Abstract
Excrement scarcity is one of the main historical factors leading dung beetles to adapt to other food resources. In the Caatinga, a seasonally tropical dry forest, harsh environmental conditions seem to restrict the availability of food resources. In this scenario, the aim of our study was to experimentally investigate the attractiveness of different potential food resources to these insects in the Caatinga. Field samplings were performed, and we tested five resources in pitfall baited traps: excrement, carrion, fruits of two species of columnar cacti, and seeds of one species of Euphorbiaceae (Jatropha mollissima (Pohl)). In a controlled setting, we tested dietary preferences of Deltochilum verruciferum Felsche by offering one or two resources simultaneously. In the field experiments, 297 dung beetles (9 species) were recovered from the traps, and D. verruciferum was the most abundant species. Carrion and excrement were the most attractive resources. Controlled dietary preference tests with D. verruciferum evidenced that these beetles used all tested food resources, excrement and carrion most pronouncedly. Our findings support copro-necrophagy as the main feeding habit of D. verruciferum, but also suggest that alternative resources might be utilized if preferred resources are scarce.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sharing of resources among species may lead to strong selective and evolutionary pressure (Raven & Johnson 2002). To avoid competition, some species have converged towards more specialized niche occupation strategies, thus utilizing resources that are less contested by syntopic consumers (Hutchinson 1957). Other species might adapt to more generalistic habits, increasing their chances to obtain food, even under competitive scenarios of resource scarcity (Devictor et al 2008, Halffter & Halffter 2009). Shifts on dietary preferences may occur even within populations of a given species, with some individuals presenting distinct diets (Costa et al 2015). Thus, on the present evolutionary configuration, generalist species are often called “winners” under the current scenario of natural habitat anthropization, which hinders the maintenance of more specialized habits (McKinney & Lockwood 1999). However, from a phylogenetic point of view, it seems that specialized organisms may not always be vulnerable to habitat modification, because on some situations, the disappearance or depletion of a resource might allow the rise of a new one (Colles et al 2009). In addition, specific interactions among certain taxa (e.g. specialized herbivores) that are associated with deforested habitats can also be favored by environmental disturbances (Maia et al 2010).
The evolutionary history of dung beetles (Scarabaeinae) evidence dietary adaptations influenced by food scarcity (Davis et al 2002, Halffter & Halffter 2009). Coprophagy, often interpreted as the main trophic guild of the group, is constantly replaced or complemented by other alternative feeding habits (Hanski & Cambefort 1991, Scholtz et al 2009). On a general context, dung beetles feed on small particles, bacteria, and the liquids filtered from excrement and other types of decomposing resources, comprising a diet rich on proteins and carbohydrates (Scholtz et al 2009). Apparently early on their evolution, during the lower Cretaceous, these beetles presented a saprophagous diet based on decaying plant matter (Ahrens et al 2014). The emergence of large-sized vertebrate herbivores led to hugely increased availability of excrement, which arguably exhibited attractive characteristics (odor and nutritional composition) similar to those found on decaying plant matter, favoring the adaptation of dung beetles to coprophagy (Davis et al 2002, Ahrens et al 2014).
Depending on the region of the world, the feeding habits of the dung beetles can be wider than the strict coprophagy (Monteith & Storey 1981, Hanski & Cambefort 1991, Halffter & Halffter 2009). In the neotropics, for instance, dung beetles exhibit a wide variety of alternative feeding habits, such as necrophagy, carpophagy, even predation (Larsen et al 2009, Halffter & Halffter 2009). Such broad assortment is associated with some factors, such as (1) biogeographical scarcity of large mammals, (2) availability of varied potentially exploitable alternative food resources, and (3) trophic niches that historically were not excessively fought over (e.g. carrion), favoring their utilization (Halffter & Matthews 1966).
Arid and semi-arid tropical environments are strongly restrictive regarding resource availability (Ma et al 2008, Valera et al 2011). Due to harsh conditions, such as high temperatures and reduced humidity, the biodiversity in these regions is relatively low, although represented by well-adapted species (Leal et al 2003, Holter et al 2009). To overcome the scarcity of food in these habitats, dung beetles present some strategies, such as a generalist habit, as well as the utilization of uncommon resources for this group, such as dry excrement and plant matter (Hernández 2007, Halffter & Halffter 2009, Holter et al 2009).
The Caatinga is a strongly seasonal semi-arid Brazilian ecoregion, characterized by a long period of drought and an irregular and short period of rainfall through the year (Leal et al 2003). During the drought period, both fauna and flora activity is extremely reduced, whereas the scenario is inverted during the rainy period (Leal et al 2003, Amorim et al 2009). The geological establishment of the Caatinga is related to processes of contraction and expansion of neighboring rainforests. Approximately 11,700 years ago, it started on the course of becoming a seasonally dry forest (Auler et al 2004).
