Abstract
This field ecological study, based on the chironomid pupal exuvial technique (CPET), is new for the Paraná River and proposes an efficient tool to be used in future ecological approaches and biomonitoring. Drifting of pupal exuviae in a river-floodplain system of the Middle Paraná River floodplain was represented by 34 Chironomidae taxa, being the characteristic association obtained from the CPET: Lopescladius, Onconeura, Paralauterborniella, Polypedilum, and Harnischia complex. Diversity, richness, dominance, total density, and density of dominant taxa were different between the longitudinal and lateral dimensions but not between hydrologic phases, with a greater diversity and richness in the main channel of the river and higher density and dominance in floodplain habitats. The species turnover is the dominant process in structuring studied assemblages in spatial and temporal analysis, increasing in the floodplain habitats and in low-water phase. The results obtained showed that drifting exuviae in the longitudinal axis were coming from different assemblages and environments of a wider area (regional), while exuviae recorded in the connections of the floodplain environments in the lateral dimension could reflect the local assemblages. We demonstrated the ecological value of CPET studies to interpret the attributes of Chironomidae assemblage in river-floodplain systems of large rivers in an integrated way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the central issues for the integral understanding of river ecology and management of large rivers is to elucidate the diversity and distribution patterns in relation to the hydrological regime (Thomaz et al 2007). In large rivers with a floodplain like the Paraná River, besides the longitudinal upstream-downstream transport, it is fundamental to consider the interchange in the lateral dimension (Marchese et al 2002, Ezcurra de Drago et al 2007), because the hydrosedimentologic dynamics, with recurrent flood and drought pulses, generate a bidirectional interchange of matter and energy from the river to the floodplain and vice versa, determining their populations’ characteristics (structure, distribution, abundance, adaptations, etc.) (Junk et al 1989, Neiff 1999, Montalto & Paggi 2006, Ezcurra de Drago et al 2007, Mesa et al 2012).
Chironomidae are among the most abundant and diverse insect families in aquatic ecosystems. The present discussions around the ecology of larval Chironomidae assemblage was oriented to elucidate the species diversity and the mechanisms involved in processes that structure assemblages in relation to environmental and spatial factors under a metacommunity concept framework (Heino 2005, Siqueira et al 2008, 2009, Anderson & Ferrington 2013, Petsch et al 2015). In Argentina, studies on the ecology of Chironomidae from their larval stages have been oriented principally to bionomy and diversity in relation to different habitat characteristics (Montalto & Paggi 2006, Medina et al 2008, Scheibler et al 2008, Zilli & Paggi 2013, Mauad et al 2016).
After chironomid adult emergence, their pupal exuviae become part of the drifting of aquatic environments. Through their study, it is possible to know the species diversity in an environment because they show diagnostic characteristics more easily recognized than the larvae, in many cases, allowing better taxonomic determination (Hardwick et al 1995, Wilson & Ruse 2005).
The use of the chironomid pupae exuvial technique (CPET) has been mentioned by different authors in ecological studies, and its efficiency has been demonstrated in lotic and lentic environments in other regions (Raunio & Muotka 2005, Raunio et al 2007a, Raunio et al 2011). These studies have been carried out, even in coincidence, with analyses of the larvae recorded in the benthos, with a higher number of taxa associated with the pupal exuvial technique (Ruse 1995, Raunio et al 2007a, 2007b).
The CPET also has been used widely to evaluate different Chironomidae assemblage attributes in relation to environmental conditions (Fend & Carter 1995, Hardwick et al 1995, Wilson & Bright 1973, Ruse 1995, Raunio et al 2007a), trophic state of habitats (Raunio et al 2010), and for biomonitoring purposes either as a complement to or a replacement for traditional benthic samplings (McGill et al 1979, Ferrington et al 1991, Raunio & Muotka 2005, Wilson & Ruse 2005, Raunio et al 2007b). However, in the Neotropical region, there are some contributions for diversity and phenology of Chironomidae for Patagonia (Argentina) (García & Añón Suarez 2007) and São Carlos (Brazil) (Siqueira et al 2008).
