Abstract
Heterogeneous acid-catalyzed organic transformations can be classified as one of the most important aspects of catalysis mostly due to its ability in recycling. This review deals with general discussion on the preparation of sulfonic acid derivatives immobilized on inorganic supports such as silica, periodic mesoporous silica, magnetic nanoparticles, metal organic frameworks, KIT-6, ZSM-5, MCM-41, bentonite, boehmite, clay, and other inorganic supports via organic linker. In addition, application of these inorganic supports in the acceleration of organic transformation is discussed one by one. This review aims to provide an overview of the recent developments in the field of heterogenizing homogeneous catalysts with a particular emphasis on the reaction scope and advantages of heterogeneous solid acid catalysts.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many of the organic reactions can be performed in the presence of acidic catalysts. These catalysts generally are classified into two groups: homogeneous and heterogeneous acidic catalysts. A major source of waste in the chemical industry is derived from the widespread use of mineral acids as homogeneous catalysts. These acids such as H2SO4, HF, H3PO4, AlCl3, and BF3 are hazardous in handling and damaging the plant and environment through their corrosiveness, and process difficulties using quenching and separation stages, which led to the large volume of toxic and corrosive wastes. Consequently, in recent years there has been developed a great interest in using heterogeneous acid catalysts instead of those homogeneous acid catalysts, because of the possibility for recovering and recycling solids and therefore significantly reducing the environmental impact [1,2,3,4,5,6,7,8,9].
There are numerous organic and inorganic materials which can be used for supporting reagents to produce heterogeneous catalysts such as polymers [10], carbons [11, 12], zeolites [13, 14], silicas [15], hydroxyapatite [16, 17], zirconia [18], magnetite nanoparticle [19], alumina [20], etc. All of these materials have a high surface area (100–1000 m2/g) and are normally porous with average pore diameters ranging from the microporous zeolites to some macroporous silicas [21]. Our ongoing research focus is the development of catalytic applications of heterogeneous acids and bases [9]. This contribution seeks to review recent advances in the catalytic applications of sulfonic acid-based inorganic supports with diverse structures, including silicates, zeolites, SBA-15, MCM-41, MOFs, and other inorganic supports in a comprehensive manner. As mentioned in our recent report, the different activities of these solid acids including nano-, micro-inorganic support acids may be related to the variation on the surface, the pH, the length of pendant groups, and the number of acidic site in pendant groups [9].
Type of inorganic supports making organic Brønested acids as solid acid catalysts
Silica-supported Brønested acids
Silica due to its high surface area, high thermal stability and many reactive sites for functionalization is an accessible substrate for modification with acidic organic functional groups. The modification on silica gel was started by the research of Clark group’s [22,23,24]. A wide variety of novel materials were prepared through the chemical modification of silica gels with organic and inorganic functionalities. Beside their use in chromatographic separations, these modified silicates have been increasingly used as catalysts in liquid-phase organic reactions. For example, a heterogeneous catalyst based on a chemically modified mesoporous silica gel possessing immobilized cobalt ions or solid peroxyacid based on organically modified silica has been prepared and successfully applied to the epoxidation of alkenes. Chemically modified mesoporous materials can be prepared as robust catalysts suitable for application in liquid-phase processes such as Friedel–Crafts reactions, selective oxidations, nucleophilic substitutions, aromatic brominations, etc. [22,23,24,25,26]. In this review, the application of those sulfonic acid derivatives including sulfonic acid, sulfamic acid, sulfur sulfonic acid, and sulfonate sulfuric acids which bonded through an organic linker to a support will be discussed.
Silica-bonded propyl sulfonic acid
Silica-functionalized propyl sulfonic acid was used as an efficient solid acid catalyst in various organic reactions.
Briefly, there are three methods for functionalization of silica with propyl sulfonic acid including: (1) Post-synthesis or grafting: In this method, the calcined SiO2 was refluxed with (3-mercaptopropyl)trimethoxysilane (MPTMS). Then, thiol groups of obtained product were oxidized to sulfonic acid using hydrogen peroxide (Scheme 1) [27].
(2) Direct synthesis or co-condensation: According to this method, tetraethyl orthosilicate (TEOS) was reacted with MPTMS. Then, the obtained thiol groups were oxidized by the same procedure described before (Scheme 2) [28].
(3) In situ oxidation method (sol–gel technique): In situ oxidation method is the same as described co-condensation method except that MPTMS and hydrogen peroxide were added at the same time (Scheme 3) [29].
For the first time, Babak Karimi et al. reported a procedure for dithioacetalization of various types of carbonyl compound. 0.15 g of the catalyst was enough to proceed the reaction with 1:1.1 mmol ratio of the carbonyl compound to 1,2-ethanedithiol when water was used as a solvent at 80 °C for 100 min (Scheme 4) [30].
Karimi and Zareyee [31] showed sulfonic acid-functionalized ordered nanoporous silica as an effective catalyst in deprotection of TBDMS-protected alcohols. Higher loading of sulfonic acid on the nanoporous silica makes it to catalyze the deprotection of TBDMS ethers with a trace amount of catalyst [31].
Two years later, they used silica-bonded propyl sulfonic acid in selective tetrahydropyranylation of a variety of alcohols and phenols. Characterization of the synthesized catalyst showed higher thermal stability in which the synthesized catalyst was stable up to 300 °C. In addition, reusability of the catalyst was experimentally tested and the results showed that after eight run there is no appreciable change in the catalytic activity of the synthesized catalyst. Heterogeneous nature of the catalyst was tested through a reaction in which after formation of 40% of the product, the solid acid filtered out, and the remaining mixture was allowed to react. There was no more increasing in the yield of product in reaction mixture, which indicates formation of products affected by applying solid acid catalyst [32].
Also, the same research group disclosed a route for O-trimethylsilylation of alcohols by hexamethyldisilazane (HMDS) through the aforementioned catalyst. Various alcoholic TMS ethers are produced by the corresponding silylation of primary alcohols, secondary alcohols, phenol, 4-BrC6H4OH, and 2-naphthols. Silylation of tertiary alcohols was a bit slower than that of primary and secondary ones, but it should be noted that no elimination product was observable in this case [33].
In 2015, preparation and application of this heterogeneous catalyst has been reviewed by Ziarani et al. [34].
Recently, Maggi et al. [35] used silica-supported sulfonic acid for the esterification of levulinic acid with stoichiometric amounts of alcohols under mild conditions and give good conversion of the corresponding products.
Silica-bonded 4-ethylphenylsulfonic acid (SBEPSA)
Badley and Ford [36] reported silica-supported 4-ethylphenylsulfonic acid as a heterogeneous catalyst for hydrolysis of diazinon [diethyl 2-isobropyl-6-methyl-4-pyrimidinyl phosphorothioate] and triphenylmethyl fluoride. This catalyst was prepared by tethering the amorphous silica with trimethoxy(2-phenylethyl)silane followed by sulfonation with chlorosulfonic acid (Scheme 5) [36].
Piscopo et al. [37] employed silica-bonded 4-ethylphenylsulfonic acid for oxidative coupling of xanthene and thioxanthene with methylene active compounds. The catalytic activity of the prepared catalyst was measured in the model reaction between cyclopentanone and xanthene carried out under an oxygen atmosphere for 24 h. The product was achieved in good yield (74%) and selectivity (95%). High atom economy, mild reaction conditions, metal-free reaction and easy workup was some advantage of this method (Scheme 6) [37].
The mechanism that was purposed involving autoxidative formation of a xanthene hydroperoxide (2), which would react in an acid-catalyzed SN1 type reaction with nucleophiles such as various ketones (4) to form the coupling product (5) and hydrogen peroxide (Scheme 7) [37].
Silica/A123-bonded phenylsulfonic acid
Sulfonic acid functionalized silica/A123 as a solid acidic catalyst introduce for Baeyer–Villiger oxidation of cyclic ketones in the presence of H2O2 as an oxidant in which A123 defines phenyltrimethoxysilane. Schematic representation for the preparation of the catalyst was demonstrated in the following (Scheme 8). The results obtained by different catalyst compared with silica/A123-SO3H when 2-adamantanone used as a substrate. The results summarized in Table 1 [38].
Silica-bonded S-sulfonic acid (SBSSA)
Silica-bonded S-sulfonic acid (SBSSA) was prepared by the simple reaction of 3-mercaptopropylsilica with chlorosulfonic acid in chloroform (Scheme 9) [39,40,41].
Niknam and his co-authors reported aromatic aldehydes can be converted to the 1,1-diacetates by treatment with acetic anhydride under solvent-free condition at room temperature. To a mixture of aldehyde and acetic anhydride with the molar ratio of 1:15 (mmol), 5 mg of catalyst was enough to produce 72-100% of the corresponding 1,1-diacetates (Scheme 10) [39].
Also, they showed that by applying silica-bonded S-sulfonic acid (SBSSA) as a recyclable catalyst quinoxaline derivatives were synthesized from the reaction between 1,2-diamino compounds and 1,2-dicarbonyl compounds [40]. In addition, chemoselective silylation of the hydroxyl group in the presence of other functional groups using SBSSA as catalyst was reported [41]. Interestingly, 30 times recycling of the catalyst showed that there is no appreciable loss in catalytic activity.
In another work, silica-bonded S-sulfonic acid was reported for the production of trisubstituted imidazoles that has been brought in (Scheme 11) [42].
Also, a simple methodology for the synthesis of coumarins was proposed using SBSSA as an efficient heterogeneous catalyst. Two-component coupling between resorcinol and ethyl acetoacetate indicated formation of coumarins under solvent-free condition with only applying 0.1 g of catalyst per mmol of resorcinol at 80 °C (Scheme 12) [43].
One year later, they mixed 2 mmol of indole, 1 mmol of benzaldehyde with 0.1 g of silica-bonded S-sulfonic acid to produce derivatives of bis-indolymethanes in acetonitrile at room temperature. In addition, tetra-(indolyl)methanes were achieved via the condensation of indole and dialdehyde compounds (Scheme 13) [44].
SBSSA (0.03 g) was also able to catalyze two-component mixtures of aromatic aldehydes (1 mmol), and 5,5-dimethyl-1,3-cyclohexanedione (2 mmol) to form the corresponding 1,8-dioxo-octahydroxanthenes in ethanol under reflux condition. Three-component reaction of the aromatic aldehydes (1 mmol), and 5,5-dimethyl-1,3-cyclohexanedione (2 mmol) and amines (1 mmol) led to the formation of 1,8-dioxodecahydroacridines in the same reaction conditions [45].
In an another study, the role of obtained reagent was investigated in the promotion of the reaction of aromatic aldehydes with 3-methyl-l-phenyl-5-pyrazolone to produce 4,4′-alkylmethylene-bis(3-methyl-5-pyrazolones) derivatives (Scheme 14). The studies showed that the reactions were performed in ethanol under reflux conditions in good to high yields. All types of aromatic aldehydes were efficiently reacted with 3-methyl-l-phenyl-5-pyrazolone to give the related products in 75-90% yields during 1-4 h. SBSSA as an impressive catalyst could be readily recovered and reused for four cycles with slight decrease in activity [46].
Also, this solid acid was employed as a useful and recyclable catalyst for the preparation of α-amino nitriles [47].
In continue, Niknam et al. applied SBSSA as a solid acid catalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives by three-component condensation of isatoic anhydride and an aromatic aldehyde with ammonium acetate or primary amine. Optimization condition of 80 °C as a temperature of the reaction, 5 mg of SBSSA, EtOH as a solvent along with the equivalent molar ratios (1:1:1 mmmol) of the starting materials were applied to produce the desired 2,3-dihydroquinazolinones [48].
There is also another methodological report concerning synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazole derivatives using the same catalyst. 0.05 g of SBSSA is enough to catalyze the reaction of o-phenylenediamine with aromatic aldehydes at 80 °C in aqueous media [49].
Tajbakhsh et al. [50] reported a simple and new procedure for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones and thiones by condensation of aryl aldehydes, β-dicarbonyl compounds and urea or thiourea catalyzed by SBSSA in acetic acid at 110 °C. Compared to the classical Biginelli reaction conditions, the use of silica-bonded S-sulfonic acid as a catalyst offers several advantages such as high yields, short reaction times, mild reaction conditions and a recyclable catalyst with a very easy workup [50].
Silica-bonded S-sulfonic acid also successfully employed in the promotion of the synthesis of β-amino alcohol derivatives under solvent-less conditions at room temperature (Scheme 15) [51]. The generality of this method was studied using different amines and epoxides under optimal reaction condition. The results clearly showed both steric and electronic effects on the regioselectivity of the reaction. The reaction of styrene epoxide with aromatic amines afforded high ratio of regioisomer A by nucleophilic attack at the benzylic carbon, which could be due to the localized positive charge on the more highly substituted benzylic carbon. Aliphatic amines gave regioisomer B, with the preferential SN2 attack at the terminal carbon of the epoxides. The reversal regioselectivity was observed when aliphatic epoxides such as phenyl glycidyl ether and epoxy propyl methacrylate were employed in this reaction. Steric factor seems to be responsible for this regioselectivity.
Pushpalatha et al. [52] used same catalyst in microwave irradiated synthesis of bioactive pyrimidine derivatives from reaction of arylaldehydes, 1,3-diketones, ammonium acetate and urea under solvent-free conditions within short reaction times [52].
Aswin et al. reported silica-bonded S-sulfonic acid as a facile catalyst for preparation of 2-amino-5-oxo-5,6,7,8-tetrahydro-4H-chromenes using dimedone, aromatic aldehydes, and malononitrile in refluxing conditions. Also, 2-amino-4H-pyrans synthesized by same catalyst from the reaction of ethyl acetoacetate, aldehydes, and malononitrile in aqueous ethanol (Scheme 16) [53].
Already, synthesis of naphthoxazinone derivatives from one-pot, three components coupling of β-naphthol, aromatic aldehyde and urea successfully catalyzed by silica-bonded S-sulfonic acid under solvent-free conditions (Scheme 17) [54].
Sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester (SASPSPE)
Niknam and Saberi reported the preparation of sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester as a new and reusable catalyst [55]. Sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester was prepared by simple reaction of 3-(thio(propy-3-yl)silica)-propanol with chlorosulfonic acid in chloroform (Scheme 18).
Prepared acid catalyst was employed as an efficient promoter for the formylation of different types of alcohols with ethyl formate under mild condition at room temperature with good to excellent yields. Also, the catalyst was used for acetylation of alcohols with ethyl acetate under reflux conditions and acetic anhydride at room temperature. Under the selected conditions, the catalyst can be efficiently used for chemoselective esterification of primary and secondary alcohols in the presence of tertiary alcohols [55].
Sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester could be used efficiently in the acceleration of a tandem condensation reaction between aromatic aldehydes and two equivalents of 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one in refluxing ethanol to provide 4,4′-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) [56]. Also this catalyst could be applied in the silylation of hydroxyl groups with HMDS. This reaction was performed at room temperature [57].
Niknam et al. [58] described a useful method for the synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles from the reaction of o-phenylenediamine and aromatic aldehydes using SASPSPE as a heterogeneous catalyst (Scheme 19). In this procedure, the reactions performed in aqueous media at 80 °C and the corresponding products obtained in good to excellent yields [58].
Furthermore, SASPSPE utilized as a catalyst for the synthesis of severally substituted imidazoles through a four-component condensation among aldehydes, benzil, ammonium acetate, and amines under solvent-free condition at 40 °C. Elongation of the organic linker makes the catalyst to behave like homogeneous ones in which excellent yields of products were obtained with SASPSPE. In addition, reusability of the catalyst makes it to be preferable in comparison with the homogeneous ones [59].
In 2013, the same catalyst was used for the synthesis of the α-amino nitriles by applying trimethylsilyl cyanide, aldehydes and amines as reagents [60].
Niknam et al. also presented protection of different aldehydes via acetal formation. They showed that by applying 0.03 g of catalyst I or II which was shown in the following (Scheme 20), such conversion become optimum in terms of reaction time and isolated yield and by this way promote the reaction rate. Trying to synthesize 1,1-diacetates without using catalyst was unsuccessful after 12 h in the same condition [61].
Silica-bonded N-propylsulfamic acid (SBNPSA)
Silica-bonded N-propylsulfamic acid (SBNPSA) is introduced by Niknam and Saberi as a new solid acid catalyst which simply prepared via the reaction of 3-aminopropylsilica with chlorosulfonic acid in chloroform at 0 °C (Scheme 21) [62].
After the preparation and identification, they applied this reagent in acceleration of various types of functional group transformations. At first, SBNPSA was employed as an efficient and reusable catalyst for transesterification of ethyl formate with alcohols and phenols. Also, various N-alkylformamides were prepared using amidation of ethyl formate with different amines. Also, under the selected conditions, the catalyst showed that can be used for chemoselective esterification of primary alcohols in the presence of secondary or tertiary alcohols and phenols (Scheme 22).
The acetylation of alcohols, phenols and amines with acetic anhydride was also studied using this catalyst. The obtained results indicated that alcohols and amines including different types of substituents reacted well with acetic anhydride to produce the acetylated products in high to excellent yields [62].
In 2010, silica-bonded N-propylsulfamic acid (SBNPSA) was employed as a solid acid catalyst for the synthesis of several heterocycles. Synthesis of 1,8-dioxo-decahydroacridine derivatives from dimedone, aldehyde, and aryl amine, synthesis of 1,8-dioxooctahydroxanthene derivatives from dimedone and aldehyde, synthesis of bis(1,8-dioxooctahydroxanthenes) from terephthalaldehyde/isophthalaldehyde and dimedone, and finally synthesis of quinoxaline derivatives by cyclization of different 1,2-diaryldiketones with o-phenylenediamine derivatives are reported by Niknam et al. The following scheme illustrates reaction condition for each of them (Scheme 23). An amount of 0.03 g SBNPSA was selected as an optimum amount for the first three reactions, but in the case of forth one (synthesis of quinoxaline derivatives), 0.1 g of SBNPSA was required to catalyze the reaction in optimum condition [63].
Also, SBNPSA was used in the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives via a three-component coupling reaction of isatoic anhydride and aldehydes with amines or ammonium acetate in ethanol under reflux conditions (Scheme 24). In this reaction, a wide range of aromatic aldehydes and amines were reacted well and the corresponding products were obtained in good to high yields in appropriate times. It is interesting to note that the recovered catalyst showed the same efficiency as the freshly prepared catalyst even after four times recycling [64].
In another work, Xie and Yang applied silica-bonded N-propylsulfamic acid as an efficient heterogeneous catalyst for transesterification of soybean oil with methanol [65].
Already, Jetti et al. applied silica functionalized N-propylsulfamic acid (SBNPSA) for the multicomponent Biginelli reaction of thiourea/urea, ethyl acetoacetate and different aromatic aldehydes in an environmentally friendly procedure to produce 3,4-dihydropyrimidin-2-(1H)-ones and thiones. The main feature of this catalyst was its ability to tolerate various derivatives of each starting materials. Reusability of the catalyst also studied to show its high performance catalytic activity after eight times recycling. Simplicity of this procedure has been shown in Table 2 to compare it with other reported catalysts by other groups [66].
Shakeri et al. [67] used SBNPSA for the acceleration of the reaction of alcohols with nitriles to produce the corresponding amides. In the presence of this catalyst, different types of alcohols and nitriles bearing a variety of substituents employed and the corresponding amides obtained in very good yields [67].
