Abstract
Nivolumab is an anti-programmed cell death-1 antibody that is utilized as an immune checkpoint inhibitor for several malignancies. However, this agent is associated with immune-related adverse events (irAEs), mainly in the spectrum of autoimmune disease including interstitial pneumonia, colitis, type 1 diabetes, and renal impairment. We herein present the case of a 59-year-old man with renal cell carcinoma who developed worsening renal function approximately 4 months after initiation of nivolumab. Urinalysis showed proteinuria and microscopic hematuria along with increase levels of N-acetyl-β-d-glucosaminidase. Renal biopsy revealed acute tubulointerstitial nephritis and thickening of the glomerular basement membranes. Immunofluorescence showed granular IgM deposits in capillary loops. We initiated high-dose prednisolone therapy with nivolumab, which improved renal function and achieved complete remission of proteinuria. Although renal irAEs are considered to be rare and glomerulonephropathy is not typical presentation, physicians need the close monitoring of renal function and urinalysis in patients under immunotherapy with this agents. In addition, our case provides a possible link between nivolumab and immune-mediated glomerulonephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) are attracting attention as novel cancer therapeutic agents against multiple cancer species, such as melanoma, non-small-cell lung carcinoma, and renal cell carcinoma [1,2,3]. These agents are monoclonal antibodies targeting anti-cytotoxic T-lymphocyte-associated protein-4 and anti-programmed death-1 (PD-1) signaling pathways. Nivolumab (anti-PD-1 antibody) is considered to enhance tumor-directed immune response by reactivation of cytotoxic T cell, leading to tumor cell lysis [4].
On the other hand, ICIs have been associated with numerous unique side effects, termed immune-related adverse events (irAEs). IrAEs occur in up to 60% of treated patients, usually mild to moderate in grade. The pathophysiology of irAEs is possibly mediated through non-specific immune activation against self-antigens [5]. The most commonly reported irAEs include skin rash, colitis, hepatitis, hypophysitis, interstitial pneumonia, and type 1 diabetes [5]. According to previous reports, renal irAEs are less frequent than other organ involvement [6]. Furthermore, most cases of renal irAEs present as acute tubulointerstitial nephritis; however, IgM deposition on glomerular capillary walls triggered by nivolumab has not been previously reported [7].
Herein, we present the case of a patient with metastatic clear cell renal cell carcinoma treated with nivolumab, who developed acute tubulointerstitial nephritis and immune-mediated glomerulonephropathy.
Case report
A 59-year-old Japanese man was referred and admitted to our department owing to progressive deterioration of renal function and new onset proteinuria after immunotherapy with nivolumab.
With regard to the patient’s history, he was referred to our medical center with kidney tumor and nephrotic syndrome 4 years previously. Left nephrectomy was performed for treatment of renal cell carcinoma (cT2N2M0) diagnosed using positron emission tomography–computed tomography. Histopathological analysis of the nephrectomy specimens confirmed clear cell renal cell carcinoma of the left kidney and minor glomerular abnormalities (Fig. 1). Immunofluorescence study demonstrated absence of deposits of immunoglobulins and complements. Electron microscopy showed extensive foot process effacement without any immune complex deposits (Fig. 1). Surgical resection of the tumor resulted in complete remission of proteinuria without corticosteroid therapy. After operation, the patient received several adjuvant therapies, including sunitinib, sorafenib, and pazopanib. However, 3 years after the initial diagnosis, the cancer progressed to stage IV (T0N2M1) with multiple metastases to the bone, adrenal grand, and spleen. He was then started on biweekly treatments with nivolumab (3 mg/kg, by intravenous drip infusion). Although nivolumab was effective, an acute increase in serum creatinine (Cre) levels (from 1.13 to 2.39 mg/dL) was observed approximately 4 months after initiation of nivolumab. His medications were rosuvastatin for dyslipidemia, esomeprazole for gastroesophageal reflux disease, and pregabalin for chronic pain.
