Abstract
In this work, a green and efficient procedure has been reported for the synthesis of pyrimido[1,2-a]benzimidazole and tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-one derivatives using Brönsted acidic ionic liquid supported on rice husk ash (RHA-[pmim]HSO4) as the catalyst. These reactions were performed at 100 °C under solvent-free conditions with high yields in very short reaction times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterocyclic compounds as widely distributed compounds in nature are essential to life. Quinazolinone derivatives are an important class of these types of compounds showing a broad scope of pharmacological and biological activities [1, 2]. For example, they can be found as important motif in a variety of potent commercial drugs such as Quazinone (a cardiotonic and vasodilator drug which was developed and marketed in the 1980s), Anagrelide (a drug used for the treatment of essential thrombocytosis (ET), or overproduction of blood platelets), Alfuzosin (an α1 receptor antagonist used to treat benign prostatic hyperplasia) and Prazosin (a sympatholytic drug used to treat high blood pressure) [3]. Tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-ones are an important class of N-fused heterocyclic quinazolinones which can be prepared via the condensation of substituted aldehydes with 2-aminobenzimidazole as amine sources and dimedone. For this purpose, a variety of conditions have been reported [4–11].
Pyrimido[1,2-a]benzimidazoles are another group of N-containing heterocycles which have two biologically active heterocyclic cores. These compounds show antidiabetic [12], antioxidant, antimicrobial [13], antibiotic and antiarrhythmic [14] properties. Additionally, they act as calcium antagonistic [15], and as lipid peroxidation inhibitor [16]. In the literature, a few methods for the synthesis of these compounds have been reported [17–19].
Although the reported procedures used for the synthesis of the above-mentioned heterocyclic compounds provide an improvement, they suffer from disadvantages such as long reaction times, harsh reaction conditions, need to excess amounts of the reagent, use of organic solvents, use of toxic reagents and non-recoverability of the catalyst. Therefore, there is still a demand for introducing simple, efficient and mild procedures with easily separable and reusable solid catalysts to overcome these problems.
Very recently, we have reported the preparation and characterization of a new Brönsted acidic ionic liquid supported on rice husk ash (RHA-[pmim]HSO4) (Fig. 1), and its applicability in the promotion of the formylation of amines and alcohols [21], and the synthesis of 1-(benzothiazolylamino)phenylmethyl-2-naphthols [22]. In continuation of these studies and in order to overcome the above-mentioned restrictions, we were interested to investigate the applicability of this reagent in the promotion of the synthesis of pyrimido[1,2-a]benzimidazole and tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-one derivatives, which need the acidic catalyst to speed-up.
Experimental
General
All chemicals were purchased from Merck, Aldrich and Fluka Chemical Companies and used without further purification. Products were characterized by their physical constant and comparison with authentic samples. The purity determination of the substrates and reaction monitoring were accompanied by TLC using silica gel SIL G/UV 254 plates.
Preparation of 1-methyl-3-(trimethoxysilylpropyl)-imidazolium hydrogen sulfate supported on RHA (RHA-[pmim]HSO4)
A mixture of 10 mmol 1-methylimidazole and 10 mmol (3-chloropropyl)trimethoxysilane was refluxed at 90 °C for 30 h. Then, the reaction mixture was cooled down. The crude product was washed with Et2O (2 × 5 mL) and dried under vacuum to obtain [pmim]Cl as a slightly yellow viscous oil. Then, 0.6 g (2 mmol) of [pmim]Cl was dissolved in 25 mL of CH2Cl2 and treated with 2 g of RHA. The reaction mixture was refluxed with stirring for 3 days. Then, the reaction mixture was cooled to room temperature and the solid was isolated by filtration and washed with 20 mL of boiling dichloromethane to remove the unreacted ionic liquid. In the next step, the material was dried to give 2.5 g RHA-[pmim]Cl as a gray powder. In continue, 3 g of RHA-[pmim]Cl was suspended in 20 mL of dry CH2Cl2. Under vigorous stirring and in an ice bath (0 °C), 2.9 mmol of concentrated H2SO4 (97 %) was added dropwise to this mixture. The mixture was then warmed to room temperature and heated under reflux for 30 h. When the formed HCl was completely distilled off, the solution was cooled and CH2Cl2 was removed under vacuum to afford RHA-[pmim]HSO4 as the product (Fig. 1).