Currently, there are approximately 38 species of dung beetles documented for Caatinga environments, with species richness values fluctuating between 13 to 23 species in a number of focal studies (Hernández 2007, Lopes et al 2006, Liberal et al 2011, Medina & Lopes 2014a, 2014b). The diversity of dung beetles in this ecoregion is composed mainly from species that are adapted to open and drier areas of neighboring Atlantic and Amazon rainforests (Hernández 2007, Costa et al 2013, Medina & Lopes 2014a, 2014b, Silva et al 2014), with few endemics (Lopes et al 2006, Hernández 2007). Studies with dung beetles in the Caatinga are mainly focused on the structure of assemblages across landscapes (Lopes et al 2006, Hernández 2007, Liberal et al 2011). Research aiming at the evaluation of dietary preferences of dung beetles has been usually restricted to samplings with traps baited with carrion and excrement (Hernández 2007, Liberal et al 2011). Curiously enough, Medina & Lopes (2014a) used pitfall traps baited with rotten banana, which tentatively simulated the attractiveness of locally available decomposing fruits to dung beetles. Although the majority of captures were associated to carrion and excrement, which were also simultaneously offered in baited pitfall traps, few species were also attracted to decomposing fruits. This observation suggests that under the harsh conditions of the Caatinga, at least selected species of dung beetles can be attracted to (and likely utilize) alternative feeding resources. The biota of the Caatinga exhibit several adaptations and behavioral strategies to overcome limited resource availability (Leal et al 2003). Native treehoppers (Membracidae) present temporal segregations, while bees exhibit low levels of niche overlap, both strategies associated with scenarios of resource scarcity (Creão-Duarte et al 2012, Aguiar et al 2013).
The aim of our study was to analyze the attraction of dung beetles to actual and potential feeding resources in a Caatinga environment. More specifically, we evaluated the attractiveness in situ and in the laboratory of the following substrates: (1) carrion and (2) excrement, used due to their proven attractiveness to Neotropical dung beetle species (Hanski & Cambefort 1991, Filgueiras et al 2009); fruits of native species of columnar cactus from Caatinga, (3) Cereus jamacaru DC and (4) Pilosocereus gounellei (A. Weber ex K. Schum.) Bly. ex Rowl, selected due to the known utilization of species of this family of plants by dung beetles on other regions of the world (Halffter & Halffter 2009); (5) seeds of Jatropha mollissima (Pohl), selected due to recent records of their utilization by dung beetles (Iannuzzi et al 2013). Our hypothesis is that selected species of dung beetles native to the Caatinga will both recognize and feed on resources other than excrement and carrion. Nonetheless, we expect that the utilization of excrement will be predominant over that of other offered feeding resources, as it is the resource to which the dung beetles are most commonly associated.
Material and Methods
Study area
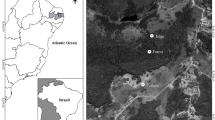
The study was conducted in the Caatinga ecoregion, which is situated in northeastern Brazil and comprises approximately 800,000 km2. Fieldwork was carried out in two fragments, which presented similar ecological structure (Velloso et al 2002). Area 1: located at Boqueirão da Onça (10°9′48″S 41°21′11″W), north of the state of Bahia, Brazil. Area 2: located at the municipality of Parnamirim (8°5′26″S 39°34′42″W), at the center of the state of Pernambuco, Brazil (Fig. 1). Both areas are dominated by hot semi-arid climate (type “BSh”) according to the Köppen climate classification (Velloso et al 2002). The average annual temperature at Area 1 is 30°C, whereas at Area 2, it is 26°C (Velloso et al 2002).
Average annual rainfall is 650 and 431 mm in Area 1 and Area 2, respectively. The rainy period occurs from October to April (Velloso et al 2002). Dung beetle collection and observations were conducted in May 2012 and February and March 2013 in Area 1 and April 2013 in Area 2. Most of the local annual rainfall is concentrated between February and April, during which interval the dung beetles are most active (Velloso et al 2002, Hernández 2007, Liberal et al 2011).
Field attractiveness tests
We used baited pitfall traps to test the attractiveness of different feeding resources to dung beetles in field conditions. The baits were inserted in small plastic bottles and attached to the interior of the traps, which once set were only recovered after approximately 24 h. All the captured dung beetles were collected and kept inside aerated plastic bottles (Hernández 2007). Individuals of Deltochilum verruciferum Felsche were kept alive for laboratory experiments, and the other species were incorporated to “Coleção Entomológica da UFPE” (CE-UFPE).
