) Last but not least, as the most widely used biopesticide, Bt with lead(II) bioremediation capacity and insecticidal activity is expected to be widely applied for achievement the dual effect of pest control and lead-contaminated restoration.
Taken together, the present work provided useful information on the mechanism of lead(II) uptake by bacteria, highlighting the potential of Bt in developing cost-effectively, environmental-friendly in situ bioremediation or long-term management strategies of lead(II) contaminated repositories.
References
Aiking H, Govers H, Vantriet J (1985) Detoxification of mercury, cadmium, and lead in Klebsiella aerogenes NCTC 418 growing in continuous culture. Appl Environ Microbiol 50:1262–1267
Akar T, Tunali S (2006) Biosorption characteristics of Aspergillus flavus biomass for removal of Pb(II) and Cu(II) ions from an aqueous solution. Bioresour Technol 97:1780–1787. doi:10.1016/j.biortech.2005.09.009
Akar ST, Gorgulu A, Anilan B, Kaynak Z, Akar T (2009) Investigation of the biosorption characteristics of lead(II) ions onto Symphoricarpus albus: Batch and dynamic flow studies. J Hazard Mater 165:126–133. doi:10.1016/j.jhazmat.2008.09.089
Al-Qadiri HM, Al-Alami NI, Al-Holy MA, Rasco BA (2008) Using Fourier transform infrared (FT-IR) absorbance spectroscopy and multivariate analysis to study the effect of chlorine-induced bacterial injury in water. J Agric Food Chem 56:8992–8997. doi:10.1021/jf801604p
Brar SK, Verma M, Tyagi RD, Valero JR, Surampalli RY (2009) Concurrent degradation of dimethyl phthalate (DMP) during production of Bacillus thuringiensis based biopesticides. J Hazard Mater 171:1016–1023. doi:10.1016/j.jhazmat.2009.06.108
Bravo A, Gill SS, Soberon M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435. doi:10.1016/j.toxicon.2006.11.022
Bueno B, Torem ML, Molina F, de Mesquita L (2008) Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: Equilibrium and kinetic studies. Miner Eng 21:65–75. doi:10.1016/j.mineng.2007.08.013
Cheng YJ, Yan FB, Huang F, Chu WS, Pan DM, Chen Z, Zheng JS, Yu MJ, Lin Z, Wu ZY (2010) Bioremediation of Cr(VI) and Immobilization as Cr(III) by Ochrobactrum anthropi. Environ Sci Technol 44:6357–6363. doi:10.1021/es100198v
Cho DH, Kim EY (2003) Characterization of Pb2+ biosorption from aqueous solution by Rhodotorula glutinis. Bioprocess Biosyst Eng 25:271–277. doi:10.1007/s00449-002-0315-8
Dave SR, Dave RH (2009) Isolation and characterization of Bacillus thuringiensis for Acid red 119 dye decolourisation. Bioresour Technol 100:249–253. doi:10.1016/j.biortech.2008.05.019
De J, Ramaiah N, Vardanyan L (2008) Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar Biotechnol 10:471–477. doi:10.1007/s10126-008-9083-z
Edris G, Alhamed Y, Alzahrani A (2014) Biosorption of cadmium and lead from aqueous solutions by Chlorella vulgaris biomass: equilibrium and kinetic study. Arab J Sci Eng 39:87–93. doi:10.1007/s13369-013-0820-x
Fowler BA (1998) Roles of lead-binding proteins in mediating lead bioavailability. Environ Health Perspect 1066:1585–1587. doi:10.1289/ehp.98106s61585
Gordon JN, Taylor A, Bennett PN (2002) Lead poisoning: case studies. Br J Clin Pharmacol 53:451–458. doi:10.1046/j.1365-2125.2002.01580.x
Huang TP, Xiao Y, Pan JR, Chen Z, Li LF, Xu L, Zhang LL, Guan X (2014) Aerobic Cr(VI) reduction by an indigenous soil isolate Bacillus thuringiensis BRC-ZYR2. Pedosphere 5:652–661. doi:10.1016/S1002-0160(14)60051-5
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Bioresour Technol 61:221–227. doi:10.1016/S0960-8524(97)00055-2
Karaduman AB, Yamac M, Pat Z, Amoroso MJ, Cuozzo SA (2011) Lead(II) biosorption by a metal tolerant Streptomyces strain. Environ Eng Manag J 10:1761–1771
Kebria DY, Khodadadi A, Ganjidoust H, Badkoubi A, Amoozegar MA (2009) Isolation and characterization of a novel native Bacillus strain capable of degrading diesel fuel. Int J Environ Sci Technol 6:435–442. doi:10.1007/BF03326082
Mire CE, Tourjee JA, O’Brien WF, Ramanujachary KV, Hecht GB (2004) Lead precipitation by Vibrio harveyi: evidence for novel quorum-sensing interactions. Appl Environ Microbiol 70:855–864. doi:10.1128/AEM.70.2.855-864.2004
Muneer B, Rehman A, Shakoori F, Shakoori A (2009) Evaluation of consortia of microorganisms for efficient removal of hexavalent chromium from Industrial wastewater. Bull Environ Contam Toxicol 82:597–600. doi:10.1007/s00128-009-9662-3
