Abstract
An efficient and facile synthesis of indazolo[2,3-α]quinoline derivatives has been described which occur through Povarov reaction and tandem process of visible light-promoted intramolecular N–N bond formation using choline chloride/CuCl. This protocol is highly efficient and of low cost due to its mild reaction conditions, operational simplicity, applying inexpensive photoredox catalyst and overall good to excellent yields of various derivatives. Scale-up preparation of these heterocycles is also carried out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The indazoles and related derivatives constitute an important class of building block because such moieties are found in a wide range of biologically active compounds. For examples, indazole core structures can be found in commercial drugs with several biological activities including antipyretic [1], analgesic [2], antiviral [3], antiangiogenic [4], anti-inflammatory and anticancer [5,6,7].
In the past few decades, the indazole synthesis has received considerable attention and numerous synthetic strategies have been reported [8,9,10]. Existing synthetic approaches mainly involve cyclization of 2-azidobenzylidene Schiff bases [11, 12], base-promoted cyclizations of arylamino oximes [13, 14], cycloadditions [15,16,17] and coupling of organometallic reagents with aryldiazonium salts [18, 19]. However, the most of these synthesis approaches suffer from some drawbacks such as availability of starting material, using either toxic reagents or harsh reaction conditions and prolonged reaction times.
Because of the biological importance of indazole frameworks and limitations of traditional methods, considerable efforts to find new efficient and facile methodologies to improve their synthesis would be logical. While various synthesis strategies have been reported, to the best of our knowledge, N–N bond formation to preparation indazole derivatives using copper in visible light photoredox catalysis (VLPC) has not been reported previously.
The VLPC is a fundamental and interesting process [20] and have many vast applications in organic synthesis [21]. The most common VLPC catalysts included ruthenium and iridium polypyridyl complexes [22] have been successfully applied in functional group conversions [23, 24], carbon–carbon bond coupling, [2 + 2] cycloaddition [25, 26] and C–H activation reactions [27, 28]. The tradition photochemical catalysts have some limitations such as toxicity and cost. These problems can be largely overcome by carrying out the reactions using other transition metal such as copper catalyst which is non-toxic, inexpensive and available and exhibit high catalytic activity [29,30,31]. Therefore, we have focused our attention on copper which has been successful in a number of photoreactions, generally carried out under visible light irradiation. We were especially intrigued by the report of Reiser et al. that described the visible light photoredox for carbon–carbon bond-forming reactions in the presence of [Cu(dap)2Cl] as an efficient catalyst. Moreover, this copper complex provided a more economically viable synthesis method alternative to the widely used ruthenium and iridium catalysts.
Many different catalytic systems, including copper complex, have been applied for photoredox reactions [32, 33], but the use of effective a facile preparation and inexpensive systems would obviously be much more interesting in view of modern organic synthesis, because of the higher cost of applied ligands and complicated complex synthesis approach.
Ionic liquids (ILs) have been successfully applied as green reaction media, which can attend as a catalyst and/or solvent in organic synthesis [34, 35]. A series of low-cost and moisture-stable Lewis acidic ionic liquids have been synthesized from choline chloride and ZnCl2, FeCl3 and SnCl2 and used in Diels–Alder and Fischer indole reactions [36,37,38].
Therefore, in this work, we report the use of (choline chloride)·CuCl as an efficient catalyst for facile synthesis of a wide range of substituted indazolo[2,3-α]-quinolines via N–N photoredox coupling of intermediate. The intermediate was achieved by the Povarov reaction of aromatic amine, 2-nitrobenzaldehyde and the electron-donating group (EDG)-substituted alkene at room temperature applying Ox-MWCNTs (oxidized multiwalled carbon nanotube).
In recent years, carbon nanotubes have aroused significant interests of scientists all over the world since; it exhibits unique features such as large surface, intrinsic low mass and easy surface modifications which might be promising candidates as catalysts or supports [39, 40]. Modification of its activity, including oxidation, makes a unique and recoverable catalyst. Based on the fascinating structure of CNTs material, as well as recoverability and efficiency, we reason that the Ox-MWCNTs can be used as solid acid catalyst in Povarov reaction.
In continuation of our studies in the application of heterogeneous catalysts in coupling reactions [41,42,43,44,45,46,47,48], we wish to report the use of two highly active catalysts, Ox-MWCNTs and Choline chloride·CuCl, for indazole synthesis through intramolecular N–N photoredox coupling reaction in the tandem process which has remarkable environmental advantages such as reducing the number of synthetic steps and consumption of energy and waste producing [49, 50].