Recently, Karimzadeh et al. used SBNPSA as catalyst for the benzylation of 1,3-dicarbonyl compounds using secondary aromatic alcohols or styrenes as alkylating agents in high yields and short reaction times [68].
Silica-bonded propylpiperazine-N-sulfamic acid (SBPPSA)
Silica-bonded propylpiperazine-N-sulfamic acid (SBPPSA) as a solid acid was synthesized from the reaction of 3-piperazine-N-propylsilica (3-PNPS) and chlorosulfonic acid in chloroform (Scheme 25) [69].
The catalytic activity of this reagent was tested for synthesis of 1,2,4,5-tetrasubstituted imidazoles. Various aliphatic and aromatic amines and arylaldehydes were applied in this method and in each case imidazole derivatives were obtained in excellent yields in short reaction times (Scheme 26). The simplicity of the procedure, stability, high reactivity and reusability of the catalyst are the most important advantages of this study [69].
Also, SBPPSA was used as an affective catalyst in synthesis of α-aminonitriles from the reaction of aromatic or aliphatic aldehydes, primary or secondary amines and trimethylsilyl cyanide under mild reaction conditions at room temperature. The results showed that under the optimized conditions, recovered catalyst could be reused five times without appreciable loss in its catalytic ability [70].
Silica-bonded N-propylpiperazine sulfamic acid (SBPPSA) introduced as an active catalyst for one-pot tandem Knoevenagel–Michael condensation of phenylhydrazine, ethyl acetoacetate and aldehydes. Five times recycling of the catalyst from the reaction medium indicated the same efficiency of catalyst when it used for the first time (Scheme 27) [71].
Silica-bonded N-propyl diethylenetriamine sulfamic acid (SBPDSA)
Silica-bonded N-propyl diethylenetriamine sulfamic acid as a new heterogeneous catalyst was reported in 2011. This reagent synthesized via the reaction of 3-diethylenetriamine-propylsilica (DTPS) and chlorosulfonic acid in chloroform (Scheme 28) [72].
The prepared catalyst was successfully used in protection of aromatic aldehydes by acetic anhydride. The obtained results showed that different types of aromatic aldehydes containing different functional groups were protected at room temperature and solvent-free conditions with good to high yields [72].
In another study, SBPDSA applied in the promotion of the synthesis of α-aminonitrile derivatives via a one-pot condensation of aldehydes, amines, and trimethylsilyl cyanide under mild reaction conditions at room temperature (Scheme 29). A wide range of substrates, including aromatic or aliphatic aldehydes and primary or secondary amines, were reacted under the selected conditions and the corresponding α-aminonitriles were obtained in good to excellent yields in short reaction times. This catalyst showed the same efficiency when used in consecutive reaction runs [73].
Silica-bonded n-propyldiethylenetriamine sulfamic acid (SBPDSA) was detected as a suitable acidic catalyst in coumarins and bis-coumarins formation (Schemes 30, 31) [74].
Also, SPDTSA was used as a heterogeneously catalyst benzopyrano[2,3-d]pyrimidines via three-component synthesis of malononitrile, secondary amines and salicylaldehydes. For this synthesis, 2 mmol of 2-hydroxybenzaldehyde derivative, 1 mmol from each of malononitrile and secondary aliphatic amine was mixed with 0.03 g of catalyst under solvent-free condition at room temperature. Several recyclability of the catalyst along with the milder reaction condition makes this catalyst suitable for such synthesis (Scheme 32) [75].
Silica-bound N-propyl triethylenetetramine (SBPTETSA)
Another novel catalyst which named as silica-bound N-propyl triethylenetetramine sulfamic acid (SBPTETSA) was prepared via the reaction of silica-bound N-propyl triethylenetetramine with chlorosulfonic acid in chloroform at room temperature [76] (Scheme 33).
SBPTETSA was employed as an efficient catalyst for the preparation of 2-amino-4,6-diarylnicotinonitriles (Scheme 34). The best results were obtained in the presence of 0.07 g of the catalyst at 100 °C under solvent-free conditions. A variety of aromatic aldehydes were used and the results showed that the different types of substituents in each position of arylaldehydes afforded the products in high yields in short reaction times [76].
N-(propylcarbamoyl)sulfamic acid (SBPCSA)
A new silica-bonded N-(propylcarbamoyl)sulfamic acid (SBPCSA) catalyst was designed to apply it in Knoevenagel condensation of 2-thiopheneacetonitrile with various aromatic/heterocyclic aldehyde having different substitution in their structures. Schematic synthesis pathway for the desired catalyst has been shown in the following (Scheme 35). As it is depicted in Scheme 35, 1,3-chloropropylsilica was formed by adding SiO2 to the solution of (3-chloropropyl)-trimethoxysilane in toluene. After separation of 1,3-chloropropylsilica, this compound was added to the solution of urea in ethanol and the mixture refluxed to obtain urea functionalized propylsilica. As-functionalized compound separated from the reaction mixture and chlorosulfonic acid was added drop-wise in room temperature. After evolution of HCl gas and monitoring pH, filtering and then washing led to the desired SBPCSA. Synthesized catalyst was fully characterized with XRD, SEM–EDX, FT-IR and TGA/DTA. Elemental mapping of catalyst was also taken to show its good dispersion. Investigation of the catalyst in the above-mentioned reaction showed that only very small amount of catalyst, 0.04 g of SBPCSA per 1 mmol mixture of each component, is required to catalyze the reaction in a few minutes (Scheme 36) [77].
Optimization of the reaction was surveyed to study the effect of temperature, different reaction media and different catalyst loading, and then, optimized result achieved using SBPCSA were compared with other reported catalysts. For more information, such comparison has been shown in Table 2. As it is clear in Table 2, the order of activity of catalyst based on the reaction time and also the yield of product was as follows: SBPCSA > NH2SO3H > SiO2–HClO4 > SBNPU > SiO2–NH4SO4 > SiO2–H2SO4 > SiO2–Cl > SiO2 > without catalyst [77].
Amino-1-naphthalene sulfonic acid immobilized silica nano particles (RHANPSO3H)
7-Amino-1-naphthalene sulfonic acid immobilized silica was introduced as a new heterogeneous catalyst with was obtained from rice husk (RHA). The synthesis of this reagent carried out by adding 7-Amino-1-naphthalene sulfonic acid to the suspension of activated 3-chloropropyl silica in dry toluene (Scheme 37) [78].
RHANPSO3H showed good catalytic activity toward esterification of n-butanol with acetic acid. The conversion of n-butanol was 88% with 100% selectivity toward n-butyl acetate. The catalyst could be reused many times after a simple regeneration procedure [78].
Zolfigol et al. 79 reported the preparation of modified silica sulfuric acid (MSSA) as a new type of silica sulfuric acid, and effectively it was used in the conjugate addition of indole, pyrrole, and thiols with Michael acceptors under mild conditions at room temperature (Scheme 38). Also, MSSA was used as a catalyst for the synthesis of 1,1,3-tri-indolyl compounds in good to excellent yield at room temperature [79].
In another study, they reported the preparation of silica phenylsulfonic acid (SPSA) as an effectively solid acid catalyst, and it was used in the one-pot synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles from o-phenylenediamine with aldehydes in water in the presence of tetrabutyl ammonium bromide with good to high yield [80]. Also, SPSA was used as a catalyst for the synthesis of bis(indolyl)methanes in water (Scheme 39).
Later, they reported the preparation of nano-sphere silica sulfuric acid (NS-SSA). They used NS-SSA as catalyst for the highly efficient synthesis of 1,2,3,4-tetrahydropyridines in good yields by one-pot multicomponent reaction (MCRs) [81]. The reagent nano-sphere silica sulfuric acid (NS-SSA) has several advantages, such as easy workup, nontoxicity, convenience and high yields of products (Scheme 40).
In another study, nanometasilica disulfuric acid (NMSDSA) and nanometasilica monosulfuric acid sodium salt (NMSMSA) as two nanostructured novel, green and heterogeneous catalysts were designed, synthesized and fully characterized by FT-IR, energy-dispersive X-ray spectroscopy, X-ray diffraction patterns, scanning electron microscopy, transmission electron microscopy and thermal gravimetric analysis. Then their catalytic applications were studied in the Biginelli-type reaction for the synthesis of 3,4-dihydropyrimidin-2(1H)-one derivatives via one-pot three-component condensation reaction between several aldehydes, ethyl acetoacetate and urea or thiourea [82]. In 2014, Sudha and Pasha reported the preparation of the silica sulfuric acid (Si-OSO3H) and used as catalyst for the synthesis of 1,3-oxazines [83].
Moosavi-Zare et al. [84] reported the preparation of a novel nanostructured heterogeneous catalyst, namely silica-bonded 1,4-diaza-bicyclo[2.2.2]octane-sulfonic acid chloride (SBDBSAC) as an acidic ionic liquid based on 1,4-diaza-bicyclo[2.2.2]octane ring bonded to silica and fully characterized by several techniques such as Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), thermogravimetric analysis (TGA), differential thermogravimetric (DTG), scanning electron microscope (SEM), transmission electron microscopy (TEM) and energy-dispersive X-ray analysis (EDX) [84]. The nanostructured catalyst has been successfully used as reusable nanostructured catalyst for green, simple and efficient synthesis of spiropyrans by the one-pot tandem Knoevenagel-Michael-cyclization reaction of isatin derivatives or acenaphthenequinone with barbituric acid derivatives, and 1,3-dicarbonyl compounds under aqueous media (Scheme 41).
In 2017, they used this nanostructured heterogeneous catalyst, {silica-bonded 1,4-diaza-bicyclo[2.2.2]octane-sulfonic acid chloride (SBDBSAC)}, for the synthesis of bis-coumarin derivatives by the condensation reaction of arylaldehydes with 4-hydroxycoumarin at 70 °C under solvent-free conditions [85].
Acidic ionic liquid-modified silica
Yokoyama et al. [86] developed another catalytic procedure for esterification of alcohols using acetic acid and nitration of aromatic compounds using nitric acid. To do this, they initially prepared 1-allylimidazolium involving acidic ionic liquids through the reaction of 1-allyimidazole and 1,3-propanesultone or 1,4-butane sultone, then acidifying it with trifluoroacetic acid. Afterward, this Brønsted acid was immobilized on the surface of as-modified silica using 3-mercaptopropyltrimethoxysilane (MPS). Immobilization of acidic ionic liquid has been performed by applying AIBN as radical initiator to connect as-synthesized acidic ionic liquid covalently to the surface of as-modified silica. The whole procedure is demonstrated in Scheme 42 [86].
Silica-supported–SO3H functionalized imidazolium-based ionic liquid (AIL-SiO2)
In 2010, silica-supported–SO3H functionalized imidazolium-based ionic liquid was reported as a new solid acid catalyst (Scheme 43). This reagent was prepared by two steps involving nucleophilic substitution reaction of 3-chloropropyl silica with imidazole anion and then condensation of the alkylimidazole silica with 1,3-propane sultone [87].
Obtained catalyst showed to be an impressive promoter in the hydrolysis of cellulose dissolved in 1-n-butyl-3-methylimidazolium chloride at 70 °C [87]. In another study, they compared the catalytic activity of the prepared reagent with sulfonic acid silica (SiO2- SO3H) and n-propyl sulfonic acid silica (SiO2- PrSO3H) for the hydrolysis of cellulose in water. The results showed that cellulose samples heated with the new catalyst produced significantly higher amount of TRS and glucose than the others [88].
N-(3-silicapropyl) imidazolium hydrogen sulfate ([Sipim]HSO4)
After one year, Niknam et al. synthesized silica-grafted N-propyl-imidazolium hydrogen chloride ([Sipim] Cl) by reaction of 3-chloropropyl silica with imidazole followed by quenching with concentrated H2SO4 (97%) (Scheme 44) [89]. The prepared catalyst was successfully applied for the synthesis of α-aminonitriles by a one-pot condensation of aldehydes, amines, and trimethylsilyl cyanide at room temperature. The catalyst showed high thermal stability and could be recycled for several times without any additional treatment (Scheme 44).
They also investigated catalytic activity of prepared catalyst [Sipim] HSO4 in the synthesis of pyrano[3,4c]pyrazoles and pyrano[c]chromenes. Equivalent molar ratio of 4-hydroxycoumarin, malononitrile, and aldehyde mixed with 0.1 g of [Sipim] HSO4 to obtain the corresponding dihydropyrano[c]chromenes under solvent-free conditions at 100 °C. Reusability of the catalyst tested for four times and no appreciable loss in the catalytic activity was observed. In addition, a bit higher amount of [Sipim] HSO4 compared to the previous reaction (0.15 g) was used to catalyze multicomponent reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one, malononitrile, aldehyde under solvent-free conditions at 110 °C for 90 min (Scheme 45) [90, 91].
In another study, Tajik et al. was prepared N-(3-silicapropyl) imidazolium hydrogen triflate from the reaction of silica propyl imidazolium chloride ([Sipim]Cl) with CF3COOH in dry dichloromethane (Scheme 46) [92].
In this study, silylation of different compounds having hydroxyl functional moiety in their scaffold was performed with 1,1,1,3,3,3-hexamethyldisilazane (HDMS) in the presence of three different immobilized acidic ionic liquids including; silica propyl imidazolium chloride ([Sipim]Cl), pyridinium 2,2,2-trifluoracatate ([Py][Tfa]), and silica propyl-imidazoliumtrifuoro acetate ([Sipim]Tfa) [92].
Recently published work by Niknam et al. describes the synthesis of spiro[indoline‑3,4′ pyrano[2,3‑c]pyrazole] and spiro[indoline‑3,4′‑pyrano[2,3‑c]chromene] derivatives using silica-bonded ionic liquids as a reusable catalyst in refluxing aqueous medium in good to excellent yields [93]. Various silica-supported ionic liquids were as follows: silica propyl imidazolium triflate ([Sipim]OTf), silica propyl imidazolium chloride)[Sipim]Cl), N-(3-silicapropyl) imidazolium hydrogen phosphate, and ([Sipim]H2PO4)N-(3-silicapropyl) imidazolium hydrogen sulfate ([Sipim]HSO4). Schematic diagram of the reported catalysts was shown in the following (Scheme 47).
Also, applicability of the catalysts was investigated by applying 0.03-0.07 g of the catalyst for condensation of equivalent molar rations of isatin, malononitrile with 3-methyl-l-phenyl-5-pyrazolone. Good to excellent yields (75-95%) of products obtained using [Sipim]Cl as catalyst. There was no observable loss in catalytic activity after three times recycling (Scheme 48).
In addition, some other spiroxindoles were synthesized through the reaction of isatin, reactive methylene compound and 1,3-dicarbonyl compounds using 0.05 g [Sipim]Cl in the presence of water as a solvent heating in an oil bath. In this case, 70-90% yields of products showing good catalytic activity of [Sipim]Cl (Scheme 49) [93].
Silica-supported–SO3H functionalized benzimidazolium-based ionic liquid (SILC)
Silica-supported–SO3H functionalized benzimidazolium-based ionic liquid (SILC) was simply synthesized from the reaction of 3-(1-benzimidazole)propyl silica and 1,3-propane sultone in acidic solution (Scheme 50) [94].
After the preparation, this reagent was used as a stable and general catalyst for the solvent-less synthesis of 1-amidoalkyl naphthols (Scheme 51). A wide range of substrates, including aromatic or aliphatic amines and aromatic aldehydes with substituent’s carrying electron-donating or electron-withdrawing groups, were reacted under the optimized conditions and the corresponding products were obtained in good to excellent yields in short reaction times. The heterogeneous catalyst was recycled for five runs on the reaction of 3-nitrobenzaldehdye, acetamide and 2-naphthol without losing its catalytic activity [94].
Silica-bonded imidazolium-sulfonic acid chloride (SBISAC)
Moosavi-Zare et al. [95] reported a new heterogeneous acidic ionic liquid (ILs) catalyst, named silica-bonded imidazolium-sulfonic acid chloride, which was simply prepared by the reaction of propylimidazol silica with chlorosulfuric acid in chloroform [95]. The novel catalyst was fully characterized by several techniques including Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction, thermal gravimetric analysis, differential thermal gravimetric, transmission electron microscopy and energy-dispersive X-ray analysis (Scheme 52).
This reagent exhibited high catalytic activity for the synthesis of 12-aryl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-ones via the one-pot three-component condensation reaction of aromatic aldehydes with 2-naphthol and dimedone under mild and solvent-free conditions.
To assess the efficiency of SBISAC in the preparation of tetrahydrobenzo[a]xanthene-11-ones, various aromatic aldehydes (including electron-releasing and electron-withdrawing substituents) were reacted with β-naphthol and dimedone under optimized conditions and the corresponding products obtained in good to excellent yields in short reaction times (Scheme 53).
Also, they employed SBISAC as an impressive solid acid catalyst for the one-pot multi-component condensation reaction between arylaldehydes, β-ketoesters, dimedone and ammonium acetate to provide hexahydroquinoline derivatives. Short reaction times, high yields, solvent-free conditions and reusability of the catalyst were some advantages of this study [96]. Moreover, they reported the application of this catalyst as heterogeneous and reusable catalytic system for the solvent-free condensation of arylaldehydes with β-naphthol and alkyl carbamates leading to α-carbamato-alkyl-β-naphthols [97].
Silica-supported poly (styrene sulfonic acid) brush
A new class of potentially water-tolerant solid acid, silica-supported poly (styrene sulfonic acid) brushes, was prepared via surface initiated atom transfer radical polymerization (ATRP) of styrene followed by sulfonation of the polymer brush for use as acid catalysts containing highly accessible acid sites with high loading (Scheme 54) [15].
The catalytic activity and recyclability of the polymer brush sulfonic acid catalysts demonstrated in the hydrolysis of ethyl lactate. Polymer brush sulfonic acid catalysts displayed similar activity to theirs homogeneous analogue-p-toluenesulfonic acid, and a much higher reaction rate compared to an acidic polymer resin such as Amberlyst-15. A new ATRP initiator designed to be more hydrolytically stable and the resulting polymer brush catalyst, SiO2@alkyl-PS-SO3H, shown to have improved stability relative to the catalysts made with a traditional ATRP initiator containing an ester group, SiO2@ester-PS-SO3H. In addition, Chen et al. [98] reported silica hollow nanospheres with sulfonated polystyrene and octyl groups dispersed in nanopores as efficient solid acid catalysts for esterification and transesterification reactions.
Mesoporous silicates
Mesoporous silicates have attracted considerable attention since they first reported for potential application as catalysts, supports, adsorbents as well as nano-reactors for making new materials [7, 99,100,101,102,103,104,105,106,107].
These materials have relatively uniform pore sizes and high void volumes and surface areas as compared with nonordered amorphous silica. The pore sizes of these materials can be tailored depending on the synthesis method used, ranging from about 15 to about 100 Å. Moreover, larger pore sizes from 50 to 300 Å are also shown by the SBA family of solids, e.g., SBA-15 [108, 109]. Chemical surface modification of mesoporous silicas via covalent bonding of organic molecules has been achieved using two general strategies: grafting methods (post-synthesis procedure) and co-condensation reactions (direct synthesis) [110, 111].
Grafting procedures are based on modification of the silica surface with organic groups through silylation reactions occurring on isolated and geminal silanol groups using trichloro- or trialkoxyorganosilane and silylamines as organic precursors. In contrast, direct synthesis consists of the co-condensation of siloxane and organosiloxane precursors in the presence of various types of surfactants. Functional groups have been placed selectively on the internal or external pore surfaces or even within the walls of the mesoporous solids. Organic functionalization of these solids permits tuning of the surface properties (hydrophilicity, hydrophobicity, binding to guest molecules). Field of research about organic Brønsted acid-functionalized mesoporous silicates, and their application in organic chemistry has been tremendously expanded and reviewed several times [112,113,114].