General procedure for the synthesis of pyrimido[1,2-a]benzimidazole derivatives
A mixture of aldehyde (1 mmol), 2-aminobenzimidazole (1 mmol), malononitrile (1.1 mmol), RHA-[pmim]HSO4 (10 mg, 0.8 mol %) was heated in an oil bath at 100 °C under solvent-free conditions for the appropriate time. After completion of the reaction (monitored by TLC), EtOH was added and the catalyst was separated by filtration. Then water was added, and the precipitated product was separated by filtration in high purity. The spectral data of the new compound are as follows:
Compound 1i
1H NMR (DMSO-d6, 400 MHz): δ = 2.43 (3H, s, SCH3), 5.18 (1H, s, CH), 6.83 (2H, s, NH2), 7.0 (1H, t, J = 7.6 Hz), 7.11 (1H, t, J = 7.2 Hz), 7.20–7.25 (5H, m), 7.62 (1H, d, J = 8 Hz), 8.56 (1H, s, NH) ppm; 13C NMR (DMSO-d6, 100 Mz): δ = 15.1, 53.3, 62.3, 112.8, 116.5, 119.6, 120.3, 123.8, 126.6, 127.0, 129.7, 138.2, 139.8, 144.0, 149.6, 152.1.; IR (KBr, cm−1): 3462, 3323, 3218, 3060, 2912, 2188, 1677, 1598, 1521, 1461, 1402, 1347.
Compound 1j
1H NMR (DMSO-d6, 400 MHz): δ = 5.43 (1H, s, CH), 6.89 (2H, s, NH2), 7.01 (1H, td, J 1 = 8 Hz, J 2 = 1.2 Hz), 7.11 (1H, td, J 1 = 8 Hz, J 2 = 1.2 Hz), 7.24 (1H, d, J = 7.6 Hz), 7.46-7.53 (3H, m), 7.68 (1H, d, J = 8.0 Hz), 7.79 (1H, s), 7.87-7.89 (2H, m), 7.93 (1H, d, J = 8.8 Hz), 8.72 (1H, s, NH) ppm; 13C NMR (DMSO-d6, 100 Mz): δ = 54.0, 62.3, 112.9, 116.5, 119.6, 120.3, 123.8, 124.8, 125.0, 126.7, 126.9, 128.0, 128.3, 129.1, 129.7, 133.0, 140.5, 144.1, 149.7, 152.2; IR (KBr, cm−1): 3436, 3301, 3229, 3056, 2913, 2187, 1679, 1600, 1464, 1398.
General procedure for the synthesis of tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-one derivatives
A mixture of aldehyde (1 mmol), 2-aminobenzimidazole (1 mmol), dimedone (1 mmol), RHA-[pmim]HSO4 (50 mg,4 mol%) was heated in an oil bath at 100 °C under solvent-free conditions for the appropriate time. After completion of the reaction (monitored by TLC), EtOH was added and the catalyst was separated by filtration. Then water was added, and the precipitated product was separated by filtration in high purity. The spectral data of the new compound are as follows:
Compound 2j
1H NMR (DMSO-d6, 400 MHz): δ = 0.92 (3H, s, CH3), 1.07 (3H, s,CH3), 2.07 (2H, d, J = 16.0 Hz), 2.16 (2H, d, J = 16.0 Hz), 6.56 (1H, s), 6.98 (1H, td, J 1 = 7.6 Hz, J 2 = 0.8 Hz), 7.08 (1H, td, J 1 = 7.6 Hz, J 2 = 0.8 Hz), 7.24 (1H, d, J = 8.0 Hz), 7.54 (2H, dd, J 1 = 5.0 Hz, J 2 = 1.6 Hz), 7.75 (2H, dd, J 1 = 5.0 Hz, J 2 = 1.6 Hz), 11.26 (1H, s, NH) ppm.
