Abstract
Stomoxys calcitrans is widely adopted in experiments due to their relevance in animal health. Nevertheless, the rearing of S. calcitrans under laboratory conditions is still a challenge. In this manuscript, we show a new method for rearing the immature stages of S. calcitrans using plant material as only substrate source and sterilized sand for pupae to mimic their natural environment in order to maintain the colonies in the laboratory and for other biological tests. For this purpose, 65 eggs were collected from the colony established in the laboratory and placed into Petri dishes (80 × 15 mm) containing 50 grams of a mixture of alfalfa hay (100 g) and distilled water (200 ml), in a controlled environment (27 ± 1 °C and 70 ± 5 % UR), and the experiment was repeated six times. The mean number of days for egg hatching was 1.4 (± 0.5), the larval period from L1 to L3 was 11.33 days (± 1.75) and the pupal period was 5.83 days (± 0.51). The complete cycle from egg to adult was 18.66 days (± 2.06). The mean larval survival rate, pupation rate, and emergence rate (pupa to adult) were 91.03 % (± 3.14), 88.71 % (± 3.17), and 91.13 % (± 3.0), respectively. The results obtained in the present work indicated that the use of a larval substrate with alfalfa hay allowed the development of immature stages of S. calcitrans in laboratory, which has been demonstrated by the good percentages of larval development, pupation, and adult emergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The stable fly, Stomoxys calcitrans (Linnaeus 1758), is a cosmopolitan mandatory blood-sucking Diptera, responsible for the spread of pathogenic microorganisms to humans and domestic animals, such as bacteria, protozoa, helminths, and viruses, including Trypanosoma evansi (Steel 1885), Trypanosoma vivax (Ziemann 1905), West Nile fever virus, equine infectious anemia virus, and others (Valgode 1992; Green et al. 1996; Cugloviti et al. 2010; Doyle et al. 2011; Rodriguéz et al. 2014). It affects mainly horses and cattle, causing stress due to their flight habit and painful bites, inducing defensive/escape movements, resulting in loss of energy and reduction of feeding time/total consumption, which negatively influences horse performance and productivity gains in cattle (Baldacchino et al. 2013).
Endemic in several regions of Brazil, stable fly outbreaks have significant socio-economic impacts, resulting in annual losses of up to US$ 335 million in Brazilian livestock (Grisi et al. 2014; Kassab et al. 2012). Outbreaks in both Southeastern and Mid-Western of Brazil occur mostly due to its proliferation in organic sugarcane waste and by-products of ethanol production (Côrrea et al. 2013; Dominghetti et al. 2015). As we observe frequent outbreaks of stable flies, there is an increasing need to conduct bioassays to establish alternative of parasite control methods, making it necessary to maintain viable colonies in the laboratory. However, the rearing of stable flies in laboratory remains a challenge. In Brazil, a few laboratories have viable colonies to perform bioassays, such as Embrapa Gado de Corte, which has immature and adult forms of this Diptera (Barros et al. 2014).
Although it is known that S. calcitrans has a preference for laying eggs and developing in decomposing organic, vegetable matter and dung of vertebrate herbivore animals (Baleba et al. 2019; Soulsby 1987), most larvae diets for S. calcitrans consist of wheat flour, meat flour, bicarbonate, and sugar cane (Macedo 2001). Larvae diet should mimic their natural environment, containing adequate moisture and fermentation, so usually, those substrates require baking soda and water for proper control of these variables (Barros et al. 2014). Stable fly rearing protocols do not consider many conditions found naturally in the fly environment, such as the substrate and the possibility of larvae migrating to a drier ground when ready for pupation. Furthermore, it is common to recommend different temperatures for the different ontogenetic stages of the fly, between 25 ºC (± 1) for adults and 26.7 °C (± 1) for immature stages (Bailey et al. 1975; Gilles 2005; Parr 1962).
Thus, the objective of this study was to evaluate the development of colonies created with the supply of alfalfa hay as the only larval substrate, sterilized sand for pupation, and stabilization of a temperature for all stages, given the lack of studies that use vegetable matter and to mimic the conditions found in their natural environment. The present study was part of JBD master degree dissertation and is partially available as a pre-print in the Universidade Federal de Santa Maria repository (see Dillmann 2018).
Materials and methods
The assay was carried out in the Laboratório de Parasitologia Veterinária (Laboratory of Veterinary Parasitology) of the Departamento de Microbiologia e Parasitologia (Department of Microbiology and Parasitology) from Universidade Federal de Santa Maria (UFSM), Santa Maria, Rio Grande do Sul, Brazil. As in Dillmann et al. (2020), the initial colony of S. calcitrans was collected manually using Falcon® tubes, from equines located at Escola de Equitação Universitária de Santa Maria (EQUSM), Universidade Federal de Santa Maria (UFSM), in the municipality of Santa Maria, Rio Grande do Sul, Brazil, in October 2016.
