Abstract
Two new semiliquid artificial diets (A and B), the aphid Rhopalosiphum padi L. (Hemiptera: Aphididae) and combinations of these were evaluated to improve the rearing of the Neotropical lacewing predator Chrysoperla externa (Neuroptera: Chrysopidae). Additionally, a nutritional analysis of the aforementioned diets was conducted. The developmental time, pupal weight, preoviposition period, fecundity, and fertility were used to evaluate the impact (i.e., potential benefit) of these artificial diets on the predator. We recorded the shortest development time in the treatment group that was given diet A + prey or prey alone ad libitum. Pupal weight was significantly higher with diet A + prey. No difference was found in the female preoviposition period between the five different diets. Fecundity was higher with diet A + prey or prey ad libitum, while fertility on the first day was higher with diet A + prey, cumulative fertility was optimal with diet A and B as food. In conclusion, diet A proved to have the optimal nutritional properties (proteins and carbohydrates) to improve lacewing rearing when used as a nutritional supplement. Furthermore, use of this diet allows a reduction in the use of prey for rearing, and is suitable to be used for mass-rearing programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biological control of pests is one of the most ecologically relevant strategies within the framework cited for integrated pest management and organic crops (van Driesche et al. 2009). Augmentative biological control, where large numbers of natural enemies are periodically released in the crops, is being implemented successfully in several crops worldwide (van Lenteren 2000; van Lenteren and Bueno 2003). As of now, more than 125 species of natural enemies are on the market for biological control of pests (van Lenteren 2003). The production of these organisms—mostly predators and parasitoids—should be cost-effective, requires adequate nutrients to be provided through the diet, and requires an adequate reproductive capacity (Cohen 2004).
Nowadays, the production of predators for biocontrol is commonly based on the use of factitious prey, i.e., the eggs of Sitotroga cerealella (Lepidoptera: Tineidae) or Ephestia (Anagasta) kuehniella (Lepidoptera: Pyralidae) for Chrysoperla carnea (Neuroptera: Chrysopidae), Macrolophus pygmaeus (Heteroptera: Miridae), Orius laevigatus (Hemiptera: Anthocoridae) among others (Bonte and De Clercq 2008; Vandekerkhove et al. 2008), involving high costs in the mass rearing of natural enemies for field-releasing (the market price of the factitious prey eggs of E. kuehniella is around 650 $US kg−1, Vandekerkhove et al. 2008). In other instances, biocontrol producers need to rear the prey of the natural enemy and the corresponding host plant in a tritrophic system. This approach entails high labor costs for the operation and maintenance of separate spaces and the appropriate equipment to produce each trophic level (Riddick 2009). In contrast, the use of an artificial diet allows a reduction in or elimination of plant materials and prey colonies in the rearing system, leading to lower production costs (Bonte and De Clercq 2010).
The subsistence of healthy laboratory insects depends on the quality of their diets, which must provide nutritional support for basic metabolism, development, and reproduction. The nutritional needs vary with the stage and the physiologic state and are generally higher for larvae than for adults (Grenier 2012). Within this framework, a completely artificial diet must contain a nitrogen source, lipids, carbohydrates, vitamins, and minerals in addition to stabilizers and preservatives. Proteins are used as the principal source of nitrogen by most insects, and after the degradation of those proteins to amino acids the latter are reutilized for the synthesis of the insect-specific proteins of muscles, enzymes, and certain hormones and for structural elements. Likewise, lipids are essential for the structure of cell membranes, hormones, nutrient transporters, and sources of energy and as raw materials for building other molecules. Finally, carbohydrates function as receptors and channels for the movement of materials into and out of the cells and furthermore serve not only as structural elements and energy sources but also as constituents of glycoproteins (Cohen 2004).
