Abstract
Urban–industrial contamination in streams affects the functional structure of chironomid assemblages. The aims of this study were to determine whether water quality affects the functional diversity of Chironomidae, and which biological traits are related to water quality variables. We characterized species according to larval and pupal traits and used linear models to test whether functional richness and diversity were related to urban–industrial impact. We performed an RLQ and fourth-corner analysis to evaluate the relationship between traits and environmental variables. Functional richness and diversity were related negatively to nutrients and depth, and positively to temperature, macrophyte cover and dissolved oxygen in the different seasons. Body size, thoracic horn shape and current velocity preferences were significantly related to environmental variables. Our results suggest that nutrients may be an environmental filter for chironomids. The pupal thoracic horn was associated with highly impacted sites, suggesting that oxygen depletion could be an environmental challenge for pupae. Our study shows that urban–industrial impact decreases functional richness and diversity. Furthermore, because some characteristics of the pupae were sensitive to urban–industrial impact, the use of pupal traits is suggested in future biomonitoring analyses of aquatic environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urbanization is one of the most important processes that impairs the integrity of rivers (Allan, 2004; Vörösmarty et al., 2010; Wang et al., 2011). For example, the increase in impermeable surfaces intensifies flooding events, and runoff alters the loads of sediments, nutrients, and pollutants. These changes, jointly referred to as “urban stream syndrome”, lead to a decrease in biodiversity as tolerant species become dominant (Paul & Meyer, 2001; Meyer et al., 2005; Walsh et al., 2005; Cuffney et al., 2010; Mcgoff et al., 2013). In this context, biological traits analysis may explain how the environmental conditions of urban rivers determine the dominance of certain species. Biological traits are measurable features that influence species’ performance and roles in the ecosystem (Violle et al., 2007; Villeger et al., 2008). Moreover, the value and range of species traits in biological assemblages determine the functional diversity (Tillman, 2001). While species responses to environmental conditions depend on their biological traits, the functional diversity is considered to reveal more information about ecosystem functioning than taxonomic diversity.
Chironomids are one of the most important components of benthic assemblages in South American lowland rivers. They may be dominant in soft bed rivers, where they play a key role in processing organic matter (Sanseverino & Nessimian, 2008; Saigo et al., 2016) and in connecting basal resources to higher trophic levels (Saigo et al., 2015). Chironomids include species with contrasting biological traits such as feeding habits, habitat preferences, reproductive strategies, and life cycles (Maasri et al., 2008; Roque et al., 2010). Most studies on aquatic macroinvertebrates have focused on the larval stages of Chironomidae. The pupal stage of chironomids links two active phases of the insect's life: the larva and the adult. The pupal stage of chironomids is short, lasting from a few hours to several days. The value of the pupal stage was appreciated when pupal exuviae were studied as an aid to species identification and, later, in providing additional or alternative characters to larvae and adults in estimating phylogenetic relationships. Pupal exuviae have been used as a quick, simple way to obtain ecological information on aquatic habitats, and data on autoecology and geographic distribution. The chironomid pupal exuviae technique (CPET) has been used in a variety of studies to investigate the composition of the Chironomidae community (García & Añón Suárez, 2007; Anderson & Ferrington, 2012; Anderson et al., 2014; Mestre et al., 2018), and to monitor surface water quality (Wilson & Bright, 1973; Wilson & McGill, 1979; Raunio et al., 2007; Ruse, 2011) and phenology (Coffman, 1973, 1974; Zanotto Arpellino et al., 2022). Several studies report that relatively high percentages of species or genera can be detected with moderate effort associated with sample processing (Rufer & Ferrington Jr, 2008; Anderson & Ferrington, 2011; Bouchard & Ferrington, 2011). In addition, pupal exuviae represent taxa that have originated in a wide range of microhabitats (Ferrington et al., 1991; Wilson, 1994; Coffman & de la Rosa, 1998). Despite their numerous advantages, pupal exuviae have not been included in most biomonitoring studies. Coffman (1973), Wilson & Bright (1973) and Wilson (1980) list several advantages, including the fact that it is usually relatively simple to identify the genus, and generally also the species, by using appropriate keys and descriptions. Diagnostic characters in pupal structures can generally be seen even if the exuviae are mounted. In the case of larvae, it is necessary to clarify the specimen to see the diagnostic characters, and the incorrect position of structures or worn parts often makes it difficult to determine the status of the character of interest.
Some pupal traits can play a key role in determining the success of a species in an aquatic system. Species with a multibranched thoracic horn are better adapted to tolerating oxygen scarcity thanks to the increased surface area to volume ratio (Int Panis et al., 1996; Rossaro et al., 2007). Swimming structures help individuals to escape from the flow when the absence of stable substrates reduces shelter availability (Statzner, 2008). The lower the oxygen concentration in the habitat, the more extensive is the fringe—a structure that improves the respiratory movements to drive water through the tube produced in the larval stage (Langton, 1995). Tube construction in the larval stage provides advantages such as an adequate supply of oxygen and food in soft sediments, elimination of unneeded metabolites and carbon dioxide, and making the organism less visible to predators (Cranston, 1995; Van Kleef et al., 2015). Armament structures are used by the pupa to remain inside the tubes in environments with high energy flow (Langton, 1995), though in some species they may be used as an anchor within the tube to facilitate respiratory movements (Humphries, 1937).
The close relationship between the environmental conditions of the habitat and the biological traits of the species (river habitat templet; Townsend & Hildrew, 1994), allows predicting changes in invertebrate communities in environmental gradients (natural or anthropic) (Statzner et al. 2005). According to Bonada et al. (2006), using biological traits allows obtaining functional information, since many of the traits used are directly or indirectly related to ecological functions, they can be used in studies at different spatial scales, since most of the traits they are independent of taxonomic aspects and, therefore, also of the biogeographical region that is considered. And finally, simple hypotheses can be established to predict changes in communities due to changes in the environment. Despite these advantages, the use of biological traits requires detailed biological information for all species in a region, which is still incomplete (Statzner et al., 2007). An analysis of the relationship between water quality and the functional features of chironomids in Pampean rivers could provide important information about how this group responds to one of the main environmental threats in the region. Furthermore, focusing on pupae rather than larvae could result in novel insights about the environmental constraints that affect the aquatic stages of chironomids.
The present study analyzed traits of Chironomidae larvae and pupae in streams exposed to urban and industrial pollution. The aims were to (1) determine if there is an effect of natural spatial and/or temporal variation in the community of Chironomidae in the Pampean streams, together with the assemblage description using diversity indices, (2) determine whether water quality affects the functional diversity of Chironomidae and (3) determine which biological traits are related to water quality variables. According to these objectives, we hypothesized that (1) there is a natural variation among the streams studied, which can be quantified and taken into account to explain possible changes in the assemblages and functional traits at each sampling site, (2) urban and industrial uses decrease the functional diversity of chironomids and (3) pupal biological traits are filtered by environmental variables. In this study, the pupal exuviae technique is applied for the first time in chironomids, providing information on the characteristics of the chironomid community of first-order Pampas streams. This type of study is scarce worldwide and particularly in our region, and given the previously explained benefits of functional traits, we consider this work as a good contribution to understand the ecological dynamics of Chironomidae and its possible applications in bioassessment of contaminants and other fields such as climate change and conservation.
