Abstract
In this work, a new generation of hydrophobic deep eutectic solvents (DESs) was prepared using eugenol (as hydrogen bond donor) and benzyltriethylammonium bromide, benzyltributylammonium bromide, benzyltriethylammonium chloride and benzyltributylammonium chloride (as hydrogen bond acceptor) in different molar ratios. These DESs were applied to vortex-assisted dispersive liquid-liquid microextraction of Sudan dyes from food samples, followed by high-performance liquid chromatographic determination. The influencing parameters, including the type of DES, amount of DES, extraction time, solution pH and salt addition, were investigated and optimized. Under the optimized conditions, a linear range of 2–1000 ng mL−1 with determination coefficients of <0.999 was obtained. Limits of detection and limits of quantification were in the range of 0.5 to 1 ng mL−1 and 2 to 3 ng mL−1, respectively. The proposed method was successfully used in the determination of Sudan dyes in chili sauce, chili powder and ketchup, and satisfactory recoveries of between 89.9 and 119.3% were obtained, with relative standard deviations in the range of 0.1–6.8%. The proposed method is simple, green and efficient, and can be applied to determine Sudan dyes in complex matrices.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sudan dyes are a family of phenyl-azoic derivatives, commonly used as colorants or polishing agents in plastics, oils, floor polishes, wax, and so on due to their low cost and color fastness [1]. Toxicological studies show that Sudan dyes and their metabolites may induce DNA damage, respiratory problems, liver, spleen and bladder cancers [2,3,4]. Therefore, the International Agency for Research on Cancer has notified Sudan dyes as category 3 carcinogens [5]. However, illegal addition of Sudan dyes to foodstuffs, particularly chili-containing in foodstuffs has been reported [6]. In this regard, development of an effective method for the determination of Sudan dyes in food samples is of great importance.
High-performance liquid chromatography (HPLC) with ultraviolet-visible detection has been widely used for the determination of Sudan dyes. However, this instrument is not sensitive enough for the determination of Sudan dyes in food samples due to the complexity of the sample matrices and low concentration of the analytes [7, 8]. Therefore, various sample preparation methods have been developed for the extraction of Sudan dyes, including solid-phase extraction [9], dual solvent–stir bar microextraction [10], U-shaped hollow fiber–liquid-phase microextraction [10] and molecularly imprinted matrix solid-phase extraction [11]. However, some of the methods are tedious and time-consuming, while others require large amounts of organic solvents. In recent years, significant efforts have been focused on developing simple, green and rapid techniques for the extraction and pre-concentration of Sudan dyes in food samples [12,13,14,15]. Dispersive liquid-liquid microextraction (DLLME) has attracted considerable attention because of its low cost, simplicity, high extraction efficiency and speed [16]. The major drawback of the method is the use of highly toxic and polluting extraction solvents, namely chloroform, carbon tetrachloride and chlorobenzene. As a result, alternative environmentally friendly and greener solvents have been identified for use as extraction solvents in DLLME [17].
Ionic liquid (IL) have been used as a potential and powerful alternative to conventional organic solvents [18, 19]. However, deficiencies of ILs include high cost, complex preparation and in some cases toxicity. Therefore, their applications in sample preparation still present some great challenges. Recently, deep eutectic solvents (DESs) have been used as a substitute for ILs in the extraction of trace amounts of analytes in DLLME [20]. A DES is synthesized by mixing a hydrogen bond acceptor (HBA) and a hydrogen bond donor (HBD) in a certain molar ratio, which then form a liquid with a melting point lower than any of the individual components [21,22,23]. Physicochemical properties of DESs are similar to those of ILs, such as low flammability, low vapor pressure, and good solubility for both organic and inorganic chemicals, but with benign characteristics, including low cost, easy synthesis, and biodegradability. [21,22,23,24]. The use of DESs in sample preparation has shown extraordinary performance as compared with most of the organic solvents and ILs [19, 24,25,26,27,28,29,30]. However, most DESs in sample preparation are water-miscible, which limits their use in the liquid samples. Development of hydrophobic DESs can result in low volumes of DES usage as well as the elimination of the use of a disperser or emulsifier organic solvents. Preparation and application of hydrophobic DESs in sample preparation has gained considerable attention of researchers in recent years [19, 25,26,27,28,29,30,31]. For example, hydrophobic DES formed from trioctylmethylammonium chloride and 2-octanol for the extraction of sulfonamides in fruit juices has been reported [26]. Zhang et al. synthesized a hydrophobic DES, composed of trioctylmethylammonium chloride and 4-cyanophenol, as the extraction solvent for the vortex-assisted DLLME of formaldehyde in biological and indoor air samples [25]. However, only a few hydrophobic DESs have been used so far to extract analytes from aqueous solution.
