Abstract
A simple, rapid, selective in situ ionic liquid dispersive liquid–liquid microextraction and determination of Au(III) by flame atomic absorption spectrometry has been first proposed for the extraction of Au(III). In the developed method, NH4PF6 aqueous solution was added to the sample solution containing [CnMIM]Br to form a water-immiscible ionic liquid, [CnMIM]PF6 as extraction solution. The formation of hydrophobic ionic liquid and subsequent extraction of gold(III) were achieved in one step without the need for dispersive solvent and chelating agent. The effects of various experimental parameters on gold extraction were investigated in detail. At optimum conditions, the linear range of 0.9–400 ng/mL of Au(III), the limit of detection 0.13 ng/mL for Au(III) along with enrichment factors of 23.7 and the extraction capacity of 97.5 mg/g by the proposed method for Au(III) were obtained. The proposed method was applied to extract trace amounts of Au(III) in water samples with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an important precious metal, gold is widely used in industry and economics due to its unique physical and chemical properties [1, 2]. The concentration of gold in environmental, geological and metallurgical materials is usually too low to be determined directly by flame atomic absorption spectrometry (FAAS) owing to insufficient sensitivity and matrix interferences [3, 4]. Hence, preconcentration technique has been an important aspect in the determination of gold besides the effect of other trace metal ions in complex matrices [5–7].
Many preconcentration methods have been developed for the determination of gold. Liquid–liquid extraction (LLE) and solid-phase extraction (SPE) have been the most classic and common alternative methods [8–10]. However, the extraction or elution procedure often requires a large amount of toxic organic solvent, which is hazardous to operators and the environment.
Recently, several microextraction (ME) approaches, aimed at miniaturizing the procedures and consuming less solvent and less time, etc., have been established for the analysis of gold in environmental samples. For example, different types of solid-phase microextraction (SPME) and liquid-phase microextraction (LPME) have been developed as alternative techniques to the classical LLE and SPE [8, 11].
Compared with other ME, LPME is better due to its advantages: fast and simple operation, low cost, low consumption of sample and solvent, and swiftness. Some novel sample preconcentration techniques have been developed based on LPME, including hollow-fiber LPME (HF-LPME) [12], single-drop microextraction (SDME) [13] and dispersive liquid–liquid microextraction (DLLME) [14, 15].
When compared with the SDME and HF-LPME, DLLME exhibits a more wide application area and a relatively high extraction efficiency, improved stability and possibly enhanced sensitivity and simplification of the extraction procedure. The DLLME technique streamlines the sample preparation step and can be used for isolation of both metal and organic analytes [16].
Room temperature ionic liquids (RTILs) are being recently considered as replacement solvents in sample preparation, because of their negligible vapor pressure, chemical and thermal stability, and good solubility in both organic and inorganic molecules. There has been enormous interest in the development of analytical methods that exploit the unique physicochemical properties of ILs. Several pretreatments combined with ME technique have been introduced and developed in recent years to exploit alternative solvent systems containing ILs, such as ionic liquid (IL)-based headspace microextraction [17] and IL-based hollow-fiber LPME (IL HF-LPME) [18, 19]. The DLLME technique based on ILs has become increasingly popular as a successful model of LPME. This technique has been applied for the trace analysis of metal ions, organic compound and other analytes. However, organic dispersive solvent is required, not only in typical DLLME but also in modified DLLME, to assist in the extraction of solvents (ILs) to form fine droplets within an aqueous solution.
In the present study, in situ metathesis reaction combined with DILLME in conjunction with FAAS was introduced and applied to determine Au(III) in water samples. In the extraction procedure, a hydrophilic IL (+, [CnMIM]Br) and an ion-exchange reagent(NH4PF6) were added to the aqueous solution in sequence and a hydrophobic IL (l-alkyl-3-methylimidazolium hexafluorophosphate, [CnMIM]PF6) was formed as extraction solvent (Scheme 1). Infinite amounts of fine droplets guarantee the huge contact area between Au(III) and extractant. Au(III) was transferred easily into [CnMIM]PF6 without adding chelating agent and sedimented in the [CnMIM]PF6 droplet after centrifugation. Furthermore, experimental factors, such as type of [CnMIM]Br, vortex time and centrifugation times, the quantity of [CnMIM]Br and NH4PF6, volume of the sample solution, etc., were assessed and optimized.