Materials and methods
Study area
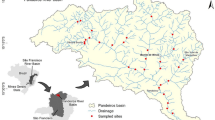
The study area comprises the southern sector of the Argentine Pampean grasslands. Rainfall and groundwater feed into the streams, which flow at low current velocity over a relief of gently sloping plains (0.3 m/km). The streambeds present a hard, homogeneous substratum, with high calcium carbonate content, upon which thin sediments are usually deposited (mainly silt and clay), without rocks or pebbles. The water in the Pampean streams is shallow, and has high conductivity (1,000–6,000 µS/cm) and slightly alkaline pH. However, most of the physical and chemical variables vary widely due to the occurrence of floods and seasonal changes in flow. High levels of nutrients have been recorded in these streams, related to lithology (Feijoó & Lombardo, 2007). It must be considered that areas with water erosion contribute additional concentrations of nutrients and sediments that also modify the quality of the water. A wide range of macrophytes grows on the streambeds, though macrophyte presence is variable and their coverage heterogeneous, depending, among other factors, on the season and the occurrence of heavy rainfall. The lack of autochthonous riparian forest determines high irradiation, even in the upper reaches of the streams, while the low current velocity and high nutrient level in the Pampean water bodies lead to regular eutrophication (Giorgi et al., 2005; Feijoó & Lombardo, 2007). According to Köppen’s classification, the climate in the study sector is temperate with oceanic influence. The summer is warm, and the winter is cool with frequent frosts, but without snow. The average annual temperature is 17 °C, with high humidity in all seasons. Rainfall is concentrated in the spring and summer months (source: Argentine National Weather Services).
To reduce natural variability, similar Pampean streams were selected. All the streams are first order and belong to the ecoregion Tributaries of the Paraná and Río de la Plata Rivers (Feijóo & Lombardo, 2007). The selected control sites are El Pescado Stream (P1 and P2), Cajaravilla Stream (CJ) (tributary of the El Pescado Stream), and Juan Blanco Stream (JB1; JB2 and JB3). Disturbed sites are the Rodríguez Stream (R1; R2 and R3) and Carnaval Stream (C1; C2 and C3) (Fig. 1).
Field design
At each sampling station, three samples were collected on the same day in every season, for two consecutive years (September 2016–August 2018). A D-net 25 cm in diameter and 250 μm mesh size was used to collect pupal exuviae. Sampling was active because of the low current in the streams. Three 30 m2 samples were collected from each stream, and fixed in situ with 80% ethanol. Pupal exuviae were separated under stereoscopic microscope at × 10 magnification and preserved in 80% ethanol. Pupal exuviae were mounted on permanent microscope slides following the technique proposed by Pinder (1986) for subsequent taxonomic identification to the lowest possible level using specific literature for the region and the corresponding keys (Wiederholm, 1986; Wiedenbrug, 2000; Wiedenbrug & Ospina-Torres, 2005; Merrit et al., 2008; Prat et al., 2014; among others). The specimens are deposited in the collection at Instituto de Limnología “Dr. Raúl A. Ringuelet” (ILPLA-CONICET-UNLP).
Along with the collection of exuviae, the following physical and chemical parameters were measured in the field: current velocity (Cvel; m/s) (measured mid-channel, average of three measurements) by timing a float as it moved over a known distance (Gordon et al., 1994), depth (cm), water temperature (Tw, °C), dissolved oxygen (DO, mg/l) (dissolved oxygen meter Lutron YK-22DO), pH (Universal pH Test Paper Strips), conductivity (cond; mS) (AD204 Standard conductivity pocket tester), and transparency (Tran; cm) (Secchi disk). Water samples were taken for laboratory determination of nitrate (N-NO3; mg N/l), nitrite (N-NO2; mg N/l), ammonium (N-NH4; mg N/l), total phosphorus (P-PO4; mg P/l), biological oxygen demand (BOD5) and chemical oxygen demand (COD), following standard methods (APHA, 1998). At each sampling session, the percentage of macrophyte coverage (macrop) was weighted by means of the Mapping Technique (Feijoó & Menéndez, 2009), which consists of establishing transects at known distances along the section under study and estimating the percentage of total macrophyte coverage. Greater macrophyte coverage provides many microhabitats and refuge sites for macroinvertebrates.
Data analysis
Assemblage description and natural variation analysis
For each sample site, we calculated density (number of exuviae/m2), species richness (S), diversity (Shannon–Wiener) and equitability, using program PAST 3.0 (Hammer et al., 2001). All community parameters between sample sites were analyzed using ANOVA. Because the assumptions of normality and homoscedasticity of the variance were not met, non-parametric Kruskal–Wallis tests were performed. This analysis was performed with R statistical software with the Agricolae package (de Mendiburu, 2019).
We used canonical correspondence analysis to evaluate the relationship between physical and chemical variables and species composition. Environmental variables were standardized (ter Braak, 1986) and selected according to their significance (p < 0.05). Multicollinearity was controlled by requiring a variance inflation factor (VIF) to be less than 20. These analyses were performed with the software CANOCO Windows version 4.5.
Seasonal differences in Chironomidae have been shown to vary naturally and must be used cautiously in environmental assessment (Lenat, 1983; Milošević et al., 2013, 2022). We performed two different multivariate analyses to assess the natural variability and discriminate it from variability caused by anthropogenic influence on both chironomid diversity and biotic metrics on the spatial and temporal scale. We performed a similarity analysis (ANOSIM) by applying the Bray–Curtis similarity index (Clarke, 1993) to examine whether there were significant differences in the density of species between streams in each season and between sites. Significance was computed by permutation of group membership with 999 replicates. We applied SIMPER (Similarity Percentage) analysis to samples which presented significant differences (p < 0.05), to assess which taxa are primarily responsible for the observed difference between groups of samples (Clarke, 1993). All these analyses were carried out in the statistical program PAST 3.0 (Hammer et al., 2001).
The natural variation in environmental variables on the spatial and temporal scale were assessed using the Kruskal–Wallis test, and the analyses were performed with R statistical software, applying the Agricolae package (de Mendiburu, 2019).
Biological traits analysis
We characterized species according to 3 larval and 7 pupal traits (see Online Resource 1—Supplementary Material). The affinity of each taxon with each trait modality was encoded using a fuzzy variable ranging from 0 to 3, where a score of 0 indicates non-affinity, while a score of 3 indicates strong affinity of a taxon for a trait modality. The information of the affinity of each taxon for each trait was obtained from the literature. All categories were evaluated at species level. When no information was available at species level, we used data at the genus level. Information on larvae was obtained from Saigo et al. (2016) and Ocón et al. (in press), based on studies conducted in Neotropical environments. Missing information was completed with data obtained by Vieira et al. (2006), and Serra et al. (2016).
