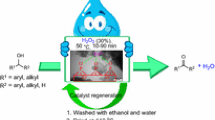
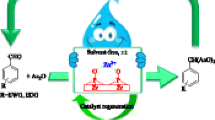
Abstract
Nickel zirconium phosphate nanoparticles have been used as an efficient catalyst for the acetylation of a wide range of alcohols and phenols with acetic anhydride in good to excellent yields under solvent‐free conditions. The steric and electronic properties of the different substrates had a significant influence on the reaction conditions required to achieve the acetylation. The catalyst used in the current study was characterized by inductively coupled plasma optical emission spectroscopy, X‐ray diffraction, N2 adsorption–desorption, scanning electron microscopy, and transmission electron microscopy. This nanocatalyst could also be recovered and reused at least six times without any discernible decrease in its catalytic activity.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
α‐Zirconium phosphate (ZP) is one of the most important compounds in inorganic chemistry, and the layered structure of this material has been used in a variety of different fields [1–3]. ZP behaves as a unique ion exchanger because of its exceptionally poor aqueous solubility, high thermal stability, resistance to radiation and abrasive properties [4, 5]. The H+ of the P–OH moiety in ZP can be exchanged for various other ions, which results in the enlargement of the interlayer distance [6–9]. Several studies pertaining to the successful exchange of the H+ of the P–OH group in ZP with various divalent and trivalent cations have been reported in the literature [10–14]. It has also been reported that ZP possesses excellent selectivity towards Pb2+, Zn2+, and Fe3+ as an ion exchanger [15–17]. Furthermore, ZP has been reported to exhibit antibacterial activity when it was loaded with Cu2+, Zn2+, or Ce3+ [5, 6, 13, 14]. Several reports have also appeared in the literature concerning the catalytic activities of ion exchanged materials of this type, including the use of zinc zirconium phosphate (ZPZn) as a catalyst in the acetylation of alcohols and phenols and the use of a copper zirconium phosphate (ZPCu) as catalysts in selective oxidation of alcohols [18–24].
Protection and deprotection of organic functions are important processes during multi-step organic synthesis. The choice of a method for the functional group’s transformations depends on its simplicity, high yields of the desired products, short reaction times, low cost of the process, and ease of the work-up procedures [25, 26]. The acetylation of alcohols, phenols, thiols, and amines is one of the most important and frequently used transformations in organic synthesis, especially in the synthesis of natural compounds, biologically active compounds, and polyfunctional molecules such as nucleosides, carbohydrates, chalcones, flavanones, naphthoquinones, pesticides, and steroids. Acetylated groups are also commonly found in cosmetics and foodstuffs, as well as solvents, perfumes, plasticizers, flavors, polymers, and pharmaceuticals [25–27]. One of the most common examples of a compound containing an acetylated group is aspirin, which is produced by the acetylation of salicylic acid with acetic anhydride (AA) in the presence of an acid catalyst [28]. The acetyl group is one of the most inexpensive and commonly used protecting groups in organic chemistry for the protection of –OH, –SH, and –NH2 functional groups because the resulting acetylated compounds are stable to various reaction conditions and reagents. Furthermore, the acetyl group can be readily introduced using inexpensive reagents and easily removed using mild alkaline hydrolysis [29–31]. A variety of different procedures have been developed for the acetylation of alcohols, phenols, amines, and thiols using both homogeneous and heterogeneous catalysts such as VIV(TPP)(OTf)2 [27], La(NO3)3 6H2O [29], B(C6F5)3 [30], CuSO4 5H2O [31], ZnCl2 [32], borated zirconia [33], ZnO2 [34], Ce(OTf)3 [35], SiO2–ZnCl2 [36], H3PW12O40 [37], DMAP HCl [38], Cu(BF4)2 [39], silica‐bonded sulfamic acid [40], Cp2ZrCl2 [41], [TMBSA][HSO4] [42], [bmim][OTs] [43], [MMPPA][HSO4] [44], SaSA [45], SBNPSA [46], SuSA [47], P(4‐VPT) [48], acylimidazolium acetate [49], polyvinylpolypyrrolidoniume tribromide [50], ZnAl2O4@SiO2 [51], P2O5/Al2O3 [52], [Hmim]HSO4 [53], Yttria‐zirconia [54], CoCl2 [55], MWCNTs–C–PO3H2 [56], NiCl2 [57], Ni/SiO2 [58], DBSA [59], Rice husk [60] anhydrous NiCl2 [61], LaFeO3/SiO2 [62], and