Abstract
In the present study, a new ion-pair solvent including tetra-n-butyl-ammonium iodide and 1-pentanol was prepared for the first time and it was used for microextraction and UV–Vis spectrophotometric determination of tartrazine (E102), sunset yellow (E110), ponceau 4r (E124), allura red (E129) and brilliant blue (E133). Analytical parameters of the procedure such as pH, concentration of ion-pair solvent and its volume, times of vortex and centrifugation were optimized. Interference effect of matrix ions and dyes were investigated after optimization of the parameters. Limits of detection between 24 and 82 μg L−1 and limits of quantification in the range of 80–275 μg L−1 were determined for the examined dyes. Preconcentration factor was obtained as 15 for each of the dyes. Relative standard deviations were found between 3.2 and 6.1%. Linear dynamic ranges were obtained between 0.28 and 20 µg mL−1 for the determined dyes. Procedure was applied to various food samples including energy drinks, powdered juice samples, syrups and candies. Analyte addition-recovery studies were also performed both for validation of procedure and determination of dye concentrations in the real samples. Food dye contents of real samples were determined between 5.9 and 52.4 μg mL−1 for liquid samples and 6.2 and 135.2 μg g−1 for solid samples with satisfactory recovery results ranging from 93 to 103%. Finally, the greenness of the developed procedure was assessed using two tools, the Green Analytical Procedure Index and Analytical Eco-Scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthetic colourants, being indispensable additives for food products such as confectioneries and energy drinks, have posed potential risk factor which may threaten human health [1,2,3]. The most of synthetic food dyes are petroleum originated and they are manufactured by distillation of petroleum and its derivatives [4, 5]. Amongst the artificial food dyes, all food dyes contain aromatic benzene rings and some of them contain azo group (–N=N–) [6]. These food dyes have been denoted with E code as colouring food additives on the foodstuff packs and/or bottles. Tartrazine (TZ), sunset yellow (SY), ponceau 4r (P4R), allura red (AR) and brilliant blue (BB) are the synthetic food dyes which have known as E102, E110, E124, E129 and E131, respectively [7,8,9]. When they are consumed excessive amount by eating and drinking through different food products, they can lead to undesired human health problems, such as skin irritation, redness, eczema, hyperactive behaviour defects, distractibility, etc. [10, 11]. Therefore, they are potentially hazardous chemicals which have limited per day consumable amounts. Acceptable daily intake values of E102, E110, E124, E129 and E131, defined by World Health Organization (WHO), are 7.5, 4, 4, 7 and 6 mg kg−1, respectively [12, 13]. When restrictions and harmful effects of the dyes are taken into account, it is necessary and important to monitor dye contents of food products by determining dye concentration with sensitive, reliable and simple analytical procedures.
Food samples containing synthetic colourants have been detected and determined various instrumental techniques such as high performance liquid chromatography (HPLC) [14], spectrofluorometry [15], liquid chromatography and mass spectrometry (LC–MS) [16], voltammetry [17], capillary electrophoresis [18] and UV–Vis spectrophotometry [19]. The used instruments in these techniques are sophisticated, expensive, not available in many laboratories and implementation of these techniques have highly time consuming and required high operation experience, knowledge and highly skilled analyst except UV–Vis spectrophotometry. The usage of UV–Vis spectrophotometer is simple and its operation cost is cheap and moreover it is available in many laboratories. Spectrophotometer presents fast determination and easy operation. However, target dye in the samples containing interfering species may not be detected and determined sensitively and accurately by UV–Vis spectrometer. Due to the interference effect of matrix, the device loses its advantages, such as selectivity and precision [20]. In order to keep advantages of the spectrophotometer, applications of one or more separation and preconcentration steps are necessary prior to spectrophotometric determination.
For the preconcentration and separation processes of the hazardous synthetic chemicals and dyes, analytical chemists have attempted to overcome this problem by eliminating interference effect of matrix with using conventional separation and preconcentration methods such as solid-phase extraction (SPE) [21], cloud point extraction (CPE) [22] and liquid–liquid extraction (LLE) [23]. On the other hand, the large volumes of solvents are used in these traditional methods. In order to avoid usage of large volumes of toxic organic solvents, analytical chemists have tended to green, environmentally friendly and miniaturized extraction methods such as dispersive liquid–liquid microextraction (DLLME) [24], ionic liquid-based dispersive liquid–liquid microextraction (IL-DLLME) [25], supramolecular solvent-based liquid phase microextraction (SS-LPME) [26], dispersive solid-phase microextraction (DSPME) [27], liquid phase microextraction (LPME) [28] and deep eutectic solvent-based liquid–liquid microextraction (DES-LLME) [29,30,31].