Experimental
Materials and chemicals
An atomic absorption spectrometer model TAS-990(Beijing Purkinje General Instrument Co., Ltd, China) was used for the determination of gold in air–acetylene flame. The instrumental settings of the spectrometer were as follows: wavelength, 242.8 nm; slit width, 1.0 nm; lamp current, 4 mA; acetylene flow, 1.5 L min−1; and air flow, 3.5 L min−1 All pH values were measured by a PHS-25B pH-meter (Shanghai Precision and Scientific Instrument Co., Ltd., Shanghai, China). Shaking for all extractions was performed using an XK80-A vortex mixer from Jiangsu Xinkang Medical Apparatus (Jiangyan, china), and the phase separation of the sample solution was assisted with centrifuging (Model TDL80-2B, Shanghai Anting Science Instrument Factory, Shanghai, China).
The standard solution of Au(III) (1.0 mg/mL) (GSB 04-1715-2004) and the standard reference materials (GBW(E)080175, analysis gold of standard reference materials in water) were purchased from Beijing standard material net (Beijing, China, http://www.biogbw.com/Default.htm). Working solution was diluted with doubly distilled deionized water prior to use. 10.0 mg/mL solutions of other metal ions (Cu2+, Ni2+, Co2+, Zn2+, Mn2+, Ca2+, Al3+, Pb2+, Fe3+) were obtained by dissolving the corresponding metal salt in double-distilled water. Clark–Lubs buffer solution with pH 1.0 to 1.8 and Britton–Robinson buffer solutions with pH 1.8 to 12.0 were prepared according to the literature [20].
All materials were of analytical grade and spectral purity for all experiments. The doubly distilled deionized water was used throughout.
ILs of [CnMIM]Br (n = 4, 6, 8,10) were synthesized as described in the literature [14]. The main structure information of these ionic liquids was confirmed by FT-IR (KBr pellet) at room temperature. As seen from Fig. 1, the characteristic absorption peaks of RTILs are at 3,170, 3,125, 2,930, 2,858, 1,573, 1,466, 1,169, 840 and 742 cm−1. These results were in good agreement with the publications [14]. 100 mg/mL [CnMIM]Br aqueous solution of these ionic liquids was prepared before use.
In situ IL-DLLME procedure
A screw cap glass test tube with conical bottom was filled with 40 mL working standard solution containing 3.6 μg of Au(III) (or sample solution). Then, 5.0 mL Britton–Robinson buffer solutions of pH 1.8 and 1.20 mL 100 mg/mL [CnMIM]Br were added to the solution and completely dissolved into the solution after gently shaking by hand for 30 s. A cloudy solution was formed as soon as 4.00 mL 40 g/L NH4PF6 was added. Then the suspension was kept in a vortex for 4 min and subsequently centrifuged at 3,000 rpm for 4 min to accomplish separation of the aqueous phase and the IL phase. The supernatant aqueous phase was then decanted completely by a syringe centered at the tube, and the IL phase settled at the bottom of the tube was about 420 μL (Fig. 2).
The extraction efficiency of gold
The concentration of Au(III) in the aqueous phase could be determined by FAAS against the same concentration of gold in the extraction system, but without [CnMIN]Br added. The extraction efficiency of Au(III) was therefore calculated with Eq. (A.1),
where c aq(f)Au , A aq(f)Au and c aq(i)Au A aq(i)Au are the final (equilibrium) concentration and absorbance of Au(III) in the aqueous phase and the initial concentration of Au(III) in the aqueous phase, respectively.
The determination of gold in the IL phase
After in situ DLLME extraction, the IL phase was diluted to 2.0 mL with methanol containing 0.2 mL of 1.0 mol/L HCl solution. Then this solution was directly aspirated into the FAAS and the absorbance was calculated as concentration of gold from the working curve. All extraction experiments were performed in triplicate.
Results and discussion
The effect of alkyl chain lengths
The characteristics of ILs, such as solubility in water, viscosity and extraction capacity, play a key role in influencing recovery and enrichment factor [16]. A water-immiscible IL with very low solubility must be formed after the in situ IL-based DLLME procedure. Four kinds of ILs with different alkyl chain lengths, including [C4MIM]Br, [C6MIM]Br, [C8MIM]Br and [C10MIM]Br, were selected in this study. The extraction efficiency of gold affected by the alkyl chain lengths of hydrophobic IL of [CnMIM]PF6 (n = 4,6,8,10), which was produced from the anion-exchange reaction between [CnMIM]Br as the initial hydrophilic IL and NH4PF6 as anion exchange, was investigated. As seen in Fig. 3, the results indicated that the extraction efficiency obtained with [C10MIM]Br was higher than those obtained with [C4MIM]Br [C6MIM]Br and [C8MIM]Br. It was also found that [C4MIM][PF6] was almost entirely dissolved in the sample solution at the same experimental condition. The reason could be that the water solubility of these hydrophobic IL [CnMIM][PF6] was increased with the decreasing carbon chain length of alkyl group in the imidazolium cation [21, 22]. Therefore, [C10MIM][PF6] was selected as the extraction solvent for the following study.