The nutritional plasticity of certain predator species—as demonstrated by the consumption of non prey foods such as nectar, honeydew, and pollen—is a positive characteristic that makes them more easily reared on an artificial diet (Specty et al. 2003). Those alternative natural components increase the survival when the prey is scarce, reduce the mortality during diapause, and enhance the reproductive capacity of the insect (Lundgren 2009). This same plasticity is also modulated by the reproductive strategy of the species. One example is the occurrence of proovigenic females whose eggs are already mature before adult emergence (Jervis et al. 2007), whereas with the alternative strategy, referred to as synovigeny, the egg complement is formed after emergence of the adult (Jervis and Ferns 2005). Intermediate states exist between these two strategies. Therefore, in a proovigenic species, larval nutrition during mass rearing is critical since the fat bodies of the larval stages are responsible for vitellogenesis, i.e., the materials used for egg maturation in the imago derive mostly or entirely from previously stored larval resources.
The Chrysopidae—among the most extensive families of neuropterans and the most valuable ones in economic terms (Senior and McEwen 2001)—consists of a large group of insects with a wide geographic distribution that feeds on a diversity of prey such as aphids, coccids, and other soft-bodied pests, thus diminishing their populations in agroecosystems below the economic-injury level (Tauber et al. 2000). For this reason, several species of Chrysoperla Steinmann have received special attention as biological control agents of pests (Díaz-Aranda and Montserrat 1995) and are all produced for commercial use by feeding on a factitious prey such as S. cerealella (Albuquerque et al. 1994; Legaspi et al. 1994; Soto and Iannacone 2008) or E. kuehniella eggs (Boregas et al. 2003; Cohen and Smith 1998; Hilbeck et al. 1998). Nevertheless, this type of rearing could make predator production too expensive for large-scale use and open-field agricultural settings. Considerable progress has been made in the manipulation of Chrysoperla spp. colonies for biological control programs through the development of several artificial diets. None of these, however, have been incorporated into a large-scale production system, and different explanations have been given for this lack of success, e.g., the complexity of manufacturing and production expense of the diet along with the higher performance of Chrysoperla reared on the natural or factitious prey compared with diet-reared insects (Cohen and Smith 1998).
Chrysoperla externa Hagen is widely distributed from the southeastern United States and the Antilles to southern South America (Albuquerque et al. 1994). The species has been considered as an excellent potential biological control agent because of its high adaptive capacity to different ecosystems and a promiscuous feeding ability, involving a broad range of prey (Albuquerque et al. 1994). In Argentina, C. externa—located as far south as northern Patagonia—is associated with crops, fruit trees, and grasses adjacent to cultivated land (González et al. 2011). In the Buenos Aires province, the species has been recorded as a relevant predator in the protection of extensive (i.e., soybean) and intensive (i.e., sweet pepper) crops (Haramboure et al. 2014; Rimoldi et al. 2008). The three larval instars exhibit predatory behavior, thus being effective natural enemies of mites and insects such as coccids, aphids and eggs of lepidopterans and thrips (Bastidas et al. 2010; Soto and Iannacone 2008). C. externa can easily be reared in the laboratory, thus enabling the development of improved techniques for its production in view of the demands for rearing and maintenance exacted within the context of research or commercialization (Boregas et al. 2003).
The aims of this research were: (1) to evaluate the nutritional quality of two new semiliquid artificial diets for the development and reproduction of C. externa over the species’ different biological stages compared with that on different densities of prey, and (2) to analyze the biochemical composition of the diets in relation to their nutritional value in supporting reproductive performance. Finally, we discuss the potential use of these semiliquid artificial diets in the mass rearing of this commercially significant predator.
Materials and methods
Insects and plant materials
Adults of C. externa were collected from vegetable crops within the environs of La Plata (34° 59′ 10.06′′S; 57° 59′ 52.77′′W), Argentina. In the laboratory, the adults were conditioned in ventilated plastic containers (15 cm diameter, 9 cm high) covered with a fine mesh and quarantined to minimize parasitism and disease prior to rearing. Once quality and health of collected material were corroborated in quarantine, their progeny was used to initiate the laboratory colony of the species. Chrysopid adults, whose diet is not prey but based on pollen and nectar, were reared on an artificial diet based on honey, wheat germ, and brewer’s yeast, which is commonly used for adults of the species (Vogt et al. 2000). The colony reproduced for several generations, and the wild stock (between 50 and 80 adults), collected yearly from the same geographical origin, was crossbred to help maintain its genetic variability.
