Abstract
A series of novel 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamides have been synthesized by the reaction of N-benzyl-2-cyanoacetamide with 2-naphthol and aromatic aldehydes in the presence of piperidine as a base. The chemical structures of all the compounds were confirmed by spectroscopic methods as well as elemental analysis. In addition, the antibacterial activities of some target compounds against Gram-negative and Gram-positive bacteria in vitro were determined. Among these compounds, 4b, 4c, 4h and 4i exhibited antibacterial effects. The advantages of the present work reaction are clean reaction, metal-free transition, environmentally friendly approach to prepare different benzo[f]chromene-2-carboxamide derivatives, good yields, low cost, readily available starting materials and in addition does not need cumbersome procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multi-component reactions (MCRs) have proven to be a unique and versatile tool in organic and medicinal chemistry due to their ability to synthesize diverse chemical libraries of pharmaceutical agents with higher efficiency and atom economy in a single step from three or more different starting materials reacting. Additionally, multi-component protocols have the several advantages such as experimental simplicity and synthetic efficiency over conventional chemical reactions [1]. Therefore, designing of new MCRs for the synthesis of diverse groups of compounds, especially the ones that are biologically active, has gained great attention in green organic synthesis [2,3,4]. Hence, MCRs gained significant popularity as a pivotal theme in the synthesis of many important heterocyclic ‘drug-like’ libraries such as chromene derivatives currently. Chromone (4H-benzopyran-4-one) is a heterocyclic compound found primarily in plants but also found in other sources [5]. Simple chromones and their analogues are a large class of heterocycles that have attracted a great deal of interest for a long time either from a biosynthetic and synthetic point of view or owing to their interesting biological activities.
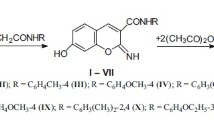
Especially, among various chromene derivatives, 2-amino-4H-chromenes have attracted great interest due to their wide range of biological and pharmaceutical properties such as antibacterial antimicrobial [6], antiviral [7], mutagenicity [8], sex pheromone [9] and antitumor [10] activities. A few exemplary biologically active 4H-chromene derivatives are collected in Fig. 1.
Recently, the synthesis of 2-amino-4H-chromenes has been achieved by the condensation of 1-naphthol, malononitrile and aromatic aldehydes in the presence of a catalyst, such as methanesulfonic acid [11], tetrabutylammonium bromide [12], TiCl4 [13], K2CO3 [14], heteropolyacid [15], basic alumina [16], 1-butyl-3-methyl imidazolium hydroxide [17], KF-Al2O3 [18], iodine and K2CO3 [19], diazabicyclo-[2.2.2]octane [20], Mg/Al hydrotalcite [21], piperidine [22], montmorillonite KSF [23], nanosized magnesium oxide [24], NaHCO3 [25], hexadecyl trimethylammonium bromide [26], cetyltrimethylammonium chloride [27], cetyltrimethylammonium bromide [28] and N, N-dimethylaminoethyl-benzyldimethylammonium chloride [29]. Due to the important aforementioned properties of chromene derivatives, considerable attentions have been focused on the synthesis of new 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamide derivatives.
Herein, the synthesis of new chromene-2-carboxamide by the reaction of N-benzyl-2-cyanoacetamide with 2-naphthol and aromatic aldehydes has been described (Scheme 1).
Experimental section
Chemicals
All chemicals were either prepared in our laboratory or purchased from Merck and Aldrich Chemical Corporation and were used without any further purification. All reactions were monitored by TLC, petroleum–ethyl acetate (3:1). Melting points were determined with a hot-plate microscope apparatus.
Apparatus
IR spectra were recorded in KBr, using a BRUKER FT-IR spectrophotometer. 1H and 13C NMR spectra were recorded on Bruker 500 MHz and 300 MHz spectrometers using DMSO, CDCl3 and TMS as solvent and internal standard, respectively. Elemental analyses for C, H and N were performed on a 204Q CHN (Heraeus, USA).