Nowadays, DES-LLME method has much attention because it offers simplicity, fast analysis, accurate determination and effective separation [32]. The method has reduced usage of toxic organic solvents and hazardous chemicals [33]. Moreover, it has minimized analysis time, energy consumption and amount of waste chemical substances. DES is new eco-friend extractant which can be prepared easily at least by two or more chemicals in many laboratories which have not equipped highly with instruments and specific chemicals [34]. The components of DES are H bond acceptor and donor [35, 36]. Mixture of two compounds at a certain mole ratio is essential to synthesize hydrophobic DES and furthermore, formed eutectic solvent gains superior extraction capability.
Amongst the different DES types, alkanol-based DES is formed by mixing a quaternary ammonium salt (H bond acceptor) and an alcohol compound (H bond donor) [37]. First one is phase transfer agent which is quaternary ammonium salt that transfers the ionic water soluble analyte from aqueous phase to hydrophobic organic liquid phase. Second one is straight and long hydrocarbon chain which contains hydrophobic alkyl groups (-CH2-) and water soluble hydroxyl (-OH) functional group. The long hydrocarbon chain of alcohol forms micelles by self-assembly process in water, resulting extraction takes place [38]. Furthermore, it was reported that alkanol-based DES exhibited effective separation and satisfactory quantitative microextraction for the food dyes [39].
In this study, tetra-n-butyl ammonium iodide (TBAI) and 1-pentanol had been used to create new DES but it was not obtained when 1-pentanol and TBAI are mixed at 1:1 mol ratio. The mixture was solid and it is therefore, ion-pair solvent (IPS) was created for the first time instead of DES with using components of DES having superior extraction capacity mentioned above. The created IPS was used separately to extract five synthetic food dyes in developed vortex-assisted ion-pair solvent based liquid–liquid microextraction (VA-IPS-LLME) procedure (see in Fig. 1). Analytical extraction parameters, affected extraction yield, were investigated in detail and optimized. Finally, UV–Vis spectrophotometric determination of E102, E110, E124, E129 and E131 in foodstuffs was performed. Analytical characteristics and performance of the procedure were determined, evaluated and also compared with previously reported studies. The envisaged possible microextraction mechanisms were explained.
Materials and method
Reagents and solutions
Chemicals mentioned in the experiments were used directly and without purification. (TBAI) (Merck, Germany) and 1-pentanol (Fluka, Germany) were used to prepare IPS. Ethanol (Merck, Germany) was used to dilute IPS rich phase. Chemicals of buffer solutions (Sigma-Aldrich, Germany) H3PO4//NaH2PO4 for pH 2, 3, HAc//NaAc for pH 4, 5 and NaH2PO4//Na2HPO4 for pH 6–8 were used. Buffer solutions used in the experiments have total concentration 0.01 mol L−1. Each of the standard stock dye solutions having 100 µg mL−1 concentrations were prepared by dissolving adequate amount of powder dye (Merck, Germany) in distilled water. More diluted solutions were prepared daily by using stock dye solutions.
Instruments
A Shimadzu UV160A double beam UV–Vis spectrophotometer (Shimadzu Corporation, Japan) equipped with 1.4 mL of quartz cell was used for scanning spectra and photometric determination of E102, E110, E124, E129 and E131 dyes. Hanna HI2211 digital pH-meter (Hanna Instruments, USA) was used to fix aqueous media pH and measure buffer solution pH values. A Velp brand ZX3 model (Velp Scientifica, Italy) vortex mixer was used to mix IPS with aqueous model solution in centrifuge tube. Centrifuge step was performed with using Nuve NF400 (Nuve Laboratory, Turkey) model centrifuge. An ultrasonic bath (Alex Machine, Turkey) was used throughout the experiments for preparation of IPS and in pretreatment steps of real samples.
Software
Adobe Photoshop (CS edition) for designing graphical abstract, ChemDraw Ultra (version 8.0) for explaining microextraction mechanisms and drawing moleculer structures of the chemicals and dyes used in the experiments, Microsoft Office (Professional Plus 2010) for writing manuscript and calculating experimental results were used.
Preparation of IPS
Total 25 mL of each IPSs having certain different concentrations (mmol L−1 of TBAI in 1-pentanol) 2.5, 5, 7.5 and 10 were prepared for IPS1, IPS2, IPS3 and IPS4, respectively. For this, theoretically calculated amounts of IPS components, 1-pentanol and TBAI, were added in a beaker and then it was subjected ultra-sonication at room temperature (25 °C) until formation of clear liquid solvent.