We used approximately 50 g of one of the following tested feeding resources as bait in each trap: (1) human excrement, (2) seeds of J. mollissima (“pinhão-brabo”), (3) carrion (bovine spleen) and decomposing fruits of (4) C. jamacaru (“mandacaru”) and (5) P. gounellei (“xiquexique”). To accelerate the decomposition of the fruits and carrion, they were kept on closed bottles for 48 h. For a faster decomposition of the fruits, we added 50 ml of sugarcane juice per 100 g of fruit (Salomão et al 2014).
In each study site, we conducted two field attractiveness experiments:
-
Experiment 1
With this experiment, we aimed to comparatively evaluate the attractiveness of the five resource types offered simultaneously in transects. Thus, we evaluated whether potential feeding resources (fruits and seeds) could be attractive to dung beetles in the presence of their preferred feeding substrates (excrement and carrion). The experiment was performed on May 2012 and February and March 2013 at Area 1 and on April 2013 at Area 2. We performed 20 repetitions, 10 in 2012 (Area 1) and 10 in 2013 (five at Area 1 and five at Area 2). Each repetition consisted of a group of five traps, installed along pre-defined transects 20 m apart from one another. Each trap was singly baited with one of the tested feeding resources and randomly set along the transects to avoid any bias. According to previous studies, the minimum distance between traps to avoid interference on attractiveness is 100 m (Silva & Hernández 2015). However, as the objective was to test the attractiveness of these resources comparatively under mutual presence, interference was desirable. Transects were defined at least 200 m apart from one another to avoid interference between replicates.
-
Experiment 2
With this experiment, we aimed to evaluate the attractiveness of the five tested feeding substrates independently. It was conducted on February and March 2013 at Area 1 and on April 2013 at Area 2. We performed 10 repetitions, five in each area. Each repetition consisted of five transects that were disposed similarly to what was described for Experiment 1, but we used one type of resource per transect; each repetition included all five tested feeding resources.
Controlled feeding resource preference test
In this experiment, we observed the feeding preference of Deltochilum verruciferum, an abundant species of dung beetle endemic to the Caatinga, when faced with the five types of resources aforementioned. Mature beetles were collected at Area 2 from pitfall traps baited with either excrement or carrion. Two-choice tests were performed inside an arena comprised of a round flat platform (total area 1060 cm2), whose bottom surface was covered with clean sheets of filter paper. For testing, we offered 15 g of any one type of resource set at one of the sides of the arena; at the opposite side, the same amount of another type of resource, or a control, was set. The controls consisted of a 4 cm2 of filter paper. After each repetition, we thoroughly cleaned the surface of the arena with alcohol 70% and replaced the sheets used to cover it with new ones. Tested individuals were not sexed. During the tests, a glass lid was used, so that the beetles could not escape.
Nine paired combinations of feeding resources were evaluated as follows: (1) resource vs. control (five combinations, one for each type of resource) and (2) excrement vs. other resources (four combinations, one vs. each type of resource). The second evaluation was performed in order to verify the preference for excrement facing the availability of other feeding resources. Each combination was repeated 16 times, and each beetle was only tested once.
Prior to the beginning of the preference tests, individuals of D. verruciferum were kept in cylindrical transparent plastic containers (45 × 30 cm) with a layer of ca. 5 cm of soil obtained from the sites of capture. The containers were maintained under permanent shade inside a room where temperatures varied between 22 and 28°C. Approximately 15 individuals were distributed per container, starved from the moment of capture until the setting of the experiment (10 to 38 h later).
The first step of the experiment consisted of placing an individual beetle at the center of the arena and keeping it for 5 min inside a transparent plastic cup for both acclimation and stress minimization due to handling. After that, the cup was removed and we observed each tested individual’s behavior for 15 min. Observations included (1) use of the resource, whether the individual contacted it with its mouthparts; (2) time elapsed between the first action of the beetle and the moment it came into contact with the resource; (3) time spent feeding on the resource. For the latter, we only considered data collected from paired tests involving excrement vs. other resources. All data was recorded between 18:00 and 00:00, the period during which beetles of this species are most active (Hernández 2007). The observations were performed with the aid of infrared light, set at a distance of 30 cm from the arena. Video recordings were taken with a digital camcorder (Sony DSC-HX 5, Sony corp., Japan).
Vouchers of D. verruciferum specimens used throughout the preference tests are deposited at CE-UFPE.
Data analysis
Field attractiveness tests
For a visual and explorative evaluation of the dung beetle assemblage that could explain patterns of segregation according to different tested feeding resources, we performed non-metric multidimensional scaling (NMDS). The tested feeding resources were used as factors and the abundance of each species sampled in the baited pitfall traps as attributes. We performed two separate NMDS ordinations, one with the data obtained from the transects where the resources were offered simultaneously (experiment 1) and another with the data from the transects where the resources were offered separately (experiment 2). For experiment 1, each trap was considered a sampling unit, whereas for experiment 2, the transects were the sampling units. All data was transformed to square root to reduce the high discrepancies among the abundant and rare species. After that, we constructed a Bray-Curtis similarity matrix and the NMDS ordinations were run with 2500 repetitions. An analysis of similarities (ANOSIM) was performed to test for segregation in the assemblage of dung beetles according to the type of resource. Both the NMDS and ANOSIM were conducted with Primer 6.0 (Clarke & Gorley 2006).
