Abstract
Advances in techniques for rearing insects on artificial diets are fundamental to solving issues of basic and applied entomology. In this study, we evaluated the development of Spodoptera albula (Walker) (Lepidoptera: Noctuidae) on three artificial diets used for other species of Lepidoptera, at three larval densities, and two densities of adult couples housed in oviposition cages of two sizes, with the aim of optimizing methodology for rearing S. albula in the laboratory. Biological parameters were recorded from S. albula, and a fitness index was calculated based on the larval survival and duration and weight of pupae. The total and daily oviposition was recorded using 5 or 10 adult couples of S. albula housed in two cage sizes. Concentrations of total nitrogen and protein in the tested diets were determined. Development of S. albula was completed in all artificial diets; however, the diet used for rearing Anticarsia gemmatalis (Hübner) larvae was the most suitable for S. albula, yielding intermediate development time and higher survival relative to the other diets. Individualization of larvae favored S. albula development by producing overall greater weights of larvae and pupae, higher survival rates, and longer adult longevity. Cage size and number of couples per cage did not influence S. albula fecundity in the experiment conditions. Spodoptera albula can be satisfactorily reared on the artificial diet used for A. gemmatalis, using one larva per tube, and either density of adults at any cage size. Additional amendments are needed in the rearing methodology to achieve optimal conditions for larval development to adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spodoptera albula (Walker) (Lepidoptera: Noctuidae) is an important pest of agricultural crops in the Americas (Pogue 2002). Due to its polyphagous feeding habit, S. albula infests approximately 30 plant families, including tomatoes, soybeans, corn, sorghum, vegetables, cotton, peas, beets, and peanuts (Montezano et al 2013) from the southern USA to Brazil (Guerrero et al 2014). Larvae of S. albula cause reductions in crop yields by extensively defoliating the host plants in addition to occasionally cutting the plant stems (Teixeira et al 2001).
Studies on insect bioecology and nutrition have resulted in the development of artificial diets, providing suitable conditions for mass rearing of insects in the laboratory, which has greatly benefited research in basic and applied entomology. Results from these studies have increased our knowledge of insect biology and found application in aspects of integrated pest management (IPM) such as biological control, sterile insect technique, pheromone synthesis (Panizzi & Parra 2012), and host plant resistance. Therefore, improvement in the methodology for rearing S. albula is necessary in order to obtain experimental individuals of sufficient quantity and quality for use in IPM studies.
One of the first steps for improving the rearing methodology for a given insect species is the development of an artificial diet that allows for insect culture in the laboratory. An artificial diet must meet the minimum nutritional requirements of the insect and also must be economically feasible (Salvadori & Parra 1990, Cohen 2004). Regardless of the insect species and feeding behavior, qualitative nutritional requirements are similar, and dietary nutrients must be properly balanced. A method of developing an artificial diet for a given insect starts with a diet successfully used for a taxonomically similar species and then adjusts it to the nutritional requirements of the insect of interest (Panizzi & Parra 2012).
Protein, as a source of nitrogen and amino acids, is essential in diets of nearly all insect species, as it is required for synthesis of proteins found in insect muscles, enzymes, hormones, and other components of the body (Cohen 2004). In addition, nitrogen plays a fundamental role in insect growth and reproduction (Elden & Kenworthy 1994). Higher protein concentrations in an artificial diet increased survival and reproduction rates of the tobacco bud worm Heliothis virescens (Fabricius), a noctuid of the same family of S. albula (Blanco et al 2009). In another study, artificial diets containing both wheat germ and yeast as protein sources provided the best development of Spodoptera cosmioides (Walker) (Bavaresco et al 2004). Thus, measurement of protein concentration in artificial diets may serve as a reliable parameter of food suitability to S. albula.