The bird-cherry aphid, Rhopalosiphum padi L. (Hemiptera: Aphididae), was used as prey. An aphid colony was initiated from clones obtained from the School of Agricultural and Forestry Sciences (National University of La Plata, Argentina) and was reared on pesticide-free wheat seedlings (Triticum aestivum L., cultivar ACA 901). These seeds were germinated in plastic pots (6 cm high, and 6 cm in diameter) with standard substrate (fertile soil and perlite 1:1 [v/v]) and infested with aphids at germination. The seedlings were maintained in ventilated plastic boxes (13 cm high, × 13 cm long, × 23 cm wide). The insect colonies and all bioassays were carried out in a growth chamber with controlled environmental conditions (23 ± 5 °C, 70 ± 5 % RH, and a 16:8-h light:dark period).
Composition analysis and cost of artificial diets
Two new semiliquid artificial diets were designed and developed under aseptic conditions in our laboratory (Schneider, unpublished data), herein after referred to as diet A and diet B (Table 1). Both diets were supplied to larvae in spherical packages which were prepared by placing approximately 0.2 g of the diet on a 2 × 2 cm square of Parafilm® and then joining the square corners together. These spheres were supplied once every two days. The ingredients of both diets were collected and afterwards the costs for making 1 kg for each diet were calculated to compare with the cost of predator rearing with factitious prey.
The two artificial diets were analyzed to determine their nutritional content in terms of the percent composition of carbohydrates, proteins, and lipids. Two samples per diet were sent to the Center of Research and Development in Food Cryotechnology, School of Exact Sciences, National University of La Plata, Argentina.
The Ratzlaff method, i.e., an acid treatment followed by extraction with an organic solvent, was used to determine the lipid content after cleaving the protein-lipid bonds in the sample. Proteins were extracted by the Kjeldahl method (conversion factor, 6.25) and carbohydrates by the Fehling-Causse Bonnans procedure.
Development and reproduction bioassays
Cohorts of neonates of C. externa (24 h after hatching) were randomly selected from the predator colony and used for the bioassays. The larvae were placed in ventilated Petri dishes with a fine brush. Five treatments involving different nutritional regimens were carried out: (a) diet A, (b) diet B, (c) adults of R. padi at a low prey density (20 aphids per larva), d) adults of R. padi ad libitum (50, 100, and 150 aphids applied to the 1st, 2nd, and 3rd larval instars, respectively), and finally e) a combination of diet A and prey at low density (20 aphids per larva). The combination of diet A + prey was chosen over diet B for this experimental condition based on pilot experiments (Haramboure M, unpublished data) where diet A had shown more favorable results than diet B. Each Petri dish was checked every two days for replenishment of the corresponding food. The nutritional quality of all diets was assessed through the following biological parameters: The developmental time of the larval and pupal stages was checked daily and recorded until the emergence of adults. In addition, the pupal weight was measured on an analytical balance (Acculab®) at a precision of 10−4 g. Once emerged, adults were identified as females or males by the external genitalia and then paired. The preoviposition period (from adult emergence to the first day of oviposition), fecundity (number of eggs laid per day), and fertility (number of hatched larvae per day) were observed daily. The last two parameters were estimated as the total over a period of five days.
Statistical analysis
Data were tested by the analysis of variance (ANOVA) after being log-transformed [log (x + 1)] when this was necessary, to insure normality and homoscedasticity of variances, or Kruskal–Wallis test when ANOVA assumptions were not met. Multiple comparisons between means or medians were done with a Fisher’s least-significant-difference or Box and Whisker plot tests, respectively and P < 0.05 was considered significant (Scheiner and Gurevitch 2001). These analyses were performed using the Statgraphic v. XVI software (STSC 2013).
Results
Composition analysis and costs of diets A and B
An analysis of the dietary macrocomponents indicated specific differences between the basic composition of the two diets A and B (Table 2): Diet A contained 2 % more proteins than diet B, whereas diet B, contained more than twice the lipids than diet A. Conversely, the hydrolyzed carbohydrates of diet B were a little over 2 % more than in diet A, but the nonhydrolyzed-carbohydrate content of the latter was over ten times that of diet B. On the basis of these macrocomponents only, we determined the caloric value of the two diets to be about 150 kcal per 100 g for diet A and 188 kcal per 100 g for diet B. For the rearing of one thousand individuals, the cost of diet A and B was around 20 and 40 $US kg−1, respectively.