General procedure for the synthesis of 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamide 4
N-benzyl-2-cyanoacetamide (1) was synthesized from benzylamine and ethyl cyanoacetate following the reported procedure [30] (Table 1).
A mixture of N-benzyl-2-cyanoacetamide (1 mmol), aromatic aldehydes (1 mmol), 2-naphthol (1 mmol) and piperidine (0.1 mmol) in absolute EtOH (5 ml) was refluxed for an appropriate time as shown in Table 2. The reaction progress was monitored by TLC. After completion of the reaction, the mixture was cooled to r.t and then H2O (5 mL) was added. The solid product was filtered, washed with cold distilled water (2 mL) to obtain essentially pure products. The solid products were recrystallized from ethanol.
The structure of the products was characterized by IR, 1H and 13C NMR spectra along with elemental analysis data.
3-Amino-N-benzyl-1-phenyl-1H-benzo[f]chromene-2-carboxamide (4a):
White solid, Yield 75%; mp: 125–126 °C. IR (KBr) (νmax, cm−1): 3425(NH), 1683 (C=O). Anal. calcd. for C27H22N2O2 (406.48): C, 79.78; H, 5.46; N, 6.89; % found: C, 79.70; H, 5.54; N, 6.84, % dH (400 MHz, CDCl3) 8.76 (s, 2H, NH 2 ), 7.66–7.86 (m, 4H, CH arom), 7.53–7.66(m, 3H, CH arom), 7.44–7.53 (d, J = 8 Hz, 2H, CH arom), 7.29–7.35 (m, 5H, CH arom and NH), 6.96 (t, J = 7.5 Hz, 1H, CH arom), 6.94 (m, 1H, CH arom), 6.84 (t, J = 7.5 Hz, 1H, CH arom), 5.20 (s, 1H, CHPh), 4.15 (d, J = 5 Hz, 2H, CH 2NH). dC (100 MHz, CDCl3) 37.18, 44.16, 87.40, 11931, 122.71, 122.99, 123.44, 124.26, 125.43, 125.67, 126.54, 128.01, 128.27, 128.43, 128.45, 128.58, 128.86, 128.91, 128.97, 129.58, 130.56, 131.05, 131.45, 134.84, 143.35, 163.38, 177.39.
3-Amino-N-benzyl-1-(4-chlorophenyl)-1H-benzo[f]chromene-2-carboxamide (4b):
White solid, Yield 70%; mp: 183–185 °C. IR (KBr) (νmax, cm−1): 3420 (NH), 1675 (C=O). Anal. calcd. for C27H21ClN2O2 (440.92): C, 73.55; H, 4.80; N, 6.35% found: C, 73.44; H, 4.90; N, 6.25. dH (300 MHz, CDCl3) 8.15 (s, 2H, NH 2 ), 7.85–7.87 (d, J = 7 Hz, 2H, CH arom), 7.64–7.69 (m, 3H, CH arom), 7.36–7.47 (m, 6H, CH arom), 7.20–7.36 (m, 5H, CH arom and NH), 5.30 (s, 1H, CHPh), 4.40 (d, J = 5 Hz, 2H, CH 2NH). dC (75 MHz, CDCl3) 35.35, 55.06, 87.50, 114.49, 121.95, 128.28, 128.74, 128.80, 130.45, 130.50, 130.98, 131.52, 134.41, 135.06, 135.81, 139.99, 147.38, 150.77, 152.79, 153.30, 154.38, 155.77, 164.41, 175.06.