Results and discussion
Choline chloride·CuCl (Scheme 1) was prepared through the reaction of choline chloride with CuCl in a 1:1 ratio. Choline chloride as an OH-functionalized ligand and also as a tetraalkylammonium salt supports and stabilizes Cu(I) species during the reaction. The quaternary salt protects the Cu(I) species against oxidation by air and therefore improves the efficiency and performance of the catalyst. In addition, the quaternary salt prevents aggregation of non-stable Cu(I) species. In the FTIR analysis, the shifting of O–H absorption band of ChCl·CuCl to a higher frequency compared to pure choline chloride was observed (Supplemental data). This shift is evidence of the choline chloride and CuCl interaction and indicates that the hydroxyl groups of choline chloride are the active sites in this system.
The efficiency of this catalytic system was evaluated in N–N bond formation reaction via visible light photoredox process in intermediate (see top reaction in Table 1) to reach the final product. The synthesis of intermediate (2-(2-nitrophenyl)-1,2,3,4-tetrahydroquinolines) was promoted using Ox-MWCNTs as a heterogeneous highly efficient Lewis acid catalyst.

In order to optimize reaction conditions, the reaction of aniline (1 mmol), 2-nitrobenzaldehyde (1 mmol) and 3,4-dihydro-2H-pyran (1 mmol) was selected as a template which was performed at room temperature, under visible light irradiation without protection of inert atmosphere (Table 1).
In general, the experimental procedure for this reaction is remarkably simple and does not require the use of expensive organic reagents.
Initially, the efficiency of both catalysts was evaluated and reactions were not occurred in the absence of each catalyst (Table 1, entries 1–3). It was also established that the irradiation of visible light has a critical role in final step of transformation and the N–N bond was not formed when reaction was performed in dark conditions (Table 1, entry 4). Various catalyst amounts were also tested (Table 1, entry 5). As shown in Table 1, the best result was obtained using 6.0 mg of cat 1 (Ox-MWCNTs) and 10 mol% of cat 2 (ChCl–CuCl). Next, investigations were carried out to define the best solvent for this transformation. Among different solvents such as ethanol, THF, NMP and acetonitrile (MeCN), the last one was shown to be the best solvent.
Encouraged by the efficiency of the reaction protocol described above, applying the optimal reaction conditions consist of optimized catalyst amounts in MeCN under household light irradiation at room temperature, the scope and specificity of this protocol were further investigated and the results are given in Table 2. A total of 12 examples were given with the isolated yields ranging from 42 to 93%. The results showed that electron-donating group (EDG)-substituted alkene with different nature and various substituent anilines containing electron-withdrawing or electron-releasing group afforded the corresponding products in moderate to good yields. It was also observed that the reactions with dihydropyran and dihydrofuran were faster than those of aliphatic aldehyde and styrene. The structures of the products were characterized by IR, 1H and 13C NMR spectroscopy and mass spectrometry, and the characterization data are given in supporting information.

Interestingly, our catalytic system (Ox-MWCNTs and ChCl–CuCl) was compatible with a wide range of functional groups and exhibited good activity in the synthesis of indazolo[2,3-α]-quinoline derivatives. Mild reaction conditions with excellent conversions and simple experimental procedure are noteworthy advantages of this method.
Moreover, the efficiency of the catalyst in scale-up synthesis is key parameters for industrial and commercial uses of any catalytic operations. The scale of the reaction was increased to 10.0 mmol, keeping the reaction stoichiometry intact. The reaction was found to proceed successfully, and the corresponding product was obtained in 69% yield via described method.
The separation and reusability of noble metal catalysts are the trends of the catalysis industry and green chemistry, not only for lowering costs, but also for avoiding pollution. To gain insight into this issue, the catalyst reusability experiments in model reactions were carried out. After first step of reaction, Ox-MWCNTs were separated from the reaction mixture through centrifugation followed by washing with diethyl ether and ethanol to remove the organic impurities. After drying, the catalyst was employed in the next run. This catalyst was successfully recovered and reused for several times without any significant losing of its activity. The table of reusability investigation results is given in supplementary data.