Number of designations has been used for mesoporous silicate structures, some of those relevant to this review include SBA-15 (2D hexagonal, acidic conditions, prepared with block-copolymer templates) [108, 109], MCM-41 (2D hexagonal, prepared under basic conditions using cationic surfactants) [115].
SBA-15-Ph-SO3H
Veisi et al. produced various 2H-indazolo[2,1-b]phthalazine-triones and triazolo [1,2-a]indazole-triones which was demonstrated in Scheme 55 [116].
SBA-15 obtained through mixing water solution of pluronic P123, adding HCl and then TEOS, stirring, filtering, washing and drying. SBA-15 dispersed in toluene and then dichlorodiphenylsilane was added to silylate SBA-15 under N2 atmosphere. Modification of SBA-15 was done using trimethylsilyl chloride (TMSC) to protect hydroxyl groups of SBA-15. The obtained trimethylsilylated phenyl-modified SBA-15 was soaked in a solution of chlorosulfonic acid to give the desired catalyst. Scheme 56 explains step-by-step formation of SBA-15-Ph-SO3H.
Catalytic surveys indicated that SBA-15-Ph-SO3H as a nano-reactor can be applied as an active and recyclable heterogeneous catalyst in three-component and one-pot synthesis of dimedone, aldehydes and phthalazine/N-phenylurazoles under solvent-less and thermal condition [116].
Functionalized 8-hydroxyquinoline-5-sulfonic acid mesoporous silica (HQS-SBA-15)
Attachment of 8-hydroxyquinoline-5-sulfonic acid (HQS) groups onto the pores of SBA-15 (HQS-SBA-15) demonstrated in Scheme 53. As it is clear, reaction of iodo-functionalized SBA-15 with 8-hydroxyquinoline-5-sulfonic gives the corresponding HQS-SBA-15 (Scheme 57). Application of the achieved catalyst investigated through the reaction of amino acid methyl esters and isothiocyanates to give the corresponding thiohydantoin derivatives under solvent-less conditions [117].
N1-(3-(trimethoxysilyl)propyl)ethane-1,2-diamine (SBA-15/PrEn-NHSO3H)
Rostamnia and Doustkhah reported a facile and efficient catalytic procedure for N-formylation of amines using SBA-15 functionalized with N1-(3-(trimethoxysilyl)propyl)ethane-1,2-diamine as solid acid catalyst. This catalyst was prepared from reaction of SBA-15/prEn-NH2 with chlorosulfonic acid as it depicted in Schemes 58 and 59.
Optimization of the depicted reaction performed under different condition; finally, 1 mol % of catalyst, solvent-free media and 50 ̊C was acquired as optimum condition. In the case of investigation on the scope of methodology, various amines were applied and the results showed that due to the higher nucleophilicity of alkyl amines and amines containing electron-donating groups in their structural motifs, higher yields of product can be obtained. Recyclability of catalyst was tested and after 12 times recycling there was only slightly loss of catalytic activity which shows its high performance after a long runs. Chemoselectivity of SBA-15/PrEn-NHSO3H was also another main advantageous of synthesized catalyst (see Scheme 60 for more details) [118].
1-(Propyl-3-sulfonate) vinylimidazolium hydrogen sulfate-[CH2)3SO3HVIm]HSO4)
1-(Propyl-3-sulfonate) vinylimidazolium hydrogen sulfate [CH2)3SO3HVIm]HSO4) as an acidic ionic liquid supported on silica gel by applying TEOS. For this purpose, 1,3-propane-sultone added to a mixture of p-hydroquinone and vinylimidazole at a cooled condition. Then, the resultant solid reacted with sulfuric acid to obtain acidic ionic liquid. In order to immobilization of the acidic ionic liquid on the surface of silica, mesoporous silica was achieved by adding TEOS to a solution of P123 (EO20PO70EO20) to proceed hydrolysis. Afterward, MPS was added to form thiol-functionalized silica. Finally, suitable amounts of (CH2)3SO3HVIm]HSO4 and AIBN added to the as-prepared silica to give the desired catalyst (Scheme 61). It is required to mention that all the procedures involving immobilization has been done under nitrogen atmosphere. Synthesized catalyst was then applied to esterification of different carboxylic acids by means of different alcohols and it gave higher yields of products when 8 wt % of catalyst used relative to the limiting reagent in approximately 90 ˚C [119].
Immobilized sulfonic acid Brønsted acidic ionic liquid on chloromethyl polystyrene-grafted silica
Another esterification reaction was reported by Guan et al. through one another Brønsted acidic ionic liquid, but this time immobilization has been done on the surface of chloromethyl polystyrene grafted silica gel. To achieve this goal, addition of 1,3-propane sultone to an ethanolic solution of imidazole was slowly performed. Separation of the resultant solid and then drop-wise addition of sulfuric acid into it makes the formation of acidic ionic liquid. In another experiment, MPS-modified vinyl benzyl chloride formed through the heating of vinyl benzyl chloride, MPS and AIBN as an initiator in toluene. Afterward, P123 dissolved in water and HCl, and desired amount of TEOS added to occur pre-hydrolysis. Then, as-functionalized polymer mixed with the latter mixture under nitrogen. Removing P123 from the reaction mixture was done in refluxing ethanol to give the desired PS-SG hybrid. Finally, mixing PS-SG hybrid with acidic ionic liquid gave the desired catalyst. Schematic representation for the described procedure has been shown as follows (Scheme 62). Different carboxylic acids have been esterified with various alcohols in high to excellent yields. In addition, reusability of the catalyst tested nine times after recycling, and there was no significant loss in catalytic activity, which shows its high performance heterogeneous nature of the catalyst [119].
Sulfonic acid-functionalized mesoporous Pt/SBA-15
One-step synthesis of xylose to furfuryl alcohol was studied using platinum supported on ordered mesoporous SBA-15 having –SO3H acidic functional groups. It was shown that such acidic sites have very significant roles in the conversion of xylose. Cooperation of booth acid and metal was proposed in this study. It was also shown that without such acidic sites, one another molecule (xylitol) will be formed as a main product on Pt/SBA-15. This study was performed with an aqueous solution, and it was confirmed that there is no more formation of furfuryl. Therefore, cooperatively playing roles of both metal and acid concluded (Scheme 63). TEOS and a symmetric triblock copolymer with poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) structure was used to create SBA-15. Post-synthetic functionalization of SBA-15 was performed by applying 3-mercaptopropyltrimethoxysilane as precursor and then oxidizing with hydrogen peroxide. Platinum supported on acidic based catalysts were achieved with impregnation and the calcination method. Then, the oxidized metal was then reduced over continuous flowing of hydrogen gas [120].
It has been shown that PrSO3H/SBA-15 can be used for esterification of acetic acid with methanol. Synthesis of this catalyst was based on the post-functionalization of mesoporous SBA-15 using mercaptopropyltrimethoxysilane. More investigation indicates that incorporation of octyltrimethoxysilane (OTMS) in the preparation of as-prepared catalyst can lead to enhancement in the hydrophobicity of the catalyst and then increasing activity of the catalyst by inhibiting reverse ester hydrolysis [121].
Magnetic nanoparticle conjugated SBA = 15 (Fe3O4@mesoporous SBA-15)
In order preparation of magnetic nanoparticle conjugated mesoporous nanocatalyst (Fe3O4@mesoporous SBA-15), vinyl modified mesoporous SBA-15 was reacted with cysteine hydrochloride via the thiol–ene click reaction. The resulting mesoporous reagents were attached with magnetic nanoparticles (Scheme 64).
Obtained new catalyst was successfully employed in the Biginelli condensation of aldehydes, acetoacetate and urea for the synthesis of a diverse range of 3,4-dihydropyrimidin-2(1H)-ones under mild conditions. This catalyst was used several times without a significant loss of activity [122].
MCM-41-bonded propyl sulfonic acid (MCM-41-Pr-SO3H)
MCM-41-Pr-SO3H as a green and stable catalyst were synthesized from a mixture consisting of (3- mercaptopropyl) trimethoxysilane (MPTS), tetramethoxysilane (TMOS), and cetyl trimethoxy ammonium bromide (CTAB), as a template or structure directing agent (SDA). Extracted mercaptopropyl-MCM-41 oxidized to the corresponding sulfonic acid derivative using HNO3 as the oxidant (Scheme 65).
Silylation of alcohols with hexamethyldisilazane (HMDS) in dichloromethane provides the corresponding silyl ethers in excellent yields at room temperature using 1–3 mol % of mesoporous silica-bonded propyl sulfonic acid. Also, the catalyst could be easily recovered and reused for at least 20 reaction runs without loss of reactivity [123] (Scheme 66).
Methyl propyl sulfonic acid-functionalized MCM-41
MCM mesoporous silica was functionalized with methyl propyl sulfonic acid groups by means of a one-step simple synthesis approach involving the co-condensation of tetraethoxysilane (TEOS) and 3-mercaptopropyl(methyl)dimethoxysiloxane (MPMDS) in the presence of cationic surfactants (CTAB) under basic conditions and then oxidation with aqueous H2O2 (Scheme 67) [124].
Karnjanakom et al. [125] applied this heterogeneous catalyst for the preparation of biodiesel production from Hevea brasiliensis oil (para rubber seed oil) under high pressure in an autoclave reactor. Four experimental parameters, namely catalyst loading, reaction time, reaction temperature, and the molar composition of 3-mercaptopropyl(methyl)dimethoxysiloxane, were investigated using the Box–Behnken design. The optimization model showed good statistical reliability with a linear correlation coefficient close to 1. Under the optimum reaction condition (5.06 wt % catalyst loading, 120 min, 153 °C, and 0.266 of MPMDS molar composition), the highest predicted and experimental fatty acid methyl ester yields were 96.6% and 95.5%, respectively. The catalyst was benchmarked against a commercial homogeneous catalyst (H2SO4) and proven to be more effective. Moreover, the catalyst could be reused up to four cycles under the optimum reaction condition without significant loss of product yield [124].
Shagufta et al. [124] reported that sulfonic acid MCM-41 catalyzed esterification and transesterification reactions for the synthesis of esters and biodiesels. Moreover, later Alrouh et al. [126] reported mesoporous silica MCM-41 and SBA-15 containing propyl sulfonic acid groups catalyzed the esterification reaction of glycerol with olive pomace oil at 110 °C in high yields.
Mesoporous MCM-41 silica functionalized with sulfonic acid groups (MCM-41–Obenzyl-SO3H)
MCM-41–Obenzyl-SO3H was prepared by etherifying the hydroxyl group on freshly calcined MCM-41 samples with benzyl alcohol in toluene. Then the benzene ring in benzyl-incorporated sample was sulfonated by ClSO3H. The more the reacting amount of ClSO3H the higher the acid amount of SO3H-MCM-41 (Scheme 68).
SO3H-MCM-41, which has the highest acid amount 8.2 mmol/g, exhibits satisfied shape-selectivity and better catalytic activity to one-pot Fischer indole synthesis of tryptophols via phenylhydrazine hydrochlorides and 2,3-dihydrofuran than H2SO4 and H2SO4-SiO2 (Scheme 69) [127].
MCM-41-N-propylsulfamic acid
MCM-41-N-propylsulfamic acid prepared by reaction of propylamine functionalized MCM-41 and chlorosulfonic acid (Scheme 70) [128].
After the preparation, Hajjami et al. applied propylsulfamic acid-functionalized MCM-41 as an efficient catalyst for the multicomponent one-pot synthesis of 1-amidoalkyl-2-naphtols under thermal solvent-free conditions. A wide range of aromatic aldehydes, acetamide and 2-naphtol was used to synthesis of corresponding products with good to excellent yields [129].
p-Phenylamino sulfonic acid ligand functionalized on MCM-41 (MCM-3-NHPhSO3H)
MCM-3-NHPhSO3H as a new organo-inorganic hybrid material was synthesized by immobilizing 3-(4-aminophenylamino)-propane-1-sulfonic acid onto functionalized mesoporous MCM-41 via simple post-synthesis method (Scheme 71).
The catalyst was tested in the solvent-free liquid-phase tert-butylation of phenol and gave a high 99.5% tert-butyl phenol conversion in 4 h with good selectivity of 67.8% 2-tert-butyl phenol and 30.8% 4-tert-butyl phenol. The catalyst was only selective to mono-alkylated products [130].
MCM-41-4-(propylamino)butanesulfonic acid
MCM-41-4-(propylamino)butanesulfonic acid as a green and useful catalyst was first prepared by anchoring (3-aminopropyl)triethoxysilane (APTES) on Si-MCM-41, and then the obtained reagent reacted with 1,4-butane sultone in toluene (Scheme 72).
The catalyst showed high catalytic activity and high selectivity in tert-butylation of hydroquinone under microwave irradiation. No leaching problem was observed after several runs, while the catalyst can be recovered and reused without loss of reactivity under the described reaction conditions [131].
MCM-41-4-(propylthio)propyl sulfonic acid (PTPSA@MCM-41)
Sulfonic acid-functionalized mesoporous MCM-41 catalyst was successfully synthesized by anchoring 3-((3-(trimethoxysilyl)propyl)thio)propane-1-sulfonic acid onto MCM-41-type silica (Scheme 73).
This catalyst found to be effective for the synthesis of 1H-pyrazolo-[3,4-b]pyridines and spiro-pyrazolo-[3,4-b]pyridines. This is interesting to note that after eight times recycling of PTPSA@MCM-41, there was no obvious change in the structure of the catalyst (Scheme 74) [132].
MCM-41 functionalized both Lewis and Brønsted acids
Incorporating both Lewis and Brønsted acids simultaneously on the surface of MCM-41 have already been studied. MCM-41, APTS-NH2-Cr(salen), and acidic ionic liquid [CPTES-IM-SO3H][HSO4]/[Cl] were mixed together to form MCM-41 functionalized both Lewis and Brønsted acids. The resulted compounds used as catalyst for excellent synthesis of HMF (83.5%) from the corresponding fructose (Scheme 75) [133].
Hydrophobic modification of propyl sulfonic acid-functionalized mesoporous silica
Yamashita et al. showed that triethoxyfluorosilane (TEFS) as a silylation reagent grafts to the SO3H functionalized mesoporous silica and causes an increasing effect in their hydrophobic properties (Scheme 76). Such newly generated catalyst was able to promote Friedel–Crafts alkylation of anisole with benzyl alcohol as it illustrated in the following (Scheme 77). The effectiveness of catalytic performance assessed based on controlling the surface hydrophobicity of SO3H-MS without negative effect on porous structure [134].
Mesoporous surface nanoparticles silica-bonded (MSNs-HPZ-SO3H) homopiperazine sulfamic acid
Kassaee et al. used new mesoporous silica nanoparticles attached to the homopiperazine sulfamic acid (MSNs-HPZ-SO3H) for the routine synthesis of 1-amidoalkyl-2-naphthols through the condensation between amides or urea, aromatic aldehydes and β-naphthols. In order to achieve MSNs-HPZ-SO3H, initially MSN was prepared by employing CTAB to make the silica mesoporous. Then, it was sequentially treated with 3-chloropropyltriethoxysilane to obtain MSN-Cl. Homopiperazine behaved like a nucleophile and substituted instead of chlorine atom. Finally, reaction of ClSO3H with the as-prepared MSN-HPZ resulted in the formation of MSNs-HPZ-SO3H. Catalyst preparation method fully illustrated in Scheme 78 [135].
Periodic mesoporous organosilica
In the late 1990s, mesoporous materials composed by hybrid inorganic–organic frameworks with ordered mesopores, designated as periodic mesoporous organosilicas (PMO’s) were first synthesized [121, 136].
The synthesis strategy of these materials is based on the condensation, in the presence of the corresponding surfactant, of organosilanes such as (R’O)3-Si-R–Si-(R’O)3 in which the organic moiety (-R-) is covalently attached to two trialkoxysilyl groups (-Si-(R’O)3). PMOs feature materials with open porous structure and high loading of homogeneous distribution of organic groups covalently bonded within the siliceous framework and inside the pore walls. This allows the easy tailoring of both the chemical and physical properties while improving the hydrothermal and mechanical stabilities of the porous framework. In 2006, preparation and application of sulfonic acids-functionalized PMOs has been reviewed by Melero et al. [112].
Sulfonic acid-functionalized PMOs are mainly categorized in two groups: (1) sulfonic acid groups tethered into organic bridges such as phenyl and ethyl and (2) sulfonic acid groups embedded into channel walls of PMO through an organosilane precursor such as 3-mercaptopropyltrimethoxysilane. Although the first group has advantages such as higher stability of sulfonic acid sites and the ability of incorporating higher loading of sulfonic acid groups, the second class benefits from higher local hydrophobicity of the sulfonic acid sites [137].
Perfluorinated alkylsulfonic acid-functionalized periodic mesostructured organosilica (PMO)
Fluorinated alkylsulfonic acid-functionalized PMO was one-pot synthesized using 1,2-bis(trimethoxysilyl)ethane and a perfluorinated alkylsulfonic acid silane in the presence of Pluronic 123 as surfactant under acidic conditions (Scheme 79). This new heterogeneous solid acid showed a high catalytic activity in self-condensation of heptanal, owing to their high acid site strength and the presence of both hydrophobic ethane bridged framework and trimethylsilane function [138].
PMO functionalized with perfluoroalkylsulfonic acid
PMO was functionalized with perfluoroalkylsulfonic acid as a useful organosilica by reaction of the PMO with 1,2,2-trifluoro-2-hydroxy-1-trifluoromethylethane sulfonic acid β-sultone for alkylation of iso-butene with 1-butene (Scheme 80). The obtained results showed this reagent to exhibit better catalytic activity as well as catalytic stability compared with some of zeolitic catalysts and hybrid organic–inorganic acid catalysts such as perfluoroalkylsulfonic acid-functionalized SBA-15 and alkylsulfonic acid-functionalized PMO [139].
A novel sulfonic acid-functionalized periodic mesoporous organosilica with well-ordered mesoporous structure was prepared in a one-step process by co-condensation of a thiol-functionalized bis-silane precursor with 1,2-bis(triethoxysilyl)ethane in the presence of a nonionic triblock copolymer (Pluronic P123) and oxidation of thiol to sulfonic acid groups. The catalytic activity of this reagent was evaluated in the esterification of acetic acid with benzyl alcohol and compared with the commercial resin Amberlyst-15. Obtained data showed in situ sulfonated PMO to be an efficient, robust, and recyclable catalyst in acid-catalyzed reactions throughout consecutive catalytic cycles (Scheme 81) [140].
Propyl sulfonic acid-anchored isocyanurate bridged to the periodic mesoporous organosilicas (PMOs) was synthesized by Karimi et al. (Scheme 82). Efficient synthesis of bis(indolyl)methane derivatives using various aldehydes and indole was performed under mild reaction condition such as ethanol as a solvent in short times (Scheme 83). In comparison with other reported methodologies, this one figures important advantages involving lower loading of the catalyst, good to excellent yield of products, shorter times, preventing from applying toxic transition metals for increasing catalytic activity, facile workup of the products, and recyclability of the catalyst [140].