Results and discussion
At first, we focused our attention to study the synthesis of pyrimido[1,2-a]benzimidazoles. For optimization of the reaction conditions, the condensation of 4-chlorobenzaldehyde, 2-aminobenzimidazole and malononitrile leading to 2-amino-3-cyano-4-(4-chlorophenyl)-1,4-dihydropyrimido[1,2-a] benzimidazole was selected as a model reaction under various conditions (Table 1). As reaction media, different solvents such as EtOH, H2O, EtOH:H2O and MeCN and also solvent-free conditions were used and the best results were obtained in the absence of solvent. Having accomplished the optimization of the reaction media, more experiments were performed to delineate the best reaction. It was observed that 10 mg of RHA-[pmim]HSO4 was the optimal catalyst loading for this reaction, and higher catalyst loading did not improve the yield of the product to a greater extent. In addition, the results indicated that the reaction at 100 °C proceeded with highest yield and shortest time. Finally, the best result was obtained using 10 mg of RHA-[pmim]HSO4 at 100 °C under solvent-free conditions (Scheme 1).
In order to establish the effectiveness and the acceptability of the method, we explored the protocol with a variety of simple readily available substrates under the optimal conditions (Table 2). It was observed that under the applied conditions, a wide range of aromatic aldehydes containing electron-withdrawing as well as electron-donating substituents including Cl, Br, F, i-Pr, SCH3 and NO2 in the ortho, meta, and para positions of the benzene ring were easily converted to the corresponding pyrimido[1,2-a]benzimidazoles (1) in good to excellent yields during short reaction times (Table 2, entries 1–9). As can be seen, the effect of the nature of the substituents on the aromatic ring did not show obvious effects in terms of yields and reaction times under the selected reaction conditions. Furthermore, 2-naphthaldehyde as a polycyclic aromatic aldehyde also provided the desired product in very good yield (Table 2, entry 10). However, under this catalytic system, application of the aliphatic aldehyde was not successful.
After the successful application of RHA-[pmim]HSO4 in the synthesis of pyrimido[1,2-a]benzimidazoles, this catalyst was also used in the three-component condensation of aromatic aldehydes and dimedone (5,5-dimethyl-1,3-cyclohexanedione) with 2-aminobenzimidazole leading to tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-ones (2). But under the same conditions used for the preparation of pyrimido[1,2-a]benzimidazoles, this reaction was not completed. Optimization of the reaction conditions clarified that 50 mg RHA-[pmim]HSO4 was adequate to accomplish the reaction effectively at 100 °C (Scheme 1). Subsequently, to reveal the generality of this method, the condensation was carried out with various aromatic aldehydes containing electron-withdrawing (Cl, Br, NO2) or electron-donating (CH3, OCH3, OH) groups, and the corresponding pyrano[2,3-d]pyrimidinones were obtained in high yields and short reaction times (Table 2, entries 11-19).
The proposed mechanism for the synthesis of pyrimido[4,5-d]pyrimidinones and pyrano[2,3-d]pyrimidinones in the presence of RHA-[pmim]HSO4 as a promoter is shown in Scheme 2. On the basis of this mechanism, RHA-[pmim]HSO4 catalyzes the reaction by the activation of the carbonyl groups against the nucleophilic attack.
An important and interesting point about the obtained products is that these compounds showed strong fluorescence emission. The changes of some of these derivatives from visible to fluorescence wavelength (at 365 nm) are displayed in Fig. 2.
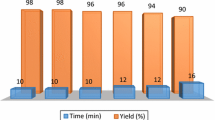
To check the reusability of the catalyst, the synthesis of 2-amino-3-cyano-4- (4-chlorophenyl)-1,4-dihydropyrimido[1,2-a]benzimidazole (1c) under the optimized reaction conditions was studied again. When the reaction completed, EtOH was added and the catalyst was separated by filtration. The recovered catalyst was washed with EtOH, dried and reused for the same reaction. These processes were carried out over five runs, and all reactions led to the desired products without significant changes in terms of the reaction time and yield which clearly demonstrates practical recyclability of this catalyst (Fig. 3).