Besides the artificial diet, other aspects of the S. albula rearing methodology are worthy of being tested. For example, the number of insects to be reared per tube must take into account the insect behavior. Indeed, many insect species are cannibalistic or can become cannibals under certain adverse conditions (Panizzi & Parra 2012). Food competition among larvae inside rearing containers can also negatively affect their growth, weight, and survival, depending on larval crowding (Agnew et al 2002, Gibbs et al 2004). In addition, characteristics of oviposition cages used for housing adults are likely to influence insect reproductive success. Oviposition cages should be properly sized because some insect species require adequate space for mating (Damiens et al 2013). Thus, the number of larvae per rearing container and the number of adult couples used per oviposition cage as well as the size of that cage may affect S. albula larval growth and reproductive success.
To our knowledge, there is little information in the literature on artificial diets suitable for rearing S. albula or optimal methods for the insect’s culture, thus warranting further investigation. In this study, we evaluated the development of S. albula on three artificial diets that have been successfully used for rearing Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), Anticarsia gemmatalis Hübner (Lepidoptera: Erebidae), and Diatraea saccharalis (Fabricius) (Lepidoptera: Crambidae), with the goal of identifying the most suitable for S. albula. These artificial diets were chosen because of the relatedness between S. albula and those three lepidopteran species, and we hypothesized that one of them would be suitable for rearing S. albula. Once the best diet was identified, the next step was to ensure that S. albula could be reared in sufficient quantity and quality for use in IPM studies. To this end, S. albula fed with the selected diet was tested at three larval densities and two densities of adult couples housed in oviposition cages of two sizes.
Material and Methods
The study was conducted in the Laboratório de Resistência de Plantas a Insetos (LARPI), Departamento de Fitossanidade, Faculdade de Ciências Agrárias e Veterinárias (FCAV/UNESP) in Jaboticabal, state of São Paulo, Brazil. Assays were conducted under controlled conditions of temperature (25 ± 2°C), relative humidity (70 ± 10%), and photoperiod (12 L:12D h).
To establish a population of S. albula in the laboratory, we collected approximately 100 larvae with the aid of a beat cloth in areas cultivated with peanuts (cv. IAC Runner 886) in the region of Jaboticabal, state of São Paulo, Brazil. The collected specimens were transferred individually into flat-bottomed tubes (2.5 cm diameter × 8.5 cm height) using a fine paintbrush. The tubes contained an adapted diet from Greene et al (1976) composed of 15 g soy protein, 30 g wheat germ, 19 g brewer yeast, 1.5 g nipagin, 1.8 g ascorbic acid, 0.9 g sorbic acid, 9 ml vitamin solution, 0.06 g tetracycline, 2 ml formaldehyde, 11 g agar, 15 g casein, 150 g common beans, and 600 ml water. After the establishment of the S. albula colony in the laboratory, assays were begun using the F3 generation.
Bioassay 1: selection of the most suitable artificial diet for S. albula development
To select the best artificial diet to culture S. albula, the larvae received three diets commonly used for rearing other Lepidoptera, as follows: (a) Diet D. saccharalis (DS), a diet originally used for rearing the sugarcane borer D. saccharalis (King & Hartley 1985); (b) diet A. gemmatalis (AG), a diet originally used for the velvetbean caterpillar A. gemmatalis (Greene et al 1976); and (c) diet S. frugiperda (SF), a diet originally used for the fall armyworm S. frugiperda (Kasten et al 1978) (Table 1). We verified that all diets contained the concentrations of nitrogen and protein described by the original developers (data not shown).
Larvae of S. albula were individualized using a fine paintbrush in flat-bottomed glass tubes (2.5 cm diameter × 8.5 cm height) previously oven-sterilized at 120°C for 2 h. Each tube contained approximately 15 ml of the solidified diet on the bottom. The tubes were plugged with hydrophobic cotton wool and placed with the opening facing down on wooden shelves with a 15% slope. We used a completely randomized design with 100 replicates, the experimental unit being an individualized larva in a tube.