Propyl sulfonic acid-functionalized PMOs and MCM-41
Ethane bridged periodic mesoporous organosilicas (PMOs) and MCM-41 having propyl sulfonic acid groups in the pore channels were synthesized by co-condensation method, using 3-mercaptopropyltriethoxysilane (3-MPTS) as the sulfur precursor and following with oxidation by aqueous H2O2 as an oxidizing agent [141, 142].
The catalytic activity of the developed materials was evaluated in the liquid-phase Claisen–Schmidt condensation reaction of aromatic aldehydes with ketones to probe the effect of mesoporous support surfaces as well as the role of preparation methods. Results showed that sulfonic acid-functionalized ethane–silica samples were more active, selective and stable than the conventional sulfonic acid containing mesoporous catalysts [143].
Zeolites
Acid-functionalized silicalite-1
Bhatia et al. introduced two types of catalytic zeolite membranes namely (1) propyl sulfonic acid acid-functionalized silicalite-1 membrane and (2) arenesulfonic acid-functionalized membrane that were prepared over α-alumina support via one-step in situ hydrothermal crystallization and subsequent post-synthesis modification. The synthesis mixture was prepared by mixing tetrapropylammonium hydroxide (TPAOH), double deionized water (DDI H2O), tetraethylorthosilicate (TEOS) and organosilane source. Two types of organosilane source used in the present study were 3-mercaptopropyltrimethoxysilane (3MP) and phenethyltrimethoxysilane (PE), respectively. The thiol-functional group present in the membrane synthesized using 3MP was oxidized to propyl sulfonic acid by H2O2 and phenethyl-functional group present in the membrane synthesized using PE was sulfonated to arenesulfonic acid group by ClSO3H. Both membranes were tested for their catalytic activity in m-xylene isomerization reaction at room temperature range of 355–450 °C. Due to higher acid density, arenesulfonic acid-functionalized silicalite-1 membrane gave higher catalytic activity compared to propyl sulfonic acid-functionalized silicalite-1 membrane [144, 145].
MOR zeolite supported Brønsted acidic ionic liquid sulfonic acid
In order to widen the application of ionic liquids as an efficient heterogeneous catalysts, Chen et al. prepared MOR zeolite supported Brønsted acidic ionic liquid sulfonic acid catalyst (BAIL@MOR) by anchoring 3-sulfobutyl-1-(3-propyltriethoxysilane) imidazolium hydrogen sulfate onto the surface of MOR zeolite (Scheme 84).
The catalytic performance tests demonstrated that the catalyst BAIL@MOR exhibited excellent catalytic activities in the ketalization of cyclohexanone with glycol, 1,2-propylene glycol and 1,3-butylene glycol under mild reaction conditions, as comparable with homogeneous catalysis of precursors [BSmim][HSO4] and H2SO4. In addition the catalyst BAIL@MOR was also found to be reusable five times without a significant loss of its catalytic activity [146].
Propyl sulfonic acid-functionalized nanozeolite clinoptilolite
In another work, the natural nanozeolite clinoptilolite (Nano CP) was successfully functionalized by propyl sulfonic acid and used as a useful heterogeneous catalyst in the synthesis of quinoxaline derivatives via the reaction of o-phenylenediamines, 1,2-diketones or phenacyl bromides in aqueous media at room temperature for an appropriate time. Also this nanocatalyst could be recycled and reused eight times without significant loss of catalytic activities [147].
Sulfonic acid-functionalized mesoporous KIT-6
Najafi et al. reported synthesis of well-ordered mesoporous KIT-6 functionalized with –SO3H groups which was then applied for the conversion of fructose to 5-hydroxymethylfurfural. For the synthesis of KIT-6, pluronic acid was used as a triblock copolymer. Afterward, this surfactant was mixed with TEOS under the desired condition and mesoporous KIT-6 was formed after heating for a while in a polypropylene bottle and then calcination at 550 ˚C. For the functionalization of KIT-6, it was added to the solution mixture of 3-mercaptopropyltrimethoxysilane and dry toluene. Finally, thiol groups oxidized to –SO3H group using H2O2 as an oxidant (Scheme 85). Dehydration of fructose to the desired HMF performed using KIT-6-Pr-SO3H as the catalyst under various conditions (Scheme 86) [148].
Sulfonic acid-functionalized mesoporous ZSM-5
Sulfonic acid-functionalized mesoporous ZSM-5 (SO3H-Meso ZSM-5) were produced by post-grafting of 3-mercaptopropyltriethoxysilane with different loading with an amount of 10–50 wt % followed by oxidation of thiolic group applying H2O2 to produce the corresponding sulfonic acid group (Scheme 87). Two-component reaction of 2-hydroxyacetophenone and benzaldehyde was improved using acid functionlized ZSM-5 compared to the non-acid-functionalized corresponding ZSM-5 (Scheme 88) [149].
Bentonite functionalized with propyl sulfonic acid
Moraes et al. [150] functionalized bentonite by the grafting of propyl sulfonic acid groups to catalyze esterification reaction of acetic acid and 1-propanol. Functionalization was accomplished by anchoring, oxidation and acid activation of (3-mercaptopropyl) trimethoxysilane (Scheme 89). In addition, the main clay mineral of the bentonite used in this work was a montmorillonite with a high iron content and structural formula K0.33Ca0.05Na0.03(Al1.34Mg0.41Fe0.26Ti0.03)[(OH)2/Al0.19Si3.81O10], collected from the Amazon (region) [150].
Boehmite functionalized with silylpropylsulfamic acid
Bahrami et al. [151] reported the synthesis of boehmite nanoparticles-silica-NHSO3H (BNPs-SiO2@(CH2)3NHSO3H) via functionalization of bohomite by the grafting of (3-aminopropyl) triethoxysilane followed by sulfonated by chlorosulfonic acid (Scheme 90).
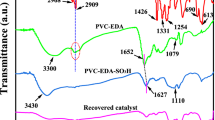
Reusable boehmite nanoparticles-silica-NHSO3H (BNPs-SiO2@(CH2)3NHSO3H) was found to be an efficient heterogeneous nanocatalyst for the selective oxidation of sulfides to sulfones in the presence of H2O2. Excellent yields, easy and quick isolation of products, short reaction times and excellent selectivity are the main advantages of this method. The catalyst was characterized using Fourier transform infrared spectroscopy, energy-dispersive X-ray analysis, X-ray diffraction, and transmission and scanning electron microscopies [151].
In another study, they reported boehmite nanoparticles-silica-NHSO3H (BNPs@SiO2(CH2)3NHSO3H) as a metal-free and environmentally friendly catalyst has been found to be effective for the one pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones and the preparation of 1,4-dihydropyridines derivatives [152]. Some features of this protocol are low cost and available materials, short reaction times, convenient catalyst separation, and no need for a neutral atmosphere. Moreover, the catalyst can be reused for at least five times with only a 7% reduction in yield. This study also shows that BNPs@SiO2(CH2)3NHSO3H is a sustainable, recoverable and effective heterogeneous catalyst for multicomponent reactions (Scheme 91).
Sulfonic acid-functionalized nano-γ-Al2O3
Wu et al. reported [153] a new sufonic acid catalyst that was supported on γ-Al2O3 by organic linker, this reagent was prepared from reaction of nano-γ-Al2O3 with 1,3-propanesultone in toluene. This catalyst was characterized by FT-IR, X-ray, XRD, TGA, SEM and TEM. In addition, the amount of sulfonic acid loaded on the surface of nano-γ-Al2O3 was determined by TG analysis and confirmed by ion-exchange pH analysis (Scheme 92).
Functionalized nano-γ-Al2O3 was used as a recyclable and environmentally benign catalyst in preparation of per-O-acetylation carbohydrate derivatives by treatment of sugars with a stoichiometric quantity of acetic anhydride under solvent-free conditions. In this work, A wide range of per-O-acetylation carbohydrate derivatives was synthesized in the presence of 50 mg/mmol of catalyst at 50 °C in high yields and in short reaction time (Scheme 93) [153].
Titanium oxide
Sulfonic acid-functionalized nanoporous titania (TiO2-Pr-SO3H)
Atghia et al. introduced sulfonic acid-functionalized nanoporous titania (TiO2-Pr-SO3H) as a new solid acid from the reaction of (3-mercaptopropyl)trimethoxysilane and TiO2, then by oxidation of thiols group with hydrogen peroxide (Scheme 94) [154].
The catalytic performance of TiO2-Pr-SO3H was studied in the N-tert-butoxycarbonylation of various aliphatic, aromatic and heterocyclic amines under solvent-free condition at room temperature. This novel method had several advantages such as high reaction rates, excellent yields, no side reactions and effective reusability of the catalyst. In continue, under the selected conditions, N-Boc protection of alcohols, phenols and thiols was also investigated and the starting material was recovered unchanged after 2 h. The selectivity of a method determines the importance of its application in organic reactions (Scheme 95) [154].
Sulfamic acid-functionalized n-TiO2 (n-TiO2-NHSO3H)
Amoozadeh et al. reported synthetic procedure for sulfamic acid-functionalized n-TiO2 (n-TiO2-NHSO3H) as follows: Isocyanate-functionalized nano-titanium dioxide (n-TiO2-NCO) prepared by covalent attachment of toluene diisocyanate (TDI) on the previously synthesized nano-TiO2 using hydrothermal method. The corresponding amino-functionalized n-TiO2 obtained by mixing n-TiO2-NCO and water–acetone (50/50) mixture. Afterward, functionalization performed with chlorosulfonic acid to give the desired n-TiO2-NHSO3H (Scheme 96) [155].
The resultant used for the synthesis of sulfoxides from the corresponding sulfides through oxidation (Scheme 97). In order to generalize the catalytic activity, sulfamic acid-functionalized n-TiO2 (n-TiO2-NHSO3H) used in other reactions to show its generality. Oxidative coupling of thiols to the corresponding disulfides selected as another reaction which is depicted in the following (Scheme 98) [155].
Zwitterionic sulfamic acid-functionalized nanoclay (MMT-ZSA)
Safari and Ahmadzadeh [156] reported the preparation of zwitterionic sulfamic acid-functionalized nanoclay (MMT-ZSA) via the functionalization of montmorillonite K10 as template with 3-aminopropyltriethoxysilane as linker and chlorosulfonic acid as a SO3H source. The physical and chemical properties of zwitterionic nanoclay were characterized by the following instrumental techniques including, FT-IR spectroscopy, elemental analysis, TGA, DTA, SEM, XRD, elemental analysis and Hammett acidity function techniques. The catalytic activity of MMT-ZSA was investigated in the synthesis of dihydropyrano[2,3-c]pyrazoles and spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives via the multicomponent reaction between hydrazine hydrate (or phenyl hydrazine), malononitrile, β-keto ester and carbonyl compounds (1,2-di ketones and benzaldehyde derivatives) under solvent-free conditions (Scheme 99).
Magnetic nanoparticles (MNPs)
Magnetic nanoparticles have received a great deal of attention because of their potential use in magnetic fluids [157], catalysis [158,159,160,161,162,163], biotechnology/biomedicine [164], magnetic resonance imaging [165], data storage [166], and environmental remediation [167].
Magnetic nanoparticles are the particles commonly consist of magnetic elements such as iron, nickel and cobalt and their chemical compounds. Among magnetic NPs, iron oxides NPs are a class of magnetic material with excellent performance. Nowadays, Fe3O4 nanoparticles, as magnetite nanoparticles, have attracted increasing interest because of their unique properties including a large surface-to-volume ratio, superparamagnetism, low toxicity, biocompatibility and their potential applications in various fields [168]. The Fe3O4 nanoparticles easily synthesized and functionalized by metal and organo-catalysts and they easily separated from the reaction mixture by external magnetic field and reused [169]. Among the four well-known crystalline polymorphs of iron(III) oxide (α-Fe2O3 as hematite, β-Fe2O3, γ-Fe2O3 as maghemite and ε-Fe2O3), maghemite has gained the greatest interest in above-mentioned applications [170]. Several methods generally been employed for iron oxide nanoparticle preparation, including co-precipitation [171], which is preferred due to its simplicity, and thermal decomposition [172] seems to give the best control of nanoparticle size and morphology [173].
Magnetite nanoparticles are unstable in air and easily agglomerated after synthesis. The surface coatings and functionalization could effectively solve these problems [174]. Silica surfaces are chemically stable, biocompatible and can be easily functionalized for bioconjugation purpose. Hence, silica-coated magnetite composite nanoparticles (Fe3O4@SiO2/core–shell) have been synthesized by many groups [175, 176].
Two different approaches have been used to generate a silica coating on magnetite nanoparticles. The first method based on microemulsion synthesis, in which micelles or inverse micelles used as mini-reactor to control the silica coating on the magnetic nanoparticles [177]. This method requires tedious steps to separate the magnetic nanoparticles from the surfactants in the microemulsion system. The other method relies on the well-known Stober process [178], which comprises the hydrolysis and the ploycondensation of tetraethoxysilane under alkaline conditions in ethanol. This method can be directly used to coat SiO2 on clay minerals, hematite [179], zirconia and titania [180] due to significant chemical affinity of these materials.
Recently, magnetic core–shell nanostructures have attracted more attention due to their unique magnetic properties. In contrast to the difficulty observed in recovering and reusing most solid catalysts, core–shell nanostructure magnetic catalysts can be easily retrieved under the influence of a magnetic field and used in subsequent reactions. Due to this property, using magnetic core–shell structure composites as catalysts recommended in literature [181, 182].
Nano n-propylsulfonated γ-Fe2O3 (NPS- γ-Fe2O3)
Nano n-propylsulfonated γ-Fe2O3 as a useful sulfonated nanomagnetic iron oxide was synthesized directly through ring opening reaction of sulfolane with maghemite NP (Scheme 100) [183, 184].
After the preparation, NPS- γ-Fe2O3 applied in the synthesis of β-phosphomalonic acid derivatives under solvent-free conditions. The catalytic system kept its performance at least over five runs (Scheme 101).
Also, Nano n-propylsulfonated γ-Fe2O3 was used in reactions of indoles with Michael acceptors or carbonyl compounds giving high yields of a variety of 3-substitution products under solvent-free conditions. As demonstrated in the reaction of indole with β-nitrostyrene, the catalyst could be recycled four times without a significant decrease in yield [183, 184].
A magnetic nanoparticle supported dual acidic ionic liquid (AIL@MNP)
In 2011, Luo and co-workers [185] prepared a novel magnetic nanoparticle supported dual acidic ionic liquid catalyst by anchoring 3-sulfobutyl-1-(3-propyltriethoxysilane)imidazolium hydrogen sulfate onto the surface of silica-coated Fe3O4 nanoparticles (Scheme 102).
After the preparation, the synthesized catalyst was employed as a green and useful catalyst in one-pot three-component condensation of various aromatic aldehydes with dimedone and 2-naphthol to obtain benzoxanthenes in excellent yields and in short reaction times. Owing to the combination of nano-support features and soft imidazolium linkers, the active sites of the supported catalyst are more free and have a good “solubility” in the reaction system to facilitate the condensation effectively. Also, this catalyst could be easily recovered by an external magnet and reused six times without significant loss of catalytic activity [185].
In continue, Khalafi-Nezhad et al. used AIL@MNP as an efficient catalyst for the one-pot synthesis of novel spirooxindole derivatives. Due to the combination of nano-support features and flexible imidazolium linkers, this catalyst acted as a “quasi-homogeneous” catalyst to effectively catalyze the one-pot synthesis of spirooxindoles by three-component reaction of wide variety of substituted isatins, 1,3-dimethyl-2-amino uracil, and barbituric acid, thiobarbituric acid, and dimedon as 1,3-dicarbonyl compounds at mild conditions and in good yields. Operational simplicity, low cost, high yields, environmental friendliness, wide applicability, reusability, and easy recovery of the catalyst using an external magnet are the key features of this method (Scheme 103) [186].
N-Propylsulfamic acid supported on HAp-encapsulated-γ-Fe2O3 [γ-Fe2O3@HAp-Si-(CH2)3-NHSO3H]
Heydari et al. [187] reported the preparation of the titled reagent. In this study and in the first step, hydroxyapatite (HAP) encapsulated γ-Fe2O3 was reacted with 3-amino propyl trimethoxy silane. Then, the obtained solid ([γ-Fe2O3@HAp-Si-(CH2)3-NH2]) allowed react with chlorosulfonic acid to afforded n-propylsulfamic acid supported on hydroxyapatite-encapsulated as a new magnetic green catalyst (Scheme 104).
After preparation, they in order to the development of benign methods in the synthesis of biologically important heterocycles, and the valuable catalytic properties of magnetic nanoparticles employed Hap-encapsulated-g-Fe2O3 [g-Fe2O3@HAp-SO3H] as an efficient catalyst in the preparation of quinolines via the reaction of 2-aminoaryl ketones and ketones having α-methylene group under solvent-free conditions. In this protocol, the use of nanocatalyst provided a green, useful and rapid method to generate the products in short reaction times and excellent yields. The nanocatalyst was separated from the reaction medium simply by an external magnetic field and reused for the subsequent reactions.
Also, they employed this reagent as a magnetic catalyst for the synthesis of α-aminophosphonates. This coupling reaction which involves various aldehydes, amines and dialkylphosphite, proceeded well at room temperature, and the products were obtained in high to excellent yields in appropriate times (Scheme 105) [13, 187].
[γ-Fe2O3@Hap-Si(CH2)3-AMP]
The magnetically inorganic–organic hybrid nanocatalyst supported on hydroxyapatite-encapsulated γ-Fe2O3 ([γ-Fe2O3@Hap-Si(CH2)3-AMP]) was prepared from the reaction of magnetic hydroxyapatite [γ-Fe2O3@HAp] with aminopropyltrimethoxysilane, 2-hydroxybenzaldehyde and NaBH3CN by Shafee and co-workers, in 2012 [188] (Scheme 106).
[γ-Fe2O3@Hap-Si(CH2)3-AMP] was found to be excellent and clean catalytic system for the preparation of 4H-benzo[b]pyrans and dihydropyrano[c]chromenes from the reaction of various aromatic aldehydes, malononitrile and 4-hydroxycoumarin or dimedon in aqueous media. The catalyst was recovered without obvious significant loss of activity. Due to water-resistant and superparamagnetic nano-nature of the catalyst, it easily separated by the application of an external magnetic device and reused conveniently (Scheme 107) [188].
Fe3O4/SMPA
Fe3O4/SMPA as a novel acid magnetic reagent was prepared by Zamani et al. [189]. To obtain this solid acid, Fe3O4 nanoparticle coated with 3-mercaptopropanoic acid (MPA) through a simple in situ method and subsequently oxidized by H2O2/H2SO4 (Scheme 108) [189].
The synthesized nanocatalyst in this work provided a green and useful method for Biginelli reactions of various aldehydes with ethyl acetoacetate and urea (or thiourea) to obtain 3,4- dihydropyrimidin-2(1H)-one/thiones derivatives under mild and solvent-free conditions. In all cases, the three-component reaction with aromatic aldehydes carrying electron-withdrawing or electron-donating groups gave the corresponding products in good to excellent yields and high purity. Thiourea also provided the Biginelli products in reasonable yields. In this study, easier catalyst preparation, high level of reusability and simple reaction workup was the major advantages of the new reported catalyst (Scheme 109) [189].
Sulfonated-phenylacetic acid coated Fe3O4 nanoparticles (Fe3O4/PAA-SO3H)
Fe3O4/PAA-SO3H was prepared by a very simple and inexpensive method. According to this procedure, Fe3O4 nanoparticle was coated with phenylacetic acid under nitrogen atmosphere to obtain Fe3O4/PAA. Then, the synthesized Fe3O4/PAA sulfonated using chlorosulfonic acid at room temperature (Scheme 110) [190].