As Chironomidae pupal traits have not yet been used, we proposed several characters to be analyzed in this study. Some pupal attributes were obtained de novo from the material collected for this study, and others from Zanotto Arpellino et al. (2022). To quantify the body size of pupae, we measured the length of the abdomen from segment 1 to the tip of the anal lobe, including the accessory structures such as anal macrosetae and/or the anal fringe. We did not consider the cephalothorax because the emergent adult splits the dorsal pupal thorax in a Y shape, deforming this segment and making measurement inaccurate. Although the analysis focused on the study of Chironomidae pupal traits, we decided to include larval traits to analyze and compare against pupal traits. All the traits used in this study are summarized in Table 1.
At each site in each season, we calculated two functional diversity measures: functional richness (FRic) and Quadratic Rao’s entropy (RaoQ) using the FD R package (Laliberté & Legendre, 2010). FRic is a measure of the overall spread of traits in a particular community (Villeger et al., 2008). RaoQ considers both the distribution of traits and species abundances, combining functional richness and divergence (Rao, 1982). To assess the relationship between functional diversity measures and environmental variables, we used linear models with an Akaike-based forward selection procedure. In these models, FRic and RaoQ were the dependent variables and environmental variables were the predictors. Finally, we performed an RLQ and fourth corner analysis (Dray et al., 2014) to evaluate the association between individual traits and environmental variables. The RLQ is an ordination method that combines the information of three matrices, one containing the values of environmental conditions in each site (called “R”) one containing the abundances of species in each site (called “L”) and finally one with the information about the biological traits of each taxon (called “Q”). Thus, the RLQ analysis explores the relationships between the environment and each biological trait. Moreover, the fourth corner method is a permutation method that evaluates the statistical significance of these relationships. All these analyses were performed in R statistical software, available in the Ade4 library.
Results
Natural variation analysis
The ANOSIM of the temporal variation in species richness showed seasonal differences in every stream except for El Pescado Stream (p < 0.0118), which established the independence of the biological functional traits analyzed in each season. SIMPER analysis enables examination of the species which contribute to the dissimilarity between the seasons, and was consistent with a previous study on the phenology and voltinism of chironomids for the streams analyzed (Zanotto Arpellino et al., 2022). Out of the four seasons, spatial variation was significant (p < 0.01) only in Spring (Table 2).
The Kruskal–Wallis test of environmental variables revealed significant differences among sites for all variables (Online Resources 2 and 3—Supplementary Material). The environmental variables in Juan Blanco Stream and El Pescado Stream were within the typical values for Pampean streams. Urban streams had the highest transparency and flow velocity (0–0.41 cm/s), and the lowest vegetation cover and depth. Rodríguez Stream had the highest levels of conductivity, pH, dissolved nutrients, BOD5, and COD.
Assemblage description
We collected a total 107,676 pupal exuviae belonging to 54 species and 31 genera. Most species were of the subfamily Chironominae (27), while Tanypodinae (14) and Orthocladiinae (13) were less represented.
At control sites, density ranged from 0.01 to 58.39 ind/m2 and the dominant taxa were Cricotopus sp.2, Parachironomus sp.2 and Rheotanytarsus sp.1. At highly impacted sites, average density ranged from 0.01 to 189.4 ind/m2 and the dominant taxa were Chironomus calligraphus Goeldi, 1905 and Cricotopus sp.1, which accounted for 92% of the total abundance. The highest species richness, diversity, and equitability were observed at sites C2, P2, and JB3, and the highest density was recorded in Rodríguez Stream (Table 3).
The first and second axis of the CCA explained 90.6% of the variance in species–environment relationship (Eigenvalue 0.478 and 0.428), and both were different from randomization (p < 0.002). The first ordination axis showed a human impact gradient, where control sites were displayed on the right side of the axis, while the more highly impacted sites were on the left. The DO, macrophyte cover and depth were on the right side, while dissolved nutrients, transparency, BOD5 and COD were on the left. Regarding species, C. calligraphus, Cricotopus sp.1 and Dicrotendipes embalsensis Paggi, 1987 were on the left side, while the rest of the species were on the right (Fig. 2).
Biplot showing distribution of sites vs. environmental variables (A) and species vs. environmental variables (B) with respect to the physical, chemical and hydraulic variables. Carnaval Stream (C1, C2, C3); Rodríguez Stream (R1, R2, R3); El Pescado Stream (P1, P2), Cajaravilla Stream (CJ), Juan Blanco Stream (JB1, JB2, JB3). Species acronyms are provided in Online Resource 1—Supplementary Material
Functional richness and diversity
In winter, both FRic and RaoQ were significantly related to environmental variables. There was a negative relationship between FRic and COD and between RaoQ and PO4 (p < 0.05). In autumn, there was no significant relationship between FRic and any environmental variable. Conversely, RaoQ showed positive significant relationships with DO, temperature, depth and macrophyte cover, and negative relationships with current velocity, transparency, NO3 and COD (p < 0.05). In spring, Fric was positively related to transparency, and negatively related to PO4 and NH4. RaoQ was positively related only to DO (P < 0.05). In summer, FRic was negatively related to PO4 and BOD, while RaoQ was positively related to DO and negatively related to NH4 and NO2 (p < 0.05, Table 4).
Biological traits vs. environmental variables
The fourth corner analysis revealed that the traits body size, thoracic horn shape and current velocity were significantly related to environmental variables (Table 5).
Notably, most of these traits refer somehow to the pupal stage. The traits plumose shape of the thoracic horn, larger mean abdominal length, and preference for medium current velocity showed a positive relationship with the variables associated with human impact (dissolved nutrients, conductivity, BOD5, and COD) and a negative relationship with the variables associated with control sites (Fig. 3).
Discussion
This study explored how water quality affects the functional diversity of Chironomidae assemblages. Our results showed that both taxonomic and functional structures of chironomid assemblages differed among streams with different water quality. The streams analyzed had high levels of nutrient concentration and organic matter, especially the Rodriguez Stream. This is typical of rivers that run through urban areas (Alexander et al., 2000; Rabalais, 2002).
The density and dominance metrics were higher in impacted rivers, while the opposite trend was observed for species richness and diversity. This is consistent with previous studies that reported a decline in taxonomic diversity in freshwater ecosystems subjected to change in water quality (Bini et al., 2014; Wang et al., 2016; Paz et al., 2022). Previous studies have reported that macroinvertebrate densities could be high in impacted rivers because of tolerant species thriving. Species composition also differed between rivers. Previous studies have concluded that the taxonomic composition of chironomid assemblages is sensitive to nutrient enrichment (Maasri et al., 2008; Stewart et al., 2014). Our results showed that C. calligraphus and Cricotopus sp.1 were dominant in the highly impacted stream, while in the rest of the rivers, these taxa were scarce or absent. This is in line with previous studies that reported high dominance of these taxa in impacted rivers (Marques et al., 1999; Vos et al., 2000; Rosa et al., 2014). Several authors have noted that several species of the genus Chironomus are tolerant and dominate many freshwater systems (Calle-Martínez & Casas, 2006; Chaib et al., 2011), and are therefore used as indicators of anthropic impact (Moller Pillot, 2009; Cortellezi et al., 2011).