Fe/SBA-15 [63]. However, most of these catalysts have advantages and limitations. Despite extensive interest in the development of new methods of acetylation, there is still scope for the development of simple, efficient, inexpensive, widely applicable, reusable, and environmentally benign catalysts and procedures capable of promoting the acetylation process. With growing environmental concerns, one of the most promising ways to achieve these goals seems to be the use of green and insoluble catalysts or of ecofriendly solvent-free conditions. When an insoluble catalyst is used, it can be easily recovered from the reaction mixture by simple filtration and recycled and can be reused several times, making the process more economically and environmentally viable. Furthermore, the reported examples have demonstrated that heterogeneous catalysts typically require easier work-up procedures. Also, solvent-free synthetic methods are valuable for environmental and economical reasons [22, 24]. With this in mind, and as part of ongoing work towards the development of efficient green catalysts for organic transformations [64, 65], with particular emphasis on the acetylation and acylation of aromatic compounds [52, 53], we report herein the use of nickel zirconium phosphate (ZPNi) as an efficient catalyst for the mild and convenient acetylation of alcohols and phenols under solvent‐free conditions. This new ZPNi catalyst was characterized by inductively coupled plasma optical emission spectroscopy (ICP‐OES), X‐ray diffraction (XRD), N2 adsorption–desorption, scanning electron microscopy (SEM), and transmission electron microscopy (TEM).
Experimental
Catalyst synthesis
All of the reagents and solvents used in the current study were purchased from Merck Chemical Company and used without further purification. The catalyst was prepared according to previously published procedures, with minor modifications [2, 8–10]. ZP was prepared according to the following procedure. ZrOCl2 8H2O (5 g) was heated at reflux in a solution of H3PO4 (50 ml, 12 mol/L) for 24 h. The resulting mixture was cooled to ambient temperature to give a suspension, which was filtered, and the filter cake was then washed with a solution of H3PO4 (0.1 mol/L) until the filtrate was free of chloride ions. The filter cake was then washed several times with distilled water until the pH of the filtrate was neutral. The solid was then collected and dried in an oven at 110 °C for 24 h [2]. ZPNi was prepared through an ion‐exchange reaction [8–10]. Briefly, ZP (3 g) was dispersed in deionized water (50 ml) at 50 °C, and the resulting suspension was treated with a solution of Ni(OAc)2 (100 ml, 0.1 mol/L) in water (excess amount of Ni2+). This mixture was then heated at reflux for 4 days. It is noteworthy that the acetate ion performed effectively as a base to keep the hydrogen ion concentration in solution sufficiently low to achieve high loadings of the catalyst [7]. A complete exchange between the cations and the hydrogen ions of the P–OH groups could not be achieved in less than 3 days or at temperatures below 80 °C [13]. The resulting slurry was filtered hot to give a light green solid, which was washed with distilled water until no Ni2+ ions could be detected in the filtrate (i.e., until the filtrate was colorless). The solid product was then dried at 100 °C for 24 h before being calcined at 600 °C for 4 h to give the final product, ZPNi, as a pale green solid (Scheme 1).
Catalyst characterization
The chemical composition of the ZPNi catalyst was evaluated at different stages of the reaction (i.e., before and after the catalytic reaction) by ICP-OES using an Optima 7300 V ICP-OES spectrometer (PerkinElmer). The samples were ground into a fine powder and analyzed by XRD on a Philips X’pert X-ray diffractometer. The specific surface areas of the samples were determined from their N2 adsorption–desorption isotherms using the Brunauer-Emmett-Teller (BET) method on a Quantachrome ChemBET 3000 instrument. Each sample was degassed at 400 °C for 2 h before being analyzed to remove any adsorbed species from their surfaces. The BET surface areas of the materials were estimated from their N2 adsorption–desorption isotherms. The surface morphologies of the ZP and ZPNi materials were studied by SEM on a Philips XL scabbing electron microscope (Philips). TEM images of ZPNi were obtained on a CENTRA 100 TEM system (Zeiss).