Experimental individuals were observed daily for the duration and survival of each biological stage. The larvae were weighed when 12 days old using an analytical scale to the nearest 0.0001 g (Model AR 2140, Ohaus Corporation, Barueri, Brazil). When the larvae reached the pupal stage, they were weighed after 24 h and then transferred to 50-ml plastic cups with lids. The pupae were sexed by noting the abdomen morphology, and the sex ratio was calculated. After emergence, longevity was recorded from unfed adults. Thus, during the development of S. albula, the following biological parameters were recorded: duration and survival of larval, pupal, and larva-to-adult stages, weights of 12-day-old larvae and 24-h-old pupae, sex ratio, and longevity of unfed adults.
To determine the nutritional value of the three artificial diets, 10 samples of each were separated, oven-dried at 60°C for 72 h, and then re-weighed. Dried samples were processed in a knife mill (Model TE-650, Tecnal, Piracicaba, Brazil) with 1-mm mesh size. Crude protein content was quantified according to the procedures described by AOAC (1990) and nitrogen was determined by thermal conductivity cell using a LECO nitrogen/protein determinator (Model FP-528, LECO Corporation, Michigan, USA).
Bioassay 2: development of S. albula reared at different larval densities
Larval densities of one to three S. albula larvae per rearing tube were compared. We used the same flat-bottomed glass tubes as in bioassay 1, prepared as described above. Larvae of the F6 generation were obtained from a stock colony on diet A. gemmatalis, as that diet provided the best development of S. albula. Methodology of assay installation and the biological parameters recorded were similar to those described for bioassay 1. We used a completely randomized design with 100 replicates, with the experimental unit consisting of the larva(e) in a tube.
Bioassay 3: influence of density of adults and cage size on S. albula fecundity
Adults from the F7 generation of the S. albula stock culture were divided into couples and placed in oviposition cages for testing. Only males and females that had emerged on the same day were chosen as couples. We evaluated four conditions for oviposition, including two types of cages and two densities of adult couples per cage. The cages consisted of 10-cm-diameter (small) or 20-cm-diameter (large) PVC tubes, both 20 cm high. We evaluated densities of 5 and 10 couples of S. albula per cage.
The cages were internally coated with paper used for seed germination. To facilitate mobility, small and large cages were arranged on plastic dishes 15 cm or 25 cm in diameter, respectively. The dishes were lined with the same paper used as oviposition substrate, and cage tops were capped with voile fabric. Adults of S. albula were fed with 10% honey solution provided on cotton wool and placed in a plastic cup on the cage bottom. Food was replaced on a daily basis. After the initiation of oviposition, the paper was replaced daily to quantify the number of eggs laid by S. albula. In this test, we used a completely randomized design replicated five times. Each experimental unit consisted of a small or large cage containing 5 or 10 adult couples of S. albula.
Statistical analysis
Data obtained from the three bioassays were subjected to the Kolmogorov-Smirnov test (α = 0.05) and Levene test (α = 0.05) to check for normality of residuals and homogeneity of variances, respectively. Data that did not meet these assumptions were square root-transformed and analyzed by one-way analysis of variance (ANOVA) with post hoc mean comparisons by Tukey’s honest significant difference test (α = 0.05). Those parameters that did not meet the ANOVA assumptions even when transformed were analyzed by the Kruskal-Wallis non-parametric test. We used a biological fitness index (rI) estimated from the larval development time, weight of pupae, and larval survival as an indicator of overall offspring performance. The fitness index was computed as rI = lx. mx/tl, where lx is the larval survival, mx is the mean pupal weight, and tl is the larval duration (Jallow & Zalucki 2003, Boregas et al 2013). The significance of the fitness index was determined by ANOVA. Statistical analysis was performed in the software Statistica version 7 (Statsoft 2004).
Results
Bioassay 1: selection of the most suitable artificial diet for S. albula development
As shown in Table 2, duration of the larval stage of S. albula was significantly different among the three artificial diets (H 2,210 = 151.70; P < 0.0001). Larval duration was the longest for S. albula fed the SF diet, with a value 1.8-fold greater than that of DS diet, which yielded the shortest duration. Larval duration for the AG diet was intermediate but significantly different from those of the other two diets.