In this study, a new generation of hydrophobic DESs composed of eugenol and different HBAs including benzyltriethylammonium bromide (BTEAB), benzyltributylammonium bromide (BTBAB), benzyltriethylammonium chloride (BTEAC) and benzyltributylammonium chloride (BTBAC) were designed and prepared. The prepared DESs were used as extraction solvents in vortex assisted-DLLME (VA-DLLME) for the extraction of Sudan dyes, followed by high-performance liquid chromatography with diode array detector (HPLC-DAD) determination. To the best of our knowledge, this is the first report on the preparation of these hydrophobic DESs and their application in VA-DLLME to extract Sudan dyes. The parameters influencing the extraction efficiency were investigated and optimized. The proposed method was successfully applied in the determination of Sudan dyes in different food samples.
Materials and methods
Reagents and chemicals
Sudan III, Sudan IV and Sudan red G were purchased from Aladdin (Shanghai, China). HPLC-grade methanol and acetonitrile were supplied by Merck (Darmstadt Germany). BTEAB, BTBAB, BTEAC, BTBAC, sodium chloride (NaCl) and sodium hydroxide (NaOH) were obtained from TCI (Shanghai, China). Ultrapure water was obtained on a Milli-Q water purification system (Millipore, Burlington, MA, USA).
Stock solutions of each analyte were prepared at a concentration of 250 μg mL−1 in acetonitrile and stored at 4 °C. Working solutions were prepared by diluting the stock solutions with ultrapure water at different known concentrations.
Instruments
The separation and quantification of Sudan dyes were performed on an Agilent 1260 HPLC system. The chromatographic separation was performed on a Zorbax Eclipse C18 column (5 μm, 4.6 mm × 150 mm, Agilent). The sample injection volume was 10 μL and column temperature was controlled at 30 °C. The mobile phase was composed of acetonitrile and water (95:5, v/v) and the flow rate was set at 1 mL min−1. Sudan Red G and Sudan III were analyzed at a wavelength of 480 nm, and Sudan IV was detected at a wavelength of 515 nm. The structure of the DESs was characterized by Fourier transform infrared (FT-IR) spectroscopy (Nicolet iS10, Thermo Fisher Scientific, Waltham, MA, USA) and proton nuclear magnetic resonance spectra (1H NMR) (Bruker Ascend™ 400, Germany) in chloroform. Differential scanning calorimetry (DSC) (Netzsch DSC 200 F3, Germany) was used to determine the melting/freezing points of the DESs. The sample amount was 10 mg, and closed aluminum pans were used. The following temperature scheme was used: initial temperature of −30 °C, increased to 50 °C at 5 °C min−1. The determination was under a nitrogen atmosphere at a flow rate of 25 mL min−1.
Initial sample pretreatment
In this study, chili sauce, chili powder and ketchup were obtained from a local supermarket randomly. For each sample, 0.5 g of the sample was weighed and mixed with 15 mL methanol. After ultrasonication for 10 min, the mixture was centrifuged for 15 min at 1676×g. Finally, 1.0 mL of the resulting solution was further diluted to 10 mL by adding ultrapure water and subjected to the extraction procedure.
Preparation of DESs
In this work, BTEAB, BTBAB, BTEAC and BTBAC were selected as HBAs and eugenol was used as HBD to prepare the DESs. DESs were synthesized by weighing a proper amount of HBA and HBD in a round-bottom flask with a stopper. They were then placed in a water bath at 70 °C until a homogeneous liquid was obtained. The prepared DESs were stored in desiccators until use. The prepared DESs were hydrophobic in nature due to the HBAs containing long carbon chains and being highly hydrophobic.
The VA-DLLME procedure
An aliquot of deionized (8 mL) water spiked with 80 ng mL−1 of each Sudan dye or food sample was added to a 10 mL centrifuge tube with a conical bottom. Then, 75 mg of the extraction solvent was injected into the sample solution, which was subsequently vortexed for 1 min to obtain a cloudy solution. After centrifugation for 5 min at 1676×g, two phases were obtained and the DES phase (lower phase) was collected. Finally, the DES phase was mixed with 50 μL of methanol and 10 μL of the mixture was introduced into the HPLC system for analysis.