After identification, the flies were transferred to entomological cages (30 × 30 × 50 cm) and kept in an air-conditioned room 27 (± 1 °C), under a light/dark cycle 12:12 and 70 (± 5 %) relative air humidity. Blood was collected weekly in 4-ml tubes of 0.38 % sodium citrate, to avoid coagulation, and stored at 4ºC for up to one week. An 18 years old gelding reared at EQUSM, which was not treated with topical or systemic insecticides within three months before the start of the study, provided the blood. Adults were fed once a day with the citrated equine blood, preheated in a water bath at 38ºC, supplied in a gauze wrapped cotton placed in glass available in a top corner of the cage and water at will. A supplementary feed composed of brown sugar mixed with tap water was provided daily, in the proportion of 50 grams for every 200 ml, disposed of the same way as the blood.
The alfalfa hay was previously placed in identified brown paper bags and autoclaved at 127º C for 15 minutes for proper sterilization. Afterward, the bags were stored in an oven at 37 °C for drying the leaves. The larval substrate was prepared in the proportion of 100 g of alfalfa hay to 200 ml of distilled water and stored in a partially closed plastic container, kept in the fly breeding room at a temperature of 27 °C ± 1 and 70 % ± 5 RH for adequate fermentation, during 6 days before use. For posture, a Petri dish (80 × 15 mm) containing the moist alfalfa hay was placed in the center of the cage, although oviposition was also observed to occur in the equine blood-soaked gauze. Eggs were carefully removed through a moistened brush and transferred to Petri dishes (80 × 15 mm) containing the larval substrate, then placed above a layer of sieved and sterilized sand (100 g) in a clear plastic container (350 ml).
The larval substrate was daily moistened through syringe jets containing distilled water (10 ml) and 50 g of alfalfa hay substrate was added on top of the existing one. The container was sealed with organza fabric and tied with a rubber band for proper ventilation and observation of the larvae. After completing their development, third instar larvae (L3) directed themselves to the sand for pupation, from where they were collected by sieving and properly relocated in a Petri dish (120 × 20 mm). Pupae that remain in the larval substrate were manually collected with a spatula.
For the assay, eggs of the third generation of adult flies kept in the laboratory were collected and maintained as described above. Six replicates were made, each of which had 65 eggs. Larvae were analyzed daily and as they got to L3 (Fig. 1) they were removed from the substrate and proceeded to count. After this procedure, the larvae were returned to the substrate for pupation to occur. The pupae were removed from the sand, counted, and placed in Petri dishes (120 × 20 mm) in the center of a new entomological cage. After hatching, adult flies were counted and analyzed visually and under a stereomicroscope to identify morphological changes.
The efficiency of alfalfa hay substrate diet was observed through the hatching time of the eggs in days (d), the larval development in days (d), the interval between pupation and adult emergence in days (d), viability (%), larval transformation to pupae (%) and pupal viability/emergence of adults (%).
Results and discussion
In order to establish an S. calcitrans colony, replicating conditions found in their natural environment, we opted to use leguminous hay, widely used as a food supplement for both livestock and horses, as a single source for immature rearing. Alfalfa hay is available in troughs or distributed on the field, and it remains in contact with humidity, forms a favorable environment for flies, such as S. calcitrans, to carry out their life cycle. In addition to a new substrate, other modifications were made to previously breeding protocols, such as using sterile sand for pupa, covering with organza fabric for protection against invaders, temperature stabilization, and providing a supplementary feed.
Supplementary feeding of adults with a mixture of brown sugar and water was chosen as an alternative option to honey, commonly used to maintain the basal metabolism of the flies (Salem et al. 2012). The alternative arose because it is common to occur outbreaks of stable flies in Brazil’s sugar cane fields. Moreover, the supply of blood once a day was sufficient for sexual maturation and consequently mating and laying of eggs.
The mean egg hatching time was 33.6 hours (± 12 h) (Fig. 2), similar to results found by Mello and Garcia (1988) under the same environmental conditions. A favorable hatch performance, according to Cançado et al. (2014), would be 72 hours. The majority of authors have found better results at higher temperatures than at lower temperatures, such as Valgode and Azevedo (1992), who obtained an incubation period of 69.90 and 42.28 hours at 20ºC and 25ºC (55–75 % relative humidity) respectively, and Florencio et al. (2020) who obtained good results at 30ºC. However, it is essential to note that post-embryonic development becomes unsustainable at temperatures above 35ºC (Valgode and Azevedo 1992).
