Abstract
A selective separation and preconcentration method for the determination of gold ions in water and ore samples has been developed using dispersive liquid–liquid microextraction, followed by flame atomic absorption spectrometry. 4-Ethyl-1(2-(4-(4-nitrophenyl)piperazin-1-yl)acetyl)thiosemicarbazide) (NPPTSC) has been used for the first time as new chelating reagent. A mixture of ethanol (dispersive solvent) and carbon tetrachloride (extraction solvent) was used. Some parameters affecting the extraction procedure including the type and volume of the extracting and dispersive solvents, HNO3 concentration, the chelating agent amount, volume of sample, and foreign ions have optimized. Also, the complex formation between gold ions and the ligand has been investigated in a methanol–water solution (1:1) using UV–visible spectrometry. The spectrophotometric titration data showed that of Au–NPPTSC complex composition was found to be 3:2. After optimizing the instrumental and experimental parameters, we achieved a detection limit of 1.5 µg L−1, a preconcentration factor of 50, and a linear dynamic range of 10.0–400.0 µg L−1. The relative standard deviation obtained 2.1% at 50 µg L−1 for gold ions (n = 10). The proposed method was successfully performed for the determination of gold in certified reference material, environmental water, and ore samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gold is a very valuable metal found in very low levels on earth. Due to unique physical and chemical properties, the use of gold has considerably increased in various fields such as catalytic converters, jewelry, electronics, metallurgy, energy, health, and environmental applications [1,2,3]. This element can be released to our environment because of many industrial activities. Therefore, determination of trace amounts of gold in environmental samples is of great importance. There are many analytical techniques presented to determine gold in real samples. Graphite furnace atomic absorption spectrometry (GF-AAS) [4], flame atomic absorption spectrometry (FAAS), [5], and inductively coupled plasma mass spectroscopy (ICP-MS) [6] have been widely used to determine gold in a variety of natural samples. FAAS is very common technique for detection of metals present in samples because of the low costs, easy operation, and requirement of inexpensive equipment [7]. However, due to unsatisfying sensitivity and matrix effects, it is difficult to directly determine metal ions at trace levels with these advanced techniques. For this reason, a separation/preconcentration step is required before their analysis.
Several sample preparation procedures have been used to separate gold ions from various matrices before its determination, such as cloud point extraction (CPE) [8], coprecipitation [9], solid phase extraction (SPE) [10], and liquid–liquid extraction (LLE) [11]. Traditional LLE is the oldest and effective technique in analytical chemistry for the extraction of a series of organic and inorganic components. Unfortunately, it is time consuming and needs large quantities of toxic organic solvents [12]. Recently, the developments in the extraction methods have gained increasing attention from the analytes for its miniaturization, effectiveness, cheapness, simplicity, and minimized reagent consumption. Assadi and his co-workers have developed and established dispersive liquid–liquid microextraction (DLLME). It is highly efficient and highly powered at preconcentrating and determining traces of inorganic and organic species in water samples [13]. Simplicity, low sample volume, ease of operation, low organic solvent cost, high recovery, and high enrichment factors are some of the outstanding advantages of DLLME. This microextraction method has also been applied to determine trace gold ions in various types of environmental samples [14].
The DLLME is a simple microextraction technique that requires an appropriate mixture of an extraction and a dispersive solvent are injected into an aqueous sample and a cloudy solution is then formed because of the formation of fine micro droplets of the extraction solvent fully dispersed in aqueous phase. At this point, extraction solvent distributed homogeneously in the whole aqueous solution in a short time by the help of the dispersive solvent, resulting in a large contact area between extraction solvent and aqueous sample. Subsequently, equilibrium state is obtained quickly, resulting in a very short extraction time, which is the major advantage of the method compared with those of other preconcentration procedures. After the cloudy solution is centrifuged, the fine remaining organic phase is recovered, in the bottom of the conical test tube [15,16,17]. The certain volume of recovered solvent can be analyzed using a variety of analytical devices.
In this work, we present a DLLME as a simple, fast, and selective method for the determination of gold ion at trace levels in ore and water samples using FAAS. Gold preconcentration was mediated by chelation with the new reagent 4-ethyl-1(2-(4-(4 nitrophenyl)piperazin-1-yl)acetyl)thiosemicarbazide) (NPPTSC), and carbontetrachloride was selected as the extraction solvent to extract a hydrophobic complex. Also, the complex composition of gold ion with the new chelation agent was observed using of spectrophotometric titration. After optimization of various experimental parameters and analytical features, the received results demonstrated that DLLME is an effective technique for analyzing gold in real samples with both a well preconcentration factor and a low detection limit.