Results and discussion
Effect of the composition of DES
In the present work, DESs were prepared from BTEAB, BTBAB, BTEAC or BTBAC as the HBA and eugenol as the HBD. The prepared DESs are listed in Table 1. The composition of DES is an important parameter in the DES-VA-DLLME procedure. The extraction efficiency of the target analytes by DES 1–4 mentioned in Table 1 was investigated. It can be seen from Fig. 1 that the DES prepared from BTEAB and eugenol (DES-1) provided the highest extraction efficiency for Sudan dyes. Br− as HBA components provided larger volume and lower electrostatic force than Cl−, consequently exhibiting higher hydrophobicity ability. Therefore, DES-1 had higher extraction efficiency for the hydrophobic target analytes than DES-3. On the other hand, the increase in alkyl chain length of HBA led to a decrease in the peak areas. The possible reason for this phenomenon is that an increase in the alkyl chain length of HBA led to increasing difficulty for Sudan dyes to interact with DESs. Therefore, the longer chain length of HBA reduced the extraction efficiency. Hence, DES composed of BTEAB and eugenol was used for further optimization.
Characterization of the prepared DESs
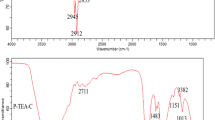
The structural characterizations of DES-1 were investigated using 1H NMR and FT-IR. The 1H NMR spectra of BTEAB, eugenol and DES-1 are shown in Fig. 2a (1H NMR spectra and FT-IR spectra of DES-2, DES-3,DES-4, DES-5 and DES-6 are given in Supplementary Information (ESM)). The peaks of DES seen can be attributed to BTEAB and eugenol, and no additional peaks were found. Figure 2b depicts the FT-IR spectrum of BTEAB, eugenol and DES-1. The DES was formed from intermolecular hydrogen bonding between BTEAB and eugenol. This can be confirmed by the broad band at 3525 cm−1 related to the stretching vibration of the O-H group in eugenol changing to 3262 cm−1 in DES-1. Based on the results obtained above, it could be said that DES-1 was prepared successfully.
The densities of the prepared DESs were determined by measuring the mass of a certain volume, and the densities of the prepared DESs were in the range of 1.01–1.17 g mL−1. The melting/freeing point of the DESs was determined using a DSC, and they ranged from −17.24 °C to 1.22 °C. A DSC thermogram of DES-1 is included in the ESM (Fig. S11).
Effect of the molar ratio of DES
The extraction efficiency of the DESs varies depending upon the molar ratio of the HBA and HBD. Therefore, DES-1 with molar ratios from 1:2 to 1:3 was prepared and investigated. DESs with a higher portion of BTEAB were not investigated due to their crystal precipitation. From the results shown in Fig. 3a, DES at a molar ratio of 1:2 had the highest chromatographic signals for all of the analytes. Thus, a molar ratio of 1:2 of DES-1 was chosen as the extraction solvent.
Amount of the extraction solvent
The amount of the extraction solvent has a significant influence on the extraction efficiency in the VA-DLLME procedure. In general, the amount of the extraction solvent should be as low as possible so as to obtain the highest enrichment factor and lowest toxicity to the environment. The amount of DES-1 was investigated to be in the range of 50–200 mg (see ESM Fig. S12). The chromatographic signals increased with the DES-1 amount from 50 mg to 75 mg, and then decreased when the DES-1 amount exceeded 75 mg. Higher amounts of DES-1 did not increase the extraction efficiency due to the dilution effect. Therefore, the amount of DES was determined to be 75 mg.
Effect of salt addition
The addition of salt to the aqueous solution has two different roles in the microextraction procedure. On the one hand, the availability of analytes for extraction can be improved by increasing the amount of salt due to the salting-out effect. On the other hand, the amount of extraction solvent could increase after extraction, thus leading to a decrease in the concentration of the analytes. To investigate the effect of salt addition, different amounts of NaCl (0–10%, w/v) were added to the aqueous solution. Results showed that the extraction efficiency was the highest with the addition of 0.5% NaCl, and then the chromatographic signals decreased in salt concentrations greater than 0.5%. The possible reason for this phenomenon is that salting-out effect played a predominant role at lower salt concentrations. However, higher salt concentrations led to higher viscosities of the aqueous samples, and therefore resulted in poor extraction efficiency. Thus, the optimal salt concentration was 0.5% (w/v).