The purpose of this study was to evaluate the application of chironomid pupal exuvial technique (CPET) for an ecological analysis in a Neotropical large river system, considering longitudinal and lateral dimensions and hydrological phases.
Material and Methods
Study area
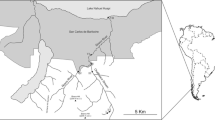
This study was carried out in five environments of the Middle Paraná River floodplain, located in the transversal section of Santa Fe and Paraná cities, Argentina (31°41′S, 60°43′W) (Fig 1). The regional climate is humid subtropical with hydric excess during the entire year and with a seasonal hydrograph influenced mainly by rainfall pattern, a high-level stage during the first 6 months of the year and maximum peaks in March–April. Low-water phase occurs in the second half of the year, with minimum flows in September–October. The Middle Paraná is included in the geographical region Chaco-Pampa plain (Marchese et al 2002, Iriondo 2007).
Study area showing sampling sites in the Paraná River system (Argentina). Abbreviations: USP Paraná River main channel, upstream from the connection with the floodplain lake channel; DSP Paraná River main channel, downstream from the connection with the floodplain lake channel; P-Ch beginning of the connection channel of the Paraná River main channel with the floodplain lake; Ch-L connection between the channel and the floodplain lake; L-SCh floodplain lake and the secondary channel connection.
The Paraná River is 4400 km long from the headwaters of the Grande River in Brazil to the Río de la Plata, constituting the second longest river in South America, the second in relation to its area, 1.51 million km2, and the third in relation to its mean annual flow into the ocean, approximately 470 km3, with 16,000–19,000-m3/s mean flow, reaching 60,000 m3/s maximum (Paoli & Schreider 2000). Middle Paraná River has built a fringing floodplain with a surface of circa 20,000 km2 including different habitats principally regulated by the flood pulse regime and the hydrological connectivity (Drago 2007). The environments studied include the right margin of the main channel of the Paraná River, the channel linking with a floodplain lake, and the connection of this lentic environment with a secondary channel.
The studied floodplain lake is connected permanently to the main channel and presents an irregular shape with a maximum depth of 4.6 m and a surface of 0.39 km2. The connection channel with the main channel is 0.74 km long (Mesa et al 2012). Mini River is a secondary channel of the Paraná River system with ≈ 100-m3/s flows.
The studied environments are characterized by dissolved oxygen ranging between 4.6 and 9.8 (ppm), conductivity of 65–540 μS/cm, pH of 6.2–7.6; temperature ranged between 13 and 28°C with Secchi disc of 0.07–0.65 m. The current velocity (m/s) ranged between 0.57 and 1.77 m/s for the main channel and < 0.8 m/s for minor secondary channels (Ezcurra de Drago et al 2007).
The five sampling stations were as follows: 1. right bank of the main channel of the Paraná River upstream from the connection with the channel to the floodplain lake (USP); 2. right bank of the main channel Paraná River downstream from the connection with the channel to the floodplain lake (DSP); 3. beginning of the connection channel of the Paraná River main channel with the floodplain lake (P-Ch); 4. connection between the channel and the floodplain lake (Ch-L); and 5. connection between the floodplain lake and the secondary channel (L-SCh) (Fig 1).
Collection and processing of chironomid pupal exuviae
Nets of 250 μm mesh, 0.10 m in diameter, and 0.30 m long, on the water surface, were used for sampling for 10 min (Ferrington 1987, Fend & Carter 1995, García & Añón Suarez 2007). At each station, three samples (replicates) were taken in the sites located in the longitudinal axis (USP, DSP) and lateral axis (P-Ch, Ch-L, L-SCh). Samplings were carried out during the high- and low-water phases (March and December 2010), respectively. For high-water phase, L-SCh could not be sampled.
Because chironomids have different daily emergency rhythms with changes in the frequency drift (Raunio & Muotka 2005), samples were all collected in the same time period (8–16 h) to minimize variability between samples. Drifting exuviae were fixed in situ with 10% formalin.
At each sampling station, depth (with a hand-held probe), transparency (Secchi disk), pH, temperature, conductivity, and dissolved oxygen (Horiba U-10) were determined in situ. To assess the flow velocity (m/s), an AOTT flowmeter was used; then, the volume of sampled water at each time interval was calculated.