Development time
The development time of each larval instar of C. externa varied with the different treatments (Table 3). However, we observed no differences between diet A + prey and prey ad libitum: the predator reached the pupal stage the earliest when the larvae were fed on diet A + prey and with prey ad libitum. Diet A alone or a low prey density resulted in a significantly longer developmental time than diet A in combination with the prey, while diet B resulted in the latest pupal formation of all treatments.
Pupal weight
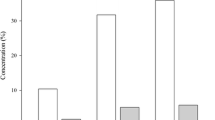
Pupal weights differed among the four diets (Fig. 1). The largest pupae resulted from feeding on diet A + prey, followed by prey ad libitum and diet A alone. Diet B and prey at low density gave rise to significantly smaller pupae.
Preoviposition period, fecundity and fertility
The preoviposition period was not affected by the different larval diets (Table 4): the time between the emergence of the adult females and their subsequent oviposition was five days under all dietary treatments. However, we noted significant differences in the adult-female fecundities on the first day of oviposition after having been fed on the different diets as larvae (Fig. 2). Females from the larvae fed on prey ad libitum exhibited a significantly lower fecundity on the first day, whereas the highest oviposition rate was observed with diet A + prey. Cumulative number of hatched eggs was lowest on diet B, and highest with prey ad libitum or diet A + prey.
Fecundity of Chrysoperla externa females from larvae reared on different diets. Fecundity on the first day (FecFD: black part of the bars) and cumulative fecundity over the first five days (AFec: entire bar length). The data are the mean ± SE. Different letters denote significant differences between nutritional treatments (ANOVA, Fisher LSD, P < 0.05) (FecFD: F = 3.39; df = 4, 36; P = 0.019; AFec: F = 3.04; df = 4, 36; P = 0.029)
The percentage of eggs hatched on day 1 (Fig. 3) was low when the larvae had been fed on a low prey density or diet B. The percentage increased significantly after having fed on diet A + prey, whereas feeding on diet A or prey ad libitum resulted in intermediate hatching percentages. The cumulative fertility followed a different pattern in relation to the cumulative fecundity with a significantly higher number of hatched eggs with diets A and B, intermediate values for diet A plus prey or prey ad libitum, and the lowest number with low prey density as food.
Fertility of Chrysoperla externa females from larvae reared on different diets. Fertility on the first day (FerFD; black bars) and cumulative fertility over the first five days (AFer; white bars). The data are the mean ± SE. Different letters denote significant differences between nutritional treatments (ANOVA, Fisher LSD, P < 0.05) (FerFD: F = 3.39; df = 4,36; P = 0.018; AFer: F = 2.71; df = 4, 36; P = 0.045)
Discussion
In recent years, the rearing of the natural enemies (predators and parasitoids) of pests in the laboratory has been of pressing, albeit limiting, concern in the mass production of those biological control agents, along with their implementation in the framework of integrated pest management (IPM) programs. Because the main problem of mass rearing of predators is the high economical costs of natural or factitious prey, the aim of our work was to evaluate a low-cost artificial diet with all the required nutritional properties. For the feeding of one thousand larvae, for example, we would need 2 kg of diet A alone (35 $US), 3.2 kg of diet B alone (112 $US), or 1.2 kg of diet A (21 $US) if we combine it with prey at low density (taking into account the differences in development time with each regime. See Table 3). These values are much lower than those reported by Vandekerkhove et al. (2008) when commercialized factitious prey were used for mass rearing of several predators (650 $US kg–1). According to our calculations, these artificial diets are about 85 % cheaper than the use of factitious prey.