Solution pH
The pH of the solution is one of the most important parameters in the extraction of Sudan dyes. The effect of solution pH was investigated in the range of 3–12, as the Sudan dyes are unstable in strongly acidic or basic media. As shown in Fig. 3b, the chromatographic signals increased with the increase in the solution pH and reached a maximum at solution pH of 9, then remained constant when the pH increased to 10, and finally, at further higher pH values. According to these observations, a solution pH of 9 was chosen in further experiments.
Method validation
To evaluate the performance of the proposed method, its linearity, precision, limit of detection (LOD), limit of quantification (LOQ), enrichment factor (EF), and recovery are investigated under the optimized conditions and the results are listed in Table 2. The method showed good linearity in the range of 2–1000 ng mL−1 for Sudan Red G and 3–1000 ng mL−1 for Sudan III and Sudan IV with satisfactory coefficients (r2) higher than 0.994. The LODs and LOQs were determined as- signal-to-noise ratios of 3 and 10; the LODs ranged from 0.5 ng mL−1 to 1 ng mL−1 and the LOQs ranged from 2 ng mL−1 to 3 ng mL−1. The precision, illustrated with the relative standard deviations (RSDs, calculated for the lowest concentration in the linear range, n = 6), was in the range of 1.4–4.6% for Sudan dyes. EF was calculated by the following equation: \( EF=\frac{C_{DES}}{C_{aq}} \) [CDES was the analyte concentration in the separated DES phase and Caq was the initial concentration of analytes within the real sample (after the initial sample pretreatment process)]. The extraction recoveries (ERs) were calculated according to the following equation: \( ER\%= EF\times \frac{V_{DES}}{V_{aq}}\times 100 \) (VDES and Vaq were the DES volume and the solution volume, respectively). Extraction recoveries and EFs were calculated for three replicate determinations of solutions containing 10 ng mL−1 of each analyte; ERs and EFs were in the range of 86.3–90.9% and 92–97, respectively.
Analysis of food samples
The proposed method was used to detect Sudan dyes in different food samples (chili powder, chili sauce and ketchup) and the obtained results are summarized in Table 3. As can be seen, no analytes were detected in the real samples, indicating that Sudan dyes were not present in all the samples or below the LODs for the method. To investigate the matrix effects, the real food samples were spiked with three concentrations (10, 100 and 250 ng mL−1) of the Sudan dyes. As shown in Table 3, the relative recoveries (RR) (defind as the ratios of HPLC peak areas of the respective spiked real sample extracts to those of the of spiked ultrapure water extracts) ranged from 89.9% to 119.3% with RSDs% ranging from 0.1% to 6.8%. Figure 4 shows the chromatograms of the ketchup sample, and the ketchup sample spiked with 5 ng mL−1 of each Sudan dye after performing the VA-DLLME procedure. The results indicate that the proposed method is an efficient method for the determination of Sudan dyes in real food samples.
Comparison of DES-VA-DLLME method with other reported methods
In this section, a comparison between the proposed method with those of other reported methods was performed in terms of extraction solvent, LOD, linear range, matrices and extraction time, and the results are summarized in Table 4. As the results show, the RSD% and LOD are comparable with those of the other mentioned methods [32,33,34,35,36]. It can be seen that VA-DLLME is one of the fastest procedures in terms of extraction speed, which could be attributed to the large contact area between the DES droplets and the aqueous solution due to vortexing [32,33,34,35,36]. Moreover, the hydrophobic DES can be deemed as environmentally friendly alternatives to the conventional organic solvents and some ILs [32, 33, 35, 36]. Therefore, the proposed method can be applied to determine Sudan dyes in food samples with satisfactory accuracy, precision and fast extraction.
Conclusions
For the first time, four hydrophobic DESs based on eugenol were designed and prepared. The DES formed from BTEAB and eugenol at a molar ratio of 1:2 was used as the microextraction solvent in VA-DLLME, followed by HPLC-DAD for the determination of Sudan dyes in food samples. Under the optimized conditions, high recoveries, low LODs and good precision were obtained. Using the DESs as extraction solvent, problems associated with the traditional extraction solvents were overcome. The proposed method has significant features such as low cost, simplicity and high extraction efficiency. Finally, this method showed satisfactory clean-up for the extraction of Sudan dyes from food samples.
References
Yan H, Qiao J, Pei Y, Long T, Ding W, Xie K. Molecularly imprinted solid-phase extraction coupled to liquid chromatography for determination of Sudan dyes in preserved beancurds. Food Chem. 2012;132:649–54.