Survival of S. albula larvae was significantly affected by diet (H 2,297 = 15.63;P = 0.0004). The AG and SF diets yielded the highest (81%) and lowest (57%) rates of larval survival, respectively. The DS diet yielded an intermediate larval survival rate (75%) that did not differ from those of the other diets (Table 2).
Duration (F 2,172 = 241.28; P < 0.0001) and survival (H 2,297 = 23.97; P < 0.0001) during the complete larva-to-adult period of S. albula differed significantly among the artificial diets. The SF diet yielded an increase in the duration of 1.5 times relative to the DS diet and 1.3 times relative to the AG diet. The AG diet resulted in the highest overall survival rate, yielding an increase in the survival of 1.6 times relative to the DS diet and 1.7 times relative to the SF diet, while values for the latter two diets did not differ significantly from each other (Table 2).
Pupal weight was 1.1-fold greater for larvae fed the DS diet compared with those fed the SF diet (H 2,210 = 21.52; P < 0.0001), but neither differed significantly in pupal weight from larvae fed the AG diet (Table 3).
The fitness index was significantly different among the three artificial diets (F 2,209 = 277.07; P < 0.0001), and the DS diet had the highest value of the fitness index (Table 3).
To evaluate the quality of the tested diets, their concentrations of total nitrogen and protein were determined. The DS diet had the lowest concentrations nitrogen and protein. The SF and AG diets had similar concentrations of these components, with values 3- to 3.5-fold higher than those of the DS diet (Fig 1).
Concentrations (%) of total protein and nitrogen in three artificial diets used for Spodoptera albula larval development. DS diet diet originally used for rearing Diatraea saccharalis, AG diet diet originally used for rearing Anticarsia gemmatalis, SF diet diet originally used for rearing Spodoptera frugiperda .
Bioassay 2: development of S. albula reared at different larval densities
Table 4 shows the effect of larval density on S. albula larval development. Larval density did not significantly affect the duration of the larval stage (H 2,265 = 0.16; P = 0.9235) of S. albula reared with one to three larvae per tube. However, larval survival of S. albula reared singly (94%) was 3.1- and 7.4-fold higher than that obtained with densities of two and three larvae per tube, respectively (H 2,297 = 85.88; P < 0.0001).
Duration of the S. albula larva-to-adult period was significantly longer (F 2,94 = 4.45; P = 0.0142) for the larval density of three larvae per tube compared to one larva per tube, while the duration for two larvae per tube did not differ significantly from those of the other two densities. The larval density of one larva per tube resulted in the highest total survival (H 2,297 = 14.89; P = 0.0006), being 2.8–4.8 times higher than survival of S. albula reared at the two higher densities (Table 4).
Larval and pupal weights of S. albula were significantly affected by larval density. Larval (H 2,297 = 7.22; P = 0.0053) and pupal (H 2, 167 = 10.47; P = 0.0053) weights were highest when S. albula was reared singly, differing significantly from the weights of larvae and pupae reared at three to a tube, while the results for the intermediate larval density did not differ from those of the other two densities (Table 5).
The results indicated that the larval densities used had a great effect on the fitness index (F 2,164 = 77.67; P < 0.0001) of S. albula, which was the highest for the one larva density (8.48) and lowest for the three larvae density (4.35) (Table 5).
Bioassay 3: influence of density of adults and cage size on S. albula fecundity
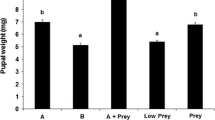
The number of eggs laid by S. albula was affected by cage size when there were 5 adult couples per cage (H 3,16 = 8.67; P = 0.0340) but not when there were 10 couples per cage. The number of eggs laid was the highest in cages containing 10 couples regardless of cage size. The large cage with 5 couples had an intermediate number of eggs, and the small cage with 5 couples had the fewest number of eggs (Fig 2a). The number of eggs laid per S. albula female was not significantly affected by either number of couples or cage size (Fig 2b).
In general, in cages with 10 adult couples, the total number of eggs laid per day and the number of eggs per S. albula female per day were the highest between the second and fifth days after initiation of oviposition. In contrast, in cages with 5 couples, oviposition peaked on the third day and then abruptly declined (Fig 3a, b).