VA-IPS-LLME procedure
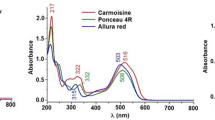
Each of the model test solutions including 5 mL of pH 5 acetate buffer solution and adequate amounts of each of the dyes for sensitive detection (AR: 2 µg, SY: 2 µg, TZ: 4 µg, BB: 1 µg and P4R: 4 µg) were prepared. The precision amounts of each dye were added to test tubes by transferring dye solutions with aid of automatic micro pipette. Solutions were diluted with distilled water up to total volume of 10 mL. Each of the model dye solutions were vortexed for 30 s after addition of 500 µL IPS4. Cloudy solutions were immediately centrifuged at 4000 rpm for 2 min to accelerate and complete phase separation. Aqueous phase at the bottom of centrifuge tube was simply decanted with help of syringe. Remaining IPS4 phase containing the dyes (AR, SY, TZ, BB and P4R) in the centrifuge tube was completed up to 1000 µL of final volume by addition of ethanol. Concentrations of diluted IPS4 phases were determined photometrically at 425, 485, 515, 506 and 630 nm, for TZ, SY, P4R, AR and BB, respectively. Experimental analysis steps of VA-IPS LLME/UV–Vis procedure are given in Fig. 1.
Preparation of real samples to analysis
Commercially available real samples including powdered fruit juices, energy drinks, candies, syrups and Turkish delight known to be contained E102, E110, E124, E129 and E131 were purchased in Turkish markets.
The certain amounts of solid powder samples between 0.15 and 2.48 g were weighed and dissolved in 100 mL distilled water. Formed colourful mixtures (solution and/or suspension) filtered through filter paper. Necessary dilutions were applied to sample solutions obtained, by taking account contents of each sample food additives. 250 µL of each of the solutions was subjected to the procedure.
0.148 g of Turkish delight sample was dissolved and/or dispersed in 100 mL distilled water. Suspension was filtered through filter paper. 100 µL of filtrate was analysed by the procedure.
The certain volumes (100 µL) of energy drink samples were subjected directly to the optimized procedure.
For the analysis of syrup samples, certain amounts (0.12 and 0.34 g) of viscous syrups were weighed and then necessary dilutions (total volume: 100 mL) were performed by distilled water. The certain volumes (100 µL) of syrup solutions were applied to the procedure to determine their dye contents.
Candy tablets (blue candy: 0.2348 g and red candy: 0.2432 g) were weighed directly and dissolved in 10 mL distilled water and then necessary dilutions (total volume: 150 mL) were done. The certain volumes (500 µL) of each of the candy solutions were analysed with the procedure.
Results and discussion
As notes for the readers: (1) Results in Fig. 2a–d were deliberately given in reverse order to show better the recovery values. (2) In this study, it had been aimed firstly to synthesize new DES with using TBAI and 1-pentanol but it was not obtained when 1-penatnol and TBAI are mixed at 1:1 mol ratio. The solid mixture was obtained instead of liquid DES and it is therefore, ion-pair solvent (IPS) was created for the first time instead of DES with using components of DES because the components of alkanol-based DES have superior extraction ability and they are also environmentally friendly green chemicals.
Effects of pH a extraction conditions: IPS: 10 mmol L−1 TBAI in 1-pentanol; volume of IPS: 500 µL; vortex: 30 s. at 2000 rpm; sample volume: 10 mL; centrifugation: 2 min. at 4000 rpm, IPS type b pH: 5; volume of IPS: 500 µL; vortex: 30 s. at 2000 rpm; sample volume: 10 mL; centrifugation: 2 min. at 4000 rpm, IPS volume c pH: 5; IPS: 10 mmol L−1 TBAI in 1-pentanol; vortex: 30 s. at 2000 rpm; sample volume: 10 mL; centrifugation: 2 min. at 4000 rpm, vortex time d pH: 5; IPS: 10 mmol L−1 TBAI in 1-pentanol; volume of IPS: 500 µL; sample volume: 10 mL; centrifugation: 2 min. at 4000 rpm, N = 3
In order to perform successful microextraction and quantitative determination of the target dyes, the parameters of VA-IPS-LLME procedure were investigated in detail and then optimized by changing one variable at one time. All extraction procedures were performed in three replicates (N = 3).
Effect of pH
Influence of pH was examined separately at pH range between 2 to 8 for each of the dyes. Results are given in Fig. 2a with 3D graphic. Recoveries for investigated dyes are quantitative at almost studied pH values. These results showed that VA-IPS-LLME procedure could be applied for microextraction of food dyes used in the experiments at investigated wide pH range. Alkanol-based IPS and the dyes operated together in superb harmony as independent of aqueous media pH. Therefore, pH 5, which is weak acidic media, was selected as an optimum for the extraction procedure and it was applied all further extraction studies.