On admission, his height was 172 cm, body weight was 60.9 kg, blood pressure was 123/84 mmHg, and pulse rate was 93/min. Physical examination revealed no abnormal clinical signs, such as ophthalmologic findings suggesting uveitis. Urinalysis showed proteinuria (1.5 g/day), mild hematuria (10–19/HPF), and a few granular casts. N-acetyl-β-d-glucosaminidase activity and β-2 microglobulin were elevated at 36.3 U/L and 7754 μg/L, respectively. The results of laboratory blood tests were as follows: white blood cell count 9900/μL, with 71.6% neutrophils, 2.4% eosinophils, hemoglobin 12.1 g/dL, platelet count 26.2 × 104/μL, serum albumin 3.7 g/dL, serum Cre 3.09 mg/dL, blood urea nitrogen 35.8 mg/dL, C-reactive protein 1.9 mg/dL, IgG4 33.5 mg/dL (0.9%), and angiotensin-converting enzyme 9.6 U/L. Furthermore, tests were negative for anti-nuclear, anti-neutrophil cytoplasmic, anti-Sjogren syndrome (SS)-A, and anti-SS-B antibodies (Table 1).
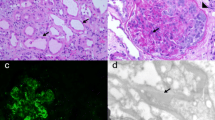
Renal biopsy was immediately performed to clarify the cause of worsening renal function. It contained total 17 glomeruli, of which 4 demonstrated global sclerosis. Light microscopy revealed thickening of the glomerular basement membranes with a bubble-like appearance, tubulitis, and focal interstitial inflammatory cell infiltration (Figs. 2, 3). The infiltrates were primarily composed of CD8+ T cells and CD68+ macrophages with admixed eosinophils and neutrophils (Fig. 3). Immunofluorescence staining showed only granular IgM deposition in the capillary loops. Staining for IgG, IgA, C3, C4, and C1q was negative (Fig. 4). Electron microscopy showed intra-membranous electron-dense deposits (arrow) in glomerular capillary wall, but not in subendothelial or mesangial area (Fig. 5). In addition, immunohistochemical staining revealed the slightly PDL-1 expression in tubular epithelial cells but not in glomeruli (Fig. 6). Based on these findings, we considered this patient as having acute tubulointerstitial nephritis and immune-mediated glomerulonephropathy.
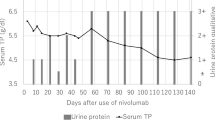
After biopsy-proven diagnosis, steroid therapy was initiated with intravenous methylprednisolone 1 g/day for 3 days, followed by oral prednisolone at 40 mg/day. Although a majority of the previous reports suggested that immunotherapy should be withdrawn when there was the possibility of irAEs, we continued nivolumab by considering the survival benefit. Steroid therapy led to rapid improvement in his renal function (serum Cre 1.32 mg/dL) along with complete remission of urinary protein excretion (0.17 g/gCre), thus allowing him to continue with nivolumab therapy. The summarized clinical course is shown in Fig. 7.
Discussion
This case report highlights that ICIs could induce not only acute tubulointerstitial nephritis, but also immune-mediated glomerulonephropathy as renal irAEs. In a previous report, two different types of ICI-related kidney injury have been reported: acute tubulointerstitial nephritis and glomerular disease [8]. Most cases of renal irAEs present as acute tubulointerstitial nephritis [9]. In the largest case series of renal irAEs [7], subepithelial and intra-membranous deposits were observed in only one patient treated with ipilimumab. As shown in Fig. 6, PDL-1 was observed in tubular epithelial cells but not in glomeruli. This finding suggests that interstitial nephritis occurred locally within the kidneys triggered by nivolumab, whereas glomerulonephropathy developed as a result of the systemic formation of immune complexes following the administration of nivolumab. To the best of our knowledge, this is the first report of IgM deposits on glomerular capillary wall following nivolumab treatment confirmed on biopsy. Similar cases would increase henceforth, and the accumulation of cases is important for elucidation of underlying mechanism and proper management of renal irAEs.