Discussion
Knowledge on the optimal method of insect rearing is essential for its continuous maintenance over extended periods of time under controlled conditions and for nutritional and biological uniformity required for IPM research. In the present study, we found that the diet commonly used for A. gemmatalis larvae was the most suitable for rearing S. albula and the density of one larva per rearing tube resulted in greater fitness compared to higher larval densities. Finally, we observed that the number of adult couples and the size of the oviposition cages tested in this experiment did not influence the fecundity of S. albula reared with the selected diet.
The duration of the larva-to-adult period for S. albula fed the DS diet was 4.72 days shorter than that obtained when larvae were fed the AG diet, and 14.8 days shorter than when larvae received the SF diet. However, the survival rate of individuals fed the DS diet was low; only 50% survived until the end of the cycle. In contrast, although the AG diet yielded an intermediate duration of the immature period (35.5 days), it produced a higher overall survival rate (78%) than the other artificial diets; therefore, the AG diet was considered the most suitable diet for rearing S. albula.
Usually, there is a positive correlation between the fitness index, pupal weight, and fecundity (Leuck & Perkins 1972). Therefore, the fitness index is regarded as a useful indicator of offspring performance and host suitability. According to our results, the highest and lowest values of larval fitness index of S. albula were obtained for the DS and SF diets, respectively.
The DS diet produced the greatest larval and pupal weights of S. albula, but its rate of larva-to-adult survival was one of the lowest. These results may have been due to the high carbohydrate content found in the DS diet. Carbohydrates are major phagostimulants for insects (Schoonhoven et al 2005, Panizzi & Parra 2012). Thus, excess carbohydrates ingested by S. albula larvae may have been converted into fat and stored into the insect body, resulting in heavy larvae and pupae. The absence or excess of some other components in the DS diet may explain its low rate of larva-to-adult survival. Crude protein analysis of the artificial diets showed a low protein concentration in the DS diet, and this may have contributed to the associated low survival rate of S. albula in light of the importance of proteins to insects.
There are alternative reasons for the observed variation in growth and survival of S. albula reared on the artificial diets tested in this study. Dietary protein: carbohydrate ratio is known to affect the performance of insect species (Roeder & Behmer 2014). For example, the optimal diet for larvae of Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) had a 79:21 (protein:carbohydrate) ratio (Waldbauer et al 1984), while a 50:50 ratio produced optimal growth of nymphal Locusta migratoria (Simpson & Raubenheimer 2001). Mixtures of inorganic salts and B-complex vitamins are necessary for insect growth and survival (Wang et al 1984), and these factors may explain the variation observed in weight gain and survival of S. albula fed different diets. Water content and activity (Cohen 2004) and even the amount of agar used (Assemi et al 2012) can modify diet quality as well. In our study, because the three artificial diets contained or lacked particular constituents, it would be purely speculative if we attempted to explain which specific nutrients or which nutrient concentrations provided the most suitable conditions for S. albula growth; therefore, this issue would be important if addressed in a future study. Nevertheless, we can conclude the AG diet was more suitable for S. albula relative to the other tested diets, providing balanced nutrition to sustain insect development during all stages.
Longevity of S. albula adults was affected by the diet consumed during the larval stage. The DS and AG diets produced similar results for the adult longevity, with values 25% higher than that yielded by the SF diet. Again, the nutritional quality of the diets probably affected S. albula larval growth and consequently the adult life span. The SF diet was simpler in its composition relative to the other artificial diets and proved unsuitable for culturing S. albula as it yielded long duration of the immature period and low survival. The key adult roles of reproduction and dispersion depend on energy consumption and accumulation in earlier stages of insect development, and longer longevity can all own more dispersion and reproduction of adults, ensuring population growth and success of the species. Therefore, the choice of an appropriate artificial diet as larval food is fundamental to S. albula reproductive success in the laboratory.