The separation of the pupal exuviae was performed under a 10× stereomicroscope and the material was preserved in 70% alcohol for qualitative and quantitative analysis. All pupal exuviae were dehydrated by means of successive immersion in 80%, 96% and 100% ethanol and mounted in Euparal® (Paggi 2009). The exuviae were stained with fuchsine acid when necessary. Exuviae were identified under an optical microscope at the lowest possible taxonomic level following the keys of Wiederholm (1986), Cranston (1996), and Wiedenbrug (2000).
Data analysis
For drifting samples, the density of pupal exuviae (exuviae.m3/h) was calculated as the quotient between the number of exuviae in a sample and the volume of sampled water. This volume was calculated by multiplying the area of the submerged net, the current velocity at the net mouth, and the sampling time.
Chironomid density, density of dominant taxa, taxonomic diversity (H, Shannon-Wiener), rarefied richness values, and dominance (D, Simpson) between the different sampling dates, and between the high- and low-water phases at the longitudinal and lateral axes were analyzed as descriptors of Chironomidae assemblage. Richness was rarefied by individuals (density values) using Past (ver 1.89) (Hammer et al 2009).
The beta diversity partitioning the spatial turnover and nestedness was performed (Baselga 2010). Analyses were carried out in R betapart package (R Development Core Team) using the functions beta-multi.R to obtain β SOR (total beta diversity), β SIM (spatial turnover), and β NES (nestedness) components (Baselga & Orme 2012).
The Mann-Whitney test was applied to compare total density, density of dominant taxa, rarefied richness, and diversity according to the hydroperiod (low- and high-water phases) and between the main channel (USP, DSP, P-Ch) and floodplain habitats (Ch-L, L-M) (longitudinal-lateral dimensions) (Past ver 1.89, Hammer et al 2009).
In order to relate the environmental parameters and assemblage metrics, Spearman correlation (Rho) was performed. Previously, we tested environmental multicollinearity and selected environmental variables for analysis (Past ver 1.89, Hammer et al 2009).
Results
Spatial and temporal analysis of Chironomidae exuviae
In general, main channel habitats were characterized by higher depth, current velocity, and dissolved oxygen. In the high-water phase, the lowest values of current velocity, pH, and dissolved oxygen (included two anoxia values in floodplain habitats) were recorded. Moreover, during the low-water phase, we observed a reduction in Secchi disc and conductivity values (Table 1).
The total richness of the Chironomidae taxa recorded was 34, with dominance of Chironominae (23 taxa, 20 Chironomini and 3 Tanytarsini), followed by Orthocladiinae (6 taxa) and Tanypodinae (4 taxa). Table 2 shows the occurrence (%Oc) and dominance (%N) of all the taxa in the different studied sites of the Paraná River during high- and low-water phases. An occurrence frequency of 100% of the studied environments in both phases was represented by Paralauterboniella sp., Polypedilum sp. 3 or 5, and Onconeura sp. 1. The highest dominance (%N) was represented by Lopescladius sp. 1 and 2 and Paralauterborniella sp. In the present study, a pupal exuviae of Robackia was reported for the first time in the main channel of the Middle Paraná River (Table 2).
For rarefied richness and Shannon diversity values, significant differences were recorded between longitudinal and lateral dimensions (river/floodplain) (p = 0.05 and p = 0.028, respectively) but not between hydrologic phases (p > 0.05). The stations that showed the highest rarefied richness values were upstream Paraná River (USP) in both phases. Shannon diversity values ranged between 0.74 (Ch-L in high water) and 2.74 (USP in low water) (Table 3). No significant differences (p > 0.05) were found in the dominance (D) values between the hydrologic phases, although significant differences were recorded between river and floodplain environments (p = 0.028), with higher values in the floodplain habitats (Table 3).