The effect of acidity
Previous research has indicated that acidity has a great important effect on the speciation of gold [23]. [AuCl4]− is stable in acid solution, readily hydrolyzed and exists as an equilibrium mixture which contains Au(OH) −4 , AuCl(OH) −3 , AuCl2(OH) −2 and AuCl3OH− if the solution is neutral or alkaline [24].
AuCl4 − + H2O → AuCl3OH− + H+ +Cl− K1 = 10−6.07
AuCl3OH− + H2O → AuCl2(OH) −2 + H+ +Cl− K2 = 10−7.00
AuCl2(OH) −2 + H2O → AuCl(OH) −3 + H+ +Cl− K3 = 10−8.06
AuCl(OH) −3 + H2O → Au(OH) −4 + H+ +Cl− K4 = 10−8.51.
To evaluate the effect of pH on the extraction of Au(III), the solutions were adjusted to a range of different acidities with the corresponding HCl solution [25], Clark–Lubs buffer solution (pH 1.0–1.8) and Britton–Robinson buffer solution (pH 1.8–12.0). The results indicated that Au(III) could be effectively extracted (E % > 95 %) by [C10MIM][PF6] when the pH was less than 4.0. The extraction efficiencies of gold decreased with the increase of pH, which declined to zero in alkaline solution (Fig. 4). Clark–Lubs buffer and Britton–Robinson buffer could both offer high extraction efficiency when the pH was set at 1.8. Under the experimental conditions, PF6 − ion remains stable and is not readily hydrolyzed to release HF [26, 27]. Therefore, Britton–Robinson buffer solution of pH 1.8 was determined for further studies.
The effect of volume of [C10MIM]Br
The formation of the insoluble IL [C10MIM]-PF6 by metathesis, which helped in the dispersion of fine droplets generating enhanced extraction efficiencies, was mainly affected by the mixing molar proportions of the soluble IL [C10MIM]Br and the counterion salt (NH4PF6). Variable amounts of [C10MIM]Br (from 0.10 to 1.40 mL) with a fixed amount of 4.0 mL 40 g/L NH4PF6 were investigated to ensure a different molar ratio. Experiments were carried out three times at each [C10MIM]Br volume. Figure 5 showed that the extraction efficiency of Au(III) increased when the volume of [C10MIM]Br increased from 0.10 to 1.0 mL and remained almost constant at the other volume levels. The reason was that the volume of [C10MIM]PF6 drop formed after shaking was increased with increasing amount of [C10MIM]Br. To obtain lower volumes of the final drop for the higher preconcentration and quantitative extraction, a [C10MIM]Br volume of 1.20 mL was chosen in further experiments.
The effect of volume of NH4PF6
In the in situ IL-DLLME method, salt is added to promote a metathesis reaction in which the counterion of the salt substitutes the counterion of the IL. This is accomplished ensuring that the formed IL, containing a different counterion after reaction, is water insoluble. Different concentrations/volumes of NH4PF6 on the extraction of gold was studied. For a selected value of 1.20 mL of [C10MIM]Br, the effect was investigated by varying the molar ratio of NH4PF6(40 g/L) to [C10MIM]Br from 0.2:1 to 3.7:1. As shown in Fig. 6, the extraction efficiency increased and reached a constant level (95 %) when excess NH4PF6 of 3.50 mL was added for the extraction (mole ratio 1.9:1). It was also found that the volume of [C10MIM][PF6] remains constant (about 420 μL) when excessive NH4PF6 was added (mole ratio > 2:1). Meanwhile the water solubility of [C10MIM][PF6] could be decreased with excessive NH4PF6 added. Based on these results, 4.0 mL NH4PF6 was chosen for the subsequent investigations and the molar ratio was fixed to 3:1.