Larvae completed their development in 11.33 days (± 1.75) (Fig. 2), and the mean emergency pupal to adult was 5.83 days (± 0.51) (Fig. 2), with an average number of 18.66 (± 2.06) days between egg to adult. Although we consider the larval period to be high, the pupa period was reduced, generating similar results compared to other methodologies in the same environmental conditions of this study, such as Valgode and Azevedo (1992), 16.35 (± 2.88) days at 25 °C, and Salem et al. (2012), 19.2 (± 1.7) days.
It seems that the subtle increase in temperature plays an important role in shortening the life cycle of S. calcitrans, by reducing the average length of development from egg to adult to 12 days at 35 °C, compared to 70 days at 15 °C (Gilles 2005). Although it appears as a good option, the increase in temperature, as previously mentioned, is critical for immature stages and leads to a decrease in the number of larvae that pupate. In our work, we maintained immature stages at 27 ºC ± 1, the average temperature between the protocols already described, which proved to be efficient, as demonstrated in the results.
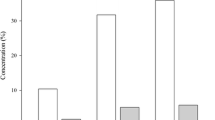
The percentage of hatching was not verified, since this has a direct relationship with the adult fly, by the process of copulation and oviposition. Another factor that affects the hatchability of eggs is the chosen methodology for collecting and handling, in this case, a fine moistened tip brush is crucial to assure a more outstanding efficient colony production. Thus, to verify the development in the larval substrate, larval viability was verified by the percentage of S. calcitrans eggs submitted to the substrate of alfalfa hay that reached third instar larvae. Results showed that an average of 91.03 % (± 3.14) of the eggs turned into third instar larvae (Fig. 3).
Nutrition acts as a limiting factor in the development of immature forms of S. calcitrans, transferring the inhibitory effect from one stage to the next (Sutherland 1978). The use of alfalfa hay, in adequate fermentation and humidity, proves to supply the larvae’s nutritional needs. Gilles et al. (2008) demonstrated that S. calcitrans larvae have a great ability to digest cellulose and take advantage of it, as well as bacteria resulting from this digestion, providing enough nutrients for an adequate larval development, which corroborates with our findings. In addition, replacing a part of the meat flour used in the standard larval diet by bovine feces, Moura (2015) reached 92.8 % (± 6.1) of larva viability, in contrast with 70 % found by Valgode and Azevedo (1992), which used a larval diet of sugar cane and wheat bran.
Good results were also observed in pupation through adequate nutrition, with a mean of 88.71 % (± 3.17) (Fig. 3). Even with a high percentage, it was observed that the period between larva and pupa was the one that obtained the most losses, being the most sensitive phase of the fly. However, when using the diet formulated by Macedo (2001) composed of sugar cane (330 g), soybean meal (125 g), meat flour (40 g), sodium bicarbonate (5 g), and distilled water (250 ml), Moura (2015) obtained only 55.9 % (± 21.3) of pupation, which contrasts with the results obtained in the present work.
In this case, we also noticed that an important factor was the availability of dry sterilized and sifted sand near the substrate for the larvae to migrate when it reached the pupation stage since the moisture requirements for this period differ from those required for the larvae. According to Mello (1989), larvae seek in their developmental substrate a place with adequate humidity conditions to initiate pupation. In our experience, it was rare to find pupa in the larvae substrate, since it contains a higher degree of humidity than that present in the sand, not being conducive to pupation. However, it is crucial to replace the sand whenever it presents high humidity due to the movement of larvae before the pupation period.
Viability of pupae, or the emergence of adults, averaged 91.13 % (± 3.0) (Fig. 3), results similar to those found by Sutherland (1978), who also states that the low quality of the larval diet produces a decrease in the size of the pupa and consequently low emergence of adults. Studies using flour-based larvae diets, under environmental conditions similar to the present study, obtained an average of 88 % (Gingrich 1960). In general, the pupae stage hardly suffers significant losses, reaching 95 % emergency levels, as described by Kunz et al. (1977). However, it is important to emphasize that in temperatures above 35 °C this percentage can reduce to 13 %.
A recurrent problem in the breeding of S. calcitrans, which even causes a decrease in larvae’s conversion into pupae, is the invasion of opportunistic insects. Researchers like Florencio et al. (2020) and Salem et al. (2012), observed significant mortality of larvae by an invasion of Drosophila sp. and Macrocheles sp., being the last a natural predator of S. calcitrans (Kinn 1966). To avoid invaders, our entomological boxes were made with plastic bags, having only one entrance, which remained closed with a rubber band, and micro holes for ventilation. All phases of the fly remained inside the cages, and in addition, the pots containing the larvae were closed with organza fabric and a rubber band.