To estimate the degree of diet specificity of the dung beetles linked to different resources, we used the Levins standardized index (BA) (Levins 1968). We selected species with n > 30, thus showing a clear degree of specificity. The species with BA >0.5 were considered generalists, and those that presented BA ≤0.5 were classified as specialists (Filgueiras et al 2009). For the calculus of this index, we used the Ecological Methodology software.
Controlled feeding resource preference test
To evaluate the attractiveness of different feeding resources to D. verruciferum, we performed Chi-square tests using the number of individuals that fed on the offered resources as the response variable to verify if there was (1) difference in the attractiveness of the resources when offered against paired controls and (2) distinction in the attractiveness of excrement when offered against other tested resources. Categorical variables were the type of feeding resource and the combination of excrement vs. resource in the first and second tests, respectively. For both analyses, we only evaluated the resources that were utilized by at least five individuals. For the analysis that presented statistical difference of resource utilization, we performed a simple Chi-square test with correction through the Bonferroni index as post-hoc. We performed a Mann-Whitney U test to verify if there was difference on feeding resource attractiveness, comparing the scenarios in which they were offered individually or simultaneously with other resource. The mode by which the feeding resources were offered (vs. control or vs. other resource) was the independent variable. The analysis was performed on Statistica 7.0 (Statsoft 2004).
To compare the time taken by the beetles to start feeding on different resources, we performed a Kruskal-Wallis analysis in which only the data from the combination resource vs. control were used. We also used a Kruskal-Wallis analysis to compare how long the beetles spent feeding on each tested resource. For this analysis, we used resource vs. resource data. The time elapsed for starting feeding activity and the time spent feeding on the resource were the dependent variables. The types of resources were the independent variables. We used data from the resources utilized by at least five individuals. For post-hoc comparisons, we performed the Dunn test. All analyses mentioned in this paragraph were performed with the aid of BioEstat 5.0 (Ayres et al 2007).
To verify if there was difference of time elapsed for start feeding on different resources when they were offered vs. control or vs. other resource, we performed a Mann-Whitney U test. The time elapsed for start feeding was the response variable. The combination of the resource offers (resource vs. control and resource vs. other resource) was the independent variable. The test was performed with the Statistica 7.0 software (Statsoft 2004).
Results
Field attractiveness tests
A total of 297 dung beetles belonging to nine species and five genera were collected. The dominant species were D. verruciferum, Canthon carbonarius Harold, and C. melancholicum Harold, which represented together 86.5% of the collected beetles. Deltochilum veruciferum was the most abundant species by far, accounting for 60.9% of the total number of captures (Table 1). The data of the dung beetle collected in each area are available as supplementary material.
Traps baited with all tested resources yielded captures, with exception of those baited with seeds of J. mollissima. The traps baited with carrion and excrement yielded most captures with 66.3 and 32.6% of the total abundance of dung beetles, respectively. Traps baited with fermented C. jamacaru and P. gounellei yielded a low number of captures: two individuals of C. carbonarius were recovered from traps baited with C. jamacaru, and a single individual of Dichotomius geminatus (Arrow) was recovered from a trap containing baited with P. gounellei (Table 1).
None of the species of sampled dung beetles were associated with traps of all five different types of resources. The majority of species were only recovered in traps baited with carrion and excrement. Among captures of the most abundant species, the largest number of individuals of D. verruciferum and C. melancholicum were recovered in traps baited with carrion, which accounted for 69.1 and 76.5% of their total collected numbers, respectively (Table 1). On the other hand, the number of individuals of C. carbonarius recovered from traps baited with either carrion or excrement was similar (41.9 and 53.5% of total captures, respectively). According to the Levins index, D. verruciferum (BA = 0.24), C. melancholicum (BA = 0.43), and C. carbonarius (BA = 0.36) were classified as specialists, being almost exclusively attracted to traps baited with either carrion or excrement (Table 1).
When evaluating the attractiveness of the five types of feeding resources offered simultaneously and independently on the transects, there was a significant, although weak, distinction of the dung beetle assemblages (simultaneously—R-Global 0.115; P = 0.001; independently—R-Global 0.425; P = 0.001). However, the NMDS ordination did not evidence a clear segregation among the tested resources (Fig. 2).