3-Amino-N-benzyl-1-(4-bromophenyl)-1H-benzo[f]chromene-2-carboxamide (4c):
White solid, Yield 72%; mp: 127–128 °C. IR (KBr) (νmax, cm−1): 3435(NH), 1670 (C=O). Anal. calcd. for C27H21BrN2O2 (484.08): C, 66.81; H, 4.36; N, 5.77% found: C, 66.79; H, 4.11; N, 5.88%. dH (500 MHz, DMSO, d6) 8.26 (s, 2H, NH 2), 7.86–7.87 (d, J = 8 Hz, 3H, CH arom), 7.61–7.66 (m, 5H, CH arom and NH), 7.42 (s, 1H), 7.39–7.40 (d, J = 8 Hz, 3H, CH arom), 7.30–7.33 (t, J = 7.5 Hz, 2H, CH arom), 7.24–7.27 (t, J = 7.5, 2H, CH arom), 5.30 (s, 1H, CHPh), 4.49 (d, J = 5 Hz, 2H, CH 2NH). dC (125 MHz, DMSO, d6) 35.21, 58.04, 87.51, 114.33, 121.95, 128.33, 128.87, 128.92, 129.79, 130.01, 130.11, 130.98, 131.46, 131.58, 135.14, 135.87, 137.21, 137.46, 147.61, 150.79, 152.80, 153.37, 163.46, 179.46.
3-Amino-N-benzyl-1-(4-nitrophenyl)-1H-benzo[f]chromene-2-carboxamide (4d):
White solid, Yield 80%; mp: 227–230 °C. IR (KBr) (νmax, cm−1): 3405 (NH), 1680 (C=O). Anal. calcd. for C27H21N3O4 (451.15): C, 71.83; H, 4.69; N, 9.31; % found: C, 71.68; H, 4.40; N, 9.78%. dH (300 MHz, DMSO, d6) 8.42 (s, 2H, NH 2 ), 7.86–7.87 (d, J = 8 Hz, 2H, CH arom), 7.61–7.66 (m, 4H, CH arom and NH), 7.42–7.44 (d, J = 8 Hz, 3H, CH arom), 7.40 (m, 3H, CH arom), 7.30–7.33 (t, J = 7.5 Hz, 2H, CH arom), 7.24–7.27 (t, J = 7.5 Hz, 2H, CH arom), 5.32 (s, 1H, CHPh), 4.32 (d, J = 5 Hz, 2H, CH 2NH). dC (125 MHz, DMSO, d6) δ 33.88, 42.33, 88.33, 113.57, 113.63, 117.21, 117.74, 118.42, 119.30, 129.72, 129.78, 129.88, 131.03, 131.07, 133.78, 133.86, 133.99, 134.06, 134.74, 156.05, 156.09, 179.90.
3-Amino-N-benzyl-1-(3-nitrophenyl)-1H-benzo[f]chromene-2-carboxamide (4e):
White solid, Yield 75%; mp: 148–149 °C. IR (KBr) (νmax, cm−1): 3420(NH), 1677 (C=O). Anal. calcd. for C27H21N3O4 (451.15): C, C, 71.68; H, 4.40; N, 9.78%; % found: C, 71.38; H, 4.47; N, 9.80%. dH (500 MHz, DMSO, d6) 8.89 (s, 2H, NH 2), 7.52–7.57 (m, 6 H, CH arom), 7.48–7.50 (m, 5H, CH arom and NH), 7.28–7.29 (t, J = 8 Hz, 2H, CH arom), 6.93–6.98 (m, 2H, CH arom), 6.58–6.60 (t, J = 7.5 Hz, 1H, CH arom), 5.37 (s, 1H, CHPh), 4.57 (d, J = 5 Hz, 2H, CH 2NH). dC (75 MHz, DMSO, d6) 35.59, 57.22, 89.50, 112.89, 121.94, 123.38, 123.92, 125.09, 131.26, 131.63, 132.93, 132.99, 135.32, 136.04, 139.57, 147.95, 150.77, 152.77, 153.45, 163.30, 172.77.