At the functional level, we found a negative relationship between functional richness, Rao's quadratic entropy, and environmental variables that indicate human impact, such as dissolved nutrients, BOD5, and COD (Tilmann et al., 2001). This result is consistent with previous studies that reported lower functional diversity in impacted rivers (Flynn et al., 2009; Kuzmanovic et al., 2017; Gutierrez et al., 2020; Liu et al., 2021). In this context, numerous authors have suggested that urbanization could imply an environmental filter for species by means of habitat simplification, eutrophication, and pollution. This, in turn, could exert a homogenization effect on biological assemblages (Kuzmanovic, 2017), severely impairing their functions, stability, and resilience (Olden et al., 2004). However, the relationship between urban impact and community homogenization may be complex and context-dependent (Petsch et al., 2021). Our results suggest that in the systems studied, nutrient concentration and organic matter could imply an environmental filter for chironomids. This is consistent with the results reported for the Yangtze River, where the functional diversity of chironomids was negatively affected by eutrophication (Jiang et al., 2019), and natural forested areas promoted the functional diversity of macroinvertebrates (Liu et al., 2021). Similarly, an analysis of rivers impacted by trout farming in Serbia concluded that nutrient concentration (NH4 and NO3) was one of the main environmental drivers of Chironomidae assemblages (Milošević et al., 2018). A recent study performed in Mongolia showed that land use intensity led to a decline in functional diversity and that NO3 was one of the main drivers of biological traits of macroinvertebrate assemblages (Yadamsuren et al., 2020). In South America, Paz et al. (2022) also concluded that the functional diversity of macroinvertebrates was negatively affected in urban–industrial streams.
Our results revealed some relationship between water quality and individual biological traits. The plumose thoracic horn, which is a respiratory structure, was associated with highly impacted sites, showing a positive relationship with COD. The morphology of the thoracic horn provides an example of correspondence between morphological characters and physiological adaptation (Rossaro et al., 2007). Orthocladiinae species, which are mostly intolerant to low oxygen concentration, have a respiratory thoracic horn that is small or even absent (Marziali & Rossaro, 2005). Some Chironominae species which are tolerant to low oxygen levels have a well-developed thoracic horn with multiple branches. This could be an ecological adaptation to oxygen depletion as it increases the surface area for oxygen exchange by presenting a larger area/volume ratio than other types of thoracic horns (e.g., simple, branched) (Armitage et al., 1995). In a previous study, Paz et al. (2022) concluded that aerial respiration through spiracles in Chironomidae is a trait that is sensitive in urban–industrial streams. Nonetheless, in Chironomidae the spiracles are predominantly absent (apneustic), although the metapneustic condition (with posterior spiracles only) occurs in some Podonominae (Cranston, 1995).
This is in line with the fact that urban impact often causes dissolved oxygen deficit in rivers due to the input of oxygen-demanding substances, including dissolved organic matter (Sánchez et al., 2007; De Oliveira et al., 2016). In this context, Chironomidae larvae have traditionally been considered as tolerant to organic pollution (Rosenberg, 1992). Our results suggest that oxygen depletion could be a challenge to some Chironomid pupae.
The preference for medium flow velocity (1.7–3.3 cm/s) was related to many of the environmental variables that indicate urban impact. This is consistent with the fact that stream course modifications are known to alter river discharge (Dewson et al., 2007). However, this effect is context-dependent, as different land uses may exert contrasting effects on river discharges. On the one hand, Walsh et al. (2005) concluded that urban impact tends to increase river flow velocity by means of channel rectification and macrophyte removal. Because of their very low gradient, Pampean rivers are typically characterized by extremely low flow velocities (nearly 0 m/s) (Feijoó & Lombardo, 2007). The fact that species adapted to medium flow velocities were positively associated with nutrient concentrations and COD suggest a relationship between urban impact and river flow velocity in the study system.
Another trait associated with urban impact is larger body size, expressed in the trait mean abdominal length. In our results, mean abdominal length was associated positively with the variables indicating urban impact. Similar results were found by Serra et al. (2017).
Analysis of the biological traits of chironomid pupae was effective in detecting impacts due to changes in water quality in streams. Our results agree with previous studies performed in the region and elsewhere. However, whereas most of the studies neglected the pupal stage of aquatic insects, the present study provides novel information on this overlooked issue. The advantages of this approach are many. First, even though the pupa is the last aquatic stage in the chironomid life cycle, it determines whether an aquatic ecosystem is suitable for certain species (Wentsel et al., 1978). Moreover, the pupa is generally immobile and therefore a particularly vulnerable stage of the life cycle. Thus, focusing on larvae and neglecting pupae could lead researchers to underestimate the environmental challenges that chironomids face during the aquatic period of their life cycle. Moreover, the sampling method applied in this study has logistic advantages. While larvae sampling requires a great effort to cover the diversity of microhabitats in a river, exuviae sampling integrates the information of the entire river reach (Ferrington et al., 1991; Wilson, 1994; Coffman & de la Rosa, 1998).
Although chironomids are amongst the most diverse insect families (Armitage et al., 1995; Epler, 2001), in most studies they are identified at a relatively coarse level, conditioning the analyses of the ecological processes (Saulino & Trivinho-Strixino, 2018). In our study, at impacted sites, the subfamily Orthocladiinae was represented by only one taxon (Cricotopus sp.1). Two other Cricotopus were highly abundant at the reference sites. The genus Cricotopus is one of the Orthocladiinae genera with the largest number of species and found in a wide range of habitats and water quality conditions (Harrison, 1992; Anjos & Takeda, 2010; Drayson et al., 2015). The utilization of functional traits at genus level, or even family level, may be insufficient to explain the relation between functional diversity and biological traits. The use of chironomid exuviae enhances the precision of taxonomic determinations (Coffman, 1973; Wilson & Bright, 1973; Wilson, 1980) and may therefore lead to new insights into the ecological aspects of the species of Chironomidae for the Neotropics.
Finally, our results support previous studies that have shown the benefits of using chironomids in different bioassessment programs, estimation of specific types of contamination, as well as improvement and increase in bioassessment accuracy (Lenat, 1983; Koperski, 2009; Milosević et al., 2018). Although there is temporal variation of the species, our results show that such variation is determined mainly by phenology and voltinism, in agreement with Serra et al. (2017) and Zanotto Arpellino et al. (2022), and that this natural variability is a factor that should be considered in environmental evaluations (Kerans & Karr, 1994; Lindegaard & Brodersen, 1995; Langton & Casas, 1998; Rossaro et al., 2006).