Controlled feeding resource preference tests
Beetles behaved in two distinct manners in the beginning of this experiment: they either (1) remained immobile at the center of the arena (79 individuals; 54.9% of the total) or (2) moved as soon as the experiment started (65 individuals; 45.1%). Among the individuals that initially did not move, 41.8% remained immobile until the end of the experiment, whereas the remainder typically extended their antennal lamellae before initiating movement. When beginning to move, the majority of the individuals (>50%) repeatedly circled the borders of the arena, and only 11 (7.6%) directly approached the feeding resources to feed on them. It was commonly observed that the beetles touched their antennae over a particular resource but did not feed on it. While feeding on the resource, the beetles generally kept their bodies in direct contact with the surface of the arena, only touching the resource with their mouthparts. In less frequent cases, the individuals grabbed the resource with all the legs before and during feeding activity (figure available as supplementary material).
All the resources evaluated in the experiment were attractive to individuals of D. verruciferum. However, the higher frequency of resource utilization was verified in the associations with excrement and carrion, with 65 and 50% positive interactions, respectively (Table 2). Among the other resources, fermented fruits of P. gounellei were most attractive, with 28% of the beetles feeding on it.
In the resources vs. control tests conducted inside the arena, there was a difference in the frequency of utilization (χ2 = 14.38; df = 4; P < 0.05). The seeds of J. mollissima were less attractive than were carrion (χ2 = 8.12; df = 1; P < 0.005), excrement (χ2 = 8.12; df = 1; P < 0.005), and fermented fruits of C. jamacaru (χ2 = 9.25; df = 1; P < 0.005). Analyzing the combinations of excrement vs. other resources, the number of individuals that fed on excrement did not differ among distinct paired resource combinations (χ2 = 4.12; df = 3; P > 0.2). Comparing the frequency of occasions in which the beetles fed on different resources in the combinations resource vs. control and resource vs. other resource, there was no distinction for the majority of the resources (excrement P = 0.41; C. jamacaru P = 1; P. gounellei P = 0.69; J. mollissima P = 1). The exception was carrion (P < 0.05), which was foraged by 12 individuals when offered alone and by four when offered simultaneously with another resource (carrion vs. excrement).
The time taken by the beetles to start feeding on a given resource was similar among the different options, independently of the offered combination (resource vs. control H = 1.44; df = 2; P = 0.48 and resource vs. other resource—excrement P = 0.42; carrion P = 0.09; P. gounellei P = 0.75). On the other hand, the average time beetles spent feeding was distinct among tested resources, being longer on excrement, both when offered independently or simultaneously with another resource (Table 2). Excrement vs. carrion paired tests led to beetles spending the longest time feeding on the former. The time beetles spent feeding differed among tested resources (H = 12.09; df = 3; P < 0.05). The time beetles took using either carrion or excrement was longer than the time spent with any of the other resources (P < 0.05) (Table 2).
Discussion
Throughout our field experiments, traps baited with carrion yielded the highest number of captures among a set of traps baited with five different potential feeding resources to dung beetles. This result could arguably be linked to the particularly diverse necrophagous dung beetle fauna that inhabit the neotropics (Halffter & Matthews 1966, Halffter & Halffter 2009). Although many species of Neotropical dung beetles can use carrion for feeding and nesting, coprophagous and copro-necrophagous species are still most diverse in the region (Hanski & Cambefort 1991, Halffter & Halffter 2009). In a study evaluating coprophagy and necrophagy among dung beetles in the Caatinga, a sheer dominance of the copro-necrophagous habit was documented; some species were strictly coprophagous and only one, Coprophanaeus pertyi (d’Olsoufieff), was necrophagous (Hernández 2007). Contrastingly, Medina & Lopes (2014a) depicted a well-balanced distribution of feeding habits in the dung beetle assembly in the Caatinga, from the more specialized to the more generalist.
Decomposing carcasses are nutritious, rich on microorganisms, and bear an optimal doughy texture favored by dung beetles (Favila 1993, Halffter & Halffter 2009). As a consequence of the high temperatures and evapotranspiration rates of most Caatinga environments, the resources used by dung beetles tend to be ephemeral when compared to the availability common to habitats under milder climatic conditions (Sampaio 1995). Therefore, carrion, a resource that remains attractive and exploitable for longer periods than others associated to dung beetles, appears to be an efficient dietary alternative for these insects (Mayer & Vasconcelos 2013).
The abundance and richness of the dung beetle fauna in our study were considerably low when compared to those documented in similar studies conducted in the Caatinga (Hernández 2007, Liberal et al 2011, Medina & Lopes 2014a). Our results may be associated to two key factors: (1) the majority of traps used on the study were baited with what could be arguably interpreted as “alternative” resources, proved to be less attractive than excrement and carrion in the field; (2) the short period that the traps remained active in the field. Usually, studies aimed at the evaluation of dung beetle assemblages apply a 48-h active trapping interval in the field (Liberal et al 2011, Medina & Lopes 2014b). Because we needed live and healthy individuals for bioassays, we decided on uninstalling the traps after 24 h.