General experimental procedure for the acetylation of substrates under solvent‐free conditions
ZPNi (1 mol %) was added to a mixture of alcohol (1 mmol) and AA (2 mmol), and the resulting mixture was stirred at 40 °C for the specified time (Scheme 2). Upon completion of the reaction (as determined by GC), the catalyst was separated from the reaction mixture by centrifuge, then the supernatant was collected and diluted with 10 % NaHCO3 solution (10 ml) before being extracted with Et2O (2 × 10 ml). The combined organic extracts were washed and then dried over anhydrous CaCl2 before being evaporated to dryness under vacuum to give the desired product. In some cases, it was necessary for the product to be purified by column chromatography over silica gel eluting with a mixture of cyclohexane and ethyl acetate.
Recyclability studies of catalyst
To examine the recyclability of the catalyst, the used ZPNi was recovered from the reaction media and re-used. For recycling, after the first use, the catalyst was separated from the reaction mixture by centrifugation, washed sequentially with ethanol and water before being dried at 110 °C for 2 h, and then activated at 450 °C for 2 h.
Results and discussion
Catalyst characterization results
The ICP-OES analyses of ZP and ZPNi are shown in Table 1. The results obtained in the current study for ZPNi were compared with those reported previously in the literature [8–10]. Our results revealed that there was a negligible leach of nickel ions into the reaction media after the reaction (i.e., following the first use of the catalyst).
Figure 1 shows the powder XRD patterns of the ZP and ZPNi materials. The results show some characteristic reflections in the 2θ range of 5°–40°. The diffraction peak in ZP at 2θ–12° was assigned to a d 002 basal spacing of 7.5 Å between the planes, which was consistent with the patterns previously reported for ZP and its derivatives with a hexagonal crystal system [2]. It shows that the d-spacing of the (002) plane of ZPNi had increased, which indicated that the Ni2+ ions had intercalated into the interlayer of ZP and increased the d 002 basal interlamellar spacing of ZP from 7.5 to 9.8 Å. It is well known that the ion radii of Ni2+ (0.69 Å) and hydrated Ni2+ (4.04 Å) are smaller than the basal spacing of ZP (7.5 Å) [66, 67].
These results therefore indicated that Ni2+ ions had inserted into the interlayer of ZP and increased the basal spacing of the modified ZP after the exchange [8–10]. Taken together, these data indicated that ZPNi had been formed successfully. The XRD pattern of the ZPNi catalyst after the 7th run showed that the basal spacing of ZP was about 10.1 Å, which was only a little larger than that of the fresh ZPNi catalyst. This increase may have occurred because of the presence of less Ni2+ on the surface of ZP, and an increase in the number of water molecules between the layers following the seventh run (i.e., Ni2+ ions may have been washed off during the regeneration of the catalyst, see “General experimental procedure for the acetylation of substrates under solvent-free conditions” and Table 1). Figure 2 shows the N2 adsorption–desorption isotherm of ZPNi, as a representative example, in the relative pressure range (p/p 0) of 0.1–1.0. The surface area of ZPNi was determined to be 103.1 m2/g. The isotherm for ZPNi shows three adsorption stages. The first of these stages was observed at p/p 0 < 0.37, whereas the second stage was observed in the range of 0.37 < p/p 0 < 0.93, and the third stage was observed at higher relative pressures (p/p 0 > 0.93). The N2 adsorption–desorption isotherm of ZPNi exhibited a typical “type IV” isotherm shape with a distinct hysteresis loop, which is characteristic of a mesoporous material [68].
The hysteresis loop (type H3) is associated with the occurrence of capillary condensation in the mesopores, which indicates the presence of a mesoporous structure in the ZPNi catalyst. The observed increase in adsorption at the higher p/p 0 value indicated the presence of larger mesopores in the sample [9].
The surface area of ZPNi after the 7th run was found to be 85.4 m2/g. The SEM image of ZP (Fig. 3a) revealed the presence of hexagonal plates with well-defined shapes and very smooth surfaces. Figure 3b and c show the SEM images of ZPNi. These images revealed that the structure of ZPNi was much less ordered than that of ZP, and that the ZPNi particles had aggregated to form both sheets and spheres of different shapes and sizes [9]. Figure 4 shows the TEM images of ZPNi. It shows that ZPNi catalyst retained the original morphology of ZP (layered structure) and that the particles were approximately 150 nm in size. These images also showed nanoparticles of different sizes on the smooth surface of the ZP. The presence of metallic crystal nanoparticles on the surface of ZP indicated that the nickel deposited on the surface of the ZP had agglomerated. Similar observations have also been reported for zinc and cerium with ZP [6, 14]. Figures 3d and 4c show the SEM and TEM images of the catalyst following its 7th run, respectively. Both of these images showed that the sheets and particles had conglomerated to a much greater extent following the 7th run because of the process used to regenerate the catalyst.