This catalyst was effectively employed as a novel acid magnetic catalyst for Biginelli reactions of various aldehydes with ethyl acetoacetate and urea (or thiourea) under solvent-free conditions. The results show, in all cases, the three-component reaction proceeded smoothly to give the corresponding 3,4-dihydropyrimidin-2(1H)-ones/thiones in moderate to good yields. The reaction with aromatic aldehydes carrying electron-withdrawing or electron-donating groups gave the corresponding products in good yields and high purity. Also Fe3O4/PAA-SO3H showed excellent level of reusability in this method [190].
In another study, Dastmalchi et al. have reported the synthesis of a magnetically heterogeneous catalyst based on the immobilization of sulfosalicylic acid onto Fe3O4 nanoparticles (Fe3O4@sulfosalicylic acid MNPs) [191]. Scanning electron microscopy, transmission electron microscopy, energy-dispersive X-ray spectroscopy, X-ray diffraction, thermogravimetric analysis, dynamic light scattering, vibrating sample magnetometry, Fourier transform infrared spectroscopy, UV–visible absorption, and Brunauer–Emmett–Teller (BET) techniques confirmed the successful synthesis of the catalyst.
The bis-coumarin analogs were synthesized in high yield using the reaction of 1 equivalent of aryl aldehydes with 2 equivalents of 4-hydroxycoumarin in water under microwave irradiation conditions (Scheme 111).
Fe3O4@SiO2@Et-PhSO3H and Fe3O4@SiO2@Me&Et-PhSO3H
Mobaraki et al. in order to evaluation of hydrophobicity of magnetic sulfonic acids in promoting some reactions elected Fe3O4@SiO2@Et-PhSO3H and Fe3O4@SiO2@Me&Et-PhSO3H as magnetic nanoparticles bearing both sulfonic acid and terminal organic groups.
Fe3O4@SiO2@Et-PhSO3H was prepared from the reaction of silica-coated magnetic nanoparticles with 2-(4-chlorosulfonylphenyl)ethyltrimethoxysilane (CSPETS, 0.4 g, 1.23 mmol) in dry toluene. Another reagent, Fe3O4@SiO2@Me&Et-PhSO3H, was synthesis by co-condensation of CSPETS and trimethoxymethylsilane (TMMS) on the silica-coated magnetic nanoparticles in dry toluene (Scheme 112) [192].
The catalytic activity of Fe3O4@SiO2@Me&Et-PhSO3H was investigated by a three-component, Strecker reaction of a series of aldehydes or ketones, amines, and trimethylsilyl cyanide for the synthesis of α-aminonitriles under solvent-free conditions. This catalyst with a combination of hydrophobicity and acidity, as well as its water-resistant property, enabled easy mass transfer and catalytic activity in the Strecker reaction. Fe3O4@SiO2@Me&Et-PhSO3H was recovered and reused for at least 6 reaction times without any significant loss of activity. The clean reaction conditions and high selectivity affording exclusively α-aminonitriles in excellent yields, no observation of undesired side products, and utilizing a green and magnetically separable heterogeneous catalyst were the advantages of this method [192].
In continue, Movassagh et al. employed Fe3O4@SiO2@Me&Et-PhSO3H as an efficient and hydrophobic catalyst in two convenient green protocols for the synthesis of β-amino ketones involving one-pot aza-Michael-type and Mannich-type reactions of a series of aldehydes, ketones and amines at room temperature. The high reactivity of the catalyst was probably due to synergistic effects between sufficient hydrophobicity and acidity of siliceous networks which in turn results in: (a) remarkable shielding effects against polar molecules, good accessibility of the active sites, easier diffusion of reaction partners within the network resulting from the presence of organic methyl groups on the surface of catalyst and (b) mild acidic conditions for the preparation of β-amino ketones (Scheme 113) [193].
[Fe3O4@SiO2@(CH2)3S-SO3H]
Zolfigol et al. reported [Fe3O4@SiO2@(CH2)3S-SO3H] as a magnetically separable catalyst for the production of different polynitrogenated heterocycles. Catalyst preparation involves mixing 3-(trimethoxysilyl)-1-propanethiol as drop-wise with as-synthesized silica-coated magnetic nanoparticles. Sonication and then adding chlorosulfonic acid as drop-wise led to the defined catalyst (Scheme 114) [194].
Then, equivalent molar ratios of starting materials involving aldehyde, malononitrile and 2,4-diamino-6-hydroxypyrimidine mixed with 2 mg [Fe3O4@SiO2@(CH2)3S-SO3H] at 100 °C to achieve the corresponding pyrido[2,3-d]pyrimidine derivatives (Scheme 115) [194].
In addition, exactly the same procedure applied for the synthesis of 2-amino-4,7-dioxo-5-phenyl-3,4,5,6,7,8-hexahydropyrido[2,3-d]pyrimidine-6-carbonitrile derivatives with only one exception in which methyl or ethyl cyanoacetate was used instead of malononitrile (Scheme 116) [194].
Sulfamic acid-functionalized Fe3O4-NPs (SA-MNPs)
Kassaee et al. [195] coated Fe3O4-NPs with 3-aminopropyltriethoxysilane to prepare amino-functionalized Fe3O4. Then, they obtained sulfamic acid-functionalized Fe3O4-NPs (SA-MNPs) from the reaction of amino groups of this reagent with chlorosulfuric acid (Scheme 117) [195].
They employed this catalyst as a novel organic–inorganic hybrid heterogeneous catalyst in one-pot synthesis of α-amino nitriles via three-component coupling reactions of aliphatic and aromatic aldehydes (or ketones), amines and trimethylsilyl cyanide in aqueous media, at room temperature with high yields and in short reaction times. The heterogeneous catalyst could be recovered easily by external magnet and reused many times without significant loss of its catalytic activity [195].
Safari and Zarnegar [196] used N-propylsulfamic acid supported onto magnetic Fe3O4 nanoparticles as a mild and effective heterogeneous catalyst in environmentally friendly approach for the synthesis of biologically active tri-substituted imidazoles via condensation of 1,2-diketone, various aromatic aldehydes and ammonium acetate under ultrasound irradiation. Corrosiveness, safety, less waste, ease of separation and recovery, replacement of liquid acids with solid acid are all among desirable factors for the chemical industry, which they have considered in this work [196].
In another work, a useful and green method was reported for the synthesis of a variety of tetraketone derivatives via the Knoevenagel condensation and Michael addition reactions of aromatic aldehydes to dimedone, 1,3-indanedione, and 1,3-dimethyl barbituric acid using Fe3O4-NPs (SA-MNPs) as an efficient solid acid nanocatalyst in good to excellent yields. The catalyst was readily separated using an external magnet and reusable without significant loss of their catalytic efficiency [197, 198].
Sulfamic acid immobilized diethylenetriamine functionalized Fe3O4 nanoparticles
Sulfamic acid immobilized diethylenetriamine functionalized Fe3O4 nanoparticles (SA-DETA-Fe3O4) was prepared as follows: At first magnetic Fe3O4 nanoparticles were produced based on chemical co-precipitation procedure. Afterward, grafting 3-chloropropyltrimethoxysilane (CPTMS) on the previously prepared nanoparticles was performed followed by the reaction of the as-prepared CPTMS loaded on Fe3O4 with diethylenetriamine in toluene as a solvent under reflux condition for 24 h. Finally, immobilizing chlorosulfonic acid on as-synthesized DETA-Fe3O4 performed at room temperature (Scheme 118). Three different reactions such as oxidative coupling of thiols (Scheme 119), sulfide oxidation (Scheme 120), and Knoevenagel condensation of aromatic aldehydes with active methylene compounds (Scheme 121) performed using this acidic, cheap and magnetically separable catalyst and good to high yields of products obtained [199].
Later, they used sulfamic acid immobilized on amino-functionalized magnetic nanoparticles (MNPs/DETA-SA) as an efficient and magnetically reusable catalyst for the synthesis of 2,3-dihydroquinazoline-4(1H)-one and polyhydroquinoline derivatives in high yields [200].
In another study, they reported the preparation of immobilized sulfuric acid on magnetic Fe3O4 nanoparticles (Fe3O4 MNPs-OSO3H) as a new solid acid nanocomposite [201]. The catalytic activity of Fe3O4 MNPs-OSO3H was investigated in a series of condensation reactions (Scheme 122). High catalytic activity, simple separation from reaction mixture by an external magnet and good reusability are several eco-friendly advantages of this catalytic system. It is noteworthy that this catalytic system is applicable to a wide range of spectrum of aromatic aldehydes, and the desired products were obtained in good to excellent yields under mild conditions.
Aminopropylsilyloxy-modified magnetite NP
Aminopropylsilyloxy-modified magnetite NP were also used as starting material for magnetic nanoparticle-supported dendritic sulfamic acids by first performing addition of the amino group to methyl acrylate, followed by reaction with 1,2-diaminoethane and final sulfamic acid formation (Scheme 123) [202].
This catalyst allowed synthesizing α-arylaminophosphonates, which are interesting in pharmaceutical chemistry. Recycling of the catalyst was showed in seven subsequent runs.
Simple preparation for the synthesis of MNP-l-Proline-SO3H depicted in Scheme 124. Co-precipitation method applied to synthesize l-proline supported magnetic nanoparticles (Fe3O4@l-proline) using l-proline, FeCl3 and FeCl2 solution. Then, Fe3O4@l-proline functionalized with SO3H groups using chlorosulfonic acid in n-hexane to give MNP-l-Proline-SO3H. The structure of the resulted nanoparticles investigated by scanning electron microscopy (SEM), vibrating sample magnetometry (VSM), thermaogravimeter analysis (TGA), X-ray diffraction (XRD), energy-dispersive X-ray analysis (EDXA) to confirm its structure [203].
The resultant solid was then used for preparation of 3,4-dihydrpyrimidine-2-[1H]thione through condensation of thiourea, aromatic aldehydes, and t-butyl acetoacetate under thermal and solvent-less condition (Scheme 125) [203].
The preparation of a new magnetic catalyst was reported by Ghorbani group’s through the reaction of silanol groups, on the surface of silica-coated Fe3O4 magnetic nanoparticles, with (3-chloropropyl)triethoxysilane followed by hexamethylenetetramine and chlorosulfonic acid (Scheme 126) [204]. The catalytic activity of Fe3O4@SiO2-HMTA-SO3H MNPs was investigated in the synthesis of pyranopyrazole compounds, and the results were excellent regarding high yield of the products and short reaction time (Scheme 127).
In the same year (2018), they have reported the preparation of 7-aminonaphthalene-1,3-disulfonic acid-functionalized magnetic Fe3O4 nanoparticles (Fe3O4@SiO2@Propyl–ANDSA) (Scheme 128), and its catalytic activity was investigated in the one-pot synthesis of new derivatives of tetrahydrotetrazolo[1,5-a]quinazolines and tetrahydrobenzo[h]tetrazolo[5,1-b]-quinazolines from the reaction of aldehydes, 5-aminotetrazole, and dimedone or 6-methoxy-3,4-dihyronaphtalen-1(2H)-one at 100 °C in H2O/EtOH as the solvent (Scheme 129) [205].
In another study, Zolfigol et al. [206] reported the preparation of sulfonic acid-functionalized 1,4-diaza-bicyclo[2.2.2]octane (DABCO)-based magnetic nanoparticle Fe3O4 [Fe3O4@SiO2@Pr-DABCO-SO3H]Cl2. Then, this catalyst was examined for the convenient synthesis of spiropyran derivatives, resulting in high reaction yields, short reaction times, and the recovery and reusability of the catalyst (Scheme 130).
Sajjadifar and Gheisarzadeh reported [207], isatin-SO3H coated on amino propyl modified magnetic nanoparticles (Fe3O4@APTES@isatin-SO3H) is found to be a novel, efficient, and reusable magnetic nanocatalyst, for the synthesis of pyrano[2,3-d] pyrimidines derivatives via one-pot three-component reaction of various aromatic aldehydes, malononitrile, and barbituric acid under reflux conditions in mixture of H2O:EtOH (1:1) as solvent (Scheme 131).
Acidic triphenylphosphonium salt functionalized magnetic Fe3O4 nanoparticles
The simple two-step preparation procedure of a novel magnetic nano-solid acid catalyst is described, which includes grafting an ionic liquid onto Fe3O4 nanoparticles, by reaction of magnetite NP with 3-chloropropyltrimethoxysilane and triphenylphosphine to the formation of triphenylphosphonium salt and then the sulfonation of phenyl groups with H2SO4 (Scheme 132).
The obtained catalyst was applied in acetalizations of ketones and aldehydes with ethylene glycol affording excellent yields, which were maintained over 5 runs [208].
Fe3O4@SiO2-2mimSO3H
Rezayan et al. [209] reported a highly efficient magnetic Brønsted acid catalyst, namely Fe3O4@SiO2-2mimSO3H, that was synthesized based on immobilization of 2-methyl-imidazole functionalized by chlorosulfonic acid on the surface of silica-coated magnetic nanoparticles (Scheme 133).
They applied Fe3O4@SiO2-2mimSO3H as a green and useful catalyst for the synthesis of α-aminophosphonates from the reaction of various aliphatic and aromatic aldehydes, amines and triethyl phosphite at room temperature in solvent-free conditions. The catalyst had an excellent activity and recyclable for at least 6 reaction runs. The facile recovery of the catalyst was carried out by applying an external magnet device. It should be noted that, High activity of Fe3O4@SiO2-2mimSO3H catalyst may be due to direct connection of sulfonic groups to the positive nitrogen atom of imidazolium aromatic ring which increases the activity of this catalyst [209].
Fe3O4 particles with imidazole-based acidic ionic liquid tag
Synthesis of 1,8-dioxo-octahydroxanthenes and dihydropyrano[2,3-c]pyrazole derivatives catalyzed by newly prepared nanoparticles having acidic ionic liquid tag. Procedure for the construction of solid acidic catalyst was as follows: The as-coated Fe3O4 with tetraethylorthoosilicate were then grafted with (3-chloropropyl(triethoxysilane in dry toluene under nitrogen atmosphere. Subsequently, desired amount of imidazole poured into the as-prepared Fe3O4@SiO2@(CH2)3Cl and the mixture was refluxed for 12 h. After filtration and washing of the synthesized Fe3O4@SiO2@(CH2)3-imidazole, chlorosulfonic acid was added drop-wise to the as-prepared solid to create [MNP-PIm-SO3H]Cl as an heterogeneous acidic catalyst (Scheme 134). Afterward, molar ratios of the aldehydes, malononitrile and 3-methyl-1-phenyl-2-pyrazoline-5-one along with 7 mg of the catalyst were mixed to form dihydropyrano[2,3-c]pyrazole derivatives under solvent-less condition at room temperature. Also, reaction of two equivalent of dimedone with one equivalent of aldehyde led to the formation of 1,8-dioxo-octahydroxanthene derivatives with only 0.01 g of the prepared catalyst under non-solvent condition at 80 ˚C (Scheme 135) [210].
In another study, they reported that the Fe3O4@SiO2/(CH2)3-[imidazolium-SO3H]Cl has been shown robust promoting capability in the synthesis of arylbispyranylmethane derivatives under mild and green conditions. In addition, arylbispyranylmethanes were synthesized via efficient three-component reaction of various aromatic aldehydes with 4-hydroxy-6-methyl-2H-pyran-2-one. The nanomagnetic core–shell catalyst presented effective potential of at least eight times recycling applicability in the described synthetic procedure in this report [211].
Moreover, in 2017 they studied the catalytic activity of Fe3O4@SiO2/(CH2)3-[imidazolium-SO3H]Cl for the synthesis of henna-based xanthenes (aryl-5H-dibenzo[b,i]xanthene- 5,7,12,14(13H)-tetraones) and bis-coumarins as an acidic and reusable catalyst [212].
Sajjadifar et al. [213] reported the preparation of 1-methyl-imidazole-based ionic liquid-stabilized silica-coated Fe3O4 magnetic nanoparticles [Fe3O4@SiO2@(CH2)3-1-methyl-imidazole]HSO4 as a solid acid magnetic nanocatalyst was explored in the synthesis of pyrano[2,3-d]pyrimidine derivatives (Scheme 136).
Fe3O4 particles with thiourea-based acidic ionic liquid tag
Zolfigol et al. used a newly synthesized environmental based nanomagnetic solid acid catalyst having a sulfonic acid tag. The synthesis procedure for the stepwise synthesis of this heterogeneous catalyst has been shown in the following. To cover the surface of Fe3O4 nanoparticles, different amounts of double distilled water, ethanol, ammonia and tetraethylorthosilicate were added to as-prepared Fe3O4. Silica-covered Fe3O4 was then mixed with (3-chloropropyl)-triethoxysilane in toluene under reflux and nitrogen atmosphere. The resultant solid was functionalized with thiourea and then with chlorosulfonic acid (Scheme 137). Then, they successfully applied the same catalyst in the synthesis of 1,1,3-tri(1H-indol-3-yl) alkane derivatives using two-component reaction of α,β-enal or enones with various indoles and also with different molar ration in 60 ˚C under solvent-less condition (Scheme 138) [214].
In another study, they used the same catalyst in the formation of 4,4′-(arylmethylene)-bis(1H–pyrazol-5-ol) and pyrano[3,2-c]pyrazole derivatives in 90 ˚C applying 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-ones, aromatic aldehydes and malononitrile as starting materials (Scheme 139) [215].
Nanostructured {Fe3O4@-SiO2@(CH2)3Im}C(CN)3
Zolfigol and Yarie [216] reported a new silica-coated magnetic nano particle immobilized ionic liquid ({Fe3O4@-SiO2@(CH2)3Im}C(CN)3) using reaction of Fe3O4@SiO2@(CH2)3-Imidazole with tricyanomethan in toluene. Obtained catalyst was characterized by several analysis such as IR, X-ray diffraction patterns (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM), thermal gravimetry (TG) and vibrating sample magnetometer (VSM) (Scheme 140).
They applied novel catalyst at the synthesis of polyhydroquinoline from condensation of various aromatic aldehydes, dimedone, β-ketoester and ammonium acetate under solvent-free and mild conditions [216].
Also, {Fe3O4@-SiO2@(CH2)3Im}C(CN)3 as a magnetic solid acid was employed in the preparation of 2-amino-3-cyano pyridines from the corresponding aldehydes, acetophenone, malononitrile, and ammonium acetate under solvent-less conditions. The main feature of this article indicates anomeric based oxidation instead of aerobic oxidation (Scheme 141) [217].
Fe3O4@SiO2@(CH2)3-Urea-SO3H/HCl
Recently, biological-based nanomagnetic catalysts based on using urea as ionic liquids and molten salts have received attended [218,219,220,221]. Zolfigol group’s reported the preparation and catalytic application of [Fe3O4@-SiO2@(CH2)3-Urea-SO3H/HCl] as a magnetically recoverable solid acid catalyst in the synthesis of 2-aminobenzothiazolomethylnaphthol derivatives, as portrayed in Scheme 142 [221].
In another study, they developed a novel and reusable biological urea-based nanomagnetic catalyst namely Fe3O4@SiO2@(CH2)3-urea-benzimidazole sulfonic acid. Then, the catalytic performance of Fe3O4@SiO2@(CH2)3-urea-benzimidazole sulfonic acid was successfully inspected toward the multicomponent synthesis of 2-amino-3-cyano pyridine derivatives through a vinylogous anomeric based oxidation pathway Scheme 143 [222].