The dominant species in our field trappings was D. verruciferum, classified as a specialist, being attracted exclusively to carrion and excrement. However, our controlled preference tests demonstrated that individuals of this species could potentially approach and feed on a broader assortment of resources, thus behaving as generalists. Deltochilum Eschscholtz exhibits a mainly Neotropical distribution (Davis et al 2002). A number of its species are known to apply infrequent feeding strategies, such as strict necrophagy and predatory habits, as observed in individuals of D. valgum Burmeister, specialized on the predation of millipedes (Larsen et al 2009).
Traps baited with fermented fruits of cacti native to the Caatinga yielded few captures of only two species, C. carbonarius and D. geminatus. It is known that foraging dung beetles can inadvertently fall into unbaited pitfall traps (Silva 2011), and it would not be surprising if the traps baited with fermented fruits in our field experiments functioned mainly passively in capturing dung beetles. The traps baited with seeds of J. mollissima were the only ones that did not yield any captures, even though their use as a feeding resource by C. carbonarius has been documented (Iannuzzi et al 2013). Moreover, in a pilot experiment, two individuals of this species were collected in traps baited with this resource and later fed on the elaisome part of the seeds under laboratory conditions. The olfactory detection of potential feeding resources by dung beetles occuring through scent plumes containing attractive volatile organic compounds (Scholtz et al 2009) and “tentative interactions” with resources may not always result in feeding. The seeds of J. mollissima give off a typical fetid odor, which could be involved in the olfactory-mediated attraction of the dung beetles (Iannuzzi et al 2013), resulting in the utilization of the resource. Based on the pilot experiment and our controlled preference bioassays with D. verruciferum, we confirm that, although rare and/or poorly documented, dung beetles do interact with seeds of J. mollissima and can use them as a feeding resource. Since dung beetles are acknowledged as important secondary seed dispersers (Andresen & Feer 2005) and could perform this kind of interaction in the Caatinga, we believe that more focal studies are needed in order to better understand the details of the interaction between dung beetles and seeds of J. mollissima.
The results of the controlled feeding resource preference tests revealed that D. verruciferum can feed on a set of five different offered resources. Prior to our findings, a single study had demonstrated the direct use of native plant-derived feeding resources (excluding excrement from herbivores) by dung beetles in the Caatinga (Iannuzzi et al 2013). We present the first record of dung beetles feeding on fermented fruits of native cacti under controlled conditions. The utilization of alternative resources can be an indicator of innate dietary plasticity of D. verruciferum, which can be advantageous under conditions of scarcity of its preferred feeding resource and strong competition (Hanski & Cambefort 1991, Halffter & Halffter 2009). Similarly, the human disturbance of natural habitats may promote the utilization of different food types by dung beetles (Bourg et al 2016), a scenario in which species adapted to use alternative resources may be more resilient.
Although the utilization of plant-derived resources by D. verruciferum was confirmed under a controlled setup, carrion and excrement were nonetheless favored by the beetles. Contrastingly to the results obtained from baited pitfall trappings, excrement was more attractive than carrion. Moreover, excrement was most pursued when offered simultaneously with other feeding resources. Since the utilization of animal excrements is also associated with dung beetle breeding, preference for this resource could be thus supported (Halffter & Matthews 1966, Hanski & Cambefort 1991). Notwithstanding, higher attractiveness to carrion observed in field experiments could be mainly associated to young individuals, whose sexual maturation require a nutrient-rich diet that could debatably be more easily obtained through necrophagy. Future experiments associating sexual maturation and dietary preferences of dung beetles should further clarify these assumptions.
At the time being, we can only safely suggest that seed allocation by dung beetles in the Caatinga has been an episodic event linked to proximal sensorial responses. Our findings reinforce the hypothesis that some dung beetle species native to the Caatinga are apt for the use of alternative feeding resources, thus being potentially generalists. Although the usage of a wide variety of decaying matter resources by dung beetles was proven, there is still little question that copro-necrophagy is the main habit of local dung beetle communities. Future studies analyzing the chemical responses of the dung beetles to volatile organic compounds emitted by preferred plant-derived resources can further improve the knowledge of those still poorly understood interactions.