3-Amino-N-benzyl-1-p-tolyl-1H-benzo[f]chromene-2-carboxamide (4f):
White solid, Yield 60%; mp: 133–134 °C. IR (KBr) (νmax, cm−1): 3425(NH), 1685 (C=O). Anal. calcd. for C28H24N2O2 (420.5): C, 79.98; H, 5.75; N, 6.66% found: C, 79.99; H, 5.85; N, 6.65%. dH (500 MHz, DMSO, d6) 8.20 (s, 2H, NH 2), 7.91–7.94 (t, J = 7 Hz, 2H, CH arom), 7.69–7.80 (m, 6H, CH arom), 7.55–7.67 (m, 4H, CH arom and NH), 7.40–7.44 (m, 1H, CH arom), 7.31–7.34 (t, J = 7.5 Hz, 1H, CH arom), 7.25–7.28 (t, J = 7.5 Hz, 1H, CH arom), 7.09–7.10 (d, J = 8 Hz, 1H, CH arom), 5.24 (d, J = 5.5 Hz, 1H, CHPh), 4.27 (d, J = 5 Hz, 2H, CH 2NH), 2.20 (s, 3H). dC (125 MHz, DMSO, d6) 31.75, 37.36, 86.79, 101.50, 111.07, 121.08, 123.22, 124.58, 124.98, 125.32, 125.80, 126.31, 128.31, 128.80, 128.99, 129.27, 130.58, 139.79, 140.37, 154.19, 155.30, 179.90.
3-Amino-N-benzyl-1-(4-methoxyphenyl)-1H-benzo[f]chromene -2-carboxamide (4 g):
White solid, Yield 68%; mp: 227–230 °C. IR (KBr) (νmax, cm−1): 3400 (NH), 1685 (C=O). Anal. calcd. for C28H24N2O3 (436.5): C, 77.04; H, 5.54; N, 6.42% found: C, 77.15; H, 5.44; N, 6.53%. dH (500 MHz, DMSO, d6) 8.50 (s, 2H, NH 2), 8.03–8.04 (d, J = 8 Hz, 2H, CH arom), 7.85–7.86 (d, J = 8 Hz, 2H, CH arom), 7.61–7.69 (m, 2H, CH arom), 7.39–7.41 (d, J = 9 Hz, 3H, CH arom), 7.29–7.32 (t, J = 8 Hz, 1H, CH arom), 7.24–7.27 (t, J = 7.5 Hz, 3H, CH arom), 7.14–7.17 (t, J = 7 Hz, 3H, CH arom), 5.39 (s, 1H, CHPh), 4.31 (d, J = 5.5 Hz, 2H, CH 2NH), 3.45 (s, 3H, OCH 3). dC (125 MHz, DMSO, d6) 34.62, 46.32, 56.79, 86.82, 111.68, 121.11, 123.24, 124.50, 124.62, 125.03, 126.63, 126.86, 128.03, 128.34, 128.45, 128.61, 129.00, 130.33, 130.92, 139.35, 139.55, 154.13, 156.19.
3-Amino-N-benzyl-1-(2, 4-dichlorophenyl)-1H-benzo [f]chromene-2-carboxamide (4 h):
White solid, Yield 78%; mp: 146–148 °C. IR (KBr) (νmax, cm−1): 3425(NH), 1683 (C=O). Anal. calcd. for C27H20Cl2N2O2 (475.37): C, 68.22; H, 4.24; N, 5.89% found: C, 68.29; H, 4.13; N, 5.81%. dH (300 MHz, DMSO, d6) 8.35 (s, 2H, NH 2), 7.59–7.78 (d, J = 7.5 Hz, 4H, CH arom), 7.46–7.51 (t, J = 7 Hz, 4H, CH arom), 7.20–7.25 (t, J = 7 Hz, 4H, CH arom), 7.13–7.19 (m, 3H, CH arom and NH), 5.33 (s, 1H, CHPh), 4.36 (d, J = 4.5 Hz, 2H, CH 2NH). dC (125 MHz, DMSO, d6) δ 36.70, 44.00, 87.04, 103.04, 110.54, 113.04, 104.02, 116.77, 119.42, 122.54, 124.72, 130.25, 133.21, 134.85, 140.04, 145.51, 147.16, 152.20, 153.09, 158.20, 160.09, 164.90, 165.09.