Experimental
Reagents and materials
All reagents used were of analytical grade. A 1000 mg L−1 standard solution of H(AuCl4), HCl, HNO3, and other acids was purchased from Merck (Darmstadt, Germany). HNO3 was used to adjust the acidity of the solutions. The working standard solutions were achieved by appropriately diluting the stock standard solution. The ligand, 4-ethyl-1(2-(4-(4-nitrophenyl)piperazin-1-yl)acetyl)thiosemicarbazide) (Fig. 1), was synthesized and purified according to the literature [18]. The complexation agent solution [0.025% (w/v)] was prepared by dissolving the appropriate amount of the mixture of dimethylsulfoxide/methanol (1/24). The remaining chemicals used, including carbon disulfide (for spectroscopy Uvasol®), dimethylsulfoxide (for analysis Emsure®), dichloromethane (for analysis Emsure®), carbon tetrachloride (for analysis Emsure®), chloroform (for analysis Emsure®), methanol (for analysis Emsure®), ethanol (for analysis Emparta®), acetonitrile (for liquid chromatography LiChrosolv®), acetone (for liquid chromatography LiChrosolv®), and tetrahydrofuran (for liquid chromatography LiChrosolv®), were obtained from Merck (Darmstadt, Germany). For the accuracy tests, the sandy soil certified reference material (CRM-SA-C) used during analysis was supplied by High-Purity Standard Inc. (Charleston, USA).
Instrumentations
A Perkin Elmer model A Analyst 400 flame atomic absorption spectrometer (Norwalk, CT, USA) was equipped with a deuterium lamp for background correction. An air/acetylene burner and 10 cm of burner head were used for the determination of gold absorbance. A gold hollow-cathode lamp operated at 12 mA was used as a radiation source. Absorbance measurements were recorded at the peak height mode of 242.80 nm using a spectral bandwidth of 0.2 nm. Spectrophotometric measurements of the ligand were performed on a Specord 210 Plus model spectrophotometer (Jena, Germany) using 1.00-cm quartz microcells. The CEM Mars 6 One Technology (Matthews, USA) microwave system, an equipped with a closed-vessel, was utilized for digesting the solid samples. The program parameters were optimized at a maximum pressure of 880 psi and temperature of 200 °C at 400 W. The pH values of the aqueous solution were measured with a pH meter the Hanna pH 211, (HANNA instruments, Cluj, Romania) model digital glass electrode. A centrifuge Model Sigma 3-16P (Sigma laborzentrifugen GmbH, Germany) was employed to assist phase separation. Pure laboratory water, generated by the Direct-Q 8UV system (Merck Millipore, Germany), was used during the experimental studies.
UV–visible study of the metal–ligand complex
The presence of 10 equivalents of Au3+ ions produces modest changes in the absorption of the ligand (Fig. 2). The absorbance spectra of NNPTSC exhibit distinct changes between 300 and 450 nm in response to treatment with gold ion in methanol. The spectral behavior of NNPTSC changed with the addition of Au(III) ions into a solution of NNPTSC (2.0 × 10−5 mol L−1), and the absorbance of peak at 292 nm was gradually increased. The stoichiometry of the complex was assigned using Job’s method [19]. The solutions were prepared by mixing an equimolar concentration (2.0 × 10−5 mol L−1) of both receptor NPPTSC and H(AuCl4) in a binary solvent, i.e., methanol–ultra pure water with ratios varying from 1:9 to 9:1 and a dilution of up to 4.0 mL. Finally, the absorbance was measured at 292 nm. As we can see from Fig. 3, the breakpoint is 4.0 in the molar ratio plot for this complex, indicating the formation of the 3:2 (metal–ligand) complex in the solution between Au3+ and the ligand.