Although, aqueous media pH is very important for extraction studies on ionisable dyes [40], pH had not played important role for this procedure because the food dyes used in the experiments contain also strong sulfonic acid (-SO3) groups. Therefore, protonation of sulfo groups are not possible at applied pH range. The protonation of phenolic rings, carbocyclic acid groups and azo groups of the dyes at investigated pHs made the dye molecules more hydrophobic in the aqueous solution, resulting contribution to transfer of ionic dye molecules from aqueous to hydrophobic IPS phase [41]. The investigated food additives were negatively charged forms at applied pH values. Therefore, food additive colourants were extracted from aqueous phase to liquid immiscible IPS phase by electrostatic attractions between anionic dye molecules and cationic quaternary ammonium salt.
Effect of IPS type
In order to obtain quantitative microextraction, IPS type used as a microextraction agent is very important criteria. For this reason, various IPSs which were prepared by mixing TBAI and 1-pentanol having different concentrations 2.5, 5, 7.5 and 10 mmol L−1 were used for IPS1, IPS2, IPS3 and IPS4, respectively. When 1-pentanol is used only as extractant, extraction percentages of target dyes were between 1 and 3%. For this reason phase transfer agent (quaternary ammonium salt, TBAI) was used with 1-pentanol. Effect of IPS type on the microextraction of five food dyes is showed in Fig. 2b. It can be clearly seen that recoveries of examined food dyes increased with increasing TBAI amount in the IPS. This circumstance can be explained that electrostatic interaction between IPS and anionic dyes increased with increasing amount of TBAI in IPS. When IPS3 and IPS4 are used as microextraction solvent, quantitative microextraction of the dyes was obtained. As a result of this experiment, IPS4 was selected an optimum and it was used further extraction studies.
Effect of IPS amount
It has prime importance to optimize amount of IPS both for complete quantitative microextraction of analyte dyes and obtaining high preconcentration factor (PF) by minimizing final extractant volume containing target dyes. In order to obtain quantitative microextraction of the dyes, sufficient surface area generated by IPS which is being immiscible extractant is necessary [42]. Hence, amount of IPS4 was investigated between 200 and 700 µL. Recovery results are given in Fig. 2c. At the lower volumes than 200 µL of IPS, separate immiscible layer were not observed. Recoveries increased with increasing IPS4 volume up to 500 µL and then reached maximum quantitative value and remained constant. This circumstance showed that it was reached to enough surface area to extract complete dye molecules in the aqueous medium. In order to keep final volume of IPS4 at a low level, obtain high PF and avoid using high volume of organic solvent, 500 µL of minimum IPS4 volume, at which was obtained quantitative extraction, was used forthcoming extraction studies.
Effect of vortex time
Vortex is necessary and important parameter to shorten analysis time and obtain quantitative extraction by increasing and accelerating the interaction between analyte and IPS. During the vortex process, entire IPS droplets micronize thus micro-holes occur for the dye molecules to enter and number of IPS droplets increase by dividing to micro size herewith sufficient surface area is formed to extract quantitatively analyte from aqueous phase [29, 43]. For that reasons, vortex time was investigated at 2000 rpm between 5 and 50 s. Recovery results for the dyes are given in Fig. 2d. Recoveries of the dyes increased with increasing vortex time up to 30 s and then reached constant value. Therefore, 30 s vortex time was selected an optimum and applied all further extraction studies.
Effect of centrifugation
In LLME studies, centrifuge is important extraction step to supply effective phase separation, obtain quantitative recoveries, accelerate and complete phase separation and huddle together immiscible hydrophobic IPS droplets containing target analyte [44]. Sticky organic immiscible phase tend to adhere centrifuge tube wall because the interactions between liquid organic phase and polyethylene centrifuge tube are higher than interactions between liquid organic phase and water. In order to examine effect of centrifuge on the LLME of each of the dyes, time and rate of centrifugation were applied simultaneously between 1 and 5 min and 1000–4000 rpm. Optimum centrifugation time and rate were found that 2 min at 4000 rpm was enough to collect together alkanol based IPS droplets containing the target dyes.
Sample volume
Obtaining quantitative data at higher sample volumes is of great necessary to reach high PF and low limit of detection (LOD) in quantitative analysis [45]. Sample volume was examined between 5 and 30 mL (5, 10, 15, 20, 25 and 30 mL) by using model test solutions. Significant changes were not observed in terms of recovery values until 15 mL of sample volume. The microextraction of the examined dyes was not quantitative above 15 mL of sample volumes. Decreased on the recoveries by increasing sample volume can be explained that turbidity of IPS in the aqueous solution decreased. It was observed that droplets of IPS did not aggregate after vortex and centrifuge processes and IPS dissolved slightly in higher sample volume than 15 mL. The experimental results showed that proposed procedure can be applied up to 15 mL of maximum sample solution. Therefore, PF of the procedure was obtained to be 15 for the target dyes, according to 15 mL maximum sample volume and 1 mL final volume.