Conclusion
The present study is, as far as we are aware, the first to address the effects of urban impact on functional diversity and biological traits of chironomid pupae. In line with previous research, we conclude that urban and industrial impact tend to decrease the functional richness and RaoQ quadratic entropy. Moreover, we reported that some pupal traits were sensitive to urban impact, suggesting that pupae could be a crucial stage of the chironomid life cycle in rivers under urban and industrial impact. The main traits associated with the contamination conditions were maintained in the different environments throughout all seasons, providing an important tool for biomonitoring aquatic environments.
References
Alexander, R. B., R. A. Smith & G. E. Schwarz, 2000. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403: 758–761.
Allan, J. D., 2004. Landscapes and riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology, Evolution, and Systematics 3: 257–284.
American Public Health Association (APHA), 1998. In Eaton, A. D., L. S. Clesceri, E. W. Rice & A. E. Greenberg (eds), Standard Methods for the Examination of Water and Wastewater 20th edition, American Water Works Association; Water Pollution Control Federation, Washington, DC.
Anderson, A. M. & L. C. Ferrington, 2011. In Proceedings of 18th International Symposium on Chironomidae on Fauna Norvegica, 2011, Vol. 31.
Anderson, A. M. & L. C. Ferrington, 2012. Time-efficiency of sorting Chironomidae surface-floating pupal exuviae samples from urban trout streams in Northeast Minnesota, USA. In Stur, E. E. & K. Aagaard (eds), 18th International Symposium on Chironomidae. Fauna Norvegica, Trondheim.
Anderson, A. M., P. Kranzfelder, A. T. Egan & L. C. Ferrington, 2014. Survey of Neotropical Chironomidae (Diptera) on San Salvador Island, Bahamas. Florida Entomologist 97: 304–308.
Anjos, A. F. & A. M. Takeda, 2010. Estrutura da comunidade das larvas de Chironomidae (Insecta: Diptera), em diferentes substratos artificiais e fases hídricas, no trecho superior do rio Paraná, Estado do Paraná, Brasil. Acta Scientiarum Biological Sciences 32: 131–140. https://doi.org/10.4025/actascibiolsci.v32i2.5387.
Armitage, P. D., P. S. Cranston & L. C. V. Pinder, 1995. The Chironomidae, Springer, Dordrecht:
Bini, L. M., V. L. Landeiro, A. A. Padial, T. Siqueira & J. Heino, 2014. Nutrient enrichment is related to two facets of beta diversity for stream invertebrates across the United States. Ecology 95: 1569–1578.
Bonada, N., N. Prat, V. H. Resh & B. Statzner, 2006. Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annual Review of Entomology 51: 495–523.
Bouchard, R. W. & L. C. Ferrington, 2011. The effects of subsampling and sampling frequency on the use of surface-floating pupal exuviae to measure Chironomidae (Diptera) communities in wadeable temperate streams. Environmental Monitoring and Assessment 181: 205–223. https://doi.org/10.1007/s10661-010-1824-6.
Calle-Martínez, D. & J. J. Casas, 2006. Chironomid species, stream classification, and water-quality assessment: the case of 2 Iberian Mediterranean Mountain regions. Journal of the North American Benthological Society 25: 465–476.
Chaib, N., B. Samraoui, L. Marziali & B. Rossaro, 2011. Chironomid taxocenosis in a South Mediterranean Wadi, the Kebir-East (Algeria). Studi Trentini di Scienze Naturali 88: 61–75.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143.
Coffman, W. P., 1973. Energy flow in a woodland stream ecosystem: II. The taxonomic composition and phenology of the Chironomidae as determined by the collection of pupal exuviae. Archiv fur Hydrobiologie 71: 281–322.
Coffman, W. P., 1974. Seasonal differences in the diel emergence of a lotic chironomid community. Entomologisk Tidskrift Supplement 95: 42–48.
Coffman, W. P. & C. L. de la Rosa, 1998. Taxonomic composition and temporal organization of tropical and temperate species assemblages of lotic Chironomidae. Journal of the Kansas Entomological Society 71: 388–406.
Cortelezzi, A., A. C. Paggi, M. Rodríguez & A. R. Capítulo, 2011. Taxonomic and nontaxonomic responses to ecological changes in an urban lowland stream through the use of Chironomidae (Diptera) larvae. Science of the Total Environment 409: 1344–1350. https://doi.org/10.1016/j.scitotenv.2011.01.002.
Cranston, P. S., 1995. Morphology. In Armitage, P. D., P. S. Cranston & L. C. V. Pinder (eds), Chironomidae: Biology and Ecology of Non-biting Midges Chapman and Hall, London: 11–30.
Cuffney, T. F., G. Mcmahon, R. Kashuba, J. T. May & I. R. Waite, 2010. Responses of benthic macroinvertebrates to environmental changes associated with urbanization in nine metropolitan areas. Ecological Application 20: 1384–1401. https://doi.org/10.1890/08-1311.1.
de Mendiburu, F., 2019. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-1 [available on internet at: https://CRAN.R-project.org/package=agricolae. Accessed 15 July 2020.
De Oliveira, L. M., P. Maillard & É. J. de Andrade Pinto, 2016. Modeling the effect of land use/land cover on nitrogen, phosphorous and dissolved oxygen loads in the Velhas River using the concept of exclusive contribution area. Environmental Monitoring and Assessment 188: 333. https://doi.org/10.1007/s10661-016-5323-2.
Dewson, Z. S., A. B. James & R. G. Death, 2007. A review of the consequences of decreased flow for instream habitat and macroinvertebrates. Journal of the North American Benthological Society 26: 401–415.
Dray, S., P. Choler, S. Dolédec, P. R. Peres-Neto, W. Thuiller, S. Pavoine & C. J. F. ter Braak, 2014. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95: 14–21. https://doi.org/10.1890/13-0196.1.
Drayson, N., P. S. Cranston & M. N. Krosch, 2015. Taxonomic review of the chironomid genus Cricotopus v.d. Wulp (Diptera: Chironomidae) from Australia: keys to males, females, pupae and larvae, description of ten new species and comments on Paratrichocladius Santos Abreu. Zootaxa 3919: 1. https://doi.org/10.11646/zootaxa.3919.1.1.
Epler, J. H., 2001. Identification Manual for the Larval Chironomidae (Diptera) of North and South Carolina: A Guide to the Taxonomy of the Midges of the Southeastern United States, Including Florida. St. Johns River Water Management District.
Feijoó, C. & R. J. Lombardo, 2007. Baseline water quality and macrophyte assemblages in Pampean streams: a regional approach. Water Research 41: 1399–1410.
Feijoó, C. & M. Menéndez, 2009. La biota de los ríos: los macrófitas. In Elosegi, A. & S. Sabater (eds), Conceptos y técnicas en ecología fluvial Fundación BBVA, Barcelona: 367–386.
