Abstract
Acute rejection is a major cause of graft loss in patients with kidney transplantations. However, the appropriate timing for performing a biopsy is often difficult to gauge in a clinical settings. We encountered an 8-year-old boy in whom antibody mediated rejection (AMR) associated with de novo donor-specific antibody (DSA) developed shortly after an episode of type IA acute cellular rejection (ACR). He had received a preemptive ABO-compatible kidney transplantation due to bilateral renal hypoplasia. Type IA ACR developed 2 months after transplantation and was successfully treated with methylprednisolone pulse therapy (MPT) and gusperimus hydrochloride. However, 4 months after transplantation, his serum creatinine level increased again. We decided to perform an additional biopsy despite having done the previous biopsy only a short time ago. Marked infiltration of inflammation cells in the peritubular capillaries (PTCs) with positive C4d staining was observed. AMR associated with de novo DSA with type IB ACR was newly diagnosed because DSA was not detected and the crossmatch test was negative before transplantation. He immediately received two courses of plasma exchange (PE), three courses of MPT, and rituximab. He confessed to non-adherence and underwent a patient education program with his family again. To date, no cases of AMR associated with de novo DSA shortly after ACR have been reported. Our experience lends support to the ‘episode biopsy’ method in which a biopsy is performed for each episode of serum creatinine increase as recommended by The Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Working Group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
If a recipient is negative for donor-specific antibody (DSA) before transplantation, the kidney transplantation generally shows favorable graft survival. Antibody mediated rejection (AMR) by de novo DSA is not common in such recipients. However, de novo DSA may emerge via sensitization of donor human leukocyte antigen (HLA) mainly during the first year after transplantation [1]. Graft survival in patients with de novo DSA is reportedly poor [2,3,4]. Therefore, immediate diagnosis and prompt intervention are important. We encountered an 8-year-old boy with a kidney transplantation who developed de novo DSA-mediated AMR shortly after acute cellular rejection (ACR). Because we performed a renal biopsy for every episode of significant serum creatinine increase, his condition was immediately diagnosed and successfully treated using multidisciplinary therapy including plasma exchange and rituximab. Our experience underscores the importance of ‘episode biopsies’.
Case presentation
An 8-year-old boy with bilateral renal hypoplasia had received a preemptive ABO-compatible kidney transplantation from his 62-year-old grandmother. Before transplantation, DSA was not detected (Complement Dependent Cytotoxicity (CDC): T warm (−), B warm (−), flow cytometry cross match (FCXM): T cell-IgG (−) B cell-IgG (−), flow-panel reactive antibody (PRA) class 1 0.74%, class 2 0.12%). After transplantation, he received basiliximab, methylprednisolone, tacrolimus, and mycophenolate mofetil, and his serum creatinine level was stable (0.4 mg/dl, estimated glomerular filtration rate (e-GFR) of 95.9 ml/min/1.73 m2).
Two months after the transplantation, he suffered from cytomegalovirus infection and his renal function deteriorated (serum creatinine 0.63 mg/dl, e-GFR 61.9 ml/min/1.73 m2). A kidney biopsy revealed type IA ACR (g1, t2, i2, v0). He was treated with methylprednisolone pulse therapy (MPT) and gusperimus hydrochloride. At this point, no de novo DSA was detected (Table 1). His renal function soon normalized (serum creatinine 0.43 mg/dl and e-GFR 89.4 ml/min/ 1.73 m2).
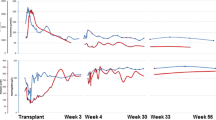
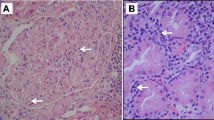
Six months after transplantation, his serum creatinine rose again (0.62 mg/dl, with e-GFR of 65.9 ml/min/1.73 m2). Episode biopsy was again performed, despite the short interval from the previous biopsy. Moderate tubulitis and C4d-positive dilated peritubular capillaries (PTCs) filled with inflammatory cells were newly observed (Fig. 1). Type II AMR and type IB ACR (g1, t3, v0, i2, ptc3, C4d3) were diagnosed. A crossmatch test (CDC-T 0%, CDC-B 30%, FCXM-T 39.06%, FCXM-B 205.82%, Flow PRA 29.65%) and de novo DSA were positive (Table 1). He received two courses of plasma exchange (PE) (1.5 plasma volume exchange for three consecutive days per course), combined with three courses of methylprednisolone pulse therapy (MPT) (30 mg/kg/day of methylprednisolone for three consecutive days per course). Finally, single-dose rituximab (375 mg/m2) was also administered. His serum creatinine level stabilized around 0.5 mg/dl (e-GFR 77 ml/min/1.73 m2) (Fig. 2). During hospitalization, he confessed that his non-adherence started around 4 months after the transplantation, much to his mother’s consternation. He and his family again underwent the patient education program based on 2009 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline on the monitoring, management, and treatment of kidney transplant recipients [5]. We identified a family conflict and attempted to resolve it.
A renal biopsy 14 months after transplantation revealed a near-complete disappearance of inflammatory cells from the PTCs and significant attenuation of C4d staining along the PTCs. The DSA had also significantly decreased (Table 1). The patient received a maintenance regimen of rituximab as recovery of his B cell after 14 months from the transplantation. Three years and 6 months after the transplantation, he received a protocol biopsy which showed no sign of AMR or ACR, but was slightly positive for C4d with PTCs. His serum creatinine level has remained around 0.6 mg/dl (e-GFR 65 ml/min/1.73 m2).