3-Amino-N-benzyl-1-(3-methoxyphenyl)-1H-benzo[f]chromene-2-carboxamide (4i):
Yellow solid, Yield 65%; mp: 101–103 °C. IR (KBr) (νmax, cm−1): 3420 (NH), 1680 (C=O). Anal. calcd. for C28H24N2O3 (436.5): C, 77.04; H, 5.54; N, 6.42% found: C, 77.10; H, 5.49; N, 6.50%. dH (300 MHz, DMSO, d6) 8.32 (s, 2H, NH 2), 7.88–7.90 (d, J = 8, 2H, CH arom), 7.67–7.72 (t, J = 8 Hz, 2H, CH arom), 7.43–7.50 (m, 4H, CH arom), 7.35 (m, 3H, CH arom and NH), 6.85–6.89 (t, J = 8 Hz, 3H, CH arom), 6.73–6.76 (d, J = 8 Hz, 2H, CH arom), 5.20 (s, 1H, CHPh), 4.40 (d, J = 5 Hz, 2H, CH 2NH), 3.71(s, 3H, OCH3). dC (75 MHz, DMSO, d6) 36.78, 43.90, 57.09, 84.98, 102.98, 110.31, 113.04, 116.67, 119.02, 122.54, 124.78, 125.57, 130.15, 133.20, 134.84, 140.78, 145.57, 147.96, 152.37, 153.99, 158.27, 159.69, 165.90.
3-Amino-N-benzyl-1-(4-hydroxyphenyl)-1H-benzo[f]chromene-2-carboxamide (4j):
White solid, Yield 70%; mp: 192–194 °C. IR (KBr) (νmax, cm−1): 3402, 1660 (C=O). Anal. calcd. for C27H22N2O3 (422.48): C, 76.76; H, 5.25; N, 6.63% found: C, 76.87; H, 5.14; N, 6.60%. dH (300 MHz, DMSO, d6) 10.30 (s, 1H, OH), 8.38(s, 2H, NH 2), 7.82–7.85 (d, J = 8 Hz, 3H, CH arom), 7.62–7.67 (t, J = 7.8 Hz, 2H, CH arom), 7.38–7.45 (m, 3H, CH arom and NH), 7.29–7.30 (d, J = 8 Hz, 4H, CH arom), 6.99–7.01 (d, J = 8 Hz, 4H, CH arom), 5.28(s, 1H, CHPh), 4.28 (d, J = 5 Hz, 2H, CH 2NH). dC (75 MHz, DMSO, d6) δ 36.23, 55.12, 84.36, 112.75, 113.95, 115.27, 116.60, 119.35, 122.52, 124.75, 128.84, 132.91, 133.46, 135.45, 141.75, 145.84, 152.16, 153.14, 157.99, 158.42, 159.58, 160.54, 169.80.
Determination of antimicrobial activity
For studying of antimicrobial effect of IS16 and IS16 L were used Escherichia coli PTCC1330, Staphylococcus aureus PTCC 1112, Pseudomonas aeroginosa ATCC 27853 and E. faecalis which isolated from clinical samples. The antimicrobial effect of benzo[f]chromene derivatives on the microorganisms was assayed by agar well diffusion method [31]. Mueller–Hinton medium (Merck) and brain–heart infusion medium (Merck) were used as the test medium. Also, the minimum concentrations of the benzo[f]chromene to inhibit the microorganisms (MIC) were determined by the micro-broth dilutions technique strictly following the National Committee for Clinical Laboratory Standards (NCCLS) recommendations [32]. The inoculum was prepared using an overnight culture of each bacterium and yeast strains (18–24 h) adjusted to a turbidity equivalent to a 0.5 McFarland standard [33].
Petri plates containing 20-ml agar test plates were seeded with microorganism strains. Wells were cut and 50 μl of the benzo[f]chromene (50 mg/ml; DMSO was used as solvent) were added. The plates were then incubated at 37 °C for 24–48 h. The antibacterial activity was assayed by measuring the diameter of the inhibition zone formed around the well. DMSO as solvent was used as a negative control, whereas media with trimethoprim–sulfamethoxazole (SXT) were used as the positive controls. The experiments were performed in triplicate. Serial dilutions ranging from 100 mg/mL to 24.41 µg/mL were prepared in medium.