Catalytic activity of ZPNi towards the acetylation of phenols and its expected mechanism
The conversion of phenol (1 mmol) to phenyl acetate was selected as a model reaction to optimize the conditions, and the reaction was conducted in the presence of ZPNi (1 mol %) and AA (2 mmol) in various solvents, as well as being investigated under solvent-free conditions. As shown in Table 2, the use of ZPNi as a catalyst under solvent-free conditions provided higher yields and shorter reaction times than those achieved under conventional conditions.
With the optimized conditions in hand, we proceeded to evaluate the scope and generality of the method using various alcohols and phenols (Table 3). Pleasingly, the hydroxyl groups of the all of different alcohols and phenols tested were converted to the corresponding acetates in good yields and short reaction times using 3 or 4 equivalents of AA (Table 3, entries 8–10, 20). Furthermore, the products were readily isolated from the reaction mixtures by a simple filtration followed by a standard work-up procedure. Phenols reacted smoothly under the optimized conditions (Table 3, entries 1–15), with the corresponding acetates being formed in yields of 83–96 %. Furthermore, no by-products (such as those resulting from a Fries rearrangement) were observed with the substituted phenols. The presence of electron-donating substituents (i.e., –CH3, –OCH3, –OH) on the phenol ring led to a significant increase in the rate of the acetylation reaction (Table 3, entries 2–10), with shorter reaction times being observed in these cases. In contrast, the presence of electron-withdrawing groups (i.e., carboxyl, nitro, and halo groups) on the phenol ring led to a decrease in the rate of the acetylation reaction (Table 3, entries 11–15), with longer reaction times being observed. The optimized reaction conditions were also successfully applied to the acetylation of benzylic alcohols bearing an electron-withdrawing or electron-donating group without the formation of any by-products resulting from oxidation reactions (Table 3, entries 17–22). For deactivated aromatic rings (Table 3, entries 21 and 22), the acetylated products were obtained in much lower yields and required longer reaction times than the corresponding activated aromatic systems (Table 3, entries 18–20). The reaction times required for the acetylation of the phenols were longer than those required for the benzylic alcohols.
This difference in the reaction times was attributed to the low nucleophilicity of phenols compared to benzylic alcohols because of the delocalization of the lone pairs of electrons on the phenolic oxygen throughout the benzene ring [43, 49, 51]. To further extend the scope of the ZPNi catalyst, we also investigated the acetylation of several aliphatic alcohols, including 1-hexanol, cyclohexanol, 3-methyl-1-butanol, and tert-butanol, under the optimized conditions (Table 3, entries 23–26). The acetylation reactions of 1-hexanol cylohexanol, 3-methyl-1-butanol proceeded much more rapidly than the acetylation of tert-butanol, most likely because of the steric hindrance provided by the tert-butyl group.
Also, we extended the use of ZPNi for direct acetylation of some thiols (Table 3, entries 27–30) and amines (Table 3, entries 31–36) with AA. Excellent results were obtained with one equivalent of AA in the presence of ZPNi. It is obvious that the acetylation rate was influenced by the electronic factors associated with the substrates. The acetylation of thiols and amines with the electron-donor group in the para-position is faster than those with electron-withdrawing groups. The acetylation of primary amines was significantly faster than that of secondary amines (Table 3, entries 31, 32). In contrast, the rate of acetylation of thiols is lower than that of phenols and amines.
A schematic representation of the ZPNi-mediated acetylation process is shown in Scheme 3. To develop a deeper understanding of the role of the ZPNi catalyst in the acetylation reaction, we investigated the acetylation of phenol, 4-hydroxyphenol, and benzyl alcohol in the absence of the catalyst. As expected, no products were formed in any of these reactions, which demonstrated the importance of the catalyst in the acetylation process.