Conclusion
In conclusion, in this study we have introduced RHA-[pmim]HSO4 as a highly powerful supported acidic ionic liquid for the simple and efficient synthesis of pyrimido[1,2-a]benzimidazole and tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-one derivatives. The obtained results show that this method is convincingly superior to other reported procedures in the terms of reaction times and yields. Simple experimental procedure and use of inexpensive and reusable catalyst with lower loading are other advantages of this procedure. Furthermore, this process avoids problems associated with organic solvents and liquid acids use, which makes it a useful and attractive strategy in view of economic and environmental advantages.
References
K. Ozaki, Y. Yamada, T. Oine, T. Ishizuka, Y. Iwasawa, J. Med. Chem. 28, 568 (1985)
W. Wang, L.M. Lagniton, R.N. Constantine, M.C. Desai, Patent US 7, 939, 539 B2 (2011)
T.P. Selvam, P.V. Kumar, Res. Pharm. 1, 1 (2011)
A. Shaabani, E. Farhangi, A. Rahmati, Comb. Chem. High. T. Scr. 9, 771 (2006)
A.E. Mourad, A.A. Aly, H.H. Farag, E.A. Beshr, Beilstein J. Org. Chem. 3, 11 (2007)
M.M. Heravi, L. Ranjbar, F. Derikvand, B. Alimadadi, H.A. Oskooie, F.F. Bamoharram, Mol. Divers. 12, 181 (2008)
M.M. Heravi, F. Derikvand, L. Ranjbar, Synth. Commun. 40, 677 (2010)
G.M. Ziarani, A. Badiei, Z. Aslani, N. Lashgari, Arab. J. Chem. 8, 54 (2015)
R.G. Puligoundla, S. Karnakanti, R. Bantu, N. Kommu, S.B. Kondra, L. Nagarapu, Tetrahedron Lett. 54, 2480 (2013)
G. Krishnamurthy, K.V. Jagannath, J. Chem. Sci. 125, 807 (2013)
M.R. Mousavi, M.T. Maghsoodlou, Monatsh. Chem. 145, 1967 (2014)
A.C.White, R.M. Black, US 3 989 709 (1997)
S.P. Vartale, P.N. Ubale, S.G. Sontakke, N.K. Halikar, M.M. Pund, World J. Pharm. Sci. 2, 665 (2014)
P.F. Asobo, H. Wahe, J.T. Mbafor, A.E. Nkengfack, Z.T. Fomum, E.F. Sopbue, D. Döpp, J. Chem. Soc. Perkin Trans. 1, 457 (2001)
R. Alajarin, J.J. Vaquero, J. Alvarez-Builla, M.F. de Casa-Juana, C. Sunkel, J.G. Priego, P. Gomez-Sal, R. Torres, Bioorg. Med. Chem. 2, 323 (1994)
C.G. Neochoritis, T. Zarganes-Tzitzikas, C.A. Tsoleridis, J. Stephanidou-Stephanatou, C.A. Kontogiorgis, D.J. Hadjipavlou-Litina, T. Choli-Papadopoulou, Eur. J. Med. Chem. 46, 297 (2011)
A. Shaabani, A. Rahmati, A.H. Rezayan, M. Darvishi, Z. Badri, A. Sarvari, QSAR Comb. Sci. 26, 973 (2007)
M.V. Reddy, J. Oh, Y.T. Jeong, C. R. Chim. 17, 484 (2014)
Y. Cui, J. Wang, J. Yin, C. Ji, C. Guo, Chin. J. Appl. Chem. 1, 53 (2010)
G. Liu, Q. Shao, S. Tu, L. Cao, C. Li, D. Zhou, B. Han, J. Heterocyclic Chem. 45, 1127 (2008)
F. Shirini, M. Seddighi, M. Mamaghani, RSC Adv. 4, 50631 (2014)
M. Seddighi, F. Shirini, M. Mamaghani, C. R. Chim. 18, 573 (2015)
Acknowledgments
We are thankful to the University of Guilan Research Council for the partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirini, F., Seddighi, M. & Goli-Jolodar, O. Facile and efficient synthesis of pyrimido[1,2-a]benzimidazole and tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-one derivatives using Brönsted acidic ionic liquid supported on rice husk ash (RHA-[pmim]HSO4). J IRAN CHEM SOC 13, 2013–2018 (2016). https://doi.org/10.1007/s13738-016-0918-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-0918-7