Moreover, they reported the preparation of semicarbazide functionalized with chlorosulfonic acid on the surface of silica-coated magnetic nanoparticles, {Fe3O4@SiO2@(CH2)3Semicarbazide-SO3H/HCl}, as a novel magnetic Brønsted acid catalyst [223]. The capability and excellent activity of this nanoparticle catalyst were exhibited in the synthesis of two series of compounds with important biological activities, namely 3,3′-(arylmethylene)bis(4-hydroxycoumarin) and 1-carbamato-alkyl-2-naphthol derivatives, under mild, green and solvent-free conditions (Scheme 144).
Shirzaei et al. [224] reported the preparation of a nanomagnetic catalyst namely [Fe3O4@SiO2@(CH2)3-Thiocarbohydrazide-SO3H]Cl. The catalytic activity of [Fe3O4@SiO2@(CH2)3-thiocarbohydrazide-SO3H]Cl was successfully inspected toward the multicomponent synthesis of furan-2(5H)-one derivatives through a three components reaction of dialkylacetylenedicarboxylate with aromatic aldehydes and aromatic amines Scheme 145 [224].
Fe3O4@MCM-41-Alkyl-SO3H
Golshekan et al. for the first time reported the preparation of organic–inorganic MCM-41 mesoporous magnetite nanoparticles (MNPs@MCM-41) as a new class of solid acid catalyst with large density of sulfonic acid groups. The specific surface area and textural properties of synthesized nanoparticles were improved through the coupling of inorganic and organic components by template synthesis on the magnetite nanoparticle surface. The new solid acid catalyst was prepared by co-condensation method. In this procedure, Fe3O4 was coated with alkylsulfonic acid groups by means of a one-step simple synthesis approach involving tetraethoxysilane (TEOS) and (3-mercaptoalkyl)trimethoxysilane in the presence of CTAB as a template and then oxidation of thiol groups to sulfonic acid (Scheme 146) [225].
Finally, the synthesized nanocomposites was applied as an efficient and useful heterogeneous catalyst for one-pot three-component synthesis of aryl benzo[α]xanthenone derivatives in room temperature and solvent-free conditions [225].
Fe3O4@TiO2@O2PO2(CH2)NHSO3H
Zolfigol and Yarie applied the as-synthesized Fe3O4@TiO2@O2PO2(CH2)NHSO3H solid acid catalyst for the synthesis of previously reported diphenylnicotinonitrile derivatives using four-component one-pot synthesis of arylbenzaldehydes, acetophenone derivatives, malononitrile and ammonium acetate at 90 ˚C. Products obtained in good to excellent yields under solvent-less, moderate condition, and in short times (Scheme 147). For the preparation of nanomagnetic catalyst, tetraethyl orthotitanate was used as a coating agent of Fe3O4. Treating Fe3O4@TiO2 with 2-aminoethyl dihydrogen phosphate and then reaction of the prepared compound with chlorosulfuric acid resulted in formation of the desired solid acid (Scheme 148) [226].
In another study, the catalytic activity of Fe3O4@TiO2@O2PO2(CH2)NHSO3H was investigated by Zolfigol group’s for the synthesis of 1,8-dioxodecahydroacridines via the condensation reaction between dimedone, aromatic aldehyde, and ammonium acetate under mild and solvent-free reaction conditions (Scheme 149) [227].
Azarifar et al. [228] reported the preparation of N-(3-silyl propyl) diethylene triamine N,N’,N’’-tri-sulfonic acid (SPDETATSA), which it was grafted on magnetic Fe3−xTixO4 nanoparticles. The structure of the resulted nanoparticles was characterized based on Fourier transform infrared (FT-IR), energy-dispersive X-ray spectroscopy (EDX), scanning electron microscopy (SEM), transmission electron microscopy (TEM), thermal gravimetric analysis (TGA), and vibrating sample magnetometer (VSM) analyses. These nanoparticles exhibited high catalytic activity as novel magnetically recyclable acid nanocatalyst in the synthesis of a diverse range of hexahydroquinolines through one-pot tandem reactions in excellent yields (Scheme 150).
In another study, they reported the preparation of sulfamic acid-functionalized Fe3-xTixO4 magnetic nanoparticles (MNPs) [229]. This catalyst was prepared through one-pot reaction of Fe3-xTixO4 MNPs with 3-chloropropyltrimethoxysilane and imidazolidine-2,4-dione followed by functionalization with chlorosulfonic acid (Scheme 151). The potential catalytic ability of this nanocatalyst was evaluated in one-pot four-component condensation reaction between aromatic aldehydes, dimedone, alkyl acetoacetates and ammonium acetate in order to synthesis of hexahydroquinoline derivatives.
Magnetic Fe3O4-grafted calix[n]arene sulfonic acids (C[n]SO3HMNPS)
In 2014, Sayin and co-workers [230] introduced three novel and magnetically recoverable Brønsted acidic calix[n]arene derivatives. These catalysts were prepared by the immobilization of the calix[n]arene sulfonic acids onto [3-(2,3-epoxypropoxy)-propyl]-trimethoxysilane coated Fe3O4 nanoparticles. The structures of calix[n]arene-grafted magnetic nanoparticles (C [4] SO3H-MN, C [6] SO3H-MN, C [8] SO3H-MN) were determined by a combination of FT-IR spectroscopy, TEM and elemental analysis (Scheme 152).
In this study, the catalytic abilities of Fe3O4-grafted calix[n]arene sulfonic acids (C [4] SO3HMNP, C [6] SO3H-MNP, and C [8] SO3H-MNP) for coupling of electron-rich arenes with two activated sec-alcohols (4,4-dimethoxybenzhydrol and 1,3-diphenyl-2-propen-1-ol) at 50 °C in aqueous media were investigated. The corresponding products were isolated in good to excellent yields (up to 99%) in short reaction times. These catalysts easily separated by magnetic decantation and the recovered catalysts were reused for several cycles without significant loss of catalytic activity (Scheme 153) [230].
Acidic poly(styrene-co-divinylbenzene) encapsulated Fe3O4
Lu et al. [231] provided a simple method for the preparation of highly active and recyclable colloidal acid catalyst namely poly(styrene-co-divinylbenzene) encapsulated Fe3O4 nanoparticles. First, 16-heptadecenoic acid (HAD)-functionalized Fe3O4 was encapsulated in monodisperse cross-linked polymer spheres. This was achieved by emulsion copolymerization technique in an aqueous phase of styrene and divinylbenzene (DVB). The obtained colloid functionalized with sulfonic acid groups to obtain magnetically catalyst (Scheme 154).
The catalytic activity and the recyclability of this magnetic catalyst were evaluated in the acid-catalyzed condensation reaction of ethylene glycol and benzaldehyde to 2-phenyl-1,3-dioxolane. The obtained results showed that corresponding product was achieved in high yield and in short reaction time and the recovered catalyst was reused for several times without significant loss of catalytic activity [231].
Acidic poly(glycidyl methacrylate) encapsulated Fe3O4 (SO3H-PGMA-MNPS)
SO3H-PGMA-MNPS, consisting of a core of iron oxide magnetic nanoparticles (MNPs), a poly(glycidyl methacrylate) (PGMA) shell, and sulfonic acid groups on the surface, was synthesized from the corresponding core–shell structured PGMA–MNPs containing epoxy surface groups by gentle sulfonation with Na2SO3. The catalyst possesses a mean size of 90 nm, high acid capacity of 2.3 mmol H+ g−1, and high superparamagnetic properties (Scheme 155).
This magnetic solid acid catalyst was examined for the esterification of free fatty acid (FFA) to develop an efficient pretreatment step for producing biodiesel (fatty acid methyl ester) from waste grease containing FFA. Esterification of FFA (16 wt %) in grease with methanol using this catalyst gave 96% conversion of FFA within 2 h. SO3H-PGMA-MNPS was easily separated under a magnetic field and showed no loss of productivity during 10 cycles. In comparison, SO3H-PS-MNPS consisting of a core of iron oxide MNPs, a polystyrene shell, and benzenesulfonic acids on the surface, was active but with little or no recyclability; SO3H-Si-MNPS, consisting of a core of iron oxide MNPs, a silica shell, and propyl sulfonic acids as surface groups, showed lower activity and poor recycling performance [232].
Sulfonic acid-functionalized magnetic-poly(divinylbenzene-4-vinylpyridine) or M-poly(DVB-4VP-SO3H)
In the first time, sulfonic acid-functionalized magnetic-poly(divinylbenzene-4-vinylpyridine) was reported by Kara and Erdem [233]. This new catalyst was prepared from the copolymerization of DVB with 4VP, and mixing this copolymer with Fe3O4 nanoparticles to produce magnetic-poly(divinylbenzene-4-vinylpyridine) [m-poly(DVB-4VP)]; finally, the synthesized reagent was functionalized by sulfonic acid (Scheme 156).
After the preparation, its catalytic performance was tested for the esterification of propionic acid with methanol and compared with the traditional catalysts such as Amberlyst and Dowex which are the best known catalysts for the esterification reactions. The results show that m-poly(DVB-4VP-SO3H) have higher reactivity than commercially available solid acid catalysts for the conversion of propionic acid to methyl ester [233].
Sulfochitosan encapsulated nano-Fe3O4 (Fe3O4@CS NPs)
Sulfochitosan encapsulated nano-Fe3O4 was synthesized simply through in situ co-precipitation of Fe3+and Fe2+ ions via NH4OH in an aqueous solution of chitosan for the formation of Fe3O4@CS NPs and followed by treatment of the obtained reagent with chlorosulfonic acid in dichloromethane (Scheme 157).
Fe3O4@CS-SO3H NPs as a green and recyclable heterogeneous catalyst was used in preparation of 2-amino-4H-chromen-4-yl phosphonates through one-pot three-component reactions of salicylaldehydes, malononitrile, and triethyl phosphite in water at room temperature. Mild reaction conditions, green solvent, high yields, short reaction time and product purity are some of the advantages of this protocol [234].
Sulfamic acid-functionalized APTES-coated Fe/Fe3O4 nanoparticles
The sulfamylation strategy was applied to obtain sulfamic acid organo-catalysts linked to MNP consisting of iron cores and magnetite shells. These systems were used to epoxide ring opening of epoxy fatty esters by nucleophilic SN2 attack of methanol. Also, the excellent selectivity of the reaction with the recyclable NPs was confirmed by 1H NMR, 1H-1H COSY NMR, and ESI–MS comparable to non-recyclable H2SO4 (Scheme 158) [192].
Sulfonic acid-functionalized silica-coated CoFe2O4
Sulfonic acids fixed to carbon skeletons were attached to cobalt spinel ferrite NP in four different ways. These useful catalysts that was reported by Jones et al. containing: (1) SiMNP–SO3H, was prepared from the reaction of SiMNP with (3-mercaptopropyl)trimethoxysilane (MPTS) and further oxidation with hydrogen peroxide (Scheme 159).
2) SiMNP–PhSO3H, was synthesized via a condensation of CoFe2O4@SiO2 and 2-(4-chlorosulfonylphenyl)ethyltrimethoxysilane (CSPTMS) in chloroform under acid silica-based catalysis (Scheme 160).
3) SiMNP-FSO3H, was achieved by ring opening of a perfluorinated sultone by CoFe2O3@SiO2 NP in toluene without oxidation step (Scheme 161).
Moreover, (4) SiMNP–SiFSO3H, was prepared from the reaction of silica-coated magnetic nanoparticle with triethoxysilyl-perfluorosulfonyl fluoride in the presence of diethyl amine (Scheme 162) [235].
These catalysts were evaluated in deprotection reaction of benzaldehyde dimethylacetal in terms of activity and recyclability and compared with commercially available heterogeneous acidic resins and homogeneous sulfonic acids. The magnetic, solid acid catalysts exhibit comparable activity to the other commercial and homogeneous catalysts. Recovery tests confirm the presence of surface-bound sulfonic acid functionalities in three of the four catalysts. These results illustrate the utility of magnetic nanoparticles as a heterogeneous support for simple recovery of sulfonic acid catalysts [235].
Surface modification of ferrite nanoparticles
As it was depicted, surface modification of ferrite inverse spinels was done by applying divergent dicarboxylic acids such as oxalic acid, succinic acid, malic acid, tartaric acid and terepthalic acid (Scheme 163). To the best of our knowledge, this was the first report of the dicarboxylic acid as modifier of nanoparticles. Application of the as-prepared catalyst in the synthesis of HMF from fructose studied carefully, which demonstrates a new methodology for having advantageous toward being environmentally friendly (Scheme 164) [236].
Sulfonic acid-functionalized zirconium phosphonate (ZrBPMSA)
Han et al. [237] reported the preparation of sulfonic acid-functionalized zirconium phosphonate via the reaction of N,N-bis (phosphonomethyl)-sulfamic acid with ZrOCl2.8H2O and its applicability in the transesterification of alcohols (Scheme 165).
In continue, this reagent exhibited high catalytic activity in synthesis of unsymmetrical organic carbonates from the reaction of various alcohols with diethyl carbonate under solvent-free conditions at 120 °C. The research results showed that even aliphatic alcohols with long chain reacted well with diethyl carbonate to give the requested unsymmetrical organic carbonates in high to excellent yields with high selectivity. The catalyst is recoverable for several times without significant loss in its activity (Scheme 166) [237].
Nanohydroxyapatite functionalized with 2-aminoethyl dihydrogen phosphate (HAP@AEPH2-SO3H)
Zarghani and Akhlaghinia [238] designed nano-HAP that was modified with 2-aminoethyl dihydrogen phosphate (AEPH2) as a ligand and then sulfonated with chlorosulfonic acid (HAP@AEPH2-SO3H). Such type of synthesis illustrated in the following (Scheme 167).
The catalytic activity of HAP@AEPH2-SO3H was investigated for the synthesis of 4,4′-(aryl methylene)bis(3-methyl-1H-pyrazol-5-ol)s from the reaction of phenylhydrazine or hydrazine hydrate, ethyl acetoacetate and aromatic aldehydes. The best results were obtained in the presence of 1.5 mol % of catalyst at 80 °C under solvent-less conditions at 80 °C. This catalyst showed notable advantages, such as environmental friendliness, excellent yields, shorter reaction time, reusability of the inexpensive catalyst and easy workup procedure [238].
RSO3H functionalized on MOFs
MIL-101(Cr)-SO3H
First report for the synthesis of MIL-101(Cr)-SO3H exhibits dual role of Lewis and Brønsted acid described by Jiang et al. at University of Science and Technology of China. Heterogeneous alcoholysis of epoxides investigated at room temperature and the results showed the catalytic performance of the aforementioned MIL-101(Cr)-SO3H far exceeds all previously published Lewis acid-type MOF catalysts. In addition to its recyclability performance, it also shows even much higher catalytic activity compared to the MIL-101 encapsulating guest Keggin phosphotungstic acid. For good understanding, the results summarized in Table 3 [239].
Conversion of furfuryl alcohol (FA) to ethyl levulinate (EL) performed with employing EtOH as a substrate and a MIL-101(Cr)-SO3H as a catalyst. The as-synthesized catalyst showed easy accessibility to SO3H Brønsted moiety functionalized on MIL-101(Cr), high thermal and chemical stability. For the synthesis of MIL-101(Cr)-SO3H, equivalent molar ratio of 2-sulfoterephthalic acid monosodium salt (2-NaSO3-H2BDC) and CrO3 mixed with desired amounts of hydrochloric acid in water. The resulting mixture was transferred to a Teflon-lined stainless steel autoclave and heated for a period of time. After washing, the desired catalyst obtained and its catalytic performance compared with other reported catalysts (Table 4).
Among the investigated multiple SO3H-functionalized solid catalysts, complete conversion obtained with MIL-101(Cr)-SO3H, Nafion NR50, and Amberlyst-15d, but the good selectivity to the corresponding EL was achieved when MIL-101(Cr)-SO3H used as a catalyst. In this work, effect of temperature, molar ratio of FA to EtOH, and different amounts of catalyst loading was studied. Synthesis of alkyl levulinates was investigated with other alcohols except EtOH. Recyclability of the as-synthesized catalyst was also tested and the results indicated that after five times recycling of the catalyst, only slight loss in the activity of the catalyst was observed. Finally, leaching processes confirmed heterogeneous nature of the catalyst and two reaction path involving 2-ethoxymethylfuran and 4,5,5-triethoxypentan-2-one intermediates was proposed for this reaction [246].
Cellulose hydrolysis by a new porous coordination polymer involving chromium in its structural motifs and its decorated form with sulfonic acid functional groups has already been reported. After the synthesis of the MIL-101(Cr) decorated with -SO3H functional group, acid content was obtained by titration with sodium hydroxide solution, and 1.8 mmol/g reported as the concentration of surface -SO3H functional group. In hydrolysis of cellulose, one of the main concerns is high thermal stability of the catalyst in water and MIL-101(Cr)-SO3H showed high performance for this experiment. The cellulose hyrdrolysis was investigated with MIL-101(Cr) and its decorated ones (Table 5). As it demonstrated in Table 5, the cellulose did not hydrolyzed without use of catalyst. Its hydrlolysis can be done only in a small amount by applying undecorated MIL-101(Cr), but its decorated form can convert cellulose to the corresponding xylose, glucose and cellobiose with 2.6%, 1.4%, and 1.2%, respectively. By this way, 5.3% of mono and disaccharides will be formed during the condition. Such conversion will be increased when the reaction was performed in higher periods of time. Levulinic acid and formic acid are the by-products of cellulose hydrolysis under strong acidic condition. These side products formed when the hydrolysis was performed with just H2SO4, but finally employing MIL-101(Cr)-SO3H indicated no observation of such side products. Scheme 168 depicts the as-prepared MIL-101(Cr)-SO3H [247].
Recently, MIL-101(Cr)-SO3H catalyzes synthesis of one-pot synthesis of 2-amino-4H-chromenes via three-component condensation reaction between resorcinol, malononitrile and diverse range of aromatic aldehydes in aqueous medium was reported by Saikia and Saikia et al. [248].
Biomass fructose valorization studied with a series of sulfonic acid-functionalized metal–organic frameworks using post-synthetic modifications. Different MOFs having organic linker in their structural motifs including MIL-101(Cr)-SO3H, MIL-53(Al)-SO3H, and UIO-66(Zr)-SO3H have been investigated in conversion of fructose to 5-hydroxymethylfurfural (HMF). The results indicated in Table 6 propose that the loading level of sulfonic acid in various MOFs as well as the contribution of Brønsted acidity in different MOFs decreases in the order of MIL-101(Cr)-SO3H (15%) > MIL-53(Al)-SO3H (9.5%) > UIO-66(Zr)-SO3H (8.2%) > MIL-101(Cr)-SO3H (6.1%) > MIL-101(Cr)-SO3H (3%) indicating that the acidity strength of MOFs corresponds with its sulfonic acid grafting level [249].
Batch and continuous mediated synthesis of 5-Hydroxymethylfurfural using a fixed bed reactor have been already published by means of MIL-101(Cr)-SO3H which indicates dual role of catalyst as Brønsted and Lewis acidic media [250].
UiO-66-SO3H
Porous UiO-66-SO3H is a metal–organic framework having both Brønsted and Lewis acidic sites in their scaffolds can act as a catalyst in the multicomponent synthesis of dihydro-2-oxopyrroles. UiO-66-SO3H is described with a structural formula of Zr6O4(OH)4(HSO3BDC)6x(BDC)6−6x, in which BDC indicating benzene-1,4-dicarboxylate (Scheme 169) [251].