All of the acetylated products formed in the current study were characterized by GC–MS (Agilent 5975C spectrometer), FT-IR (JASCO FT-IR 680 plus spectrophotometer; JASCO), and 1H NMR (Bruker-Avance AQS 400 MHz spectrometer) analyses and a comparison of these data with those of standard samples or data from the literature [28, 44, 45, 47–53]. The reusability of the ZPNi catalyst was investigated under the optimum reaction conditions for the acetylation of phenol, and the results are shown in Table 4. The elemental composition of the catalyst remained largely unchanged following its 7th run, although the amount of nickel in the catalyst was reduced by almost 50 % compared with the first run (Table 1). The recycled ZPNi catalyst gave a similar product yield to the freshly prepared catalyst up until the sixth cycle.
The catalytic efficiency of ZPNi was compared with several previously reported catalysts and protocols, and the results are shown in Table 5. Phenol was converted to acetylphenol in 91 % yield following a reaction time of less than 15 min at 40 °C using the current protocol (Table 5, entry 18). Although some of the other catalysts performed well for the same reaction (Table 5, entries 2, 6, 10), they invariably required longer reaction times to reach completion (Table 5, entries 3, 6, 7, 9, 11, 13, 14) or required the use of a solvent (Table 5, entries 4, 6, 7, 9, 12). It is noteworthy that the more reactive acetyl chloride was required in one case, where it was used at room temperature (Table 5, entry 5). Furthermore, this reaction required a longer reaction time to reach afford a similar yield of the acetylated product to the current protocol.
Some of the previously reported protocols used acetic acid as the acetylating agent, representing a much greener choice than acetyl chloride (Table 5, entries 4, 8, 15), although these reactions required long reaction times (3–15 h), high temperatures (70–110 °C), and a large excess of acid acetic in almost all cases. The acetylation of phenol was also investigated using ZrOCl2 8H2O, and ZP under our optimized reaction conditions (Table 5, entries 19 and 20). ZrOCl2 8H2O gave an excellent yield of the desired product, but was much more difficult to recover and reuse than the ZPNi catalyst. ZP gave a lower yield (70 %) than ZPNi. The use of ZPZn as a catalyst, however, provided a similar result to that of ZPNi (Table 5, entry 1), with a 20 mol % loading of ZPZn providing the acetyl phenol product in 89 % yield following a reaction time of 45 min [22]. Moreover, ZPNi was compared with two other Ni-containing catalyst, NiCl2 and Ni/SiO2 as well (Table 5, entries 16 and 17). Based on this comparison process, ZPNi was identified the best catalyst for this transformation in terms of the reaction time and the loading of the catalyst.
Benzyl alcohol was also acetylated under the optimized conditions to give the acetylated product in 91 % yield following a reaction time of 15 min at 40 °C (Table 5, entry 35). This protocol was also compared with a variety of different previously reported procedures for the same transformation, and the results are shown in Table 5. When the reaction was conducted at room temperature in the presence of a different catalyst, it generally required longer reaction times to reach completion (Table 5, entries 23, 27–29, 33). The use of ZPZn, ZrOCl2 8H2O, or ZP as a catalyst under the optimized conditions provided the desired product in yields of 91, 90, and 75 %, respectively (Table 5, entries 21, 36, 37). However, large excesses of the catalyst were required in all three of these cases, and significant difficulties were encountered during the recovery of these catalysts. These reactions also resulted in lower yields of the product. There were, however, some benefits to using these catalysts, in that they used acid acetic as an acetylating agent instead AA, but they all required longer reaction times, higher temperatures, and a large excess of acid acetic to reach completion (Table 5, entries 24 and 32).
1H NMR and FT-IR spectral data of the selected compounds from Table 3 are as follows:
C6H5OAc (Table 3, entry 1). 1H NMR (400 MHz, CDCl3): δ = 7.2 (t, J = 7.9 Hz, 2H), 7.1 (t, J = 7.5 Hz, 1H), 7.0 (t, J = 7.9 Hz, 2H), 2.3 (s, 3H); IR (KBr): υ = 3055, 2915, 1753, 1581, 1485, 1364, 1179, 1014, 916, 876, 804, 739, 675 cm−1.
4-Me–C6H4OAc (Table 3, entry 3). 1H NMR (400 MHz, CDCl3): δ = 7.04 (d, J = 7.9 Hz, 2H), 6.82 (d, J = 7.9 Hz, 2H), 2.35 (s, 3H), 2.27 (s, 3H); IR (KBr): υ = 3045, 2936, 1773, 1608, 1515, 1442, 1378, 1207, 1187, 1173, 1014, 947, 915, 832, 816 cm−1.