Pan H, Feng J, He G-X, Cerniglia CE, Chen H. Evaluation of impact of exposure of Sudan azo dyes and their metabolites on human intestinal bacteria. Anaerobe. 2012;18:445–53.
Zhou M, Che X, Xu Y, Qu J, Jiao L, Zhang H, et al. Sensitive determination of Sudan dyes in foodstuffs by Mn–ZnS quantum dots. Dyes Pigments. 2013;99:120–6.
Kujawa J, Kujawski W, Koter S, Rozicka A, Cerneaux S, Persin M, et al. Efficiency of grafting of Al2O3, TiO2 and ZrO2 powders by perfluoroalkylsilanes. Colloids Surf A Physicochem Eng Asp. 2013;420:64–73.
Mo Z, Zhang Y, Zhao F, Xiao F, Guo G, Zeng B. Sensitive voltammetric determination of Sudan I in food samples by using gemini surfactant–ionic liquid–multiwalled carbon nanotube composite film modified glassy carbon electrodes. Food Chem. 2010;121:233–7.
Kılınç E, Çelik KS, Bilgetekin H. γ-Fe2O3 magnetic nanoparticle functionalized with carboxylated multiwalled carbon nanotube for magnetic solid phase extractions and determinations of Sudan dyes and Para red in food samples. Food Chem. 2018;242:533–7.
Sun T, Wang M, Wang D, Du Z. Solid-phase microextraction based on nickel-foam@polydopamine followed by ion mobility spectrometry for on-site detection of Sudan dyes in tomato sauce and hot-pot sample. Talanta. 2020;207:120244.
Sricharoen P, Limchoowong N, Techawongstien, Chanthai S. Ultrasound-assisted emulsification microextraction coupled with salt-induced demulsification based on solidified floating organic drop prior to HPLC determination of Sudan dyes in chili products. Arab J Chem. 2019;12:5223–33.
Wen Z, Liang X, Zhang X. Interference-free simultaneous determination of Sudan dyes in chili foods using solid phase extraction coupled with HPLC–DAD. Food Chem. 2011;125:1462–7.
Yu C, Liu Q, Lan L, Hu B. Comparison of dual solvent-stir bars microextraction and U-shaped hollow fiber–liquid phase microextraction for the analysis of Sudan dyes in food samples by high-performance liquid chromatography–ultraviolet/mass spectrometry. J Chromatogr A. 2008;1188:124–31.
Yan H, Wang H, Qiao J, Yang G. Molecularly imprinted matrix solid-phase dispersion combined with dispersive liquid–liquid microextraction for the determination of four Sudan dyes in egg yolk. J Chromatogr A. 2011;1218:2182–8.
Ling X, Chen Z. Immobilization of zeolitic imidazolate frameworks with assist of electrodeposited zinc oxide layer and application in online solid-phase microextraction of Sudan dyes. Talanta. 2019;192:142–6.
Hu K, Qiao J, Zhu W, Chen X, Dong C. Magnetic naphthalene-based polyimide polymer for extraction of Sudan dyes in chili sauce. Microchem J. 2019;149:104073.
Song Y, Wu L, Li N, Hu M, Wang Z. Utilization of a novel microwave-assisted homogeneous ionic liquid microextraction method for the determination of Sudan dyes in red wines. Talanta. 2015;135:163–9.
Pochivalov A, Davletbaeva P, Cherkashina A, Vakh C, Bulatov A. Surfactant-mediated microextraction approach using switchable hydrophilicity solvent: HPLC-UV determination of Sudan dyes in solid food samples. J Mol Liq. 2018;271:807–14.
Shi X-R, Chen X-L, Hao Y-L, Li L, Xu H-J, Wang M-M. Magnetic metal-organic frameworks for fast and efficient solid-phase extraction of six Sudan dyes in tomato sauce. J Chromatogr B. 2018;1086:146–52.
Rykowska I, Ziemblińska J, Nowak I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: a review. J Mol Liq. 2018;259:319–39.
Rajabi M, Haji-Esfandiari S, Barfi B, Ghanbari H. Ultrasound-assisted temperature-controlled ionic-liquid dispersive liquid-phase microextraction method for simultaneous determination of anethole, estragole, and Para-anisaldehyde in different plant extracts and human urine: a comparative study. Anal Bioanal Chem. 2014;406:4501–12.
Rajabi M, Ghassab N, Hemmati M, Asghari A. Emulsification microextraction of amphetamine and methamphetamine in complex matrices using an up-to-date generation of eco-friendly and relatively hydrophobic deep eutectic solvent. J Chromatogr A. 2018;1576:1–9.