Possible microextraction mechanisms
It could be thought that there are three possible extraction mechanisms belong to proposed procedure. The first is hydrogen bond between azo groups (–N=N–) of the dye molecules and (–H) hydrogen atom of hydroxyl functional group of 1-pentanol. The second one is n–π interaction between oxygen atom (–O–) of hydroxyl functional group of 1-pentanol and delocalize π-electrons of hydrophobic benzene rings of the dye molecules [46]. The third mechanism that is prime driving force is strong electrostatic attractions between negatively charged sulfo groups (SO3−) of the dyes and positively charged nitrogen atom (N+) of TBAI being a quaternary ammonium salt [47, 48]. The first and the second mechanisms are weak interactions. When 1-pentanol is used only as extractant, extraction percentages of target dyes were between 1 and 3%. The schematized possible microextraction mechanisms are given in Fig. 3 with using molecule structures of the colour additives and extractant droplet of IPS.
Influence of matrix
In dye extraction studies, to investigate interfering effects of matrix dyes are more important than examination of interferences of ions because foreign dyes can intervene extraction more than ions. Adaptation of the procedure to real matrix for microextraction of real samples and selectivity of the procedure against matrix components are defined and performed at this stage [49]. Therefore, possible available anions and cations which could be found in food products and the most used food dyes in foodstuffs were preferred as model matrix components. The developed VA-IPS-LLME procedure showed effective selectivity against matrix ions at applied concentrations. On the other hand, moderate selectivity was observed against matrix dyes. Recovery results of each of the target dyes in presence of matrix ions and foreign dyes are given in Table 1.
Analytical characteristics
At the optimum conditions analytical features of the procedure were examined. LOD and LOQ values of the procedure were determined between 24 and 82 and 80–275 µg L−1, respectively. Linear dynamic ranges of the microextraction procedure for investigated dyes were obtained between 0.08 and 20 µg mL−1. 15 PF was obtained from the sample volume experiments, according to 15 mL maximum sample volume at which obtained quantitative extraction and 1 mL final volume. PF is defined as ratio between maximum sample volume and final volume. Analytical characteristics of the proposed VA-IPS-LLME procedure for each of the dyes are given comparatively in Table 2.
LOD and LOQ values were determined following equations 3 SD/m and 10 SD/m, respectively. In the equations, SD and m were used to symbolize standard deviation of 13 blank solutions and slope of calibration curve, respectively.
Linear dynamic ranges, which could be plotted as linear after applied proposed procedure, of the procedure were obtained by using final concentration of the dyes in diluted ion-pair solvent rich phase. LDRs were determined separately for each of the target dyes. Analytical characteristics of the proposed VA-IPS-LLME procedure are also compared with previously reported methods in Table 3. Calculated analytical features make the procedure that it is comparable with other previously reported studies in Table 3.
Analysis of confectioneries and beverages
Validation of the developed VA-IPS-LLME procedure was performed by analyte addition-recovery studies. Multiple certain amounts of each of the analyte dyes were added to real samples including energy drinks, candies, juice powders, syrups and Turkish delight. Satisfactory recovery results ranging between 93 and 103% were obtained. These quantitative results showed that proposed microextraction procedure can be applied reliably to determine dye contents of various foodstuffs containing E129, E133, E110, E124 and E102. Recovery results for the analysed real samples are given in Table 4.
The proposed VA-IPS-LLME procedure was also applied to determine E102, E110, E124, E129 and E131 contents of real samples after validation and analyte addition-recovery tests. Food dye contents of analysed foodstuffs were determined between 6.2 and 135.2 µg g−1 for solid and 5.9–52.4 µg mL−1 for liquid samples. Results are given in Table 5.
Greenness of the developed method
In order to assess greenness of the developed method, the parameters of Green Analytical Procedure Index (GAPI) and Analytical Eco-Scale (AES) were performed. GAPI was evaluated with green, yellow and red labels and based on 15 parameters of three categories which are instrumentation, sample preparation, reagents and solvents [64]. Greenness evaluation is presented in Table 6. Developed procedure was exhibited 1 red, 4 yellow and 10 green boxes. According to GAPI parameters defined in Table 6, developed procedure can be evaluated as green analytical procedure.
AES score was evaluated based on total 100 points and penalty points (PPs) [65]. AES assessment is presented in Table 7. AES score was found as 80. According to score calculated in Table 7, developed procedure can be evaluated as green and eco-scale analytical procedure.
Conclusions
It was proved that the developed VA-IPS-LLME procedure presented a facile, inexpensive, efficient and environment-friendly pre-treatment and it can be used for quantitative analysis of E129, E133, E110, E124 and E102 in confectioneries and drinks. The chemicals used in the study are known chemicals and they are also available in the most of laboratories and it is therefore, the developed procedure could be applied in many laboratories.