Zhao et al. reported two hierarchically porous MOFs named NUS-6 composed of either zirconium (Zr) or hafnium (Hf) clusters which they can counterpart in the conversion of fructose to HMF as a unique solid acid catalyst. NUS-6(Hf) showed higher catalytic activity compared to its counterparts NUS-6(Zr) due to the stronger Brønsted acidity contributed from Hf-μ3-OH groups and a bit smaller pore sizes makes it to restrict other reactions. Table 7 demonstrates several active catalysts contribute in the synthesis of HMF. As it can be seen, the order of activity is as follows: NUS-6(Hf) > NUS-6(Zr) > dimethyl 2-sulfoterephthalate (DMST) > UiO-6(Hf) > UiO-6(Hf) [252].
Yaghi and jiang recently published a chemical review about brønsted acidity in metal–organic frameworks involving preparation methods, characterization and application of the reported MOFs [253].
Guan et al. reported hydrogenation of γ-valerolactones (GVL) in ethanol by means of incorporated Pd nanoparticles on the surface of the previously synthesized MIL-101(Cr)-SO3H. 83% yield of ethyl valerate was enough to indicate the activity of such bifunctional catalyst. With unmodified MIL-101, GVL conversion was around 3.1% and the GVL conversion for MIL-101-SO3H(25) (entry 3) is higher at 7.8%, indicating that the Brønsted acid SO3H groups are involved in the catalytic reaction. With addition of sulfonic acid content, the GVL conversion becomes much more. Pd/MIL-101-SO3H showed similar behavioral GVL conversion as Pd/MIL-101, but with different product selectivity, in which case the main product is 4-hydroxy-ethylvalerate (Scheme 170) [254].
Al-MIL-53-RSO3H
Luan et al. demonstrated a Brønsted acidic Al-MIL-53-RSO3H catalyst derived from metal–organic framework Al-MIL-53-NH2 which is suitable for nitro-Mannich reaction. 2-amino-1,4-benzenedicarboxylate (NH2-H2BDC) was used as an organic linker for the synthesis of desired MOF. Treating the produced MOF with 1,3-propanesultone in chloroform led to the formation of octahedral Al-MIL-53-RSO3H catalyst (Schemes 171, 172) [255].
One year later, this group showed that aryl sulfonic acids and the previously prepared alkylsulfonic acid incorporated on metal organic frameworks can be applicable to the [4 +2] cycloaddition of 2-vinyl-substituted phenols (Scheme 173) [256].
Conclusion
Heterogeneous catalysis have drawn high attention in organic transformations due to its recyclability. In addition, a wide range of reactions occurs in acidic condition. In this contribution, we have comprehensively reviewed the various methods by which a range of different types of sulfonic acid derivatives can be synthesized onto the inorganic supports through an organic linker using post-synthesis or grafting method, direct synthesis or co-condensation, and sol–gel technique. All of the reported catalysts had high surface area and normal sizes with mean pore diameters ranging from the microporous zeolites to some macroporous silicas. Then, applicability of such sulfonic acids connected to inorganic supports in diverse organic reactions have also been reviewed.
References
T. Okuhara, Chem. Rev. 102, 3641–3666 (2002)
R. Jothiramalingam, M.K. Wang, Ind. Eng. Chem. Res. 48, 6162–6172 (2009)
J. Tulla-Puche, F. Albericio, The Power of Functional Resins in Organic Synthesis (Wiely, Hoboken, 2008)
M. Kidwai, R. Chauhan, S. Bhatnagar, Curr. Org. Chem. 19, 72–98 (2015)
R.H. Vekariya, K.D. Patel, H.D. Patel, RSC Adv. 5, 90819–90837 (2015)
M. Fallah-Mehrjardi, A.R. Kiasat, K. Niknam, J. Iran. Chem. Soc. 15, 2033–2081 (2018)
E. Doustkhah, J. Lin, S. Rostamnia, C. Len, R. Luque, X. Luo, Y. Bando, K.C.W. Wu, J. Kim, Y. Yamauchi, Y. Ide, Chem. Eur. J. 25, 1614–1635 (2019)
S. Khazalpour, M. Yarie, E. Kianpour, A. Amani, S. Asadabadi, J.Y. Seyf, M. Rezaeivala, S. Azizian, M.A. Zolfigol, J. Iran. Chem. Soc. 17, 1775–1917 (2020)
K. Niknam, A. Ebrahimpour, A. Barmak, G. Mohebbi, Monatsh. Chem. 149, 73–85 (2018)
C. Ying, B. Chulsung, Curr. Org. Synth. 8, 208–236 (2011)
K. Nakajima, M. Hara, ACS Catal. 2, 1296–1304 (2012)
H. Sharghi, P. Shiri, M. Aberi, Beilstein J. Org. Chem. 14, 2745–2770 (2018)
P. Gogoi, A.K. Dutta, S. Saikia, R. Borah, Appl. Catal. A Gen. 523, 321–331 (2016)
A. Brito, M.E. Borges, N. Otero, Energy Fuels 21, 3280–3283 (2007)
W. Long, C.W. Jones, ACS Catal. 1, 674–681 (2011)
J. Deng, L.-P. Mo, F.-Y. Zhao, L.-L. Hou, L. Yang, Z.-H. Zhang, Green Chem. 13, 2576–2584 (2011)
L. Ma’mani, M. Sheykhan, A. Heydari, M. Faraji, Y. Yamini, Appl. Catal. A Gen. 377, 64–69 (2010)
Y. Zhou, R. Huang, F. Ding, A.D. Brittain, J. Liu, M. Zhang, M. Xiao, Y. Meng, L. Sun, ACS Appl. Mater. Int. 6, 7417–7425 (2014)
N. Koukabi, E. Kolvari, M.A. Zolfigol, A. Khazaei, B.S. Shaghasemi, B. Fasahati, Adv. Synth. Catal. 354, 2001–2008 (2012)
M. Khoshnevis, A. Davoodnia, A. Zare-Bidaki, N. Tavakoli-Hoseini, Synth. React. Inorg. Met. 43, 1154–1161 (2013)
P. Laszlo, Homogeneous and Heterogeneous Reaction Conditions (Preparative Chemistry Using Supported Reagents, Academic Press, Cambridge, 1987), pp. 3–12
J. Butterworth, J.H. Clark, P.H. Waltona, S.J. Barlow, Chem. Commun. 1859–1860 (1996)
J.A. Elings, R. Ait-Meddour, J.H. Clark, D.J. Macquarrie, Chem. Commun. 2707–2708 (1998)
P.M. Price, J.H. Clark, D.J. Macquarrie, J. Chem. Soc., Dalton Trans. 101–110 (2000)
P. Salehi, M.A. Zolfigol, F. Shirini, M. Baghbanzadeh, Curr. Org. Chem. 10, 2171–2189 (2006)
M.B. Gawande, R. Hosseinpour, R. Luque, Curr. Org. Synth. 11, 526–544 (2014)
G. Mohammadi Ziarani, A. Badiei, A. Abbasi, Z. Farahani, Chin. J. Chem. 27, 1537–1542 (2009)
M.L. Testa, V. La Parola, A.M. Venezia, Catal. Today 158, 109–113 (2010)
M.L. Testa, V. La Parola, A.M. Venezia, Catal. Today 223, 115–121 (2014)
B. Karimi, M. Khalkhali, J. Mol. Catal. A: Chem. 271, 75–79 (2007)
B. Karimi, D. Zareyee, Tetrahedron Lett. 46, 4661–4665 (2005)
B. Karimi, M. Khalkhali, J. Mol. Catal. A: Chem. 232, 113–117 (2005)
D. Zareyee, B. Karimi, Tetrahedron Lett. 48, 1277–1280 (2007)
P. Gholamzadeh, G.M. Ziarani, N. Lashgari, A. Badiei, P. Asadiatouei, J. Mol. Catal. A: Chem. 391, 208–222 (2014)
R. Maggi, N.R. Shihu, V. Santacroce, G. Maestri, F. Bigi, G. Rothenberg, Beilstein J. Org. Chem. 12, 2173–2180 (2016)
R.D. Badley, W.T. Ford, J. Org. Chem. 54, 5437–5443 (1989)
C.G. Piscopo, S. Buhler, G. Sartori, R. Maggi, Catal. Sci. Technol. 2, 2449–2452 (2012)
W. Xing, Q. Ma, X. Peng, C. R. Chim. 18, 581–585 (2015)
K. Niknam, D. Saberi, M. Nouri Sefat, Tetrahedron Lett. 50, 4058–4062 (2009)
K. Niknam, D. Saberi, M. Mohagheghnejad, Molecules 14, 1915–1926 (2009)
K. Niknam, D. Saberi, H. Molaee, M.A. Zolfigol, Can. J. Chem. 18, 164–171 (2010)
K. Niknam, M.R. Mohammadizadeh, S. Mirzaee, D. Saberi, Chin. J. Chem. 28, 663–669 (2010)
K. Niknam, D. Saberi, M. Bagherinejad, Chin. Chem. Lett. 20, 1444–1448 (2009)
K. Niknam, D. Saberi, M. Bagherinejad, Phosphorus Sulfur Silicon 185, 875–882 (2010)
K. Niknam, F. Panahi, D. Saberi, M. Mohagheghnejad, J. Heterocycl. Chem. 47, 292–300 (2010)
K. Niknam, D. Saberi, M. Sadegheyan, A. Deris, Tetrahedron Lett. 51, 692–694 (2010)
K. Niknam, D. Saberi, M. Nouri Sefat, Tetrahedron Lett. 51, 2959–2962 (2010)
K. Niknam, M.R. Mohammadizadeh, S. Mirzaee, D. Saberi, Chin. J. Chem. 29, 1417–1422 (2011)
S.M.G. Ahmadi-Ana, M. Bagherinejad, K. Niknam, Chin. J. Chem. 30, 517–521 (2012)
M. Tajbakhsh, Y. Ranjbar, A. Masuodi, S. Khaksar, Chin. J. Catal. 33, 1542–1545 (2012)
M. Tajbakhsh, R. Hosseinzadeh, P. Rezaee, H. Alinezhad, J. Mex. Chem. Soc. 56, 402–407 (2012)
G. Sivagamisundari, A.M. Pushpalatha, S.J. Ranee, Int. J. Sci. Eng. Technol. 3, 852–855 (2014)
K. Aswin, S.S. Mansoor, K. Logaiya, S.P.N. Sudhan, V.S. Malik, H. Ramadoss, Res. Chem. Intermed. 40, 2283–2598 (2014)
K. Niknam, P. Abolpour, J. Chem. Sci. 127, 1315–1320 (2015)
K. Niknam, D. Saberi, Appl. Catal. A Gen. 366, 220–225 (2009)
S. Tayebi, M. Baghernejad, D. Saberi, K. Niknam, Chin. J. Catal. 32, 1477–1483 (2011)
F. Rohandeh, D. Saberi, K. Niknam, Iran. J. Catal. 1, 71–78 (2011)
N. Iravani, N.S. Mohammadzade, K. Niknam, Chin. Chem. Lett. 22, 1151–1154 (2011)
Z. Tavakoli, M. Bagherneghad, K. Niknam, J. Heterocycl. Chem. 49, 634–639 (2012)
S. Ghasemi, M. Bagherinejad, K. Niknam, Iran. J. Catal. 3, 165–169 (2013)
S.P. Brojeni, M. Bagherinejad, D. Saberi, K. Niknam, Green Chem. Lett. Rev. 6, 69–75 (2013)
K. Niknam, D. Saberi, Tetrahedron Lett. 50, 5210–5214 (2009)
F. Rashedian, D. Saberi, K. Niknam, J. Chin. Chem. Soc. 57, 998–1006 (2010)
K. Niknam, N. Jafarpour, E. Niknam, Chin. Chem. Lett. 22, 69–72 (2011)
W. Xie, D. Yang, Bioresource Technol. 102, 9818–9822 (2011)
S.R. Jetti, A. Bhatewara, T. Kadre, S. Jain, Chin. Chem. Lett. 25, 469–473 (2014)
M.-S. Shakeri, H. Tajik, K. Niknam, J. Chem. Sci. 124, 1025–1032 (2012)
M. Karimzadeh, H. Saberi-Asl, H. Hashemi, D. Saberi, K. Niknam, Monatsh. Chem. 149, 2237–2244 (2018)
K. Niknam, A. Deris, F. Naeimi, F. Majleci, Tetrahedron Lett. 52, 4642–4645 (2011)
T. Rahi, M. Baghernejad, K. Niknam, Chin. Chem. Lett. 23, 1103–1106 (2012)
S. Tayebi, K. Niknam, Iran. J. Catal. 2, 69–74 (2012)
M. Nouri Sefat, A. Deris, K. Niknam, Chin. J. Chem. 29, 2361–2367 (2011)
T. Rahi, M. Baghernejad, K. Niknam, Chin. J. Catal. 33, 1095–1100 (2012)
K. Niknam, S.A. Sajadi, R. Hosseini, M. Baghernejad, Iran. J. Catal. 4, 163–173 (2014)
K. Niknam, N. Borazjani, Org. Chem. Res. 1, 78–86 (2015)
K. Niknam, A. Jamali, M. Tajaddod, A. Deris, Chin. J. Catal. 33, 1312–1317 (2012)
M. Parveen, S. Azaz, F. Ahmad, A.M. Malla, M. Allam, Catal. Lett. 146, 1687–1705 (2016)
F. Adam, K.M. Hello, M.R.B. Aisha, J. Taiwan Inst. Chem. E. 42, 843–851 (2011)
M.A. Zolfigol, H. Veisi, F. Mohanazadeh, A. Sedrpoushan, J. Heterocycl. Chem. 48, 977–986 (2011)
H. Veisi, A. Sedrpoushan, M.A. Zolfigol, F. Mohanazadeh, J. Heterocycl. Chem. 48, 1448–1454 (2011)
M. Daraei, M.A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H.G. Kruger, M. Mokhlesi, J. Iran. Chem. Soc. 12, 855–861 (2015)
M.A. Zolfigol, H. Ghaderi, S. Baghery, L. Mohammadi, J. Iran. Chem. Soc. 14, 121–134 (2017)
S. Sudha, M.A. Pasha, J. Iran. Chem. Soc. 11, 1533–1536 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, E. Noroozizadeh, R. Salehi Moratab, M. Zarei, J. Mol. Catal. A: Chem. 420, 246–253 (2016)
E. Noroozizadeh, A.R. Moosavi-Zare, M.A. Zolfigol, A. Zare, M. Zarei, Can. J. Chem. 95, 16–21 (2017)
K. Qiao, H. Hagiwara, C. Yokoyama, J. Mol. Catal. A: Chem. 246, 65–69 (2006)
A.S. Amarasekara, O.S. Owereh, Catal. Commun. 11, 1072–1075 (2010)
B. Wiredu, A.S. Amarasekara, Catal. Commun. 48, 41–44 (2014)
M. Nouri Sefat, D. Saberi, K. Niknam, Catal. Lett. 141, 1713–1720 (2011)
K. Niknam, A. Piran, Green Sustain. Chem. 03, 1–8 (2013)
Y. Shao, H. Wan, J. Miao, G. Guan, React. Kinet. Mech. Catal. 109, 149–158 (2013)
H. Tajik, K. Niknam, S. Karimian, Iran. J. Catal. 3, 107–113 (2013)
K. Niknam, A. Piran, Z. Karimi, J. Iran. Chem. Soc. 13, 859–871 (2016)
D.A. Kotadia, S.S. Soni, J. Mol. Catal. A: Chem. 353, 44–49 (2012)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Zarei, A. Zare, V. Khakyzadeh, J. Mol. Liq. 211, 373–380 (2015)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Zarei, A. Zare, J. Afsar, Appl. Catal. A Gen. 505, 224–234 (2015)
A. Zare, M. Merajoddin, A.R. Moosavi-Zare, M. Zarei, M.H. Beyzavi, M.A. Zolfigol, Res. Chem. Intermed. 42, 2365–2378 (2016)
J. Chen, J. Chen, X. Zhang, J. Gao, Q. Yang, Appl. Catal. A Gen. 516, 1–8 (2016)
A. Corma, Chem. Rev. 97, 2373–2420 (1997)
C. Li, Catal. Rev. 46, 419–492 (2004)
A. Taguchi, F. Schüth, Microporous Mesoporous Mater. 77, 1–45 (2005)
C.A. Schuh, Mater. Today 9, 32–40 (2006)
B.H. Lee, J. Oh, H.H. Tseng, R. Jammy, H. Huff, Mater. Today 9, 32–40 (2006)
Y. Wang, F. Caruso, Chem. Mater. 17, 953–961 (2005)
C.T. Kresge, M.E. Leonowicz, W.J. Roth, J.C. Vartuli, J.S. Beck, Nature 359, 710–712 (1992)
V.S.Y. Lin, D.R. Radu, M.-K. Han, W. Deng, S. Kuroki, B.H. Shanks, M. Pruski, J. Am. Chem. Soc. 124, 9040–9041 (2002)
J.H. Clark, Chem. Rev. 80, 429–452 (1980)
D. Zhao, J. Feng, Q. Huo, N. Melosh, G.H. Fredrickson, B.F. Chmelka, G.D. Stucky, Science 279, 548–552 (1998)
D. Zhao, Q. Huo, J. Feng, B.F. Chmelka, G.D. Stucky, J. Am. Chem. Soc. 120, 6024–6036 (1998)
M.H. Lim, C.F. Blanford, A. Stein, Chem. Mater. 10, 467–470 (1998)
A.P. Wight, M.E. Davis, Chem. Rev. 102, 3589–3614 (2002)
J.A. Melero, R. van Grieken, G. Morales, Chem. Rev. 106, 3790–3812 (2006)
M. Alvaro, A. Corma, D. Das, V. Fornes, H. Garcia, Chem. Commun. 956–957 (2004)
G. Mohammadi Ziarani, N. Lashgari, A. Badiei, J. Mol. Catal. A: Chem. 397, 166–191 (2015)
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, J.L. Schlenker, J. Am. Chem. Soc. 114, 10834–10843 (1992)
H. Veisi, A. Sedrpoushan, A.R. Faraji, M. Heydari, S. Hemmati, B. Fatahi, RSC Adv. 5, 68523–68530 (2015)
V.F. Vavsari, G.M. Ziarani, S. Balalaie, A. Latifi, M. Karimi, A. Badiei, Tetrahedron 72, 5420–5426 (2016)