The rearing of S. calcitrans in laboratory is important for supplying healthy flies for bioassays, providing better, controlled conditions. Thus, larval substrates that mimic the natural environment conditions, such as moist alfalfa hay, are conducive to the development of immature stages of S. calcitrans, with good percentages of adult emergence. This alternative protocol in a single environment proves to be easy to handle and low-cost methodology.
References
Bailey DL, Whitfield TL, LaBrecque GC (1975) Laboratory biology and techniques for mass Producing the Stable Fly Stomoxys calcitrans (L.) (Diptera: Muscidae). J Med Entomol 12(2):189–193
Baldacchino F, Muenworn V, Desquesnes M, Desoli F, Charoenviriyaphap T, Duvallet G (2013) Transmission of pathogens by Stomoxys flies (Diptera: Muscidae): a review. Parasite 20:26–37. https://doi.org/10.1051/parasite/2013026
Baleba SBS, Torto B, Masiga D, Weldon CW, Getahun MN (2019) Egg-laying decisions based on olfactory cues enhance offspring fitness in Stomoxys calcitrans L. (Diptera: Muscidae). Sci Rep 9:3850. https://doi.org/10.1038/s41598-019-40479-9
Barros TM, Souza TF, Cançado PHD (2014) Metodologia para bioensaios com imaturos de Stomoxys calcitrans. XVIII Congresso Brasileiro de Parasitologia Veterinária, Gramado, RS
Cançado PHD, Souza TF, Oliveira H, Barros ATM, Piranda EM (2014) Efeito do período de maturação do substrato no desenvolvimento de imaturos de Stomoxys calcitrans. XVIII Congresso Brasileiro de Parasitologia Veterinária, Gramado, RS
Corrêa EC, Ribas ACA, Campos J, Barros ATM (2013) Abundância de Stomoxys calcitrans (Diptera: Muscidae) em diferentes subprodutos canavieiros. Pesquisa Veterinária Brasileira 33(11):1303–1308
Cuglovici DA, Bartholomeu DC, Reis-Cunha JL, Carvalho AU, Ribeiro MF (2010) Epidemiologic aspects of an outbreak of Trypanosoma vivax in a dairy cattle herd in Minas Gerais state Brazil. Vet Parasitol 169:320–326
Dillmann JB (2018) Stomoxys calcitrans: feno de alfafa como substrato larval e atividade adulticida do óleo de Melaleuca alternifolia com alto teor de 1,8-cineole. Dissertation, Universidade Federal de Santa Maria
Dillmann JB, Cossetin LF, Giacometi M, Oliveira D, Matos AFIM, Avrella PD, Garlet IQ, Heinzmann BM, Monteiro SG (2020) Adulticidal activity of melaleuca alternifolia (Myrtales: Myrtaceae) essential oil with high 1,8-cineole content against stable flies (Diptera: Muscidae). J Econ Entomol. https://doi.org/10.1093/jee/toaa117
Dominghetti TFDS, Barros ATMD, Soares CO, Cançado PHD (2015) Stomoxys calcitrans (Diptera: Muscidae) outbreaks: current situation and future outlook with emphasis on Brazil. Revista Brasileira de Parasitologia Veterinária 24(4):387–395
Doyle MS, Swope BN, Hogsette JA, Burkhalter KL, Savage HM, Nasci RS (2011) Vector competence of the stable fly (Diptera: Muscidae) for West Nile virus. J Med Entomol 48:656–668
Florencio M, Rosa D, de Araújo Lima KR, da Costa GA, Guedes KVG, Fampa P (2020) Establishment and quantitative measure of Stomoxys calcitrans (Linnaeus, 1758) colony production in Rio de Janeiro, Brazil. Vet Parasitol Reg 21:100434. https://doi.org/10.1016/j.vprsr.2020.100434
Gilles J (2005) Dynamique et gene tique des populations d’insectes vecteurs. Les stomoxes, Stomoxys calcitrans et Stomoxys niger niger, dans les elevages bovins reunionnais. [PhD thesis] Universite de La Reunion, France
Gilles J, David JF, Lecomte P, Tillard E (2008) Relationships between chemical properties of larval media and development of two Stomoxys species (Diptera: Muscidae) from Reunion Island. Env Entomol 37(I1):45–50. https://doi.org/10.1603/0046-225X(2008)37
Gingrich RE (1960) Development of a synthetic médium for aseptic rearing of larvae of Stomoxys calcitrans (L.). J Econ Entom 53:408–411