Simultaneous microextraction of the dyes is possible as an alternative. Simultaneous spectrophotometric determination of binary dyes including AR-BB, BB-SY, BB-P4R and BB-TZ are possible because any spectral interference were observed at the maximum absorbance wavelengths of the dyes. Nevertheless, simultaneous determinations of the mentioned binary dyes above are possible with proposed procedure, food samples containing two food additives having E code were not found in the investigated samples and Turkish markets.
Although, the procedure has presented low PF, it is sufficient for determination of dye contents of foodstuffs and it is therefore, obtained low PF value is not a disadvantage of the procedure.
If it is necessary and preferred to work at low limits of detection and obtain higher PFs than that of proposed VA-IPS-LLME procedure, an alkanol compounds having lower water solubility than that of 1-pentanol can be used without changing type of quaternary ammonium salt (TBAI) and optimized parameters of the microextraction procedure.
In liquid–liquid mixroextraction studies a trigger such as tetra hydro furan (THF) is used usually both for dispersion of extraction liquid and formation of micelles. In the proposed procedure any triggers as aprotic solvents were not used. The prime advantage of the procedure is that microextraction of the target dyes could be performed without using any dispersive solvents such as THF and acetone.
The developed ion pair solvent (IPS)-based microextraction method is green, useful and simple for the determined food dyes. The method does not require sophisticated instruments and expert analyst. Therefore, it can be used in many laboratories.
Furthermore, developed procedure can be evaluated as green and eco-scale analytical procedure according to GAPI parameters (1 red, 4 yellow and 10 green boxes) and 80 of AES score.
References
J. Chen, X. Li, A. Huang, W. Deng, Y. Xiao, Food Chem. 364, 130373 (2021). https://doi.org/10.1016/j.foodchem.2021.130373
M. Soylak, Y.E. Unsal, M. Tuzen, Food Chem. Toxicol. 49, 1183–1187 (2011). https://doi.org/10.1016/j.fct.2011.02.013
M. Ghaedi, F.N. Azad, K. Dashtian, S. Hajati, A. Goudarzi, M. Soylak, Spectrochim. Acta A 167, 157–164 (2016). https://doi.org/10.1016/j.saa.2016.05.025
A.T. Bişgin, Y. Sürme, M. Uçan, I. Narin, Int. J. Food Sci. Technol. 51, 2367–2375 (2016). https://doi.org/10.1111/ijfs.13217
N. Masoudian, M. Rajabi, M. Ghaedi, A. Asghari, Appl. Organomet. Chem. 32, e4369 (2018). https://doi.org/10.1002/aoc.4369
M.G. Ravandi, M.R. Fat’hi, New J. Chem. 42, 14901–14908 (2018). https://doi.org/10.1039/c8nj00782a
A.T. Bişgin, M. Uçan, İ Narin, M. Soylak, Food Anal. Methods 8, 2141–2149 (2015). https://doi.org/10.1007/s12161-015-0099-5
A.T. Bişgin, J. AOAC Int. 103, 1478–1485 (2020). https://doi.org/10.1093/jaoacint/qsaa056
E.A.A. Hameed, G.H. Abd-ElHamid, O.M. El-Darder, A.K. Ibrahim, R.A.A. Salam, G.M. Hadad, M.A. Abdelshakour, Food Anal. Methods 15, 3444–3457 (2022). https://doi.org/10.1007/s12161-022-02370-8
A.T. Bişgin, M. Uçan, İ Narin, J. AOAC Int. 98, 946–952 (2015). https://doi.org/10.5740/jaoacint.14-222
S. Zhu, J. Zhou, H. Jia, H. Zhang, Food Chem. 243, 351–356 (2018). https://doi.org/10.1016/j.foodchem.2017.09.141
A.T. Bişgin, J. AOAC Int. 101, 1850–1856 (2018). https://doi.org/10.5740/jaoacint.18-0089
A.T. Bişgin, Iran J. Sci. Technol. Trans. Sci. 45, 163–175 (2021). https://doi.org/10.1007/s40995-020-00989-y