Dispersive liquid–liquid microextraction procedure
As a model solution, 0.2 mol L−1 nitric acid spiked to 25 mL solution including 50 µg L−1 of Au(III). For the DLLME, the model solution was put into a 50-mL plastic centrifuge tube. A mixture of 600 µL of ethanol and 80 µL of carbon tetrachloride and 100 µL 0.025 [% (w/v)] NPPTCS was swiftly injected into the extraction solution using a plastic syringe equipped with a stainless steel needle. A cloudy solution (including ethanol, water, carbon tetrachloride) was occured in the bottom the plastic test tube. In this step, the hydrophobic complex of Au with NPPTSC was extracted into the CCl4 droplets. The solution was centrifuged for 3 min at 2000 rpm. After the decantation of the aqueous phase, the organic phase was completed to 500 µL with 0.1 mol L−1 nitric acid (containing ethanol) and then injected into the FAAS. The gold signal was recorded in the peak area mode utilizing the instrument software. The calibration was carried out on various aqueous standards and subjected to the same DLLME procedure. The blank determinations were submitted as parallel to the measurements designed both for calibration and the sample solutions.
Preparation of samples
We selected two water samples, including stream (Maçka stream, Trabzon, Turkey) and sea water (Karadeniz Technical University, Trabzon, Turkey). The water samples were acidified using 0.2 mol L−1 of HNO3 and filtered through 0.2 mm of cellulose nitrate. The presented method was applied for the determination of gold content.
All solid samples were digested using a microwave digestion system. 0.10 g of ore-1 (Akoluk, Ordu, Turkey), 0.10 g of ore-2 (Tüprak, Uşak, Turkey), 0.10 g of ore-3 (Gümüşhane, Trabzon, Turkey), and 0.10 g of the certified reference material (CRM sandy soil C) were weighed and dissolved into a mixture of HNO3 and HCl (4:4 volume ratio) in a polytetrafluoroethylene vessel, and digested under pressure at a temperature of 200 °C for 1 h. After the digestion process, all of the aqueous solutions were boiled down to near dryness. The residuals were filtered through blue-band filter paper (Whatman® Grade). Because of the matrix ions, the solutions were diluted twofold for ore-1, tenfold for ore-2, twofold for ore-3, and twofold for the standard reference material before their determination. The diluted samples were analyzed according to the given procedure. A final measurement (500 µL) was performed using FAAS in order to determine the Au ions.
Results and discussion
Microextraction efficiency highly depends on thorough optimization of various parameters affecting the complexation reaction and the system. In this work, 4-ethyl-1-(2-(4-(4 nitrophenyl)piperazin-1-yl)acetyl)thiosemicarbazide was used first time as a chelating agent, and the effect of various analytical parameters on the extraction was studied, and the method was applied to determine the amount of gold in real ore and water samples.
Effect of nitric acid concentration
Acid concentration plays a distinctive role in metal–chelate formation and its subsequent extraction throughout most of the analytical processes. The extraction of metal ions occurs after the formation of a complex with enough hydrophobicity. In order to obtain the best analytical signal for gold, the effect of HNO3 was investigated. As shown in Fig. 4, recovery is nearly constant within the range of 0.1–1.0 mol L−1 of HNO3. The recovery percentage of Au(III) decreased with an increase in the concentration of nitric acid at the 3.0 and 5.0 mol L−1, and this may be due to decomposition of metal–ligand bond. Finally, the extraction was carried out using a sample solution adjusted to 0.2 mol L−1 of HNO3.
Effect of the concentration of HNO3 from the sample solution on the recovery of gold ions. Conditions: sample volume, 25 mL; Au concentration, 50 µg L−1; volume and type of extraction solvent, 80 µL of CCl4; volume and type of dispersive solvent, 600 µL of ethanol; centrifugation time and rate, 3 min and 2000 rpm (n) = 3
Effect of amount of the NPPTSC
The amount of the NNPTSC is the most important variable influencing the formation of AuCl4–NPPTSC complex and its extraction efficiency. The effect of the ligand on the complex formation of 50 µg L−1 gold in the model solution was studied in a range of 0.0–150.0 µL [0.025% (w/v)] in Fig. 5. The extraction efficiency was stable when the NPPTSC concentration was higher than 100 µL. Therefore, a 100 µL volume of NNPTSC was chosen for the extraction of Au ions.
Selection of type and volume of extraction solvent
The selection of an appropriate solvent as a key parameter is very important to obtain high recovery for the analyte ion in the DLLME process. The extraction phase should have a density than water, water insolubility and showing appropriate extraction efficiency of the target analyte. Thus, the solvents such as chloroform (CHCl3) carbon tetrachloride (CCl4), carbondisulfide (CS2), and dichloromethane (CH2Cl2) were tested for the extraction of gold–NPPTSC complex in aqueous media. When CH2Cl2 was used as an extraction solvent, an unstable cloudy solution was obtained which made it difficult to separate the sedimented phase from the solution. In the case of CHCl3 and CS2, the recoveries were about 38% and 51%, respectively. In the case of CCl4, the extraction efficiency was about 98%. Thus, in this work, CCl4 selected as the extraction solvent.