Results and discussion
Our initial experiments were focused on a one-pot, three-component reaction of 2-naphthol, benzaldehyde and N-benzyl-2-cyanoacetamide using different catalysts under various conditions, and the results are listed in Table 1. In the absence of any catalysts, the reaction did not proceed even after prolonged reaction time and no desired product was formed (Table 1, entry 1). But, in the presence of basic catalysts such as KOH, Et3N or pyridine the desired product was obtained in 10–55% yield (Table 1, entries 2–9). In addition, the above mentioned model reaction was conducted in the presence of piperidine under various conditions (Table 1, entries 10–13).
The best result was obtained using piperidine at reflux condition (Table 1, entry 12). Therefore, piperidine was chosen as a suitable catalyst.
We found that decreasing the temperature to 25 °C (Table 1, entry 13) led to the lower yield and longer reaction time.
In order to generalize the optimum conditions, different derivatives of 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamide (4a–j) were prepared from the one-pot reaction mixture of N-benzyl-2-cyanoacetamide (1), appropriate aldehyde (2a–j) and 2-naphthol (3) in the presence of a catalytic amount of piperidine in ethanol under reflux conditions. The results have been summarized in Table 2.
The effect of antibacterial activity of six out of ten of these new benzo[f]chromenes, namely 4a, 4b, 4c, 4d, 4h and 4i, against E. coli PTCC1330, S. aureus PTCC 1112, Pseudomonas aeroginosa ATCC 27853 and E. faecalis (which were isolated from clinical samples) was assessed by evaluating the presence of inhibition zone (IZ) and MIC and MBC values. Results showed that 4b, 4c, 4h and 4i exhibited antibacterial effects. Compounds 4h and 4i were effective on S. aureus PTCC 1112, whereas 4b was effective on E. coli PTCC1330. Also 4c could inhibit growth of E. faecalis. According to the results, it seems that the antibacterial effect of the ligands is more on Gram-positive bacteria (Table 3).
The MIC and MBC values for the benzo[f]chromenes (4a, 4b, 4h and 4i) were in the range of 100 mg/mL–24.41 µg/mL. The results of our investigation showed that these compounds had bacterostatic and bactericide effects (Table 4).
The proposed mechanism
A plausible mechanism for synthesis of 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamide is shown in Scheme 2.
The reaction is supposed to proceed in a stepwise fashion. The initial reaction is conducted via a Knoevenagel condensation between compound 1 and aromatic aldehyde 2 to form the intermediate 5 in the presence of piperidine, which suffers immediate addition of compound 3 to the C=C bond of 5. The concerted cyclocondensation of hydroxy and CN of the intermediate 6 was performed to furnish the corresponding products (4a–j).
Conclusion
In conclusion, new 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamide derivatives from the reaction of N-benzyl-2-cyanoacetamide with aromatic aldehydes and 2-naphthol in the presence of piperidine as a base have been synthesized. Among these synthesized compounds, four compounds 4b, 4c, 4h and 4i exhibited potential antibacterial activity which may guarantee their future applications in a moderate antibiotic therapy.