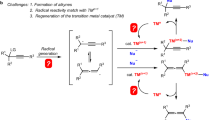
The plausible mechanism for the formation of indazolo[2,3-α]quinolines is explained in Scheme 2.
Probably, single-electron transfer (SET) from intermediate 1 to the visible light-excited Cu species to provide the strong reluctant Cu (0) and radical cation 2, after proton transfer, a radical combination between two nitrogens occurs and N–N bond forms. Finally, a single electron is transferred from Cu (I) to the radical cation 4 as a back electron transfer (BET) and concludes the products. Choline chloride acts an effective ligand and a quaternary ammonium salt, which stabilizes Cu (I) species during the reaction.
Conclusions
In conclusion, we have developed a simple and efficient route for the one-pot, three-component tandem scale-up synthesis of indazolo[2,3-α]quinoline derivatives. In this methodology, choline chloride/CuCl was applied as an inexpensive and green catalyst for visible photo redox N–N bond formation reaction to achieve indazolo[2,3-α]quinoline derivatives as very valuable frameworks in the area of drug design. In this catalyst, choline chloride, as an effective ligand and a quaternary ammonium salt, demonstrated an efficient stabilizing effect on the Cu(I) species during the reaction. The significant features of this method are good yields of products, operational simplicity, economic and environmental advantages such as performance at room temperature conditions and tandem way which diminishes considerably organic solvent usage and omits purification steps of intermediate. Various benefits of presented procedure make it a benign alternative to the existing methods.
References
M.D. Angelis, F. Stossi, K.A.B.S. Carlson, J.A. Katzenellenbogen, J. Med. Chem. 481, 132 (2005)
D. Kim, L. Wang, C.G. Caldwell, P. Chen, P.E. Finke, B. Oates, M. MacCoss, S. Mills, G.L. Malkowitz, S.L. Gould, J.A. DeMartino, M.S. Springer, D. Hazuda, M. Miller, J. Kessler, R. Danzeisen, G. Carver, A. Carella, K. Holmes, J. Lineberger, W.A. Schleif, E.A. Emini, Bioorg. Med. Chem. Lett. 11, 3103 (2001)
L.J. Huang, M.L. Shih, H.S. Chen, S.L. Pan, C.M. Teng, F.Y. Lee, S.C. Kuo, Bioorg. Med. Chem. 14, 528 (2006)
K.A. Abouzid, H.S. El-Abhar, Arch. Pharm. Res. 26, 58 (2003)
S. Brase, C. Gil, K. Knepper, Bioorg. Med. Chem. 10, 2415 (2002)
P.A. Procopiou, A.H. Miah, M.L. Morriss, D. Needham, E.B. Sheriff, R.J. Slack, C.E. Smith, J.W. Barrett, N.P. Barton, M. Begg, D.R. Clapham, C.B. Copley, A.J. Ford, R.H. Graves, D.A. Hall, A.P. Hancock, A.P. Hill, H. Hobbs, S.T. Hodgson, C.Y. Jumeaux, M.L. Lacroix, S.L. Staton, J. Med. Chem. 56, 1946 (2013)
H. Wang, H. Han, D.D. Von Hoff, Cancer Res. 66, 9722 (2006)
T. Zhang, W. Bao, J. Org. Chem. 78, 1317 (2013)
N.J. Howe, K. Blades, G.M. Lamont, Synlett 26, 228 (2015)
F.E. Marandi, M. Saeedi, M. Mahdavi, I. Yavari, A. Foroumadi, A. Shafiee, Synlett 25, 2605 (2014)
J. Hu, Y. Cheng, Y. Yang, Y. Rao, Chem. Commun. 47, 10133 (2011)
W.E. Conrad, K.X. Rodriguez, H.H. Nguyen, J.C. Fettinger, M.J. Haddadin, M. Kurth, J. Org. Lett. 14, 3870 (2012)
C.M. Counceller, C.C. Eichman, B.C. Wray, J.P. Stambuli, Org. Lett. 10, 1021 (2008)