4-(CH3)3C–C6H4OAc (Table 3, entry 6). 1H NMR (400 MHz, CDCl3): δ = 7.37 (d, J = 8.25 Hz, 2H), 7.18 (d, J = 8.25 Hz, 2H), 2.27 (s, 3H), 1.24 (s, 9H); IR (KBr): υ = 3052, 2971, 2918, 1759, 1600, 1517, 1448, 1373, 1279, 1211, 1116, 1026, 924, 841, 686 cm−1.
4-Cl–C6H4OAc (Table 3, entry 9). 1H NMR (400 MHz, CDCl3): δ = 7.43 (d, J = 8.4 Hz, 2H) 6.75 (d, J = 8.4 Hz, 2H), 2.24 (s, 3H); IR (KBr): υ = 3075, 2939, 1765, 1641, 1591, 1488, 1370, 1200, 1163, 1014, 941, 845, 798, 720 cm−1.
2-AcO–C6H4CO2H (Table 3, entry 12). 1H NMR (400 MHz, CDCl3): δ = 10.4 (s, 1H), 7.95–7.12 (m, 4H), 2.35 (s, 3H); IR (KBr): υ = 3426–2996, 2872, 1752, 1687, 1607, 1458, 1306, 1188, 917, 753, 706 cm−1.
4-MeO–C6H4CH2OAc (Table 3, entry 17). 1H NMR (400 MHz, CDCl3): δ = 7.15 (d, J = 8.5 Hz, 2H), 6.78 (d, J = 8.5 Hz, 2H), 5.0 (s, 2H), 3.65 (s, 3H), 2.2 (s, 3H); IR (KBr): υ = 3011, 2943, 2826, 1729, 1613, 1518, 1460, 1363, 1243, 1176, 1120, 1031, 960, 823 cm−1.
3-Methylbutyl acetate (Table 3, entry 23). 1H NMR (400 MHz, CDCl3): δ = 4.18 (t, J = 6.6 Hz, 2H), 2.1 (s, 3H), 1.58–1.67 (m, 1H), 1.43–1.5 (m, 2H), 0.96 (d, J = 3.4, 6H); IR (KBr): υ = 2962, 2935, 1743, 1465, 1430, 1249, 1172, 1136, 1065, 962, 857 cm−1.
4-Cl–C6H4SAc (Table 3, entry 30). 1H NMR (400 MHz, CDCl3): δ = 8.24 (d, J = 8.9 Hz, 2H). 7.61 (d, J = 8.9 Hz, 2H), 2.49 (s, 3H); IR (KBr): υ = 1745, 1503, 1222, 1198, 980, 750 cm−1.
4-NO2–C6H4NHAc (Table 3, entry 34). 1H NMR (400 MHz, CDCl3): δ = 7.43 (br s, 1H), 7.37 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.2 Hz, 2H), 2.32 (s, 3H), 2.16 (s, 3H).; IR (KBr): υ = 3361, 2916, 2849, 1702, 1685, 1610, 1597, 1443, 1408, 1368, 1314, 1253, 1175, 1118, 1000, 856, 768 cm−1.
Conclusions
ZPNi is an inexpensive, noncorrosive, and environmentally benign catalyst that can be readily prepared from simple starting materials. This catalyst was characterized using various analytical methods and the results were in agreement with those reported previously in the literature. In this study, we have developed a simple and efficient procedure for the acetylation of a variety of different alcohols in good yields over short reaction times. There are several notable advantages to this methodology, including a broad substrate scope, the use of AA as an acetylating agent, excellent product yields, and easy work-up procedure resulting from the heterogeneous conditions.