Ferrington, L. C. Jr., M. A. Blackwood, C. A. Wright, N. H. Crisp, J. L. Kavanaugh & F. J. Schmidt, 1991. Protocol for Using Surface-Floating Pupal Exuviae of Chironomidae for Rapid Bioassessment of Changing Water Quality. The 20th General Assembly of the International Union of Geodesy and Geophysics, Vienna: 181–190.
Flynn, D. F. B., M. Gogol-Prokurat, T. Nogeire, N. Molinari, B. T. Richers, B. B. Lin, N. Simpson, M. M. Mayfield & F. DeClerck, 2009. Loss of functional diversity under land use intensification across multiple taxa. Ecology Letters 12: 22–33. https://doi.org/10.1111/j.1461-0248.2008.01255.x.
García, P. E. & D. A. Añón Suarez, 2007. Community structure and phenology of chironomids (Insecta: Chironomidae) in a Patagonian Andean stream. Limnologica 37: 109–117.
Giorgi, A., C. Feijoó & G. Tell, 2005. Primary producers in a Pampean stream: temporal variation and structuring role. Biodiversity and Conservation 14: 1699–1718.
Gordon, N. D., T. A. McMahon & B. L. Finlayson, 1994. Stream Hydrology, an Introduction for Ecologists, Wiley, New York:
Gutierrez, M. F., N. R. Simões, D. Frau, M. Saigo & M. Licursi, 2020. Responses of stream zooplankton diversity metrics to eutrophication and temporal environmental variability in agricultural catchments. Environmental Monitoring and Assessment 192: 1–17.
Hammer, Ø., D. A. T. Harper & P. D. Ryan, 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4: 1–9 [available on internet at: http://palaeo-electronica.org/2001_1/past/issue1_01.htm].
Harrison, A. D., 1992. Chironomidae from Ethiopia. Part 2. Orthocladiinae, with two new species and a key to Thienemanniella Kieffer (Insecta: Diptera). Spixiana 15: 149–195.
Humphries, F. C., 1937. Neue Trichocladius-Arten. Stettiner Entomologische Zeitung 98: 185–195.
Int Panis, L., B. Goddeeris & R. Verheyen, 1996. On the relationship between vertical microdistribution and adaptations to oxygen stress in littoral Chironomidae (Diptera). Hydrobiologia 318: 61–67.
Jiang, X., B. Pan, Z. Song & Z. Xie, 2019. Do functional traits of chironomid assemblages respond more readily to eutrophication than taxonomic composition in Chinese floodplain lakes? Ecological Indicators 103: 355–362.
Kerans, B. L. & J. R. Karr, 1994. A benthic index of biotic integrity (B-IBI) for rivers of the Tennessee Valley. Ecological Applications 4: 768–785. https://doi.org/10.2307/1942007.
Kuzmanovic, M., S. Dolédec, N. de Castro-Catala, A. Ginebreda, S. Sabater, I. Muñoz & D. Barceló, 2017. Environmental stressors as a driver of the trait composition of benthic macroinvertebrate assemblages in polluted Iberian rivers. Environmental Research 156: 485–493. https://doi.org/10.1016/j.envres.2017.03.054.
Laliberté, E. & P. Legendre, 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91: 299–305. https://doi.org/10.1890/08-2244.1.
Langton, P. H., 1995. The pupa and events leading to eclosion. In Armitage, P. D., P. S. Cranston & L. C. V. Pinder (eds), The Chironomidae. The Biology and Ecology of Non-biting Midges Chapman & Hall, London: 169–193.
Langton, P. H. & J. Casas, 1998. Changes in chironomid assemblage composition in two Mediterranean mountain streams over a period of extreme hydrological conditions. Hydrobiologia 390: 37–49. https://doi.org/10.1023/A:1003589216389.
Lenat, D. R., 1983. Chironomid taxa richness: natural variation and use in pollution assessment. Freshwater Invertebrate Biology 2: 192–198. https://doi.org/10.2307/1467151.
Lindegaard, C. & K. P. Brodersen, 1995. Distribution of Chironomidae (Diptera) in the River Continuum Chironomidae: From Genes to Ecosystems, CSIRO Publications, Melbourne:, 257–271.
Liu, Z., Z. Li, D. M. P. Castro, X. Tan, X. Jiang, X. Meng, Y. Ge & Z. Xie, 2021. Effects of different types of land-use on taxonomic and functional diversity of benthic macroinvertebrates in a subtropical river network. Environmental Science and Pollution Research 28: 44339–44353. https://doi.org/10.1007/s11356-021-13867-w.
Maasri, A., S. Fayolle, E. Gandouin, R. Garnier & E. Franquet, 2008. Epilithic chironomid larvae and water enrichment: is larval distribution explained by epilithon quantity or quality? Journal of the North American Benthological Society 27: 38–51.
Marques, M. M., F. A. Barbosa & M. Callisto, 1999. Distribution and abundance of Chironomidae (Diptera, Insecta) in an impacted watershed in south-east Brazil. Revista Brasileira de Biologia 59: 553–561.
Marziali, L. & B. Rossaro, 2005. Thoracic horn structure in Orthocladiini pupae (Diptera: Chironomidae: Orthocladiinae). I. Studi Trentini di Scienze Naturali, Acta Biologica 82: 69–76.
Mcgoff, E., A. G. Solimini, M. T. Pusch, T. Jurca & L. Sandin, 2013. Does lake habitat alteration and land-use pressure homogenize European littoral macroinvertebrate communities? Journal of Applied Ecology 50: 1010–1018. https://doi.org/10.1111/1365-2664.12106.
Merritt, R. W., K. W. Cummins & M. B. Berg, 2008. An Introduction to the Aquatic Insects of North America, Kendall-Hunt, Dubuque:
Mestre, A. P., A. C. Paggi & L. Montalto, 2018. The application of chironomid pupal exuvial technique (CPET) for ecological analysis in a Neotropical large river system. Neotropical Entomology 47: 619–627.
Meyer, J. L., M. J. Paul & W. K. Taulbee, 2005. Stream ecosystem function in urbanizing landscapes. Journal of the North American Benthological Society 24: 602–612. https://doi.org/10.1899/04-021.1.
Milošević, D., V. Simić, M. Stojković, D. Čerba, D. Mančev, A. Petrović & M. Paunović, 2013. Spatio-temporal pattern of the Chironomidae community: toward the use of non-biting midges in bioassessment programs. Aquatic Ecology 47: 37–55. https://doi.org/10.1007/s10452-012-9423-y.
Milošević, D., K. Stojanović, A. Djurdjević, Z. Marković, M. S. Piperac, M. Živić & I. Živić, 2018. The response of chironomid taxonomy-and functional trait-based metrics to fish farm effluent pollution in lotic systems. Environmental Pollution 242: 1058–1066.