In the present experiments, the packaging of the diet into small spheres wrapped in stretched Parafilm® was done to resemble the size and shape of the natural prey. All Neuropteran larvae, including chrysopids, have suctorial mouthparts that consist in feeding tubes formed by the mandibles and maxillae (Monserrat et al. 2001). Since the chrysopid larvae attack their prey by inserting their jaws and injecting salivary secretions to liquefy the internal tissues through extraoral digestion (Syed et al. 2008), the Parafilm® mimicked the prey’s cuticle, needing to be perforated to extract the contents. This type of penetration and digestion allowed the larvae to attack larger prey, where a spherical body of double or triple the size of a larva is not only accessible but also becomes more suitable than small pieces of food material (Grenier 2012). This mode of feeding dictates the acceptability of artificial diets by predators (Cohen and Staten 1993; Grenier et al. 1994; De Clercq et al. 1998; Cohen 2004).
The larval development time of these insect predators is affected by their diet (Osman and Selman 1996, Principi and Canard 1984), and if the aminoacids necessary for larval growth are not acquired during the immature stages, the subsequent stages would be affected, increasing the total duration of the life cycle (Scriber and Slansky 1981; Thompson 1999; Stathas 2001). In our studies, the immature development time of C. externa was shortened when the larvae were fed on diet A + prey, which could be related to the higher protein content of this diet.
The development time of the immature stages (cf. Table 3) was shorter with prey ad libitum or diet A + prey than with the three remaining diets. Other studies have demonstrated that an artificial diet alone was not as suitable for predators as natural prey because of the presence of specific nutrients in the latter (Riddick 2009; Silva et al. 2009). Moreover, those authors observed that a diet of high prey density was comparable to one of low prey density. In contrast, and along with the present results, Atlihan et al. (2004) noted that individuals of C. carnea completed their immature stages sooner with increased prey numbers. Our results, furthermore, demonstrate that a low prey density supplemented with diet A had the same effect on the developmental time as did feeding on the prey ad libitum. Nevertheless, the presence of the prey as diet is crucial for the development of first instar. For this reason, the diets A or B alone were not of sufficient nourishment for this instar.
From the order of the pupal weights registered on the five diets (Fig. 1)—diet A + prey > diet A or prey ad libitum > diet B or low prey density—diminished prey densities did not result in optimal development of the larvae. Since diet B apparently contains insufficient protein, it might have been rejected by larvae, thus resulting in a low weight at the pupal stage. Diet A + prey resulted in much larger pupae. Their weight was similar to that obtained by Syed et al. (2008) for C. carnea fed on different nutritional regimens.
The preoviposition period, i.e., the time between the emergence of the adults and the first oviposition, did not differ with feeding on the five diets. During the period between emergence of adults and first oviposition, the pre-oviposition females could have fed on the adult artificial diet, which could have resulted in the uptake of nutrients lacking during their immature stages. In contrast, the females of C. carnea exhibited a shortened preoviposition period on a diet with an increased prey density (Atlihan et al. 2004), while Jokar and Zarabi (2014) registered a longer preoviposition period of C. carnea when those larvae were fed an artificial diet alone, and no differences on a diet consisting of a combination of S. cerealella eggs + an artificial diet or on a diet of the S. cerealella eggs alone.
The large-scale production of predatory insects for augmentative biological control is frequently restricted by a suboptimal fecundity of predators reared on artificial diets (Grenier 1994). We therefore analyzed the fecundities and fertilities on the first day and those accumulated over the first five days. These parameters, critical for the performance of C. externa as a biological control agent, are linked to oogenesis since fecundity is strongly influenced by imaginal feeding in proovigenic insects such as chrysopids (Canard and Volkovich 2001).
When larvae fed on prey ad libitum, the low oviposition rate on the first day (Fig. 2) indicated that this natural diet did not insure a high fecundity at this time. In contrast, the highest number of eggs initially laid was after feeding with diet A + prey, implying that rearing on diet A improved the reproductive development of the females. The same was observed for the cumulative fecundity, where feeding with diet A + prey resulted in a significantly higher oviposition rate than with the other diets. The higher percentage of protein in diet A compared to diet B could explain this observed difference in fecundity. Indeed, reduced egg production has previously been observed in different experiments on predatory insects reared on artificial diets, where the decreased fecundity has been attributed to a deficiency in proteins that led to a lack of mature-follicle formation (Adams 2000; Ferkovich and Shapiro 2004).