The total density of exuviae and the density of dominant taxa showed significant differences only between the longitudinal and lateral dimensions (river/floodplain) (p = 0.037 and p = 0.028). The highest total density values were recorded in the floodplain environments (Ch-L: 698.72 and 615.09 exuviae.m−3/h in high- and low-water phases, respectively) (Fig 2). The dominant taxa (density and occurrence) in the different sites and both hydrologic phases were Onconeura spp., Lopescladius spp., Polypedilum spp., Paralauterborniella sp., and Harnischia spp.; Chironomidae association characteristics of the lotic habitats were Lopescladius spp., Onconeura spp., Polypedilum spp., Harnischia spp., Paralauterboniella sp., Coelotanypus sp., Axarus sp., and Rheotanytarsus sp., while in the floodplain habitats, Lopescladius spp., Paralauterboniella sp., Polypedilum spp., Harnischia spp., and Onconeura spp. were dominant (Table 2 and Fig 3).
Density of Chironomidae pupal exuviae in sample sites in the Paraná River system during high- and low-water phases. Abbreviations: USP Paraná River main channel, upstream from the connection with the floodplain lake channel; DSP Paraná River main channel, downstream from the connection with the floodplain lake channel; P-Ch beginning of the connection channel of the Paraná River main channel with the floodplain lake; Ch-L connection between the channel and the floodplain lake; L-SCh floodplain lake and the secondary channel connection.
Relative density of dominant taxa of Chironomidae pupal exuviae in sample sites in Paraná River system during high- and low-water phases. Abbreviations: USP Paraná River main channel, upstream from the connection with the floodplain lake channel; DSP Paraná River main channel, downstream from the connection with the floodplain lake channel; P-Ch beginning of the connection channel of the Paraná River main channel with the floodplain lake; Ch-L connection between the channel and the floodplain lake; L-SCh floodplain lake and the secondary channel connection.
The beta diversity values indicated that species turnover (β SIM) was higher than nestedness (β NES) in all environments and phases. Spatial total dissimilarity (β SOR) was higher in floodplain habitats and nestedness (β NES) was lower in main channel habitats. In relation to hydrological phases, the highest values of β SIM and the lower value of β NES were obtained in the low-water phase. On the other hand, in the high-water period, higher values of β NES were recorded (Fig 4).
The chironomid total density, richness (S), and dominance (D) were not significantly correlated with the different analyzed environmental variables. However, habitat depth was positively related to Shannon diversity and negatively related to the density of dominant taxa (Rho 0.69, p = 0.044; Rho −0.67, p = 0.050).
Discussion
In relation to the taxonomic composition, the 34 taxa of Chironomidae found from drifting pupal exuviae corresponding to Chironominae, Orthocladiinae, and Tanypodinae subfamilies were the prevailing taxa in the subtropical habitats. The genera and morphotypes from larval benthic stages found in the present study coincide with those recorded for the Paraná system by other authors (Marchese et al 2002, Marchese & Paggi 2004, Montalto & Paggi 2006, Ezcurra de Drago et al 2007, Mesa et al 2012, Zilli & Paggi 2013). On the other hand, the genus Robackia recorded in the present study in the main channel was cited only in its larval stage in ecological studies from Upper Paraná (Paggi et al 1998, Pinha et al 2013). In future researches, the development of exhaustive samplings could lead to the collection of immature and adult stages which, added to laboratory breeding, would allow the detailed study of the taxonomy, distribution, and lifecycle of this genus.
In the present study, which analyzed the diversity of heterogeneous environments with a great diversity of microhabitats, the CPET application made possible the recording of an important number of taxa from these different environments, as has been registered by other authors for other latitudes (Raunio et al 2007a).
For the same system, Mesa et al (2012) recorded 33 taxa of chironomid larval assemblage, similar to 34 taxa found in this study with CPET, but considering a higher number of sampling stations (three in Paraná River, three in the connection channel, and four in the lake) in both water phases of two hydroperiods. In this sense, it is clear that, in order to find a similar number of Chironomidae taxa from the benthic larval stages, 75% more samples were required with the consequent additional effort of extraction, separation, and determination.
According to the results obtained for a river-floodplain system, the CPET more easily obtained information about the Chironomidae inhabiting different assemblages in waterbodies of different habitats (pleuston, benthos, drifting, etc.). In addition, this technique allows for the sampling of wider areas than those obtained from benthic larvae as has been observed by other authors, providing a better knowledge of the regional pool of species (Ruse 1995, Raunio et al 2007a). This constitutes a great advantage with respect to other sampling techniques because sampling larval stages from benthos, pleuston, and drifting demands a greater effort and operational costs to obtain results comparable to those attained with the CPET.