Green BE, Foil LD, Hagidus SD, Issel CJ (1996) Stability of equine infectious anemia virus in Aedes aegypti (Diptera: Culicidae), Stomxys calcitrans (Diptera: Muscidae), and Tabanus fuscicostatus (Diptera: Tabanidae) stored at -70ºC. J Am Mosq Control Assoc 12:334–336
Grisi L, Leite RC, Martins JRS, Barros ATM, Andreotti R, Cançado PHD, Léon AAP, Pereira JB, Villela HS (2014) Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet 23:150–156
Kassab SO, Gaona JC, Loureiro ES, Mota TA, Fonseca PRB, Rossoni C (2012) Novos surtos populacionais de mosca-dos-estábulos no Mato Grosso do Sul: medidas de controle e prevenção. Agrarian 5(15):84–88
Kinn DM (1966) Predation by the mite, Machroceles muscadomesticae (Acarina: Macrochelidae), on three species of fly. J Med Entomol 3(2):155–158
Kunz SE, Berry IL, Foerster KW (1977) The development of rhw immature forms of Stomoxys calcitrans . Entomol Soc 70:169–172
Macedo DM (2001) Desenvolvimento pós-embrionário de Musca domestica (Díptera: Muscidae) e Stomoxys calcitrans (Diptera: Muscidae) criadas em fezes de bovinos tratados com diferentes avermectinas. Thesis, Seropédica: Universidade Federal Rural do Rio de Janeiro
Mello RP (1989) Estudo de alguns aspectos de desenvolvimento biológico e do comportamento, em laboratório, de Stomoxys calcitrans (Linnaeus, 1758) (Diptera: Muscidae). Thesis, Itaguaí: Universidade Federal Rural do Rio de Janeiro
Mello RP, Garcia MLM (1988) Comportamento reprodutivo de fêmeas de Stomoxys calcitrans (L.) (Diptera: Muscidae) criadas isoladamente em laboratório. Mem Inst Oswaldo Cruz 83(3):385–390
Moura FVC (2015) Desenvolvimento de substratos para criação de mosca-dos-estábulos Stomoxys calcitrans (Diptera: Muscidae) em laboratório [PhD Thesis]. Universidade Federal de Mato Grosso do Sul
Parr HCM (1962) Studies on Stomoxys calcitrans (L.) in Uganda, East Africa II Notes on life-history and behavior. Bull Entomol Res 53(2):437–443
Rodríguez NF, Tejedor-Junco MT, González-Martín M, Gutierrez C (2014) Stomoxys calcitrans as possible vector of Trypanosoma evansi among camels in an affected area of the Canary Islands, Spain. Revista da Sociedade Brasileira de Medicina Tropical 47:510–512. https://doi.org/10.1590/0037-8682-0210-2013
Salem A, Bouhsira E, Lienard E, Bousquet MA, Jacquiet P, Franc M (2012) Susceptibility of two European strains of Stomoxys calcitrans (L.) to cypermethrin, deltamethrin, fenval-erate, k-cyalothrin, permethrin and phoxim. J App Res Vet Med 10:249–257
Soulsby EJL (1987) Parasitología y enfermedades parasitárias en los animales domésticos. México Interamericana 7:823
Sutherland B (1978) The suitability of various types of dung and vegetable matter as larval breeding media for Stomoxys calcitrans L. (Diptera: Muscidae). J Vet Res 45:241–243
Valgode AM, Azevedo EMWV (1992) Determinação das exigências térmicas de Stomoxys calcitrans (L.) (Diptera, Muscidae), em condições de laboratório. Mem Inst Oswaldo Cruz 87(1):11–20. https://doi.org/10.1590/S0074-02761992000500005
Ziemann H (1905) Beitrag zur trypanosomenfrage. Zentralblatt Bakteriol Parasitenkd Infekt Abt I Orig 38:307–314
Acknowledgements
We thank the anonymous reviewers for valuable comments that greatly improved this manuscript. In addition, the authors are grateful to the Escola de Equitação Universitária de Santa Maria (EQUSM) for technical support. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the institution’s ethical standards or practice at which the studies were conducted (CEUA/UFSM: 6762190917).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 14.9 KB)
Rights and permissions
About this article
Cite this article
Dillmann, J.B., Cossetin, L., dos Santos Petry, L. et al. An alternative protocol for rearing of stable fly, Stomoxys calcitrans (Diptera: Muscidae) under laboratory conditions. Int J Trop Insect Sci 41, 2453–2458 (2021). https://doi.org/10.1007/s42690-020-00422-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00422-2