Discussion
The KDIGO Transplant Working Group recommends an ‘episode biopsy’ when a patient shows a persistent or sustained elevation of serum creatinine even after empiric treatment [5]. In our patient, the creatinine level returned to baseline after treatment for ACR but soon rose again. We initially considered strengthening treatment for the ACR. However, in accordance with the KDIGO guidelines, we performed an additional biopsy instead. Surprisingly, the results disclosed AMR and we promptly initiated multidisciplinary treatment. The methodological choices that we made contributed to preserving his graft function. To date, we have been unable to find any case reports of AMR occurring shortly after ACR, especially within 6 months of a kidney transplantation.
Wiebe et al. prospectively followed 315 Canadian patients who were negative for DSA before kidney transplantation for 6.2 ± 2.9 years [6]. Forty-seven patients (15%) developed de novo DSA. In addition, 10-year graft survival in patients who developed de novo DSA was significantly poorer than in those without it (57 vs. 96%, p < 0.0001), suggesting that the presence of de novo DSA is strongly associated with graft loss.
Recently, patients with C4d-negative AMR have been reported, although the underlying mechanism remains unclear [7]. Therefore, examination of DSA is necessary for the diagnosis of AMR. Immunosuppressive therapy per protocol and the characteristics and primary diseases of the patients are irrelevant to the emergence of de novo DSA [8]. Meanwhile, non-adherence was strongly associated with de novo DSA and graft rejection [6]. Wiebe et al. studied 315 consecutive renal transplants without pretransplant DSA and reported that there was a large difference in the rate of non-adherence between patients with de novo DSA (n = 47) and without de novo DSA (n = 268) (49 vs. 8%). Furthermore, non-adherence was more prevalent in pediatric patients (24% of the pediatric cohort vs. 13% of the adult cohort) [6]. Indeed, our patient confessed to non-adherence, which might have resulted in the emergence of the de novo DSA.
For the treatment of AMR, the KDIGO Transplant Working Group recommends rituximab combined with increased immunosuppression, intravenous immuno-globulins (IVIG) and PE [5]. Prompt reduction of DSA may improve the probability of graft survival. Everly et al. demonstrated that patients who experienced a greater than 50% reduction of DSA within 14 days from the initiation of treatment had a better prognosis [9]. Genberg et al. reported that rituximab was able to prevent DSA production for long periods by eliminating B cells even in pediatric recipients [10]. For the treatment for AMR, we propose using PE to remove DSA, steroids to reduce inflammation, and rituximab to prevent DSA production. However, more cases and longer observation are necessary to establish an effective treatment against AMR due to de novo DSA especially in pediatric patients.
Although AMR occurred shortly after an episode of ACR in the present case, our patient was successfully treated. Our experience suggests that the episode biopsy is essential to assessing allograft rejection.
References
Heilman RL, Nijim A, Desmarteau YM, Khamash H, Pando MJ, Smith ML, Chakkera HA, Huskey J, Valdez R, Reddy KS. De novo donor-specific human leukocyte antigen antibodies early after kidney transplantation. Transplantation. 2014;98:1310–5.
Devos JM, Patel SJ, Burns KM, Dilloglou S, Gaber LV, Knight RJ, Daber AO, Land GA. De novo donor specific antibodies and patient outcomes in renal transplantation. Clin Transpl.2011: 351–8.
de Kort H, Willcombe M, Brookes P, Dominy KM, Santoz-Nunez E, Galliford JW, Chan K, Taube D, McLean AG, Cook HT, Roufosse C. Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transpl. 2013;13:485–92.
Ginevri F, Nocera A, Comoli P, Innocente A, Cioni M, Parodi A, Fontana I, Magnasco A, Nocco A, Taglimacco A, Sementa A, Ceriolo P, Ghio L, Zecca M, Cardillo M, Garibotto G, Ghiggeri GM, Poli F. Posttransplant de novo Donor-specific antibodies identify pediatric kidney recipients at risk for late antibody-mediated rejection. Am J Transpl. 2012;12:3355–62.
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transpl. 2009; 29(Suppl 3):S1–S155.
Wiebe C, Gibson IW, Blydt-Hansen TD, karpinski M, Ho J, Storsley LJ, Goldberg A, Birk PE, Rush DN, Nickerson PW. Evolution and clinical pathologic correlation of de novo donor-specific HLA antibody post kidney transplant. Am J Transpl. 2012;12:1157–67.
Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Verine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahpusse M, Empana JP, Hill GS, Goltz D, Legendre C, Jouven X. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–9.
Sawinski D, Forde KA, Trofe-Clark J, Patel P, Olivera B, Goral S, Bloom RD. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor specific antibodies. J Am Soc Nephrol. 2015;26:966–75.
Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Moqilishetty G, Alloway RR, Tevar A, Woodle ES. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transpl. 2009;9:1063–71.
Genberg H, Hansson A, Wernerson A, Wennberg L, Tydén G. Pharmacodynamics of rituximab in kidney transplantation. Transplantation. 2007;84(12 Suppl):S33–S36.
Acknowledgements
The authors thank Dr. J. Tang from the Department of Education for Clinical Research, National Center for Child Health and Development, for his assistance with editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from the patient included in this article.
About this article
Cite this article
Okada, M., Kamei, K., Matsuoka, K. et al. Development of antibody mediated rejection shortly after acute cellular rejection in a pediatric kidney transplantation recipient. CEN Case Rep 7, 288–291 (2018). https://doi.org/10.1007/s13730-018-0344-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13730-018-0344-z