S. Rostamnia, E. Dustkhah, J. Mol. Catal. A: Chem. 411, 317–324 (2016)
J. Miao, H. Wan, G. Guan, Catal. Commun. 12, 353–356 (2011)
S.J. Canhaci, R.F. Perez, L.E.P. Borges, M.A. Fraga, Appl. Catal. B-Environ. 207, 279–285 (2017)
B.J. Melde, B.T. Holland, C.F. Blanford, A. Stein, Chem. Mater. 11, 3302–3308 (1999)
J. Mondal, T. Sen, A. Bhaumic, Dalton Trans. 41, 6173–6181 (2012)
D. Zareyee, R. Asghari, M.A. Khalilzadeh, Chin. J. Catal. 32, 1864–1868 (2011)
W. Shagufta, I. Ahmad, R. Dhar, Catal. Surv. Asia 21, 53–69 (2017)
S. Karnjanakom, S. Kongparakul, C. Chaiya, P. Reubroycharoen, G. Guan, C. Samart, J. Environ. Chem. Eng. 4, 47–55 (2016)
F. Alrouh, A. Karam, A. Alshaghel, S. El-Kadri, Arabian J. Chem. 10, S281–S286 (2017)
X. Sheng, J. Gao, L. Han, Y. Jia, W. Sheng, Microporous Mesoporous Mater. 143, 73–77 (2011)
T.K. Khatab, K.A.M. El-Bayouki, W.M. Basyouni, W.M. Tohamy, Res. J. Pharm. Biol. Chem. Sci. 6, 816–839 (2015)
M. Hajjami, F. Ghorbani, F. Bakhti, Appl. Catal. A Gen. 470, 303–310 (2014)
F. Adam, K.M. Hello, T.H. Ali, Appl. Catal. A Gen. 399, 42–49 (2011)
E.-P. Ng, S.N. Mohd Subari, O. Marie, R.R. Mukti, J.-C. Juan, Appl. Catal. A Gen. 450, 34–41 (2013)
S. Safaei, I.M. Baltork, A.R. Khosropour, M. Moghadam, S. Tangestaninejad, V. Mirkhani, J. Iran. Chem. Soc. 14, 1583–1589 (2017)
Y. Wang, Z. Gu, W. Liu, Y. Yao, H. Wang, X.F. Xia, W. Li, RSC Adv. 5, 60736–60744 (2015)
T. Kamegawa, A. Mizuno, H. Yamashita, Catal. Today 243, 153–157 (2015)
Z. Nasresfahani, M.Z. Kassaee, E. Eidi, New J. Chem. 40, 4720–4726 (2016)
S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna, O. Terasaki, J. Am. Chem. Soc. 121, 9611–9614 (1999)
B. Karimi, H.M. Mirzaei, A. Mobaraki, H. Vali, Catal. Sci. Technol. 5, 3624–3631 (2015)
D. Dubé, M. Rat, W. Shen, F. Béland, S. Kaliaguine, J. Mater. Sci. 44, 6683–6692 (2009)
W. Shen, D. Dubé, S. Kaliaguine, Catal. Commun. 10, 291–294 (2008)
A. Yaghoubi, M.G. Dekamin, E. Arefi, B. Karimi, J. Colloid Interf. Sci. 505, 956–963 (2017)
P. Van Der Voort, D. Esquivel, E. De Canck, F. Goethals, I. Van Driessche, F.J. Romero-Salguero, Chem. Soc. Rev. 42, 3913–3955 (2013)
E. De Canck, C. Vercaemst, F. Verpoort, P. Van Der Voort, Stud. Surf. Sci. Catal. 175, 365–368 (2010)
H. Qian, D. Liu, C. Lv, Ind. Eng. Chem. Res. 50, 1146–1149 (2011)
S.-L. Wee, C.-T. Tye, S. Bhatia, Sep. Purif. Technol. 63, 500–516 (2008)
Y.F. Yeong, A.Z. Abdullah, A.L. Ahmad, S. Bhatia, Chem. Eng. J. 157, 579–589 (2010)
Z.-M. Li, Y. Zhou, D.-J. Tao, W. Huang, X.-S. Chen, Z. Yang, RSC Adv. 4, 12160–12167 (2014)
S.M. Baghbanian, Chin. Chem. Lett. 26, 1113–1116 (2015)
H. Hafizi, A. Najafi Chermahini, M. Saraji, G. Mohammadnezhad, Chem. Eng. J. 294, 380–388 (2016)
H. Jin, M.B. Ansari, S.-E. Park, Catal. Today 245, 116–121 (2015)
D.S. Moraes, R.S. Angélica, C.E.F. Costa, G.N. Rocha Filho, J.R. Zamian, Appl. Clay Sci. 51, 209–213 (2011)
K. Bahrami, M. Khodamorady, Appl. Organometal. Chem. 32, e4553 (2018)
M. Khodamorady, S. Sohrabnezhad, K. Bahrami, Polyhedron 178, 114340 (2020)
L. Wu, Z. Yin, Carbohydr. Res. 365, 14–19 (2013)
S.V. Atghia, S. Sarvi Beigbaghlou, J. Organomet. Chem. 745, 42–49 (2013)
E. Tabrizian, A. Amoozadeh, S. Rahmani, RSC Adv. 6(2016), 21854–21864 (1864)
J. Safari, M. Ahmadizadeh, J. Taiwan Inst. Chem. E. 74, 14–24 (2017)
F.C.C. Oliveira, L.M. Rossi, R.F. Jardim, J.C. Rubim, J. Phys. Chem. C 113, 8566–8572 (2009)
A.R. Hajipour, M. Karimzadeh, G. Azizi, Chin. Chem. Lett. 25, 1382–1386 (2014)
A.R. Hajipour, M. Karimzadeh, S. Ghorbani, Synlett 25, 2903–2907 (2014)
A.R. Hajipour, M. Karimzadeh, F. Fakhari, H. Karimi, Appl. Organometal. Chem. 30, 946–948 (2016)
F. Ahmadian, A.R. Barmak, E. Ghaderi, M. Bavadi, H. Raanaei, K. Niknam, J. Iran. Chem. Soc. 16, 2647–2658 (2019)
E. Pérez Mayoral, E. Soriano, V. Calvino-Casilda, M.L. Rojas-Cervantes, R.M. Martín-Aranda, Catal. Today 285, 65–88 (2017)
D. Saberi, H. Hashemi, N. Ghanaatzadeh, M. Moghadam, K. Niknam, Appl. Organometal. Chem. 34, e5563 (2020)
J. Gao, H. Gu, B. Xu, Accounts Chem. Res. 42, 1097–1107 (2009)
H.J. Chung, H. Lee, K.H. Bae, Y. Lee, J. Park, S.-W. Cho, J.Y. Hwang, H. Park, R. Langer, D. Anderson, T.G. Park, ACS Nano 5, 4329–4336 (2011)
G. Reiss, A. Hutten, Nat. Mater. 4, 725–726 (2005)
T. Dai Lam, L. Van Hong, P. Hoai Linh, H. Thi My Nhung, N. Thi Quy, L. Thien Tai, H. Phuong Thu, N. Xuan Phuc, Adv. Nat. Sci-Nanosci. 1, 045013 (2010)
S. Rajput, C.U. Pittman, D. Mohan, J. Colloid Interf. Sci. 468, 334–346 (2016)
J. Govan, Y.K. Gun’ko, Nanomaterials 4, 222–241 (2014)
J. Drbohlavova, R. Hrdy, V. Adam, R. Kizek, O. Schneeweiss, J. Hubalek, Sensors 9, 2352–2362 (2009)
W. Wu, Q. He, C. Jiang, Nanoscale Res. Lett. 3, 397–415 (2008)
V. Patsula, L. Kosinová, M. Lovrić, L. Ferhatovic Hamzić, M. Rabyk, R. Konefal, A. Paruzel, M. Šlouf, V. Herynek, S. Gajović, D. Horák, ACS Appl. Mater. Interf. 8, 7238–7247 (2016)
Â.L. Andrade, M.A. Valente, J.M.F. Ferreira, J.D. Fabris, J. Magn. Magn. Mater. 324, 1753–1757 (2012)
T. Tago, T. Hatsuta, K. Miyajima, M. Kishida, S. Tashiro, K. Wakabayashi, J. Am. Ceramic Soc. 85, 2188–2194 (2002)
A.-L. Morel, S.I. Nikitenko, K. Gionnet, A. Wattiaux, J. Lai-Kee-Him, C. Labrugere, B. Chevalier, G. Deleris, C. Petibois, A. Brisson, M. Simonoff, ACS Nano 2, 847–856 (2008)
Z. Lei, Y. Li, X. Wei, J. Solid State Chem. 181, 480–486 (2008)
W. Wei, W. Zhaohui, Y. Taekyung, J. Changzhong, K. Woo-Sik, Sci. Technol. Adv. Mater. 16, 023501 (2015)
W. Stöber, A. Fink, E. Bohn, J. Colloid Interf. Sci. 26, 62–69 (1968)
J.N. Ryan, M. Elimelech, J.L. Baeseman, R.D. Magelky, Environ. Sci. Technol. 34, 2000–2005 (2000)
L.M. Liz-Marzán, M. Giersig, P. Mulvaney, Langmuir 12, 4329–4335 (1996)
B. Kalska-Szostko, U. Wykowska, D. Satuła, Colloids Surf. A Physicochem. Eng. Asp. 481, 527–536 (2015)
Z.-M. Liu, H.-F. Yang, Y.-F. Li, Y.-L. Liu, G.-L. Shen, R.-Q. Yu, Sens. Actuators B Chem. 113, 956–962 (2006)
S. Sobhani, R. Jahanshahi, New J. Chem. 37, 1009–1015 (2013)
S. Sobhani, Z.P. Parizi, N. Razavi, Appl. Catal. A Gen. 409, 162–166 (2011)
Q. Zhang, H. Su, J. Luo, Y. Wei, Green Chem. 14, 201–208 (2012)
A. Khalafi-Nezhad, S. Mohammadi, ACS Comb. Sci. 15, 512–518 (2013)
M. Sheykhan, L. Ma’mani, A. Ebrahimi, A. Heydari, J. Mol. Catal. A: Chem. 335, 253–261 (2011)
M. Khoobi, L. Ma’mani, F. Rezazadeh, Z. Zareie, A. Foroumadi, A. Ramazani, A. Shafiee, J. Mol. Catal. A: Chem. 359, 74–80 (2012)
F. Zamani, S.M. Hosseini, S. Kianpour, Solid State Sci. 26, 139–143 (2013)
F. Zamani, E. Izadi, Catal. Commun. 42, 104–108 (2013)
Z. Zare-Akbari, S. Dastmalchi, L. Edjlali, L. Dinparast, M. Es’haghi, Appl. Organometal. Chem. 34, e5649 (2020)
A. Mobaraki, B. Movassagh, B. Karimi, ACS Comb. Sci. 16, 352–358 (2014)
B. Movassagh, L. Tahershamsi, A. Mobaraki, Tetrahedron Lett. 56, 1851–1854 (2015)
S. Moradi, M.A. Zolfigol, M. Zarei, D.A. Alonso, A. Khoshnood, Appl. Organometal. Chem. 32, e4043 (2018)
M.Z. Kassaee, H. Masrouri, F. Mocahedi, Appl. Catal. A Gen. 395, 28–33 (2011)
J. Safari, Z. Zarnegar, Ultrason. Sonochem. 20, 740–746 (2013)
F. Nemati, M.M. Heravi, R. Saeedi-Rad, Chin. J. Catal. 33, 1825–1831 (2012)
T. Cheng, D. Zhang, H. Li, G. Liu, Green Chem. 16, 3401–3427 (2014)
L. Shiri, H. Narimani, M. Kazemi, Appl. Organometal. Chem. 32, e3927 (2018)
L. Shiri, H. Narimani, M. Kazemi, Appl. Organometal. Chem. 32, e3999 (2018)
L. Shiri, S. Zarei, M. Kazemi, D. Sheikh, Appl. Organometal. Chem. 32, e3938 (2018)
Z.-H. Zhang, H.-Y. Lü, S.-H. Yang, J.-W. Gao, J. Comb. Chem. 12, 643–646 (2010)
M. Afrdai, N. Foroughifar, H. Pasdar, H. Moghanian, RSC Adv. 6, 59343–59351 (2016)
R. Ghorbani-Vaghei, V. Izadkhah, Appl. Organometal. Chem. 32, e4025 (2018)
R. Ghorbani-Vaghei, S. Alavinia, N. Sarmast, Appl. Organometal. Chem. 32, e4038 (2018)
M. Rajabi-Salek, M.A. Zolfigol, M. Zarei, Res. Chem. Intermed. 44, 5255–5269 (2018)
S. Sajjadifar, Z. Gheisarzadeh, Appl. Organometal. Chem. 33, e4602 (2019)
P. Wang, A. Kong, W. Wang, H. Zhu, Y. Shan, Catal. Lett. 135, 159–164 (2010)
M. Shakourian-Fard, A.H. Rezayan, S. Kheirjou, A. Bayat, M. Mahmoodi Hashemi, Bull. Chem. Soc. Jpn 87, 982–987 (2014)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, V. Khakyzadeh, S. Azizian, J. Mol. Catal. A: Chem. 418, 54–67 (2016)
M.A. Zolfigol, M. Navazeni, M. Yarie, R. Ayazi-Nasrabadi, Can. J. Chem. 95, 1248–1252 (2017)
M. Zarei, M.A. Zolfigol, A.R. Moosavi-Zare, E. Noroozizadeh, Iran. J. Chem. Soc. 14, 2187–2198 (2017)
S. Sajjadifar, M.A. Zolfigol, F. Tami, J. Chin. Chem. Soc. 66, 307–319 (2019)
M.A. Zolfigol, R. Ayazi-Nasrabadi, RSC Adv. 6, 69595–69604 (2016)
M.A. Zolfigol, M. Navazeni, M. Yarie, R. Ayazi-Nasrabadi, Appl. Organometal. Chem. 31, e3633 (2017)
M.A. Zolfigol, M. Yarie, RSC Adv. 5, 103617–103624 (2015)
M.A. Zolfigol, M. Kiafar, M. Yarie, A. Taherpour, M. Saeidi-Rad, RSC Adv. 6, 50100–50111 (2016)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, RSC Adv. 5(2015), 71942–71947 (1954)
M.A. Zolfigol, M. Navazeni, M. Yarie, R. Ayazi-Nasrabadi, RSC Adv. 6, 92862–92868 (2016)
B. Atashkar, M.A. Zolfigol, S. Mallakpour, Mol. Catal. 452, 192–246 (2018)
M.A. Zolfigol, M. Navazeni, M. Yarie, R. Ayazi-Nasrabadi, Res. Chem. Intermed. 44, 191–200 (2018)
M. Torabi, M. Yarie, M.A. Zolfigol, Appl. Organometal. Chem. 33, e4933 (2019)
M.A. Zolfigol, R. Ayazi-Nasrabadi, S. Baghery, Appl. Organometal. Chem. 30, 500–509 (2016)
M. Shirzaei, E. Molashahi, M.T. Maghsoodlou, M. Lashkari, J. Saudi Chem. Soc. 24, 216–222 (2020)
N. Saadatjoo, M. Golshekan, S. Shariati, H. Kefayati, P. Aziz, J. Mol. Catal. A: Chem. 377, 173–179 (2013)
M.A. Zolfigol, M. Yarie, Appl. Organometal. Chem. 31, e3598 (2017)
M.A. Zolfigol, F. Karimi, M. Yarie, M. Torabi, Appl. Organometal. Chem. 32, e4063 (2018)
D. Azarifar, Y. Abbasi, O. Badalkhani, Appl. Organometal. Chem. 32, e3939 (2018)
D. Azarifar, Y. Abbasi, M. Jaymand, M.A. Zolfigol, M. Ghaemi, O. Badalkhani, J. Organomet. Chem. 895, 55–63 (2019)
S. Sayin, M. Yilmaz, J. Chem. Eng. Data 56, 2020–2029 (2011)
M. Feyen, C. Weidenthaler, F. Schüth, A.-H. Lu, Chem. Mater. 22, 2955–2961 (2010)
G. Tan, Z. Li, Green Chem. 14, 3077–3086 (2012)
A. Kara, B. Erdem, J. Mol. Catal. A: Chem. 349, 42–47 (2011)
R. Mohammadi, M.Z. Kassaee, J. Mol. Catal. A: Chem. 380, 152–158 (2013)
C.S. Gill, B.A. Price, C.W. Jones, J. Catal. 251, 145–152 (2007)
M. Shaikh, M. Sahu, K.K. Atyam, K.V.S. Ranganath, RSC Adv. 6, 76795–76801 (2016)
J. Song, B. Zhang, S. Wu, Q. Wang, H. Fan, Z. Zhang, B. Han, Pure Appl. Chem. 84, 675–684 (2011)
M. Zarghani, B. Akhlaghinia, RSC Adv. 5, 87769–87780 (2015)
Y.X. Zhou, Y.Z. Chen, Y. Hu, G. Huang, S.H. Yu, H.L. Jiang, Chem. A Eur. J. 20, 14976–14980 (2014)
D. Jiang, T. Mallat, F. Krumeich, A. Baiker, J. Catal. 257, 390–395 (2008)
D. Jiang, A. Urakawa, M. Yulikov, T. Mallat, G. Jeschke, A. Baiker, Chem. A Eur. J. 15, 12255–12262 (2009)
A. Dhakshinamoorth, M. Alvaro, H. Garcia, Chem. A Eur. J. 16, 8530–8536 (2010)
L. He, J. Huang, T. Xu, L. Chen, K. Zhang, S. Han, Y. He, S.T. Lee, J. Mater. Chem. 22, 1370–1374 (2012)
S.M.F. Vilela, D. Ananias, J.A. Fernandes, P. Silva, A.C. Gomes, N.J.O. Silva, M.O. Rodrigues, J.P.C. Tome, A.A. Valente, P.R. Claro, L.D. Carlos, J. Rocha, F.A.A. Paz, J. Mater. Chem. C 2, 3311–3327 (2014)
L.H. Wee, F. Bonino, C. Lamberti, S. Bordiga, J.A. Martens, Green Chem. 16, 1351–1357 (2014)
X.F. Liu, H. Li, H. Zhang, H. Pan, S. Huang, K.L. Yang, S. Yang, RSC Adv. 6, 90232–90238 (2016)
G. Akiyama, R. Matsuda, H. Sato, M. Takata, S. Kitagawa, Adv. Mater. 23, 3294–3297 (2011)
M. Saikia, L. Saikia, RSC Adv. 6, 15846–15853 (2016)
J. Chen, K. Li, L. Chen, R. Liu, X. Huang, D. Ye, Green Chem. 16, 2490–2499 (2014)
Y. Su, G. Chang, Z. Zhang, H. Xing, B. Su, Q. Yang, Q. Ren, Y. Yang, Z. Bao, AlChE J. 62, 4403–4417 (2016)
R.G. Vaghei, D. Azarifar, S. Daliran, A.R. Oveisi, RSC Adv. 6, 29182–29189 (2016)
Z. Hu, Y. Peng, Y. Gao, Y. Qian, S. Ying, D. Yuan, S. Horike, N. Ogiwara, R. Babarao, Y. Wang, N. Yan, D. Zhao, Chem. Mater. 28, 2659–2667 (2016)
J. Jiang, O.M. Yaghi, Chem. Rev. 115, 6966–6997 (2015)
D. Zhang, F. Ye, Y. Guan, Y. Wang, E.J.M. Hensen, RSC Adv. 4, 39558–39564 (2014)
Z. Miao, C. Qi, L. Wang, A.M. Wensley, Y. Luan, Appl. Organometal. Chem. 31, e3569 (2017)
R. Li, Y. Jiang, J. Zhao, D. Ramella, Y. Peng, Y. Luan, RSC Adv. 7, 34591–34597 (2017)
Acknowledgements
We are thankful to Persian Gulf University Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This review is dedicated to Professor Hashem Sharghi on the occasion of his 71th birthday.
Rights and permissions
About this article
Cite this article
Niknam, K., Hashemi, H., Karimzadeh, M. et al. Recent advances in preparation and application of sulfonic acid derivatives bonded to inorganic supports. J IRAN CHEM SOC 17, 3095–3178 (2020). https://doi.org/10.1007/s13738-020-01997-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-01997-w