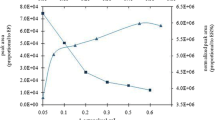
In order to obtain the highest extraction efficiency, 600 µL of ethanol and different volumes of CCl4, such as 20, 40, 60, 80, 100, and 120 µL, were used for the all experiments. By increasing the volume of CCl4 from 20 to 80 µL, the analytical signal increased steadily to 120 µL. In subsequent studies, 80 µL CCl4 was selected as the optimal volume of the extraction solvent (Fig. 6).
Selection of type and amount of dispersive solvent
The main restraint for selection of the dispersive solvent is miscible with both water solution and the extraction solvent, thus enabling the extraction solvent to be dispersed as fine particles in aqueous phase to form a cloudy solution [20, 21]. In this study, the dispersive solvents; methanol (97.6% ± 2.2), ethanol (98.6% ± 2.4), acetonitrile (55.4% ± 6.2), acetone (74.9% ± 5.9), and tetrahydrofuran (63.2% ± 7.4) were tested in this extraction procedure. The results indicate that there was no significant statistical difference using ethanol or methanol as a dispersive solvent. Ethanol was chosen as a dispersive solvent due to its lower toxicity and ease of handling.
The volume of dispersive solvent directly affects the formation of the cloudy solution. Using low volume of dispersive solvent could not disperse the extraction solvent completely, and thus cloudy solution cannot be formed properly. Conversely, at high volumes, the solubility of analytes in water increases by increasing the volume of dispersive solvent and therefore the extraction efficiency is decreased. After selecting C2H5OH as the dispersive solvent, its volume was optimized in the range of 200–3000 µL. Six hundred microliters of C2H5OH was used as an extracting solvent for the extraction of Au(III) ions in the present procedure. At a low volume, C2H5OH could not disperse CCl4 properly, and thus the cloudy solution was not completely formed. Conversely, the solubility of the ion pair (Au3+–NPPTSC) in water increased when the volume of ethanol increased. In fact, results showed that the extraction recovery increased when the volume of C2H5OH increased to 600 and 800 µL and that it decreased when the volume of C2H5OH exceeded 1000 µL. Hence, 600 µL C2H5OH was chosen as the optimum volume of dispersive solvent (Fig. 7).
Effect of the extraction time
The results obtained from many investigations show that extraction is accomplished in a very short period of time after the formation of that cloudy solution, because the finely dispersed drops of the extraction solvent provide a large surface area between the extraction solvent and the aqueous sample [22, 23]. The effect of extraction time was examined within 5–20 min under constant experimental conditions. In our research, we defined the extraction time as the time between injecting the extraction and centrifugation mixture, which is approximately 3 min.
Effect of sample volume
To obtain a high preconcentration factor, the sample volume is an important key. The effect of sample volume on the recovery of Au(III) ions was investigated in the sample volume range of 15–50 mL containing 1.0 µg Au(III). The recovery values of the analyte ions decreased when the volume of the sample solution increased. The experimental conditions were fixed and included the use of different volumes of sample: 15 mL (96 ± 2.4%), 20 mL (97 ± 2.7%), 25 mL (96 ± 2.7%), 30 mL (85 ± 2.8%), 40 mL (81 ± 2.3%), 50 mL (71 ± 4.0%) containing 100 µL [0.025 (w/v)] of NPPTSC. The recovery values were not quantitative above 30 mL of the sample volume. Thus, the optimum sample volume was determined as 25 mL for the quantitative determination of Au(III) at 0.2 mol L−1 HNO3. The enrichment factor of the analyzed ions obtained was 50, because of the final volume of 0.5 mL.
Influences of foreign ions
In order to indicate the selectivity of the DLLME method for the separation of gold, the effect of common coexistences in the water and ore samples was investigated under optimal conditions. The tolerable limit was defined as the highest amount of foreign ions that produced an error not exceeding 5% in the absorbance of the sample. Additionally, Pt2+ and Pd2+ ions were selected as competitive species with gold ions. The results showed that the presence of large amounts of cations and anions commonly present in aqueous samples have no obvious effect on the DLLME when used to determine gold. Furthermore, the vast majority of transition metals even platinum and palladium do not have any interference, and the selectivity of this method was satisfactory toward gold extraction at 0.2 mol L−1 HNO3 (Table 1).