Effect of the vortex time and the centrifugation time
The in situ IL-DLLME method is based on utilizing a hydrophilic IL as extractant solvent of the analytes contained in the aqueous solution. The hydrophilic IL is transformed into a hydrophobic IL through a metathesis reaction. This method generally requires the utilization of vortex or shaking to improve the kinetics of the metathesis reaction. In this study, vortex time and centrifugation time were evaluated to obtain higher extraction performance. The vortex time was studied from 1 to 10 min for the extraction of 3.6 μg Au(III). It was observed that short vortex times (lower than 2 min) generated a turbid solution, and the further final drop was not easy to handle, even after centrifugation times of 15 min. Hence, a vortex time of 4 min was selected. Centrifugation times were then studied between 1 and 15 min, and 4 min was selected as the lower centrifugation time which ensured a reproducible drop to handle. The overall sample treatment time, under optimized conditions, was roughly 9 min.
Effect of sample volume
An increase in the ratio of the volume of the aqueous phase to the organic phase will increase the preconcentration factor, but may reduce the extraction efficiency at a given extraction time [14]. The experiments were performed with the extraction of 100 μg Au(III) in a different sample volume ranging from 5.0 to 50.0 mL. The extraction efficiency of Au(III) was more than 95 % with the sample volume from 5.0 to 40.0 mL, which was decreased sharply after the sample volume was more than 40.0 mL. Thus, the sample volume of 40.0 mL was chosen for the experiments.
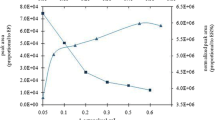
The extraction capacity of Au(III)
The extraction capacity (the maximum extraction amount of analyte on 1.00 g extraction solvent) of [C10MIM]Br with excessive addition of NH4PF6 for Au(III) was studied. As shown in Fig. 7, when the concentration of Au(III) was higher than 250 μg/mL, the extraction recovery of Au(III) arrived at its maximum value, and the extraction capacity was calculated to be 97.5 mg/g.
Effect of coexisting ions
Generally, besides gold, electronic waste water can be defined as a mixture of various metals, particularly copper, cobalt, nickel, zinc, lead and iron. The high concentration of these metal ions could interfere with the extraction of gold, limiting the applicability of the recovery process and analysis of gold. In these experiments, 10 μg Au and various amounts of interfering ions were treated according to the proposed method. The results obtained are given in Table 1. Among the ions examined, most could be tolerated up to milligram levels. Thus, this method is selective and can be used for extraction and determination of gold in the chloride medium and electronic waste aqueous solution [23].
Analytical performance
Calibration curve for the determination of gold was prepared according to the proposed procedure under the optimum conditions. The linearity was maintained in the concentration range of 0.9–400 ng/mL in the original solution. The equation of the line is A = 2.1054c + 0.0034 with the regression coefficient 0.9978 where A is the absorbance and C is concentration of Au(III) (μg/mL). The limit of detection (LOD) of the present work based on three times standard deviations of the blank was 0.13 ng/mL. The enhancement factor, defined as the ratio of slope of preconcentrated samples to that obtained without preconcentration (A = 0.0887c (μg/mL) + 0.0051, linearity range from 0.04 to 8.0 μg/mL, LOD = 0.008 μg/mL), was 23.7.
Real samples’ analysis
To assess the capability of the method for real samples with different matrices containing varying amounts of diverse ions, the method was applied to the extraction and determination of gold in the certified reference material. Au(III) in a certified reference material (GBW(E)080175, certified value: 100 ± 1.4 ppm) was determined; the result was 98.4 ± 1.5 ppm and the recovery was 98.4 %. There was no significant difference between the found value and the certified value for gold by t test.
To evaluate the accuracy of the procedure, recovery experiments were also carried out with spiked water samples and synthetic sample because certified reference materials for gold were not available. The results are given in Table 2. The recovery percentages of gold ions were evaluated and the results showed that the real sample matrixes did not affect the recovery of gold.
To investigate the applicability of the proposed extraction method, the extraction and recovery of Au(III) from electric wastes were carried out. The weight composition of metals for this electronic waste was: 0.16 %Au, 15.54 %Cu, 10.62 % Ni, 2.13 % Co, 1.56 % Mn, 16.15 % Zn, 0.79 % Cr and 7.56 % Fe. The concentration of gold in the aqueous solution before and after the extraction was 55.4 and 1.56 μg/mL, respectively, which means more than 97 % Au(III) was extracted into the RTIL phase. Hence, this study could produce a promising method to recover gold from electronic waste.