References
F. Li, M. Wei, J. He, Y. Du, P. Sun, D.G. Evans, X. Duan, Chin. J. Catal. 20, 514 (1999)
L. Sun, W.J. Boo, H.J. Sue, A. Clearfield, New J. Chem. 31, 39 (2007)
A.R. Hajipour, H. Karimi, Mater. Lett. 116, 356 (2014)
V.I. Pet’kov, A.V. Markin, I.A. Shchelokov, M.V. Sukhanov, N.N. Smirnova, Russ. J. Phys. Chem. 81, 1728 (2007)
Q. Shi, S. Tan, Y. Ouyang, Q. Yang, A. Chen, W. Li, X. Shu, J. Feng, J. Fang, Y. Chen, Adv. Matter. Sci. 150–151, 852 (2011)
X. Cai, G.J. Dai, S.Z. Tan, Y. Ouyang, Y.S. Ouyang, Q.S. Shi, Mater. Lett. 67, 199 (2012)
A. Clearfield, J.M. Kalnins, J. Inorg. Nuc. Chem. 40, 1933 (1978)
S. Allulli, C. Ferragina, A. La Ginestra, M.A. Massucci, N. Tomassini, A.A.G. Tomlinson, J. Chem. Soc. Dalton Trans. 2115 (1976)
B. Shpeizer, D.M. Poojary, K. Ahn, C.E. Runyan Jr, A. Clearfield, Science 266, 1357 (1994)
B.G. Shpeizer, P. Sylvester, R.A. Cahill, A. Clearfield, Chem. Mater. 11, 1201 (1999)
G. Alberti, M.G. Bernasconi, U. Costantino, J.S. Gill, J. Chromatogr. A 132, 477 (1977)
G. Alberti, M. Casciola, U. Costantino, R. Vivani, Adv. Mater. 8, 291 (1996)
Y. Yang, G. Dai, S. Tan, Y. Liu, Q. Shi, Y. Ouyang, J. Rare Earths 29, 308 (2011)
G. Dai, A. Yu, X. Cai, Q. Shi, Y. Ouyang, S. Tan, J. Rare Earths 30, 820 (2012)
Q.R. Zhang, W. Du, B.C. Pan, B.J. Pan, W.M. Zhang, Q.J. Zhang, Z.W. Xu, Q.X. Zhang, J. Hazard. Mater. 152, 469 (2008)
U. Costantino, L. Szirtes, E. Kuzmann, J. Megyeri, K. Lázár, Solid State Ionics 141–142, 359 (2001)
S. Khare, R. Chokhare, J. Mole. Catal. A: Chem. 344, 83 (2011)
Y. Izumi, Y. Mizutani, Bull. Chem. Soc. Jpn 52, 3065 (1979)
M. Iwamoto, Y. Nomura, S. Kagawa, J. Catal. 69, 234 (1981)
A.I. Pylinina, I.I. Mikhalenko, Russ. J. Phys. Chem. 87, 372 (2013)
A.I. Pylinina, I.I. Mikhalenko, Russ. J. Phys. Chem. 85, 2109 (2011)
A. Hajipour, H. Karimi, M. Karimzadeh, Monatsh. Chem. 145, 1461 (2014)
A.R. Hajipour, H. Karimi, Chin. J. Catal. 35, 1529 (2014)
A.R. Hajipour, H. Karimi, Chin. J. Catal. (2014). doi:10.1016/S1872-2067(14)60185-6
H.J. Yoon, S.M. Lee, J.-H. Kim, H.J. Cho, J.W. Choi, S.H. Lee, Y.S. Lee, Tetrahedron Lett. 49, 3165 (2008)
H. Sharghi, M. Jokar, M.M. Doroodmand, Adv. Synth. Catal. 353, 426 (2011)
S.A. Taghavi, M. Moghadam, I. Mohammadpoor-Baltork, S. Tangestaninejad, V. Mirkhani, A.R. Khosropour, Inorg. Chim. Acta 377, 159 (2011)
I. Montes, D. Sanabria, M. García, J. Castro, J. Fajardo, J. Chem. Educ. 83, 628 (2006)
T.S. Reddy, M. Narasimhulu, N. Suryakiran, K.C. Mahesh, K. Ashalatha, Y. Venkateswarlu, Tetrahedron Lett. 47, 6825 (2006)
S.K. Prajapti, A. Nagarsenkar, B.N. Babu, Tetrahedron Lett. 55, 1784 (2014)
M.M. Heravi, F.K. Behbahani, V. Zadsirjan, H.A. Oskooie, J. Braz. Chem. Soc. 17, 1045 (2006)
P. Yadav, R. Lagarkha, Z.A. Balla, Asian J. Chem. 22, 5155 (2010)
L. Osiglio, G. Romanelli, M. Blanco, J. Mole. Catal. A Chem. 316, 52 (2010)
F. Tamaddon, M.A. Amrollahi, L. Sharafat, Tetrahedron Lett. 46, 7841 (2005)