The progress of ICIs has revolutionized the therapy for variety of cancers, and ICIs have been approved for a number of types of cancers. ICIs enhance anti-tumor immunity by blocking co-inhibitory molecules that are expressed on both T cells and tumor cells [10]. The PD-1-blocking antibody “nivolumab” is approved by the Food and Drug Administration for the treatment of metastatic melanoma, non-small cell lung cancer, classical Hodgkin’s lymphoma, and renal cell carcinoma [11]. The incidence of renal adverse events was reported to be rare (< 1%) in randomized control trials of nivolumab [12], where the subjects were administered 3 mg/kg nivolumab every 2 weeks, similar to that in our case [12]. However, recent studies have reported that the incidence of renal toxicities might be higher than that previously reported [9]. Furthermore, these patients developed acute kidney injury without any symptoms, and pyuria was the only abnormality that was frequently observed in urinalyses. Therefore, any deterioration in renal function or abnormalities seen on urinalysis should raise a suspicion of ICI-associated nephrotoxicity, especially in the absence of background therapy with other agents that may worsen renal function. Early recognition of these renal irAEs by treating oncologists may be most important for the subsequent clinical course and recovery of renal function.
The patient had minimal change nephrotic syndrome when he underwent nephrectomy for renal cell carcinoma 4 years ago. Minimal change nephrotic syndrome is common among nephrotic syndromes found in patients with malignant lymphoma, whereas there is no evidence to suggest that renal cell carcinoma is associated with minimal change nephrotic syndrome. In this case, intra-membranous deposits may have appeared along with renal cell carcinoma; however, such findings were not observed in the resected specimen of the left kidney.
An animal study demonstrated that PD-1 knockout mice developed lupus-like glomerulonephritis with predominant IgG3 deposition [13]. This finding suggests that PD-1 signaling pathway is involved in immune-mediated renal inflammation. Although we found 41 cases of nephropathy in which IgM deposition was found in previous reports [14], these reports described mesangial deposition of IgM, which is different from our case. In addition, none of the studies performed experiments using animal models or described the possible mechanisms of IgM deposition. Although it may be difficult to prove the causal relationship between ICIs use and IgM deposits on glomerular capillary wall, we considered that nivolumab played an important role in the pathogenesis of immune-mediated glomerulonephropathy.
Steroid therapy is becoming a standard treatment in patients with acute tubulointerstitial nephritis as renal irAEs [15]. In the aforementioned case series of Cotazar et al. [7], complete or partial remission of renal damage was observed in 9 out of 10 patients with acute tubulointerstitial nephritis who received short-term steroid therapy; in contrast 2 patients who were not administered corticosteroids showed deterioration in renal function. Although the implicated ICIs were discontinued in previously reported patients, our patient was continued on biweekly treatment with nivolumab. As other anti-tumor agents were not effective so far, we emphasized the survival benefit by continuing the nivolumab therapy. At the time of writing, we could arrest the progression of cancer, and his renal function and proteinuria were maintained at baseline levels. Our case provides the possibility that the combination of corticosteroid therapy allows us to continue with the ICIs even when renal irAEs occurred.
In summary, we reported a newly diagnosed case of acute tubulointerstitial nephritis and immune-mediated glomerulonephropathy following immunotherapy with nivolumab. Because ICIs will be used more widely, careful monitoring of the renal function and proteinuria, as well as timely consideration of biopsy of the kidney is important.
References
Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23.
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82.
Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27:559–74.
Jung K, Zeng X, Bilusic M. Nivolumab-associated acute glomerulonephritis: a case report and literature review. BMC Nephrol. 2016;17:188.
Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–47.
Izzedine H, Mateus C, Boutros C, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2017;32:936–42.
Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45:160–9.
Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33:3193–8.
Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–53.
Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer: a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
Brugnano R, Del Sordo R, Covarelli C, et al. IgM nephropathy: is it closer to minimal change disease or to focal segmental glomerulosclerosis? J Nephrol. 2016;29:479–86.
Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from the patient whose case in reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Irifuku, T., Satoh, A., Tani, H. et al. Acute tubulointerstitial nephritis and IgM deposits on glomerular capillary walls after immunotherapy with nivolumab for metastatic renal cell carcinoma. CEN Case Rep 9, 48–54 (2020). https://doi.org/10.1007/s13730-019-00424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-019-00424-1