Diversity and dominance indices allowed distinguishing of the environments in relation to the longitudinal and lateral dimensions (river/floodplain), with higher values of diversity and richness in the Paraná River sites and higher dominance values in all the floodplain environments. Thus, the diversity and density of dominant taxa were related only to depth of habitats, showing differences between the main channel sites (high depth and current velocity) and floodplain sites.
None of the diversity indicators presented differences between hydrologic phases. The pupal exuviae are the result of the adult emergence, a process which develops mainly in the spring-summer months. In the studied period, the samplings of the low- and high-water phases correspond to the beginning and end of summer, respectively, with similar temperatures, which could correspond to the similar values in this attribute for both phases.
In the present study, the highest values of richness and diversity were found in the main channel, indicating that drifting exuviae in the longitudinal axis come from different environments of a wider area (regional), while exuviae recorded in the connections of the floodplain environments in the lateral dimension could reflect the local assemblages.
The beta diversity values indicate that the species turnover is the dominant process in structuring studied assemblages in spatial and temporal analysis, increasing in the lateral axis in the floodplain habitats and in the low-water phase, being higher in relation to the contribution of the turnover component in environments with very different characteristics (main channel vs. floodplain lake and secondary channel). During the low-water phase, when the connectivity between the environments was low, the sampling stations showed a higher replacement. These results are coincident with Montalto and Paggi (2006) and Marchese et al (unpublished data), who carried out studies with larval chironomid assemblages in floodplain environments under a flood pulse regime and found the greatest richness and diversity in the lateral dimension environments with a higher heterogeneity and environmental dynamics (lakes, marginal wetlands, etc.). In concordance with our results, Thomaz et al (2007) concluded that the increment of dissimilarities in different large river floodplain habitats during low water levels is due to local forces that generate high inter-habitat heterogeneity (high beta diversity), and conversely, the homogenization produced by flood is derived from increased connectivity (regional driving force) during high waters.
The results obtained through CPET application allowed ecologic interpretations, because they showed differences in the sites located in the longitudinal and lateral axes, in the main channel and its floodplain, demonstrating the efficiency of this technique for the evaluation of the attributes of the drifting pupal exuviae assemblage in an integrated way. Finally, this study based on CPET is new for the region and proposes an efficient tool to be used in ecological studies as well as in biomonitoring.
References
Anderson AM, Ferrington LC Jr (2013) Resistance and resilience of winter-emerging Chironomidae (Diptera) to a flood event: implications for Minesota trout streams. Hydrobiologia 707:59–71
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Glob Ecol Biogeogr 19:134–143
Baselga A, Orme CDL (2012) Betapart: an R package for the study of beta diversity. Methods Ecol Evol 3:808–812
Cranston PS (1996) Identification guide to the Chironomidae of the New South Wales. Australian Water Technologies Pty Ltd, West Ryde, NSW, p 376
Drago EC (2007) The physical dynamics of the river–lake floodplain system. In: Iriondo MH, Paggi JC, Parma MJ (eds) The Middle Paraná River. Limnology of a subtropical wetland. Springer, Berling Heidelberg, New York, pp 83–122
Ezcurra de Drago I, Marchese M, Montalto L (2007) Benthic invertebrates. In: Iriondo MH, Paggi JC, Parma MJ (eds) The Middle Paraná River. Limnology of a subtropical wetland. Springer, Berling Heidelberg, New York, pp 251–275