Milošević, D., A. S. Medeiros, D. Cvijanović, D. Jenačković Gocić, A. Đurđević, D. Čerba & M. Stojković Piperac, 2022. Implications of local niche- and dispersal-based factors that may influence chironomid assemblages in bioassessment. Environmental Science and Pollution Research 29: 51951–51963. https://doi.org/10.1007/s11356-022-19302-y.
Moller Pillot, H. K. M., 2009. Chironomidae Larvae – Biology and Ecology of the Chironomini, vol. II. KNNV Publishing, Zeist:
Ocon, C., A. Siri, P. Altieri & M. Donato, in press. Functional Feeding Groups of Chironomidae (Diptera: Nematocera) and Their Spatial Variation in an Intermittent Hill Stream (Ventana Stream, Buenos Aires, Argentina). Anais da Academia Brasileira de Ciências.
Olden, J. D., N. L. Poff, M. E. Douglas & K. D. Fausch, 2004. Ecological and evolutionary consequences of biotic homogenization. Trends in Ecology and Evolution 19: 18–24. https://doi.org/10.1016/j.tree.2003.09.010.
Paul, M. J. & J. L. Meyer, 2001. Streams in the urban landscape. Annual Review of Ecology and Systematics 32: 333–365. https://doi.org/10.1146/annurev.ecolsys.32.081501.114040.
Paz, L. E., M. Rodriguez, B. Gullo & A. Rodrigues Capítulo, 2022. Impacts of urban and industrial pollution on functional traits of benthic macroinvertebrates: are some traits advantageous for survival? Science of the Total Environment 807: 150650. https://doi.org/10.1016/j.scitotenv.2021.150650.
Petsch, D. K., V. S. Saito, V. L. Landeiro, T. S. F. Silva, L. M. Bini, J. Heino, J. Soininen, K. T. Tolonen, J. Jyrkänkallio-Mikkola, V. Pajunen, T. Siqueira & A. S. Melo, 2021. Beta diversity of stream insects differs between boreal and subtropical regions, but land use does not generally cause biotic homogenization. Freshwater Science 40: 53–64.
Pinder, L. C. V., 1986. The pupae of Chironomidae (Diptera) of the Holarctic region – Introduction. In Wiederholm, T. (ed), Chironomidae of the Holarctic region Keys and diagnoses. Part 2. Pupae Entomologica Scandinavica Supplement 28: 5–7.
Prat, N., J. D. Gonzalez-Trujillo & R. Ospina-Torres, 2014. Clave para la determinación de exuvias pupales de Chironomidae (Diptera: Chironomidae) de ríos tropicales del alto Andino. Revista de Biología Tropical 62: 1385–1406.
Rabalais, N. N., 2002. Nitrogen in aquatic ecosystems. AMBIO: A Journal of the Human Environment 31: 102–112. https://doi.org/10.1579/0044-7447-31.2.102.
Rao, C. R., 1982. Diversity and dissimilarity coefficients: a unified approach. Theoretical Population Biology 21: 24–43.
Raunio, J., R. Paavola & T. Muotka, 2007. Effects of emergence phenology, taxa tolerances and taxonomic resolution on the use of the Chironomid Pupal Exuvial Technique in river biomonitoring. Freshwater Biology 52: 165–176.
Roque, F. O., T. Siqueira, L. M. Bini, M. C. Ribeiro, L. R. Tambosi, G. Ciocheti & S. Trivinho-Strixino, 2010. Untangling associations between chironomid taxa in Neotropical streams using local and landscape filters. Freshwater Biology 55: 847–865.
Rosa, B. J. F. V., L. F. T. Rodrigues, G. S. de Oliveira & R. da Gama Alves, 2014. Chironomidae and Oligochaeta for water quality evaluation in an urban river in southeastern Brazil. Environmental Monitoring and Assessment 186: 7771–7779. https://doi.org/10.1007/s10661-014-3965-5.
Rosenberg, D., 1992. Freshwater biomonitoring and Chironomidae. Netherland Journal of Aquatic Ecology 26: 101–122. https://doi.org/10.1007/BF02255231.
Rossaro, B., V. Lencioni, A. Boggero & L. Marziali, 2006. Chironomids from Southern Alpine running waters: ecology, biogeography. Hydrobiologia 562: 231–246. https://doi.org/10.1007/s10750-005-1813-x.
Rossaro, B., A. Solimini, V. Lencioni, L. Marziali, R. Giacchini & P. Parenti, 2007. The relationship between body size, pupal thoracic horn development and dissolved oxygen in Chironomini (Diptera: Chironomidae). Fundamental and Applied Limnology 169: 331–339. https://doi.org/10.1127/1863-9135/2007/0169-0331.
Rufer, M. R. & L. C. Ferrington Jr., 2008. Sampling frequency required for chironomid community resolution in urban lakes with contrasting trophic states. Boletim do Museu Municipal do Funchal (História Natural) Supplement 13: 77–84.
Ruse, L., 2011. Lake acidification assessed using chironomid pupal exuviae. Fundamental and Applied Limnology 178: 267–286. https://doi.org/10.1127/1863-9135/2011/0178-0267.
Saigo, M., F. L. Zilli, M. R. Marchese & D. Demonte, 2015. Trophic level, food chain length and omnivory in the Paraná River: a food web model approach in a floodplain river system. Ecological Research 30: 843–852. https://doi.org/10.1007/s11284-015-1283-1.
Saigo, M., M. Marchese & K. M. Wantzen, 2016. A closer look at the main actors of Neotropical floodplain food webs: functional classification and niche overlap of dominant benthic invertebrates in a floodplain lake of Paraná River. Iheringia. Série Zoologia 106.
Sánchez, E., M. F. Colmenarejo, J. Vicente, A. Rubio, M. G. García, L. Travieso & R. Borja, 2007. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecological Indicators 7: 315–328.
Sanseverino, A. M. & J. L. Nessimian, 2008. The food of larval Chironomidae (Insecta, Diptera) in submerged litter in a forest stream of the Atlantic Forest (Rio de Janeiro, Brazil). Acta Limnologica Brasiliensia 20: 15–20.
Saulino, H. H. L. & S. Trivinho-Strixino, 2018. Body length determines the diet and niche specialization of non-biting midge predator (Tanypodinae) larvae in shallow reservoirs. Neotropical Entomology 48: 136–142. https://doi.org/10.1007/s13744-018-0620-9.
Serra, S. R. Q., F. Cobo, M. A. S. Graça, S. Dolédec & M. J. Feio, 2016. Synthesising the trait information of European Chironomidae (Insecta: Diptera): toward a new database. Ecological Indicators 61: 282–292. https://doi.org/10.1016/j.ecolind.2015.09.028.
Serra, S. R. Q., M. A. S. Graça, S. Dolédec & M. J. Feio, 2017. Chironomidae traits and life history strategies as indicators of anthropogenic disturbance. Environmental Monitoring and Assessment 189: 326. https://doi.org/10.1007/s10661-017-6027-y.