B.C. Wray, J.P. Stambuli, Org. Lett. 12, 4576 (2010)
Z. Liu, F. Shi, P.D. Martinez, C. Raminelli, R.C. Larock, J. Org. Chem. 73, 219 (2008)
C. Spiteri, S. Keeling, J.E. Moses, Org. Lett. 12, 3368 (2010)
Y. Fang, C. Wu, R. Larock, F. Shi, J. Org. Chem. 76, 8840 (2011)
H. Li, P. Li, L. Wang, Org. Lett. 15, 620 (2013)
K. Christopher, D.A. Rankic, D.W.C. MacMillan, Chem. Rev. 113, 5322 (2013)
S. Hirashima, A. Itoh, Green Chem. 99, 318 (2007)
N. Hoffmann, Chem. Rev. 108, 1052 (2008)
K. Kalyanasundaram, Chem. Rev. 46, 159 (1982)
W.C. Lin, D.Y. Yang, Org. Lett. 15, 4862 (2013)
S. Maity, N. Zheng, Synlett 23, 1851 (2012)
S. Cai, S. Zhang, Y. Zhao, D.Z. Wang, Org. Lett. 15, 2660 (2013)
Y. Ye, M.S. Sanford, J. Am. Chem. Soc. 134, 9034 (2012)
H. Jiang, C. Huang, J. Guo, C. Zeng, Y. Zhang, S. Yu, Chem. Eur. J. 18, 15158 (2012)
Y. Cheng, J. Yang, Y. Qu, P. Li, Org. Lett. 14, 98 (2012)
A. Sykora, J. Coordin. Chem. Rev. 159, 95 (1997)
O. Horv, Coord. Chem. Rev. 303, 135 (1994)
N. Armaroli, G. Accorsi, F. Cardinali, A. Listorti, Top. Curr. Chem. 280, 69 (2007)
R. Kore, R. Srivastava, Tetrahedron Lett. 53, 3245 (2012)
A.R. Hajipour, H.S. Nazemzadeh, F. Mohammadsaleh, Tetrahedron Lett. 55, 654 (2014)
A.R. Hajipour, Y. Ghayeb, N. Sheikhan, A.E. Ruoho, Tetrahedron Lett. 50, 5649 (2009)
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V. Tambyrajah, Green Chem. 4, 24 (2002)
R.C. Morales, V. Tambyrajah, P.R. Jenkins, D.L. Davies, A.P. Abbott, Chem. Commun. 158 (2004)
Z. Duan, Y. Gu, Y. Deng, Catal. Commmun. 7, 651 (2006)
R. Rajarao, T.H. Kim, B.R. Bhat, J. Coord. Chem. 65, 2671 (2012)
E.G. Rodrigues, S.A.C. Carabineiro, J.J. Delgado, X. Chen, M.F.R. Pereira, J.J.M. Órfão, J. Catal. 285, 83 (2012)
Q. Sun, N.A. Zorin, D. Chen, M. Chen, T.X. Liu, J. Miyake, D.J. Qian, Langmuir 26, 10259 (2010)
A.R. Hajipour, Z. Khorsandi, H. Farrokhpour, RSC Adv. 6, 59124 (2016)
A.R. Hajipour, Z. Khorsandi, Catal. Commun. 77, 1 (2016)
A.R. Hajipour, N.S. Tadayoni, Z. Khorsandi, Appl. Organomet. Chem. 30, 590 (2016)
A.R. Hajipour, Z. Khorsandi, H. Karimi, Appl. Organomet. Chem. 29, 80 (2015)
A.R. Hajipour, Z. Khorsandi, Appl. Organomet. Chem. 30, 256 (2016)
A.R. Hajipour, F. Rezaei, Z. Khorsandi, Green Chem. 19, 1353 (2017)
A.R. Hajipour, Z. Khorsandi, Chemistryselect 28, 8976 (2017)
A.R. Hajipour, Z. Khorsandi, New J. Chem. 40, 10474 (2017)
A. Schmidt, A. Beutler, B. Snovydovych, Eur. J. Org. Chem. 9, 4073 (2008)
H. Cerecetto, A. Gerpe, M. Gonzalez, V.J. Aran, C.O. Ocariz, Mini Rev. Med. Chem. 5, 869 (2005)
Acknowledgements
Financial support from the Isfahan University of Technology (IUT), Iran, is appreciated. Additional financial support from the Center of Excellence in Sensor and Green Chemistry Research (IUT) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hajipour, A.R., Khorsandi, Z. & Bahri, S. An efficient and inexpensive visible light photoredox copper catalyst for N–N bond-forming reactions: the one-pot synthesis of indazolo[2,3-α]quinolines. J IRAN CHEM SOC 15, 981–986 (2018). https://doi.org/10.1007/s13738-018-1295-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1295-1