R. Dalpozzo, A. De Nino, L. Maiuolo, A. Procopio, M. Nardi, G. Bartoli, R. Romeo, Tetrahedron Lett. 44, 5621 (2003)
R. Gupta, V. Kumar, M. Gupta, S. Paul, R. Gupta, Indian J. Chem. Sec. B 47, 1739 (2008)
R. Tayebee, F. Cheravi, Bull. Korean Chem. Soc. 30, 2899 (2009)
Z. Liu, Q. Ma, Y. Liu, Q. Wang, Org. Lett. 16, 236 (2014)
A.K. Chakraborti, R. Gulhane, Shivani, Synthesis 111 (2004)
K. Niknam, D. Saberi, Appl. Catal. A 366, 220 (2009)
M. Lakshmi Kantam, K. Aziz, P.R. Likhar, Catal. Commun. 7, 484 (2006)
W. Wang, W. Cheng, L. Shao, J. Yang, Catal. Lett. 121, 77 (2008)
Y. Liu, L. Liu, Y. Lu, Y.Q. Cai, Monatsh. Chem. 139, 633 (2008)
C. Yue, Q. Liu, T. Yi, Y. Chen, Monatsh. Chem. 141, 975 (2010)
F. Shirini, M.A. Zolfigol, M. Abedini, Monatsh. Chem. 140, 1495 (2009)
K. Niknam, D. Saberi, Tetrahedron Lett. 50, 5210 (2009)
F. Shirini, N.G. Khaligh, Chin. J. Catal. 34, 695 (2013)
M. Hajjami, A. Ghorbani-Choghamarani, M. Norouzi, Chin. J. Catal. 33, 1661 (2012)
N. Nowrouzi, S.Z. Alizadeh, Chin. J. Catal. 34, 1787 (2013)
A. Ghorbani-Choghamarani, N. Pourbahar, Chin. J. Catal. 33, 1470 (2012)
S. Farhadi, K. Jahanara, Chin. J. Catal. 35, 368 (2014)
A. Zarei, A.R. Hajipour, L. Khazdooz, Synth. Commun. 41, 1772 (2011)
A.R. Hajipour, L. Khazdooz, A.E. Ruoho, J. Chin. Chem. Soc. 56, 398 (2009)
P. Kumar, R.K. Pandey, M.S. Bodas, S.P. Dagade, M.K. Dongare, A.V. Ramaswamy, J. Mole. Catal. A Chem. 181, 207 (2002)
J. Iqbal, R.R. Srivastava, J. Org. Chem. 57, 2001 (1992)
F. Dehghani, A.R. Sardarian, M.M. Doroodmand, J. Iran. Chem. Soc. 11, 673 (2014)
V. Constantinou-Kokotou, A. Peristeraki, Synth. Commun. 34, 4227 (2004)
M. Alam, A. Rahman, N.M. Alandis, M.R. Shaik, Arabian J. Chem. 7, 53 (2014)
M. Esmaeilpour, A.R. Sardarian, Iran. J. Sci. Technol. Trans. A Sci. 38, 175 (2014)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, A.R. Aliakbar, C. R. Chim. 17, 164 (2014)
G. Meshram, V.D. Patil, Synth. Commun. 39, 4384 (2009)
S. Farhadi, K. Jahanara, A. Sepahdar, J. Iran. Chem. Soc. 11, 1103 (2014)
F. Rajabi, R. Luque, Catal. Commun. 45, 129 (2014)
A.R. Hajipour, H. Karimi, Appl. Catal. A 482, 99 (2014)
A.R. Hajipour, H. Karimi, Chin. J. Catal. 35, 1136 (2014)
T.A. Egerton, F.S. Stone, J. Chem. Soc. Faraday Trans. 1. 69, 22 (1973)
J. Sneddon, Biochem. Pharmacol. 36, 3723 (1987)
R. Pierotti, J. Rouquerol, Pure Appl. Chem. 57, 603 (1985)
Acknowledgments
We gratefully acknowledge the funding support received for this project from the Isfahan University of Technology (IUT), IR Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajipour, A.R., Karimi, H. & Kohi, A. Highly efficient and recyclable acetylation of phenols and alcohols by nickel zirconium phosphate under solvent-free conditions. J IRAN CHEM SOC 13, 55–64 (2016). https://doi.org/10.1007/s13738-015-0711-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0711-z