Statzner, B., 2008. How views about flow adaptations of benthic stream invertebrates changed over the last century. International Review of Hydrobiology 93: 593–605.
Statzner, B., P. Bady, S. Dolédec & F. Schöll, 2005. Invertebrate traits for the biomonitoring of large European rivers: an initial assessment of trait patterns in least impacted river reaches. Freshwater Biology 50: 2136–2161.
Statzner, B., N. Bonada & S. Dolédec, 2007. Conservation of taxonomic and biological trait diversity of European stream macroinvertebrate communities: a case for a collective public database. Biodiversity and Conservation 16: 3609–3632.
Stewart, E. M., R. McIver, N. Michelutti, M. S. V. Douglas & J. P. Smol, 2014. Assessing the efficacy of chironomid and diatom assemblages in tracking eutrophication in High Arctic sewage ponds. Hydrobiologia 721: 251–268.
Ter Braak, C. F. J., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
Tilman, D., J. Fargione, B. Wolff, C. D’Antonio, A. Dobson, R. Howarth, D. Schindler, W. H. Schlesinger, D. Simberloff & D. Swackhamer, 2001. Forecasting agriculturally driven global environmental change. Science 292: 281–284.
Townsend, C. R. & A. G. Hildrew, 1994. Species traits in relation to a habitat templet for river systems». Freshwater Biology 31: 265–275.
Van Kleef, H., W. C. E. P. Verberk, F. F. P. Kimenai, G. van der Velde & R. S. E. W. Leuven, 2015. Natural recovery and restoration of acidified shallow soft-water lakes: successes and bottlenecks revealed by assessing life-history strategies of chironomid larvae. Basic and Applied Ecology 16: 325–334. https://doi.org/10.1016/j.baae.2015.02.007.
Vieira, N. K. M., N. L. Poff, D. M. Carlisle, S. R. Moulton, M. L. Koski & B. C. Kondratieff, 2006. A Database of Lotic Invertebrate Traits for North America. US Geological Survey Data Series 187. US Geological Survey, US Department of the Interior, Reston.
Villeger, S., N. W. H. Mason & D. Mouillot, 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89: 2290–2301.
Violle, C., M. L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel & E. Garnier, 2007. Let the concept of trait be functional! Oikos 116: 882–892. https://doi.org/10.1111/j.2007.0030-1299.15559.x.
Vörösmarty, C. J., P. B. McIntyre, M. O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S. E. Bunn, C. A. Sullivan, C. R. Liermann & P. M. Davies, 2010. Global threats to human water security and river biodiversity. Nature 467: 555–561. https://doi.org/10.1038/nature09440.
Vos, J. H., M. A. G. Ooijevaar, J. F. Postma & W. Admiraal, 2000. Interactions between food availability and food quality during growth of early instar chironomid larvae. Journal of the North American Benthological Society 191: 58–168.
Walsh, C. J., A. H. Roy, J. W. Feminella, P. D. Cottingham, P. M. Groffman & R. P. Morgan, 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of the North American Benthological Society 24: 706–723. https://doi.org/10.1899/04-028.1.
Wang, B., D. Liu, S. Liu, Y. Zhang, D. Lu & L. Wang, 2011. Impacts of urbanization on stream habitats and macroinvertebrate communities in the tributaries of Qiangtang River, China. Hydrobiologia 680: 39–51. https://doi.org/10.1007/s10750-011-0899-6.
Wang, W., H. Wang, Y. Feng, L. Wang, X. Xiao, Y. Xi, X. Luo, R. Sun, X. Ye, Y. Huang, Z. Zhang & Z. Cui, 2016. Consistent responses of the microbial community structure to organic farming along the middle and lower reaches of the Yangtze River. Scientific Reports 6: 1–11.
Wentsel, R., A. McIntosh & W. P. McCafferty, 1978. Emergence of the midge Chironomus tentans when exposed to heavy metal contaminated sediment. Hydrobiologia 57: 195–196.
Wiedenbrug, S., 2000. Studie zur Chironomidenfauna aus Bergbächen von Rio Grande do Sul, Brasilien, Gedruckt mit Unterstützung des Deutschen Akademischen Austauschdienstes, München:
Wiedenbrug, S. & R. Ospina-Torres, 2005. A key to pupal exuviae of Neotropical Tanytarsini (Diptera: Chironomidae). Amazoniana 18: 317–371.
Wiederholm, T., 1986. Chironomidae of the Holarctic Region-Keys and diagnoses. Part 2. Pupae-Entomologica Scandinavica Supplement 28: 1–482.
Wilson, R. S., 1980. Classifying rivers using chironomid pupal exuviae. In Murray, D. A. (ed), Chironomidae – Ecology, Systematics, Cytology and Physiology Pergamon Press, Oxford: 209–216.
Wilson, R. S., 1994. Monitoring the effect of sewage effluent on the Oxford Canal using chironomid pupal exuviae. Water and Environmental Management 8: 171–182.
Wilson, R. S. & P. L. Bright, 1973. The use of chironomid pupal exuviae for characterizing streams. Freshwater Biology 3: 283–302.
Wilson, R. S. & J. D. McGill, 1979. The Use of Chironomid Pupal Exuviae for Biological Surveillance of Water Quality. Department of the Environment, Water Data Unit, Technical Memorandum 18.
Yadamsuren, O., J. C. Morse, B. Hayford, J. K. Gelhaus & P. H. Adler, 2020. Macroinvertebrate community responses to land use: a trait-based approach for freshwater biomonitoring in Mongolia. Hydrobiologia 847: 1887–1902.
Zanotto Arpellino, J. P., L. N. S. Rodríguez Catanzaro, L. Montalto, A. Siri & M. Donato, 2022. Diversity, phenology and voltinism of Chironomidae (Diptera). Neotropical streams as a study model. Anais da Academia Brasileira de Ciências 94(4): e20200314.
Acknowledgements
We would like to thank the two anonymous reviewers for comments and suggestions on the manuscript. This study was supported by Universidad Nacional del Litoral (PIC CAI+D 2016 50420150100016), Universidad Nacional de La Plata (FCNyM, #11/N785), and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP #1010). We would like to thank Lic. Jorge Donadelli for nutrient analyses, C. Connon for copyediting, and UNL-CONICET and UNLP-CONICET for their support. This article is scientific contribution number 1252 of the Institute of Limnology “Dr. Raúl A. Ringuelet” (ILPLA, CCT-La Plata CONICET, UNLP).
Author information
Authors and Affiliations
Contributions
All authors contributed to planning and manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest.
Ethical approval
Not applicable.
Consent for publication
All authors declare consent for publication.
Informed consent
All authors declare consent for publication.
Additional information
Handling editor: María del Mar Sánchez-Montoya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanotto Arpellino, J.P., Saigo, M., Montalto, L. et al. Larvae and pupae as indicators of anthropic disturbances: use of traits. Hydrobiologia 850, 4293–4309 (2023). https://doi.org/10.1007/s10750-023-05305-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05305-4