References
Aguiar CML, Santos GMM, Martins CF, Presley SJ (2013) Trophic niche breadth and niche overlap in a guild of flower-visiting bees in a Brazilian dry forest. Apidologie 44:153–162. doi:10.1007/s13592-012-0167-4
Ahrens D, Schwarzer J, Vogler AP (2014) The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proc R Soc Lond B 281:1–10. doi:10.1098/rspb.2014.1470
Amorim IL, Sampaio EVSB, Araújo EL (2009) Phenology of woody species in the caatinga of Seridó, RN. Brazil Rev Árvore 33:491–499. doi:10.1590/S0100-67622009000300011
Andresen E, Feer F (2005) The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In: Forget PM, Lambert J, Hulme P, Vander Wall S (eds) Seed fate: predation, dispersion and seedling establishment. CABI International, Wallingford, pp 331–349
Auler AS, Wang X, Edwards RL, Cheng H, Cristalli P, Smart PL, Richards D (2004) Palaeoenvironments in semi-arid northeastern Brazil inferred from high precision mass spectrometric speleothem and travertine ages and the dynamics of south American rainforests. Speleogenesis and Evolution of Karst Aquifer 2:1–4
Ayres M, Ayres Jr M, Ayres DL, Santos AA (2007) BioEstat 5.0: aplicações estatísticas nas áreas das ciências biológicas e médicas. MCT, IDSM, CNPq, Belém
Bourg A, Escobar F, MacGregor-Fors I, Moreno CE (2016) Got dung? Resource selection by dung beetles in Neotropical forest fragments and cattle pastures. Neotrop Entomol 45:490–498. doi:10.1007/s13744-016-0397-7
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Colles A, Liow LH, Prinzig A (2009) Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecol Lett 12:849–863. doi:10.1111/j.1461-0248.2009.01336.x
Costa FC, Pessoa KKT, Liberal CN, Filgueiras BKC, Salomão RP, Iannuzzi L (2013) What is the importance of open habitat in a predominantly closed forest area to the dung beetle (Coleoptera, Scarabaeinae) assemblage? Rev Bras Entomol 57:329–334. doi:10.1590/S0085-56262013000300012
Costa A, Salvidio S, Posillico M, Matteucci G, Cinti B, Romano A (2015) Generalisation within specialization: inter-individual diet variation in the only specialized salamander in the world. Sci Rep 5:1–10. doi:10.1038/srep13260
Creão-Duarte AJ, Anjos UU, Santos WE (2012) Diversity and trophic niche overlap of membracids (Hemiptera, Membracidae) in a semi-arid area. Iheringia Ser Zool 102:453–458. doi:10.1590/S0073-47212012000400012
Davis ALV, Scholtz CH, Philips TK (2002) Historical biogeography of Scarabaeinae dung beetles. J Biogeogr 29:1217–1256. doi:10.1046/j.1365-2699.2002.00776.x
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514. doi:10.1111/j.0030-1299.2008.16215.x
Favila ME (1993) Some ecological factors affecting the life-style of Canthon cyanellus cyanellus (Coleoptera Scarabaeidae): an experimental approach. Ethol Ecol Evol 5:319–328. doi:10.1080/08927014.1993.9523019
Filgueiras BKC, Liberal CN, Aguiar CDM, Hernández MIM, Iannuzzi L (2009) Attractivity of omnivore, carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera, Scarabaeidae) in a tropical Atlantic rainforest remnant. Rev Bras Entomol 53:422–427. doi:10.1590/S0085-56262009000300017
Halffter G, Halffter V (2009) Why and where coprophagous beetles (Coleoptera: Scarabaeinae) eat seeds, fruits or vegetable detritus. Bol SEA 45:1–22
Halffter G, Matthews EG (1966) The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomol Mex 12:1–312. doi:10.1002/mmnz.19690450211
Hanski I, Cambefort Y (1991) Dung beetle ecology. Princeton University Press, Princeton, p 481
Hernández MIM (2007) Besouros escarabeíneos (Coleoptera: Scarabaeidae) da Caatinga paraibana. Brasil Oecol Bras 11:356–364. doi:10.4257/oeco.2007.1103.06
Holter P, Scholtz CH, Stenseng L (2009) Desert detritivory: nutritional ecology of a dung beetle (Pachysoma glentoni) subsisting on plant litter in arid south African sand dunes. J Arid Environ 73:1090–1094. doi:10.1016/j.jaridenv.2009.04.009
Hutchinson GE (1957) Concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427. doi:10.1101/SQB.1957.022.01.039
Iannuzzi L, Leal LC, Meiado MV, Ribeiro DSC, Salomão RP (2013) First record of myrmecochorous diaspores removal by dung beetles in the Caatinga vegetation, a Brazilian semiarid ecosystem. J Arid Environ 88:1–3. doi:10.1016/j.jaridenv.2012.08.001
Larsen TH, Lopera A, Forsyth A, Genier F (2009) From coprophagy to predation: a dung beetle that kills millipedes. Biol Lett 5:152–155. doi:10.1098/rsbl.2008.0654