References
H. Bienayme, C. Hulme, G. Oddon, P. Schmitt, Chem. Eur. J. 6, 3321 (2000)
A. Nefzi, J.M. Ostresh, R.A. Houghten, Chem. Rev. 97, 449 (1997)
L.A. Thompson, Curr. Opin. Chem. Biol. 4, 324 (2000)
A. Dömling, Curr. Opin. Chem. Biol. 6, 306 (2002)
G.P. Ellis, The chemistry of heterocyclic compounds, chromenes, chromanones and chromones, vol. 31 (Wiley and Sons, New York, 2007)
M.M. Khafagy, A.H.A. El-Wahas, F.A. Eid, A.M. El-Agrody, Farmaco 57, 715 (2002)
A.G. Martinez, L. Marco, J. Bioorg. Med. Chem. Lett 7, 3165 (1997)
K. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato, K. Kikugawa, Mutat. Res. 395, 47 (1997)
G. Bianchi, A. Tava, Agric. Biol. Chem. 51, 2001 (1987)
S.J. Mohr, M.A. Chirigos, F.S. Fuhrman, J.W. Pryor, Cancer Res. 35, 3750 (1975)
M.M. Heravi, B. Baghernejad, H.A. Oskooie, J. Chin. Chem. Soc. 55(3), 659–662 (2008)
T.S. Jin, J.C. Xiao, S.J. Wang, T.S. Li, X.R. Song, Synlett 13, 2001 (2003)
B.S. Kumar, N. Srinivasulu, R.H. Udupi, B. Rajitha, Y.T. Reddy, P.N. Reddy, P.S. Kumar, J. Heterocycl. Chem. 43, 1691 (2006)
M. Kidwai, S. Saxena, M.K.R. Khan, S. Sm, Thukral. Bioorg. Med. Chem. Lett. 15, 4295 (2005)
M.M. Heravi, K. Bakhtiari, V. Zadsirjan, F.F. Bamoharramb, O.M. Heravi, Bioorg. Med. Chem. Lett. 17, 4262 (2007)
R. Maggi, R. Ballini, G. Sartori, R. Sartorio, Tetrahedron Lett. 45, 2297 (2004)
K. Gong, H. Wang, D. Fang, Z. Liu, Catal. Commun. 9, 650 (2008)
T.S. Jin, H. Xie, Y.P. Xue, T.S. Li, Asian J. Chem. 20, 5145 (2008)
Y.M. Ren, C. Cai, Catal. Commun. 9, 1017 (2008)
S. Balalaie, S. Ramezanpour, M. Bararjanian, J.H. Gross, Synth. Commun. 38, 1078 (2008)
M.P. Surpur, S. Kshirsagar, S.D. Samant, Tetrahedron Lett. 50, 719 (2009)
R.A. Mekheimer, K.U. Sadek, J. Heterocycl. Chem. 46, 149 (2009)
R. Ballini, F. Bigi, M.L. Conforti, D.D. Santis, R. Maggi, G. Oppici, G. Sartori, Catal. Today 60, 305 (2000)
D. Kumar, V.B. Reddy, B.G. Mishra, R.K. Rana, M.N. Nadagouda, R.S. Varma, Tetrahedron 63, 3093 (2007)
D. Zhou, Z. Ren, W. Cao, J. Chen, H. Deng, J. Heterocycl. Chem. 45, 1865 (2008)
T.S. Jin, L.B. Zhang, A.Q. Liu, T.S. Li, Wang, Synth.Commun. 36(14), 2009–2015 (2006)
R. Ballini, G. Bosica, M.L. Conforti, R. Maggi, A. Mazzacani, P. Righi, G. Sartori, Tetrahedron 57, 1395 (2001)
T.S. Jin, J.C. Xiao, S.J. Wang, T.S. Li, Ultrason. Sonochem. 11, 393 (2004)
L. Chen, X.J. Huang, Y.Q. Li, M.Y. Zhou, W. Zheng, J. Monatsh. Chem. 140, 45 (2009)
Z. Zhang, T. Song, X. Li, Z. Wu, Y. Feng, F. Xie, C. Liu, J. Qin, H. Chen, Eur. J. Med. Chem. 59, 141–149 (2013)
S. Irshad, M. Mahmood, F. Perveen, Res. J. Biolog. 2, 1 (2012)
H. Kubota, S. Senda, N. Nomura, H. Tokuda, H. Uchiyama, J. Biosci. Bioeng. 106(4), 381–386 (2008)
A. Cinarli, D. Gurbuz, A. Tavman, A.S. Birteksoz, Bull. Chem. Soc. Ethiop. 25, 407 (2011)
Acknowledgements
The authors express appreciation to the Shahid Bahonar University of Kerman Faculty Research Committee for its support of this investigation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pouramiri, B., Tavakolinejad Kermani, E. & Khaleghi, M. One-pot, three-component synthesis and in vitro antibacterial evaluation of novel 3-amino-N-benzyl-1-aryl-1H-benzo[f]chromene-2-carboxamide derivatives. J IRAN CHEM SOC 14, 2331–2337 (2017). https://doi.org/10.1007/s13738-017-1169-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1169-y