Murugesan A, Ravikumar L, SathyaSelvaBala V, SenthilKumar P, Vidhyadevi T, Kirupha SD, Kalaivani SS, Krithiga S, Sivanesan S (2011) Removal of Pb(II), Cu(II) and Cd(II) ions from aqueous solution using polyazomethineamides: equilibrium and kinetic approach. Desalination 271:199–208. doi:10.1016/j.desal.2010.12.029
Naik MM, Dubey SK (2013) Lead resistant bacteria: lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicol Environ Saf 98:1–7. doi:10.1016/j.ecoenv.2013.09.039
Naik MM, Khanolkar D, Dubey SK (2013) Lead-resistant Providencia alcalifaciens strain 2EA bioprecipitates Pb+2 as lead phosphate. Lett Appl Microbiol 56:99–104. doi:10.1111/lam.12026
Pan JH, Ge XP, Liu RX, Tang HX (2006) Characteristic features of Bacillus cereus cell surfaces with biosorption of Pb(II) ions by AFM and FT-IR. Colloid Surf B 52:89–95. doi:10.1016/j.colsurfb.2006.05.016
Park D, Yun YS, Park JM (2005) Studies on hexavalent chromium biosorption by chemically treated biomass of Ecklonia sp. Chemosphere 60:1356–1364. doi:10.1016/j.chemosphere.2005.02.020
Park JH, Bolan N, Megharaj M, Naidu R (2011) Concomitant rock phosphate dissolution and lead immobilization by phosphate solubilizing bacteria (Enterobacter sp.). J Environ Manage 92:1115–1120. doi:10.1016/j.jenvman.2010.11.031
Rhee YJ, Hillier S, Pendlowski H, Gadd GM (2014) Pyromorphite formation in a fungal biofilm community growing on lead metal. Environ Microbiol 16:1441–1451. doi:10.1111/1462-2920.12416
Sahin I, Keskin SY, Keskin CS (2013) Biosorption of cadmium, manganese, nickel, lead, and zinc ions by Aspergillus tamarii. Desalin Water Treat 51:4524–4529. doi:10.1080/19443994.2012.752332
Sari A, Tuzen M (2009) Kinetic and equilibrium studies of biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Amanita rubescens) biomass. J Hazard Mater 164:1004–1011. doi:10.1016/j.jhazmat.2008.09.002
Sellami S, Zghal T, Cherif M, Zalila-Kolsi I, Jaoua S, Jamoussi K (2013) Screening and identification of a Bacillus thuringiensis strain S1/4 with large and efficient insecticidal activities. J Basic Microbiol 53:539–548. doi:10.1002/jobm.201100653
Shamala TR, Divyashree MS, Davis R, Kumari K, Vijayendra S, Raj B (2009) Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by fourier transform infrared spectroscopy and scanning electron microscopy. Indian J Microbiol 49:251–258. doi:10.1007/s12088-009-0031-z
Soberon M, Gill SS, Bravo A (2009) Signaling versus punching hole: how do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol Life Sci 66:1337–1349. doi:10.1007/s00018-008-8330-9
Subhashini SS, Velan M, Kaliappan S (2013) Biosorption of lead by Kluyveromyces marxianus immobilized in alginate beads. J Environ Biol 34:831–835
Tong S, von Schirnding YE, Prapamontol T (2000) Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ 78:1068–1077
Tuzun I, Bayramoglu G, Yalcin E, Basaran G, Celik G, Arica MY (2005) Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manage 77:85–92. doi:10.1016/j.jenvman.2005.01.028
Watt G, Britton A, Gilmour HG, Moore MR, Murray GD, Robertson SJ (2000) Public health implications of new guidelines for lead in drinking water: a case study in an area with historically high water lead levels. Food Chem Toxicol 381:S73–S79. doi:10.1016/S0278-6915(99)00137-4
Zhang BG, Fan RM, Bai ZH, Wang S, Wang L, Shi JP (2013) Biosorption characteristics of Bacillus gibsonii S-2 waste biomass for removal of lead (II) from aqueous solution. Environ Sci Pollut Res Int 20:1367–1373. doi:10.1007/s11356-012-1146-z
Zhuang L, Zhou SG, Wang YQ, Liu Z, Xu RX (2011) Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: effects of heavy metals. Bioresour Technol 102:4820–4826. doi:10.1016/j.biortech.2010.12.098
Acknowledgments
This work is supported by the National High Technology Research and Development Program 863 (Grant No. 2011AA10A203), the National Basic Research Program of China (973 Program) (Nos. 2010CB933501, 2013CB934302), National Natural Science Foundation of China (21477129), the Outstanding Youth Fund (21125730), the Leading Talents of Fujian Province College (k8012012a) and the Technology Key Project of Fujian Province (2013H0058).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Z., Pan, X., Chen, H. et al. Investigation of lead(II) uptake by Bacillus thuringiensis 016. World J Microbiol Biotechnol 31, 1729–1736 (2015). https://doi.org/10.1007/s11274-015-1923-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1923-1