Fend SV, Carter JL (1995) The relationship of habitat characteristics to the distribution of Chironomidae (Diptera) as a measure by pupal exuviae collections in a large river system. J Freshw Ecol 10:342–359
Ferrington LC (1987) Collection and identification of surface floating pupal exuviae of Chironomidae for use in studies of surface water quality. EPA Standard Operating Procedure N° FW 130 A. ENSV, Kansas
Ferrington Jr. LC, Blackwood MA, Wright CA, Crisp NH, Kavannaugh JL, Schmidt FJ (1991) A protocol for using surface-floating pupal exuviae of Chironomidae for rapid bio assessment of changing water quality
García PE, Añón Suarez DA (2007) Community structure and phenology of chironomids (Insecta: Chironomidae) in a Patagonian Andean stream. Limnologica 37:109–117
Hammer Ø, Harper DAT, Ryan PD (2009) PAST−PAlaeontological STatistics, ver. 1.89
Hardwick RA, Cooper PD, Cranston PS, Humphrey CL, Dostine PL (1995) Spatial and temporal distribution patterns of drifting pupal exuviae of Chironomidae (Diptera) in streams of tropical northern Australia. Freshw Biol 34:569–578
Heino J (2005) Metacommunity patterns of highly diverse stream midges: gradients, chequerboards, and nestedness, or is there only randomness? Ecol Entomol 30:590–599
Iriondo M (2007) Geomorphology. In: Iriondo MH, Paggi JC, Parma MJ (eds) The Middle Paraná River. Limnology of a subtropical wetland. Springer, Berling Heidelberg, New York, pp 33–52
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci 106:110–127
Marchese M, Ezcurra de Drago I, Drago E (2002) Benthic macroinvertebrates and physical habitat relationships in the Paraná River flood-plain system. In: McClain ME (ed) The ecohydrology of South American rivers and wetlands. IAHS Spec Publ no. 6, pp 111–130
Marchese M, Paggi AC (2004) Diversidad de Oligochaeta (Annelida) y Cironomidae (Diptera) del Litoral Fluvial Argentino. In: Aceñolaza FA (ed) Temas de la Biodiversidad del Litoral Fluvial Argentino. INSUGEO, Ediciones Magna, Tucumán, pp 217–224
Mauad M, Siri A, Donato M (2016) Does type of substratum affects chironomid assemblage composition? A study in a river catchment in Northern Patagonia, Argentina. Neotrop Entomol https://doi.org/10.1007/s13744-016-0429-3
McGill JD, Wilson RS, Brake AM (1979) The use of chironomid pupal exuviae in the surveillance of sewage pollution within a drainage system. Water Res 13:887–894
Medina AI, Scheibler EE, Paggi AC (2008) Distribución de Chironomidae (Diptera) en dos sistemas fluviales ritrónicos (Andino- serrano) de Argentina. Rev Soc Entomol Argen 67:69–79
Mesa LM, Marchese MR, Montalto L, Zilli FL (2012) Bidirectional exchanges of benthic invertebrates in a large river–floodplain system (Paraná River, Argentina). Ann Limnol-Internat J Limnol 48:425–436
Montalto L, Paggi AC (2006) Diversity of chironomid larvae in a marginal fluvial wetland of the Middle Paraná River floodplain, Argentina. Ann Limnol-Internat J Limnol 42:289–300
Neiff JJ (1999) El régimen de pulsos en ríos y grandes humedales. In: Malvarez AI (ed) Tópicos sobre humedales subtropicales y templados de Sudamérica. UNESCO, Montevideo, pp 97–146
Paggi AC (2009) Diptera Chironomidae. In: Dominguez E, Fernandez HR (eds) Macroinvertebrados bentónico sudamericanos. Sistemática y biología. Fundación Miguel Lillo, Tucumán, pp 383–410
Paggi AC, César I, Rodrigues Capítulo A (1998) Benthic studies in the zone of islands of Yacyretá prior to impoundment of the Upper Paraná River (Argentina). Verh Internat Verein Limnol 26:1089–1094
Paoli C, Schneider M (eds) (2000) El río Paraná en su tramo medio. Contribución al conocimiento y prácticas ingenieriles en un gran río de llanura. Tomo I. Centro de Publicaciones de la Universidad del Litoral, Santa Fe, p 307
Petsch DK, Pinha GD, Dias JD, Takeda AM (2015) Temporal nestedness in Chironomidae and the importance ofenvironmental and spatial factors in species rarity. Hydrobiologia 745:181–193