Larval feeding on diet A + prey likewise produced the highest number of larvae hatched on the first day of oviposition (Fig. 3). Based on the combined fecundity and fertility results, the total number of offspring per female was higher when larvae were reared on diet A + prey. Jokar and Zarabi (2014) reported maximum fecundity values for C. carnea females when a combination of an artificial diet and S. cerealella eggs had been supplied to C. carnea larvae, but the highest percentage of hatched eggs was found when the larvae had been fed S. cerealella eggs alone.
In the present experiments, however, the cumulative fertility showed a different pattern: although lower values were obtained with prey at low density, the rest of the dietary regimens resulted in higher values (70–80 %). As alluded to above, this difference could be explained considering that adults were provided with a different type of artificial diet so that during the first five days of adult life, the reproduction parameters were affected by this regimen in a manner superimposed upon the original nutritional effects of the diets supplied during those insects’ immature development.
In conclusion, the results obtained in this study indicate that the low-cost artificial semiliquid diet A is an excellent complement to an R. padi–based diet. This combination may prove to be an optimal diet for mass-rearing of C. externa in biological-control programs. The most important characteristics of a natural enemy for any mass-rearing program are a fast developmental rate and a high rate of reproduction (Hajek 2004; Qiu et al. 2004). These are the parameters that where highest on a combination of diet A and natural prey.
References
Adams TS (2000) Effect of diet and mating status on ovarian development in a predaceous stink bug Perillu sbioculatus (F.) (Heteroptera: Pentatomidae). Ann Entomol Soc Am 93(3):529–535
Albuquerque GS, Tauber CA, Tauber MJ (1994) Chrysoperla externa (Neuroptera: Chrysopidae): life history and potential for biological control in Central and South America. Biol Control 4(1):8–13
Atlihan R, Kaydan B, Özgökçe MS (2004) Feeding activity and life history characteristics of the generalist predator, Chrysoperla carnea (Neuroptera: Chrysopidae) at different prey densities. J Pest Sci 77(1):17–21
Bastidas JS, Devia EHV, Amaya OS (2010) Breeding and test of the predatory capacity of Chrysoperla externa on Neohydatothrips signifer, a pestiferous trips of the passion fruit crop. Corpoica Cienc Tecnol Agropecu 11(1):31–40
Bonte M, De Clercq P (2008) Developmental and reproductive fitness of Orius laevigatus (Hemiptera: Anthocoridae) reared on factitious and artificial diets. J Econ Entomol 101(4):1127–1133
Bonte M, De Clercq P (2010) Impact of artificial rearing systems on the developmental and reproductive fitness of the predatory bug Orius laevigatus. J Insect Sci 10(1):1–11
Boregas KGB, Carvalho CF, Souza B (2003) Aspectos biológicos de Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) em casa-de-vegetação. Ciênc Agrotec 27(1):7–16
Canard M, Volkovich TA (2001) Outlines of lacewing development. In: McEwen P, New TR, Whittington AE (eds) Lacewings in the crop environment. Cambridge University Press, Cambridge, pp 130–155
Cohen AC (2004) Insect diets: science and technology. CRC Press, Florida
Cohen AC, Smith LK (1998) A new concept in artificial diets for Chrysoperla rufilabris: the efficacy of solid diets. Biol Control 13(1):49–54
Cohen AC, Staten RT (1993) Long-term culturing and quality assessment of predatory big-eyed bugs, Geocoris punctipes. In: Narang SK, Bartlett AC, Faust RM (eds) Applications of genetics to arthropods of biological control significance. CRC Press, Florida, pp 122–132
De Clercq P, Merlevede F, Tirry L (1998) Unnatural prey and artificial diets for rearing Podisus maculiventris (Heteroptera: Pentatomidae). Biol Control 12(2):137–142