Li G, Row KH. Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction. TrAC-Trend Anal Chem. 2019;120:115651.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003;1:70–1.
Pätzold M, Siebenhaller S, Kara S, Liese A, Syldatk C, Holtmann D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019;37:943–59.
Gajardo-Parra NF, Lubben MJ, Winnert JM, Leiva Á, Brennecke JF, Canales RI. Physicochemical properties of choline chloride-based deep eutectic solvents and excess properties of their pseudo-binary mixtures with 1-butanol. J Chem Thermodyn. 2019;133:272–84.
Ali J, Tuzen M, Citak D, Uluozlu OD, Mendil D, Kazi TG, et al. Separation and preconcentration of trivalent chromium in environmental waters by using deep eutectic solvent with ultrasound-assisted based dispersive liquid-liquid microextraction method. J Mol Liq. 2019;291:111299.
Zhang K, Liu C, Li S, Fan J. A hydrophobic deep eutectic solvent based vortex-assisted liquid-liquid microextraction for the determination of formaldehyde from biological and indoor air samples by high performance liquid chromatography. J Chromatogr A. 2019;1589:39–46.
Ji Y, Meng Z, Zhao J, Zhao H, Zhao L. Eco-friendly ultrasonic assisted liquid–liquid microextraction method based on hydrophobic deep eutectic solvent for the determination of sulfonamides in fruit juices. J Chromatogr A. 2020;1609:460520.
Farajzadeh MA, Abbaspour M, Kazemian R. Synthesis of a green high density deep eutectic solvent and its application in microextraction of seven widely used pesticides from honey. J Chromatogr A. 2019;1603:51–60.
Zarei AR, Nedaei M, Ghorbanian SA. Ferrofluid of magnetic clay and menthol based deep eutectic solvent: application in directly suspended droplet microextraction for enrichment of some emerging contaminant explosives in water and soil samples. J Chromatogr A. 2018;1553:32–42.
ZA AL, Habila MA, Yilmaz E, Alabdullkarem EA, Soylak M. A novel deep eutectic solvent microextraction procedure for enrichment, separation and atomic absorption spectrometric determination of palladium at ultra-trace levels in environmental samples. Measurement. 2020;153:107394.
Makoś P, Słupek E, Gębicki J. Hydrophobic deep eutectic solvents in microextraction techniques–a review. Microchem J. 2020;152:104384.
Farajzadeh MA, Sorouraddin SM, Mogaddam MRA. Liquid phase microextraction of pesticides: a review on current methods. Microchim Acta. 2014;181:829–51.
Hemmati M, Rajabi M. Switchable fatty acid based CO2-effervescence ameliorated emulsification microextraction prior to high performance liquid chromatography for efficient analyses of toxic azo dyes in foodstuffs. Food Chem. 2019;286:185–90.
Zhou Q, Zhao K, Xing A. Dispersive liquid-liquid microextraction combined with high-performance liquid chromatography for the enrichment and sensitive determination of Sudan red pollutants in water samples. J Sep Sci. 2014;37:3347–53.
Li W, Zhou X, Ye J, Jia Q. Development of a -aluminananoparticle-functionalized porous polymer monolith for the enrichment of Sudan dyes in red wine samples. J Sep Sci. 2013;36:3330–7.
Bazregar M, Rajabi M, Yamini Y, Arghavani-Beydokhti S, Asghari A. Centrifugeless dispersive liquid-liquid microextraction based on salting-out phenomenon followed by high performance liquid chromatography for determination of Sudan dyes in different species. Food Chem. 2018;244:1–6.
Xu B, Song D, Wang Y, Gao Y, Cao B, Zhang H, et al. In situ ionic-liquid-dispersive liquid–liquid microextraction of Sudan dyes from liquid samples. J Sep Sci. 2014;37:1967–73.
Funding
The authors acknowledge the financial support of National Natural Science Foundation of China (Grant No. 21702083), Yunnan key laboratory of food-safety testing technology and Yunnan Local Colleges Applied Basic Research Projects (2018FH001–022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 749 kb)
Rights and permissions
About this article
Cite this article
Ge, D., Shan, Z., Pang, T. et al. Preparation of new hydrophobic deep eutectic solvents and their application in dispersive liquid–liquid microextraction of Sudan dyes from food samples. Anal Bioanal Chem 413, 3873–3880 (2021). https://doi.org/10.1007/s00216-021-03337-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03337-0