A.M.S.S. Cheibub, E.S.B. de Lyra, B.J. Alves, R.A. Donagemma, A.D.P. Netto, Food Chem. 323, 126811 (2020). https://doi.org/10.1016/j.foodchem.2020.126811
A. Coloma, M. del Pozo, R. Martínez-Moro, E. Blanco, P. Atienzar, L. Sánchez, M.D. Petit-Domínguez, E. Casero, C. Quintana, Food Chem. 345, 128628 (2021). https://doi.org/10.1016/j.foodchem.2020.128628
Y.M.G.A. Shamari, A.A. Alwarthan, S.M. Wabaidur, M.A. Khan, A.A. Alqadami, M.R. Siddiqui, J. King Saud. Univ. Sci. 32, 1135–1141 (2020). https://doi.org/10.1016/j.jksus.2019.10.011
K. Pliuta, A. Chebotarev, A. Pliuta, D. Snigur, Electroanalysis 33, 987–992 (2021). https://doi.org/10.1002/elan.202060367
J. Feng, J. Li, W. Huang, H. Cheng, Z. Zhang, L. Li, Food Anal. Methods 14, 380–388 (2021). https://doi.org/10.1007/s12161-020-01894-1
A.T. Bişgin, J. AOAC Int. 102, 181–188 (2019). https://doi.org/10.5740/jaoacint.18-0073
D. Ge, Z. Shan, T. Pang, X. Lu, B. Wang, Anal. Bioanal. Chem. 413, 3873–3880 (2021). https://doi.org/10.1007/s00216-021-03337-0
Q. Han, Y. Sun, K. Shen, Y. Yan, X. Kang, Food Chem. 347, 129026 (2021). https://doi.org/10.1016/j.foodchem.2021.129026
T. Güray, B. Menevşe, A.A. Yavuz, Spectrochim. Acta A 243, 118800 (2020). https://doi.org/10.1016/j.saa.2020.118800
S.V. Smirnova, K.A. Lyskovtseva, I.V. Pletnev, Microchem. J. 162, 105833 (2021). https://doi.org/10.1016/j.microc.2020.105833
A. Asfaram, M. Ghaedi, A. Goudarzi, M. Soylak, RSC Adv. 5, 39084–39096 (2015). https://doi.org/10.1039/c5ra02214b
S. Sadeghi, Z. Nasehi, Spectrochim. Acta A 201, 134–142 (2018). https://doi.org/10.1016/j.saa.2018.04.061
M. Soylak, M. Celik, F. Uzcan, Int. J. Environ. Anal. Chem. 100, 935–944 (2020). https://doi.org/10.1080/03067319.2019.1645842
M. Hassan, F. Uzcan, U. Alshana, M. Soylak, Food Chem. 348, 129053 (2021). https://doi.org/10.1016/j.foodchem.2021.129053
U. Balcık, D.S. Chormey, M.F. Ayyıldız, S. Bakırdere, Microchem. J. 155, 104712 (2020). https://doi.org/10.1016/j.microc.2020.104712
D. Yuvali, M. Seyhaneyildizi, M. Soylak, İ Narin, E. Yilmaz, Spectrochim. Acta A 244, 118842 (2021). https://doi.org/10.1016/j.saa.2020.118842
M. Nemati, M.A. Farajzadeh, A. Mohebbi, M.R. Sehatkhah, M.R.A. Mogaddam, Microchem. J. 159, 105496 (2020). https://doi.org/10.1016/j.microc.2020.105496
M.A. Farajzadeh, M.R.A. Mogaddam, H.R. Shahbaazi, Food Anal. Methods 9, 1096–1105 (2016). https://doi.org/10.1007/s12161-015-0279-3
W. Liu, B. Zong, X. Wang, J. Cai, J. Yu, RSC Adv. 9, 17432–17439 (2019). https://doi.org/10.1039/c9ra01405e
A. Pochivalov, P. Davletbaeva, K. Cherkashina, A. Lezov, C. Vakh, A. Bulatov, J. Mol. Liq. 271, 807–814 (2018). https://doi.org/10.1016/j.molliq.2018.09.072
N.A.N.M. Yusoff, N.Y. Rahim, R.E.A. Mohammad, N. Yahaya, M. Miskam, R. Soc. Open Sci. 8, 202061 (2021). https://doi.org/10.1098/rsos.202061
M. Faraji, J. Chromotogr. A 1591, 15–23 (2019). https://doi.org/10.1016/j.chroma.2019.01.022
T.R. Sekharan, R.M. Chandira, S. Tamilvanan, S.C. Rajesh, B.S. Venkateswarlu, Biointerface Res. Appl. Chem. 12, 847–860 (2022). https://doi.org/10.33263/BRIAC121.847860
K.A. Omar, R. Sadeghi, J. Iran. Chem. Soc. 19, 3529–3537 (2022). https://doi.org/10.1007/s13738-022-02547-2
A. Wakisaka, T. Ohki, T. Iwakami, M. Nakagawa, J. Mol. Liq. 149, 45–51 (2009). https://doi.org/10.1016/j.molliq.2009.08.003
N. Altunay, A. Elik, R. Gürkan, Food Chem. 310, 125933 (2020). https://doi.org/10.1016/j.foodchem.2019.125933