Figures of merit
Linearity was obtained with gold concentration in the range of 10.0–400.0 µg L−1. For 25 mL of solution, the linear regression equation was A = 0.0003 C + 0.005 (where A is absorbance and C is the concentration (µg L−1) of gold in the final solution with a correlation coefficient of 0.998 (R2). The linear calibration equation without preconcentration was A = 0.014 C + 0.005 for Au (R2 = 0.99). The limit of detection and quantification based on 3Sb/m and 10Sb/m, (where Sb is standard deviation of the blank signals and m is the slope of calibration curve after extraction) was 1.5 and 4.2 µg L−1 for Au, respectively. Blank determinations were obtained as parallel to the measurements made for the calibration standards and sample solutions. The blank signals were 0.0019 ± 0.0002 absorbance for gold with FAAS. The method precision was studied by processing the ten replicate standard solutions of gold. The relative standard deviation (RSD) for 50 μg L−1 of gold was 2.1%. The preconcentration factor (EF) [24, 25] is defined as the ratio of the analyte concentration in the organic phase to the initial concentration in the aqueous phase as shown in equation:
where Csed and C0 are the analyte concentration in organic phase, obtained from a suitable calibration graph and the initial concentration in aqueous phase, respectively. The extraction recovery (R%) can be calculated as follows:
where Vsed and Vaq are the volumes of sedimented phase and sample solution, respectively. The enhancement factor defined based on the slope ratio of two calibration curves for gold ions with and without preconcentration was 46.6.
Comparison with other extraction procedures
A comparison of the presented method with others reported in preconcentration and extraction method for gold determination is given in Table 2. The DLLME-FAAS method has numerous advantages including simplicity, rapidness, short extraction time (not more than 5 min), high sensitively, low cost, high enrichment factor (50), low limit of detection (1.5 µg L−1), and consumption of small volumes of organic solvent (80 µL) in a green approach. In this work, the extraction capabilities of the suggested method was checked using appropriate concentration of manganese, nickel, cadmium, cobalt, copper, lead, zinc, iron, platinum, and palladium. Among all these metal ions, the method acted signally selectivity toward gold ions at high acid levels. As a result, the developed DLLME procedure using FAAS can be used as an alternative method for several environmental liquid and solid samples.
Applications of the method
The developed method was applied to sea and stream water samples as well as ore samples. The precision tests of the recommended procedure were performed with standard additions. The different amounts of gold ions were added to 25 mL of sea and stream water as well as solid samples. DLLME was then applied to the solutions. The results are provided in Table 3. There is good agreement between added and found levels of analytes for all samples. The accuracy of presented method was determined by analyzing the CRM-SA-C sandy soil C reference material. The analytical results for the tested ion are given in Table 4. Thus, the amount of gold found in the CRM using the present method was consistent with the certified values (25 µg g−1). The analytical results demonstrated that the suggested method can be reliably used for the determination of gold in the liquid and solid samples (Table 5).
Conclusion
In this work, a sensitive DLLME method combined with FAAS has been recommended for the determination of Au(III) in water and ore samples. The 4-ethyl-1-(2-(4-(4 nitrophenyl)piperazin-1-yl)acetyl)thiosemicarbazide reagent was showed excellent performance as an extractant Au(III) through DLLME when using ethanol as a dispersive solvent and carbon tetrachloride as an extraction solvent. This method (1) offers a simple, easy-to-use, and rapid alternative to current conventional sample preconcentration techniques, (2) decreases both the consumption of toxic organic solvents and the amount of secondary waste, and (3) is free from matrix interferences. Furthermore, the presented DLLME method allows for effective separation and preconcentration of gold by FAAS.