Individualization of larvae resulted in increased survival of S. albula compared to higher tested densities. Two hypotheses arose from this result. The first is that S. albula larvae cannibalize at later stages of larval development. Usually, cannibalism is associated with two main factors: lack of food and high population density. Colinvaux (1973) suggested that cannibalistic behavior occurs in extreme situations and under strong environmental pressures such as barriers to dispersion. Fox (1975) stated that when individuals feel threatened, a good strategy from both a nutritional and competitive point of view is to make the threat their food. Another possible reason for the low S. albula survival at larval densities greater than one is competition for food and a consequent lack of essential nutrients to the larvae, resulting in morphological abnormalities in the pupae. The most common deformities in S. albula pupae found in tubes with more than one larva included retention of larval morphological features, wing atrophy, and formation of a globular evagination in the integument (data not shown). According to Panizzi & Parra (2012), occurrence of morphological abnormalities at any stage of insect development is related to some nutritional deficiency.
Cage size did not affect the number of eggs laid per S. albula female, and hence, we assume that space is not a limiting factor for mating and oviposition of this insect species. Although oviposition peaked earlier and higher in cages with 5 adult couples, the oviposition period of S. albula lasted longer in cages with 10 couples, and the overall results in terms of S. albula fecundity were nearly the same. Therefore, a good option for faster egg production would be the use of 5 couples of S. albula in either small or large cages. From another point of view, using cages with 10 couples of adults would be economically advantageous by reducing the number of cages needed for S. albula breeding, space they occupy in the laboratory, and amount of materials needed for cage preparation, including PVC tubes, voile fabric, and oviposition paper substrate. Thus, in addition to the number of eggs to be produced, the choice of the number of adult couples per oviposition cage must take into account aspects related to laboratory facilities, sanitation, costs, and storage (Parra 2007).
In conclusion, our results showed that S. albula can be satisfactorily reared on the artificial diet used for A. gemmatalis at the density of one larva per tube, and at either density of adults in any cage size, with sufficient quantity and quality of individuals for use in IPM studies. To further achieve optimal survival rates from larval development to adulthood in the laboratory, additional studies using nutritional indices and fertility life tables are needed to refine qualitative and quantitative characteristics of the diet selected in this study as most suitable for rearing S. albula. Finally, observations of biological parameters of the larvae and adults during some generations in the laboratory will confirm if the methodology herein established is adequate for S. albula rearing over extended periods of time.
References
Agnew P, Hide M, Sidobre C, Michalakis Y (2002) A minimalist approach to the effects of density-dependent competition on insect life-history traits. Ecol Entomol 27:396–402
Assemi H, Rezapanah M, Vafaei-Shoushtari R, Mehrvar A (2012) Modified artificial diet for rearing of tobacco budworm, Helicoverpa armigera, using the Taguchi method and Derringer’s desirability function. J Insect Sci 12:1–18
Association of Official Analytical Chemistry (AOAC) (1990) Official methods of analysis, 15th edn. AOAC, Arlington
Bavaresco A, Garcia MS, Grützmacher AD, Ringenberg R, Foresti J (2004) Adequação de uma dieta artificial para a criação de Spodoptera cosmioides (Walk) (Lepidoptera: Noctuidae) em laboratório. Neotrop Entomol 33:155–161
Blanco CA, Portilla M, Abel CA, Winters H, Ford R, Streett D (2009) Soybean flour and wheat germ proportions in artificial diet and their effect on the growth rates of the tobacco budworm, Heliothis virescens. J Insect Sci 9:59