Conclusion
The in situ metathesis ionic liquid formation for dispersive liquid–liquid microextraction of gold without chelating agents was demonstrated. This technique can be employed for preconcentration of gold in water samples. Using in situ metathesis ionic liquid formation, the amount of organic (ionic liquid) phase was minimized. The microliter amount of [C10MIM][PF6] was employed as an extraction solvent, which was more environmentally friendly because of its low volatile and nonflammable properties and has the potential to replace traditional VOCs in DLLME procedures for gold preconcentration. The effect of complex matrices presented in the samples was tolerable by the proposed method. A low limit of detection and good precisions were achieved for the analyte. The analytical performance of the method was compared with other methods for the preconcentration and analysis of gold with FAAS reported in literatures (Table 3). The presented method exhibited a suitable preconcentration factor and higher capacities compared with the majority of the reported methods.
References
A.V. Pethkar, K.M. Paknikar, Journal of Biotechnology 63, 121 (1998)
D.F.C. Morris, M.A. Khan, Talanta 15, 1301 (1968)
W.S. El-Naggar, T.A. Lasheen, E.A. Nouh, A.K. Ghonaim, Central European Journal of Chemistry 8, 34 (2010)
S.S. Tong, Q. Jia, N.Z. Song, W.H. Zhou, T.C. Duan, C.L. Bao, Microchimica Acta 172, 95 (2011)
M. Tuzen, K.O. Saygi, M. Solyak, Journal of Hazardous Materials 156, 591 (2008)
F. Sabermahani, M.A. Taher, H. Bahrami, Arabian Journal of Chemistry (2012). doi:10.1016/j.arabjc.2012.04.053
R. Liu, P. Liang, Analytica Chimica Acta 604, 114 (2007)
K.M. Giannoulis, D.L. Giokas, G.Z. Tsogas, A.G. Vlessidis, Talanta 119, 276 (2014)
M. Behbahani, F. Najafi, M.M. Amini, O. Sadeghi, A. Bagheri, P.G. Hassanlou, Journal of Industrial and Engineering Chemistry 20, 2248 (2014)
L. Bulgariu, D. Bulgariu, Seperation and Purification Technology 80, 620 (2011)
M. Shamsipur, M. Ramezani, Talanta 75, 294 (2008)
C.J. Zeng, Y. Lin, N. Zhou, J.T. Zheng, W. Zhang, Journal of Hazardous Material 237, 365 (2012)
L. Vidala, A. Chisvertb, A. Canalsa, A. Salvadorb, Talanta 81, 549 (2010)
S.P. Wen, J. Wu, X.S. Zhu, Journal of Molecular Liquids 180, 59–64 (2013)
S. Kagaya, D. Takata, T. Yoshimori, T. Kanbara, K. Tohda, Talanta 82, 1364 (2010)
A. Zgoła-Grześkowiak, T. Grześkowiak, TrAC. Trends Analytical Chemistry 30, 1382 (2011)
Z. Gao, Y. Deng, X. Hu, S. Yang, C. Sun, H. He, Journal of Chromatography A 1300, 141 (2013)
D. Ge, H.K. Lee, Journal of Chromatography A 1229, 1 (2012)
A. Spietelun, L. Marcinkowski, M. de la Guardia, J. Namieśnik, Talanta 119, 34 (2014)
Hangzhou University, Analytical chemistry handbook: basic knowledge and safety knowledge (Chemical Industry Press, Beijing, 2003)
M. Rezaee, Y. Yamini, M. Faraji, Journal of Chromatography A 1217, 2342 (2010)
M.G. Freire, C.M.S.S. Neves, P.J. Carvalho, R.L. Gardas, A.M. Fernandes, I.M. Marrucho, L.M.N.B.F. Santos, J.A.P. Coutinho, Journal of Physical Chemistry B 111, 13082 (2007)
O. Sha, X.S. Zhu, Analytical Letters 47, 1052 (2014)
N. Bjerrum, Bulletin des Societes Chimiques Belges 57, 432 (1948)
E. Baumgartner, M. Busch, R. Fernández-Prini, Journal of Physical Chemistry 74, 1821 (1970)
D.W. Davidson, S.K. Garg, Canadian Journal of Chemistry 50, 3515 (1972)
M.R. Moghadam, S. Dadfarnia, A.M.H. Shabani, Journal of Hazardous Materials 18, 169 (2011)
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (20875082), a project funded by the Science and Technology project of Lianyungang City (SH1209) and the Fund Project of Science and Technology of Jiangsu Marine Resources Development Research Institute (JSIMR201309).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sha, O., Chen, J., Chen, L. et al. In situ ionic liquid dispersive liquid–liquid microextraction and determination of Au(III) by flame atomic absorption spectrometry. J IRAN CHEM SOC 12, 1391–1398 (2015). https://doi.org/10.1007/s13738-015-0605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0605-0