Díaz-Aranda L, Montserrat V (1995) Aphidophagous predator diagnosis: key to genera of European chrysopid larvae (Neuroptera: Chrysopidae). Entomophaga 40(2):169–181
Ferkovich SM, Shapiro JP (2004) Comparison of prey-derived and non-insect supplements on egg-laying of Orius insidiosus maintained on artificial diet as adults. Biol Control 31(1):57–64
González EV, Heredia JF, Cichón L, Fernández D, Garrido S (2011) Crisópidos (Insecta: Neuroptera) asociados a frutales de pepita en el Alto Valle de Río Negro y Neuquén (región Patagonia Norte Argentina). Hort Argent 30(73):5–8
Grenier S (1994) Rearing of Trichogramma and other egg parasitoids on artificial diet. In: Wajnberg E, Hassan SA (eds) Biological control with egg parasitoids. CAB International Pub, Wallingford, pp 73–92
Grenier S (2012) Artificial rearing of entomophagous insects, with emphasis on nutrition and parasitoids—General outlines from personal experience. Karael Sci Eng J 2(2):1–12
Grenier S, Greany PD, Cohen AC (1994) Potential for mass release of insect parasitoids and predators through development of artificial culture techniques. In: Rosen D, Bennett FD, Capinera JL (eds) Pest management in the subtropics: Biological control—A Florida perspective. Intercept, Andover, pp 181–205
Hajek AE (2004) Natural enemies: An introduction to biological control. Cambridge University Press, Cambridge
Haramboure M, Reguilón C, Alzogaray RA, Schneider MI (2014) First record of Chrysoperla asoralis and C. argentina (Neuroptera: Chrysopidae) in horticultural fields of La Plata associated with the sweet pepper (Capsicum annuum L.). Rev SEA 73(3–4):187–190
Hilbeck A, Moar WJ, Pusztai-Carey M, Filippini A, Bigler F (1998) Toxicity of Bacillus thuringiensis Cryl Ab toxin to the predator Chrysoperla carnea (Neuroptera: Chrysopidae). Environ Entomol 27(5):1255–1263
Jervis MA, Ferns PN (2005) The timing of egg maturation in insects: ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 107(3):449–460
Jervis MA, Boggs CL, Ferns PN (2007) Egg maturation strategy and survival trade-offs in holometabolous insects: a comparative approach. Biol J Linn Soc 90(2):293–302
Jokar M, Zarabi M (2014) Comparative study of different diets efficiency on some biological parameters of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Mol Entomol 5(1):1–9
Legaspi JC, Carruthers RI, Nordlund DA (1994) Life history of Chrysoperla rufilabris (Neuroptera: Chrysopidae) provided sweet potato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) and other food. Biol Control 4(2):178–184
Lundgren JG (2009) Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol Control 51(2):294–305
Monserrat VJ, Oswald JD, Tauber CA, Díaz-Aranda LM (2001) Recognition of larval Neuroptera. In: McEwen P, New TR, Whittington AE (eds) Lacewings in the crop environment. Cambridge University Press, Cambridge, pp 43–82
Osman MZ, Selman BJ (1996) Effect of larval diet on the performance of the predator Chrysoperla carnea Stephens (Neuropt., Chrysopidae). J Appl Entomol 120(2):115–117
Principi MM, Canard M (1984) Feeding habits [Chrysopidae]. Ser Entomol 27:76–92
Qiu YT, van Lenteren JC, Drost YC, Posthuma-Doodeman CJAM (2004) Life-history parameters of Encarsia formosa, Eretmocerus eremicus and E. mundus, aphelinid parasitoids of Bemisia argentifolii (Hemiptera: Aleyrodidae). Eur. J Entomol 101(1):83–94
Riddick EW (2009) Benefits and limitations of factitious prey and artificial diets on life parameters of predatory beetles, bugs, and lacewings: a mini review. BioControl 54:325–339
Rimoldi F, Schneider MI, Ronco AE (2008) Susceptibility of Chrysoperla externa eggs (Neuroptera: Chrisopidae) to conventional and biorational insecticides. Environ Entomol 37(5):1252–1257
Scheiner S, Gurevitch J (2001) Design and analysis of ecological experiments. Oxford University Press, New York
Scriber JM, Slansky FJ (1981) The nutritional ecology of immature insects. Annu Rev Entomol 26(1):183–211