Boregas KGB, Mendes SM, Waquil JM, Wilson G (2013) Estádio de adaptação de Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) em hospedeiros alternativos. Bragantia 72(1):61–70
Cohen AC (2004) Insect diets: science and technology. CRC Press, Boca Raton
Colinvaux PA (1973) Introduction to ecology. John Wiley, New York
Damiens D, Soliban SM, Balestrino F, Alsir R, Vreysen MJB, Gilles JRL (2013) Different blood and sugar feeding regimes affect the productivity of Anopheles arabiensis colonies (Diptera: Culicidae). J Med Entomol 50(2):336–343
Elden TC, Kenworthy WJ (1994) Foliar nutrient concentrations of insect susceptible and resistant soybean germplasm. Crop Prot 34:695–699
Fox LR (1975) Cannibalism in natural populations. Annu Rev Ecol Syst 6:87–106
Gibbs M, Lace LA, Jones MJ, Moore AJ (2004) Intraspecific competition in the speckled wood butterfly: Pararge aegeria effect of rearing density and gender on larval life history. J Insect Sci 4:1–6
Greene GL, Leppla NC, Dickerson WA (1976) Velvetbean caterpillar: a rearing procedure and artificial medium. J Econ Entomol 69:487–488
Guerrero A, Malo EA, Coll J (2014) Semiochemical and natural product-based approaches to control Spodoptera spp. (Lepidoptera: Noctuidae). J Pestic Sci 87:231–247
Jallow MFA, Zalucki MP (2003) Relationship between oviposition preference and offspring performance in Australian Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust J Entomol 42:343–348
Kasten P Jr, Precetti ACM, Parra JRP (1978) Dados biológicos comparativos de Spodoptera frugiperda (J. E. Smith, 1797) em duas dietas artificiais e substrato natural. Rev Agric 53:68–78
King EG, Hartley GG (1985) Diatraea saccharalis. In: Singh P, Moore RF (eds) Handbook of insect rearing. Elsevier, New York, pp 265–270
Leuck DB, Perkins WD (1972) A method of estimating fall armyworm progeny reduction when evaluating control achieved by host-plant resistance. J Econ Entomol 65:482–483
Montezano DG, Specht A, Bortolin TM, Fronza E, Sosa-Gomez DR, Roque-Specht VF, Pezzi P, Luz CP, Barros NM (2013) Immature stages of Spodoptera albula (Walker) (Lepidoptera: Noctuidae): developmental parameters and host plants. An Acad Bras Cienc 85:271–284
Panizzi AR, Parra JRP (2012) Insect bioecology and nutrition for integrated pest management. CRC Press, Brasília, Brazil, Boca Raton
Parra JRP (2007) Técnicas de criação de insetos para programas de controle biológico. FEALQ, Piracicaba
Pogue GM (2002) A world revision of the genus Spodoptera (Guenée) (Lepidoptera: Noctuidae). Mem Am Entomol Soc 43:1–202
Roeder KA, Behmer ST (2014) Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct Ecol 28:1135–1143
Salvadori JR, Parra JRP (1990) Seleção de dietas artificiais para Pseudaletia sequax (Lepidoptera: Noctuidae). Pesqu Agropecu Bras 25:1701–1713
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology. CRC Press, Boca Raton
Simpson SJ, Raubenheimer D (2001) The geometric analysis of nutrient-allelochemical interactions: a case of study using locusts. Ecology 82:422–439
StatSoft. (2004) Statistica. Data analysis software system, version 7. StatSoftInc.,Tulsa, 2004. Available in: <www.statsoft.com>. Accessed: 12 sep. 2014
Teixeira EP, Novo JPS, Stein CP, Godoy IJ (2001) Primeiro registro da ocorrência de Spodoptera albula (Walker) (Lepidoptera: Noctuidae) atacando amendoim (Arachis hypogaea, L.) no estado de São Paulo. Neotrop Entomol 30:723–724
Waldbauer GP, Cohen RW, Friedman S (1984) Self-selection of an optimal nutrient mix from defined diets by larvae of the corn earworm, Heliothis zea (Boddie). Physiol Zool 57:590–597
Wang YN, Zheng ZQ, Zhou YS (1984) Handbook of artificial diet of insect. Shanghai Scientific and Technical Publishers, Shanghai
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Eugenio E de Oliveira – UFV
Rights and permissions
About this article
Cite this article
Di Bello, M.M., Souza, B.H.S., Nogueira, L. et al. Optimization of Methodology for Rearing Spodoptera albula on Artificial Diet. Neotrop Entomol 46, 546–553 (2017). https://doi.org/10.1007/s13744-017-0490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0490-6