F. Aydin, E. Yilmaz, M. Soylak, Food Chem. 243, 442–447 (2018). https://doi.org/10.1016/j.foodchem.2017.09.154
M. Gómez, V. Arancibia, C. Rojas, E. Nagles, Int. J. Electrochem. Sci. 7, 7493–7502 (2012)
Z. Erbas, M. Soylak, J. Iran. Chem. Soc. 19, 3935–3942 (2022). https://doi.org/10.1007/s13738-022-02579-8
Z. Gholami, M.H. Marhamatizadeh, S. Yousefinejad, M. Rashedinia, S.M. Mazloomi, Microchem. J. 170, 106671 (2021). https://doi.org/10.1016/j.microc.2021.106671
H. Wu, J. Guo, L. Du, H. Tian, C. Hao, Z. Wang, J. Wang, Food Chem. 141, 182–186 (2013). https://doi.org/10.1016/j.foodchem.2013.03.015
M.S. Jagirani, F. Uzcan, M. Soylak, J. Iran. Chem. Soc. 18, 1005–1013 (2021). https://doi.org/10.1007/s13738-020-02085-9
A.T. Bişgin, Microchem. J. 187, 108420 (2023). https://doi.org/10.1016/j.microc.2023.108420
H. Song, J. Yang, X. Zhu, J. Appl. Polym. Sci. (2022). https://doi.org/10.1002/app.53423
P. Arabkhani, N. Sadeghi, A. Asfaram, Microchem. J. 184, 108149 (2023). https://doi.org/10.1016/j.microc.2022.108149
G.K. Yıldırım, Y. Sürme, Chem. Pap. 76, 3147–3154 (2022). https://doi.org/10.1007/s11696-022-02086-3
M. Soylak, F. Uzcan, J. Iran. Chem. Soc. 17, 461–467 (2020). https://doi.org/10.1007/s13738-019-01781-5
T. Oymak, Ş Tokalıoğlu, Ş Cam, S. Demir, Food Addit. Contam. A 37, 731–741 (2020). https://doi.org/10.1080/19440049.2020.1726501
S. Duman, M. Soylak, J. Iran. Chem. Soc. (2023). https://doi.org/10.1007/s13738-022-02646-0
A. Dalmaz, S.S. Özak, J. Iran. Chem. Soc. 19, 2359–2366 (2022). https://doi.org/10.1007/s13738-021-02454-y
H. Song, J. Yang, S. Wan, O. Xu, X. Zhu, Dyes Pigm. 205, 110523 (2022). https://doi.org/10.1016/j.dyepig.2022.110523
N. Kizil, E. Basaran, D. Erbilgin, M.L. Yola, F. Uzcan, M. Soylak, Microchem. J. 181, 107734 (2022). https://doi.org/10.1016/j.microc.2022.107734
S.S. Nasrollahi, Y. Yamini, A. Mani-Varnosfaderani, J. Food Compos. Anal. 106, 104339 (2022). https://doi.org/10.1016/j.jfca.2021.104339
S. Meral, A. Elik, Food Addit. Contam. A 38, 573–585 (2021). https://doi.org/10.1080/19440049.2021.1873427
F. Uzcan, M. Soylak, Int. J. Environ. Anal. Chem. 102, 1520–1530 (2022). https://doi.org/10.1080/03067319.2020.1738422
K.A. Lyskovtseva, G.B. Eldyaeva, S.V. Smirnova, I.V. Pletnev, J. Anal. Chem. 77, 1236–1246 (2022). https://doi.org/10.1134/S1061934822100100
Q. Li, Q. Gao, W. Liu, X. Zhu, J. Cosmet. Sci. 72, 347–361 (2021)
J. Guo, H. Wu, L. Du, Y. Fu, Anal. Methods 5, 4021–4026 (2013). https://doi.org/10.1039/c3ay40362a
A.T. Bişgin, Z. Nalvuran, O. Gezici, J. AOAC Int. 104, 137–147 (2021). https://doi.org/10.1093/jaoacint/qsaa125
N. Pourreza, M. Ghomi, Talanta 84, 240–243 (2011). https://doi.org/10.1016/j.talanta.2010.12.043
J. Płotka-Wasylka, Talanta 181, 204–209 (2018). https://doi.org/10.1016/j.talanta.2018.01.013
A. Gałuszka, P. Konieczka, Z.M. Migaszewski, J. Namieśnik, Trends Anal. Chem. 37, 61–72 (2012). https://doi.org/10.1016/j.trac.2012.03.013
Acknowledgements
This study was supported financially by Scientific Project Unit of Niğde Ömer Halisdemir University with project number of FMT 2023/2-BAGEP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bişgin, A.T. Ion-pair solvent-based liquid–liquid microextraction and spectrophotometric determination of E102, E110, E124, E129 and E133 in confectioneries and beverages. J IRAN CHEM SOC 20, 2089–2100 (2023). https://doi.org/10.1007/s13738-023-02830-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-023-02830-w