Senior LJ, McEwen PK (2001) The use of lacewings in biological control. In: McEwen P, New TR, Whittington AE (eds) Lacewings in the crop environment. Cambridge University Press, Cambridge, pp 296–303
Silva RB, Zanuncio JC, Serrão JE, Lima ER, Figueiredo MLC, Cruz I (2009) Suitability of different artificial diets for development and survival of stages of the predaceous ladybird beetle Eriopis connexa. Phytoparasitica 37(2):115–123
Soto J, Iannacone J (2008) Efecto de dietas artificiales en la biología de adultos de Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae). Acta Zool Mex 24(2):1–22
Specty O, Febvay G, Grenier S, Delobel B, Piotte C, Pageaux JF, Ferran A, Guillaud J (2003) Nutritional plasticity of the predatory ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae): Comparison between natural and substitution prey. Arch Insect Biochem Physiol 52(2):81–91
Stathas GC (2001) Rhyzobius lophanthae prey consumption and fecundity. Phytoparasitica 28(3):203–211
Syed AN, Ashfaq M, Ahmad S (2008) Comparative effects of various diets on development of Chrysoperla carnea (Neuroptera: Chrysopidae). Int J Agric Biol 10(6):728–730
Tauber MJ, Tauber CA, Daane KM, Hagen KS (2000) Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrysoperla). Am Entomol 46(1):26–38
Thompson SN (1999) Nutrition and culture of entomophagous insects. Annu Rev Entomol 44(1):561–592
van Driesche RG, Hoddle MS, Center TD (2009) Control of pests and weeds by natural enemies: an introduction to biological control. Blackwell, Oxford
van Lenteren JC (2000) Measures of success in biological control of arthropods by augmentation of natural enemies. In: Gurr G, Wratten S (eds) Measures of success in biological control. Kluwer Academic Publishers, Dordrecht, pp 77–103
van Lenteren JC (2003) Quality control and production of biological control agents. Theory and testing procedures. CABI Publishing, Wallingford
van Lenteren JC, Bueno VHP (2003) Augmentative biological control in Latin America. BioControl 48(2):123–139
Vandekerkhove B, Parmentier L, van Stappen G, Grenier S, Febvay G, Rey M, De Clercq P (2008) Artemia cysts as an alternative food for the predatory bug Macrolophus pygmaeus. J Appl Entomol 133(2):133–142
Vogt H, Bigler F, Brown K, Candolfi MP, Kemmeter F, Kühner CH, Moll M, Travis A, Ufer A, Viñuela E, Waldburger M, Waltersdorfer A (2000) Laboratory method to test effects of plant protection products on larvae of Chrysoperla carnea (Neuroptera: Chrysopidae). In: Candolfi MP, Blümel S, Forster R, Bakker FM, Grimm C, Hassan SA, Heimbach U, Mead-Briggs B, Reber R, Schmuck R, Vogt H (eds) Guidelines to evaluate side-effects of plant protection products to non-target arthropods. IOBC/WPRS, Reinheim, pp 27–44
Acknowledgments
This research was funded by a PICT 2011 1752 grant from the Argentine National Agency for the Promotion of Science and Technology (ANPCyT) to M. I. Schneider. M.H. was a fellow holder of the Scientific Research Commission (CIC) of Buenos Aires Government when the present research was carried out. M.H. and L.M. are also grateful to CONICET for the PhD grants. Donald F. Haggerty, a retired biologist researcher and native English speaker, edited the final version of the manuscript. The authors are grateful to Olivier Christiaens for his invaluable help and time to improve the last version of the manuscript and Guy Smagghe for his contributions. We thank also the two anonymous reviewers and editors whose contributions have helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Arne Janssen.
Rights and permissions
About this article
Cite this article
Haramboure, M., Mirande, L. & Schneider, M. Improvement of the mass rearing of larvae of the neotropical lacewing Chrysoperla externa through the incorporation of a new semiliquid artificial diet. BioControl 61, 69–78 (2016). https://doi.org/10.1007/s10526-015-9699-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9699-7