Pinha GD, Aviz D, Lopes Filho DR, Petsch DK, Marchese MR, Takeda AM (2013) Longitudinal distribution of Chironomidae (Diptera) downstream from a dam in a neotropical river. Braz J Biol 73:549–558
Raunio J, Heino J, Paasivirta L (2011) Non-biting midges in biodiversity conservation and environmental assessment: findings from boreal freshwater ecosystems. Ecol Indic 11:1057–1064
Raunio J, Ihaksi T, Haapala A, Muotka T (2007a) Within-and among-lake variation in benthic macroinvertebrate communities-comparison of profundal grab sampling and the chironomid pupal exuvial technique. J N Am Benthol Soc 26:708–718
Raunio J, Muotka T (2005) The use of chironomid pupal exuviae in river biomonitoring: the importance of sampling strategy. Arch Hydrobiol 164:529–545
Raunio J, Paasivirta L, Hämäläinen H (2010) Assessing lake thropic status using spring-emerging chironomid pupal exuviae. Fundam Appl Limnol 176:61–73
Raunio J, Paavola R, Muotka T (2007b) Effects of emergence phenology, taxa tolerances and taxonomic resolution on the use of the chironomid pupal exuvial technique in river biomonitoring. Freshw Biol 52:165–176
Ruse LP (1995) Chironomid community structure deduced from larvae and pupal exuviae of a chalk stream. Hydrobiologia 315:135–142
Siqueira T, Roque FO, Trivinho-Strixino S (2008) Phenological patterns of neotropical lotic chironomids: is emergence constrained by environmental factors? Aust Ecol 33:902–910
Siqueira T, Bini LM, Cianciaruso MV, Olivera Roque F, Trivinho-Strixino S (2009) The role of niche measures in explaning the abundance-distribution relationship in tropical lotic chironomids. Hydrobiologia 636:163–172
Scheibler EE, Pozo V, Paggi AC (2008) Distribución espacio-temporal de larvas de Chironomidae (Diptera) en un arroyo andino (Uspallata-Mendoza), Argentina. Rev Soc Entomol Argent 67:45–58
Thomaz SM, Bini LM, Bozelli RL (2007) Floods increase similarity among aquatic habitats in river-floodplain system. Hydrobiologia 579:1–13
Wiedenbrug S (2000) Studie zur Chironomidenfauna aus Bergbächen von Rio Grande do Sul, Brazilien. Ph.D Thesis, Munich, Ludwig-Maximilians-Universität
Wiederholm T (1986) Chironomidae of the Holartic region—keys and diagnoses. Part 2. Pupae-Entomol Scand Suppl 28:1–482
Wilson RS, Bright PL (1973) The use of chironomids pupal exuviae for characterizing streams. Freshw Biol 3:283–302
Wilson RS, Ruse LP (2005) A guide to the identification of genera of chironomid pupal exuviae occurring in Britain and Ireland (including common genera from northern Europe) and their use in monitoring lotic and lentic fresh waters. Special Publication No. 13. Freshw Biol Assoc. Ambleside
Zilli FL, Paggi AC (2013) Ecological responses to different degrees of hydrologic connectivity: assessing patterns in the bionomy of benthic chironomids in a large river-floodplain system. Wetlands 33:837–845
Acknowledgments
This study was supported by CAI+D 2011-535 (Universidad Nacional del Litoral) and PICT (Agencia Nacional de Promoción Científica y Tecnológica) 2012-2095. The authors are grateful to the anonymous reviewers and Mercedes Marchese for the critical reading of their article and their valuable suggestions, which contributed to improve the manuscript. We thank Mónica Cavilia and Proof Reading Service for language assistance. We are also grateful to Esteban Creus and Eduardo Lordi for their field assistance and Leticia Mesa for her assistance in the statistical analyses. This paper is Scientific Contribution No. 972 of the Instituto de Limnología “Raúl A. Ringuelet” (ILPLA, UNLP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Edison Ryoiti Sujii – Embrapa/Cenargen
Rights and permissions
About this article
Cite this article
Mestre, A.P., Paggi, A.C. & Montalto, L. The Application of Chironomid Pupal Exuvial Technique (CPET) for Ecological Analysis in a Neotropical Large River System. Neotrop Entomol 47, 619–627 (2018). https://doi.org/10.1007/s13744-017-0562-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0562-7