References
I.H. El-Sayed, X. Huang, M.A. El-Sayed, Nano Lett. 5, 829 (2005)
H. Ebrahimzadeh, E. Moazzen, M.M. Amini, O. Sadeghi, Chem. Eng. J. 215–216, 315 (2013)
C. Özdemir, Ş. Saçmacı, Ş. Kartal, M. Saçmacı, J. Ind. Eng. Chem. 20, 4059 (2014)
H. Fazelirad, M.A. Taher, Talanta 103, 375 (2013)
S. Tajik, M.A. Taher, Microchim. Acta 173, 249 (2011)
R. Juvonen, T. Lakomaa, L. Soikkeli, Talanta 58, 595 (2002)
J.J. Ma, X. Du, J.W. Zhang, J.C. Li, L.Z. Wang, Talanta 80, 980 (2009)
S. Chen, X. Zhu, Miner. Eng. 23, 1152 (2010)
M. Soylak, S. Saracoglu, U. Divrikli, L. Elci, Talanta 66, 1098 (2005)
P. Pohl, B. Prusisz, Microchim. Acta 150, 159 (2005)
M.S. El-Shahawi, A.S. Bashammakh, S.O. Bahaffi, Talanta 72, 1494 (2007)
A. Iraji, D. Afzali, A. Mostafavi, Int. J. Environ. Anal. Chem. 93, 315 (2013)
M. Rezaee, Y. Assadi, M.R. Milani Hosseini, E. Aghaee, F. Ahmadi, S. Berijani, J. Chromatogr. A 1116, 1 (2006)
S. Kagaya, D. Takata, T. Yoshimori, T. Kanbara, K. Tohda, Talanta 80, 1364 (2010)
L. Kocúrová, I.S. Balogh, V. Andruch, Microchem. J. 110, 599 (2013)
A. Zgoła-Grzeskowiak, T. Grzeskowiak, Trends Analyt. Chem. 30, 1382 (2011)
H. Yan, H. Wang, J. Chromatogr. A 1295, 1 (2013)
M. Mentese, N. Demirbas, A. Mermer, S. Demirci, A. Demirbas, F.A. Ayaz, Lett. Drug. Des. Discov. (2018). https://doi.org/10.2174/1570180814666170823163540
M.A. Qazi, Ü. Ocak, M. Ocak, S. Memon, Anal. Chim. Acta 761, 157 (2013)
L. Kocúrová, I.S. Balogh, J. Šandrejová, V. Andruch, Microchem. J. 102, 11 (2012)
P.-P. Zhang, Z.-G. Shi, Q.-W. Yu, Y.-Q. Feng, Talanta 83, 1711 (2011)
D. Nagaraju, S.D. Huang, J. Chromatogr. A 1161, 89 (2007)
H.M. Al-Saidi, Adel A.A. Emara, J. Saudi Chem. Soc. 18, 745 (2014)
E. Kazemi, S. Dadfarnia, A.M.H. Shabani, Talanta 141, 273 (2015)
S. Li, S. Cai, W. Hu, H. Chen, H. Liu, Spectrochim. Acta, Part B 64, 666 (2009)
A.S. Amin, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 77, 1054 (2010)
M. Karimi, V. Amani, F. Aboufazeli, H.R.L.Z. Zhad, O. Sadeghi, E. Najafı, J. Chem. (2012). https://doi.org/10.1155/2013/142845
S. Tong, Q. Jia, N. Song, W. Zhou, T. Duan, C. Bao, Microchim. Acta 172, 95 (2011)
D.G. Themelis, A.V. Trellopoulos, P.D. Tzanavaras, M. Sofoniou, Talanta 72, 277 (2007)
Chujie Zeng, Lifu Tang, Anal. Lett. 46, 1442 (2013)
M. Tuzen, K.O. Saygi, M. Soylak, J. Hazard. Mater. 156, 591 (2008)
E. Yilmaz, M. Soylak, RSC Adv. 4, 47396 (2014)
A. Hol, A.A. Kartal, A. Akdogan, A. Elçi, T. Arslan, L. Elci, Acta Chim. Slov. 62, 196 (2015)
Acknowledgements
The financial support of the unit of the Scientific Research Projects of Giresun University (Project No.: 101016-131) (Giresun Turkey) is gratefully acknowledged and also gratitude to Karadeniz Technical University (Trabzon, Turkey) for laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bahadır, Z., Bulut, V.N., Mermer, A. et al. The separation–preconcentration and determination of ultra-trace gold in water and solid samples by dispersive liquid–liquid microextraction using 4-ethyl-1(2-(4-(4-nitrophenyl)piperazin-1-yl)acetyl)thiosemicarbazide) as chelating agent and flame atomic absorption spectrometry. J IRAN CHEM SOC 15, 1347–1354 (2018). https://doi.org/10.1007/s13738-018-1333-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1333-z