Leal IR, Tabarelli M, Silva JMC (2003) Ecologia e Conservação da Caatinga. Editora Universitária da UFPE, Recife, p 806
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton, p 132
Liberal CN, Farias AMI, Meiado MV, Filgueiras BKC, Iannuzzi L (2011) How habitat change and rainfall affect dung beetle diversity in Caatinga, a Brazilian semi-arid ecosystem. J Insect Sci 11:1–11. doi:10.1673/031.011.11401
Lopes PP, Louzada JNC, Vaz-de-Mello FZ (2006) Organization of dung beetle communities (Coleoptera, Scarabaeidae) in areas of vegetation re-establishment in Feira de Santana, Bahia, Brazil. Sitientibus Série Ciências Biológicas 6:261–266
Ma CC, Gao YB, Guo HY, Wang JL, Wu JB, Xu JS (2008) Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia plateau. J Arid Environ 72:247–254. doi:10.1016/j.jaridenv.2007.05.009
Maia ACD, Schlindwein C, Navarro DMAF, Gibernau M (2010) Pollination of Philodendron acutatum (Araceae) in the Atlantic forest of northeastern Brazil: a single scarab beetle species guarantees high fruit set. Int J Plant Sci 171:740–748. doi:10.1086/654846
Mayer ACG, Vasconcelos SD (2013) Necrophagous beetles associated with carcasses in a semi-arid environment in northeastern Brazil: implications for forensic entomology. Forensic Sci Int 226:41–45. doi:10.1016/j.forsciint.2012.11.019
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453. doi:10.1016/S0169-5347(99)01679-1
Medina AM, Lopes PP (2014a) Resource utilization and temporal segregation of Scarabaeinae (Coleoptera, Scarabaeidae) community in a Caatinga fragment. Neotrop Entomol 43:127–133. doi:10.1007/s13744-014-0198-9
Medina AM, Lopes PP (2014b) Seasonality in the dung beetle community in a Brazilian tropical dry forest: do small changes make a difference? J Insect Sci 14:1–11. doi:10.1673/031.014.123
Monteith GB, Storey RI (1981) The biology of Cephalodesmius, a genus of dung beetles which synthetises “dung” from plant material (Coleoptera: Scarabaeidae: Scarabaeinae). Mem Queensl Mus 20:253–277
Raven PH, Johnson GB (2002) Biology, 6nd edn. McGraw-Hill, New York, p 872
Salomão RP, Lira AFA, Iannuzzi L (2014) Dominant dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) species exhibit wider trophic niches on fruits, excrement, and carrion in Atlantic forest. Brazil Coleopt Bull 68:686–688. doi:10.1649/0010-065X-68.4.686
Sampaio EVSB (1995) Overview of the Brazilian Caatinga. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 35–63
Scholtz CH, Davis ALV, Kryger U (2009) Evolutionary biology and conservation of dung beetles. Pensoft Publishers, Sofia, p 567
Silva PG (2011) Dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) of two nonnative habitats in Bagé, Rio Grande do Sul. Brazil Zool Stud 50:546–559
Silva PG, Hernández MIM (2015) Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PlosOne 10:1–18. doi:10.1371/journal.pone.0126112
Silva RJ, Coletti F, Costa DA, Vaz-de-Mello FZ (2014) Dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) of forests and pastures of southwestern Brazilian Amazon: survey of species and feeding guilds. Acta Amaz 44:345–352. doi:10.1590/1809-4392201304472
Statsoft Inc (2004) Software for data analysis Statistica 7.0. Statsoft: Making the World More Productive. http://www.statsoft.com/ Acessed 13 Jun 2013
Valera F, Díaz-Paniagua C, Garrido-García JA, Manrique J, Pleguezuelos JM, Suárez F (2011) History and adaptation stories of the vertebrate fauna of southern Spain’s semi-arid habitats. JArid Environ 75:1342–1351. doi:10.1016/j.jaridenv.2011.05.004
Velloso AL, Sampaio EVSB, Pareyn FGC (2002) Ecorregiões propostas para o Bioma Caatinga. Instituto de Conservação Ambiental—The Nature Conservancy do Brasil, Brasília, p 7
Acknowledgments
We thank Parnamirim base of “Universidade Federal Rural de Pernambuco” and the local families from “Boqueirão da Onça” for logistical support. We also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a scholarship to R. P. Salomão over the course of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Raul A Laumann – Cernagen/Embrapa
Rights and permissions
About this article
Cite this article
Salomão, R.P., Maia, A.C.D., Bezerra, B.M. et al. Attractiveness of Different Food Resources to Dung Beetles (Coleoptera: Scarabaeidae) of a Dry Tropical Area. Neotrop Entomol 47, 69–78 (2018). https://doi.org/10.1007/s13744-017-0515-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0515-1