Abstract
Background
Antibody-mediated rejection (ABMR) is a recognized cause of late kidney allograft loss. Although ABMR may occur despite appropriate chronic immunosuppressive therapy, non-adherence both facilitates and accelerates the activation of the effector phase of the humoral immune response against the donor tissue, leading in turn to progressive kidney allograft rejection. Given the poor efficacy of rescue therapies for both acute and chronic late ABMR, establishing appropriate preventive strategies at different times before and after transplantation is a critical management goal.
Case-diagnosis/treatment
In this report, we discuss the differential diagnoses and management of ABMR based on the clinical case report of a young kidney transplant recipient with progressive ABMR due to poor immunosuppressive adherence. In the absence of sensitive and specific non-invasive monitoring tools for alloimmune activation, the clinical dilemma in the management of the adolescent patient lies in differentiating between suboptimal prescribed immunosuppression and deliberate non-adherence to adequate immunosuppression dosing. Despite the advent of therapies to reduce ABMR injury, the graft is destined for untimely functional loss.
Conclusions
New biomarkers and tools for the accurate characterization of alloimmune risk before and after transplantation, and serial testing for de novo changes in circulating donor-specific alloantibodies, are urgently needed to support the delivery of optimized immunosuppression exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To date, the presence of histological signs of generalized interstitial fibrosis and tubular atrophy (IF/TA) in kidney allografts, reflecting the nonspecific clinical features of chronic allograft nephropathy, have been considered multifactorial, driven by either non-immunological or immunological mechanisms [1]. Allograft rejection mediated by donor-specific antibodies (DSA) is responsible for many cases of late graft loss [2, 3], and seems to be driven by chronic exposure to insufficient immunosuppression either by prescription or because of deliberate non-adherence to chronic immunosuppressive therapy [4, 5].
Here, we report an illustrative clinical case of a living-donor kidney transplant recipient in the transition period from adolescent to adult care. The patient presented with persistent chronic antibody-mediated rejection (ABMR) due to poor adherence to immunosuppression therapy and did not fully respond to immunosuppressive rescue treatment. We were unable to prevent premature allograft loss. In the light of this case, we discuss the possible preventive diagnostic and therapeutic strategies applicable to young transplant populations that might prevent similar outcomes in the future.
Case report
Presenting history and key features
A 19-year-old young woman with end-stage renal disease due to immunoglobulin (Ig) A nephropathy was admitted to our transplant unit to receive a kidney allograft in May 2008. IgA nephropathy was initially suspected at the age of 14 owing to recurrent episodes of macroscopic hematuria coinciding with frequent pharyngitis and tonsillitis. The diagnosis was confirmed by a renal biopsy performed when she presented with hematuria and laboratory evidence of renal dysfunction. Typical signs of IgA nephropathy were observed and confirmed in the biopsy. Despite renoprotective treatment, Kidney function gradually progressed to end-stage renal disease over the ensuing 5 years. At that point, a preemptive living-related kidney transplant from her father was proposed, and accepted.
Management
A negative cross-match test was obtained, both by flow-cytometry and by complement-dependent cytotoxicity (CDC) assay. Determinations of anti-human leukocyte antigen (HLA) antibodies both by panel reactive antibodies (PRA) and by solid-phase assays (Luminex®) were also negative before transplantation. Subsequently, we performed transplant surgery with an HLA-haploidentical kidney allograft from the patient’s father without any major surgical complications. Immunosuppression comprised anti-CD25 monoclonal antibodies (basiliximab) for induction therapy, 1 g b.i.d of mycophenolate mofetil (Cellcept®), 5 mg/day of tacrolimus (Prograf®; starting at day 1 to achieve trough blood levels between 6–8 ng/ml during the first 3 months and 4–6 ng/ml thereafter), and 500 mg of intravenous methylprednisolone during surgery that was gradually tapered to 5 mg/day of oral prednisone thereafter. Her initial clinical course was uneventful with a rapid full recovery of renal allograft function and she was discharged on day 7 after the transplantation.
After discharge, she received a 3-month course of oral valganciclovir because there was a high-risk cytomegalovirus IgG-serostatus between the donor and recipient (D+/R-), but did not experience infection. As per protocol, an allograft biopsy was performed at 6 months after transplantation, which showed excellent preservation of the kidney allograft parenchyma without evidence of acute or chronic histological lesions. Immunofluorescent staining was negative for both IgA and C4d and solid-phase assay screening detected no evidence of circulating anti-HLA antibodies. The patient decided to restart an administrative job in her family business because she felt fully recovered. Her subsequent clinical course remained stable, with optimal kidney allograft function demonstrated by an estimated glomerular filtrate rate (eGFR) >60 ml/min, and no signs of either proteinuria or microhematuria.
Early signs of non-adherence
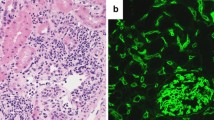
By month 10 after the transplantation, she presented with recurrent erratic trough blood levels of tacrolimus in her routine tests. Because we suspected immunosuppression non-compliance, we organized frequent meetings and talks with the patient, family, and psychologists at our center. Despite our interventions, erratic immunosuppressive trough levels persisted over subsequent months, although stable normal allograft function was maintained. At 24 months, a second kidney allograft biopsy was performed per protocol and showed mild inflammation with glomerulitis and capillaritis, with some areas showing mononuclear cells infiltrating the interstitium and some tubuli (Fig. 1). Furthermore, partial areas of interstitial fibrosis and tubular atrophy (IF/TA) were now evident. Immunofluorescent staining studies were negative for all antibodies, specifically IgA, C4d, and SV40. Screening for circulating DSA revealed some anti-HLA class II antibodies (against different DQ and DR HLA alleles), with one showing anti-donor specificity (DR11) with a mean fluorescence intensity (MFI) of 3200. Despite these changes, allograft function remained within the normal range. A diagnosis was made of subclinical acute ABMR with borderline changes suspicious of T-cell-mediated cellular rejection (TCMR), based on the revised Banff 2013 classification criteria [6].
Illustrative pictures of the second biopsy performed in the present case. a Mild pathological glomerulitis: polymorphonuclear (PMN) cells (white arrows) are evident in the capillary glomeruli. b PMN cell inflammation (white arrows) in the peritubular capillary (PTC) of the interstitium of the kidney allograft
Management of early non-adherence-mediated ABMR
Given the stability of graft function, and the biopsy changes of mild ABMR and TCMR, baseline immunosuppression levels were increased to target tacrolimus trough levels of 8–10 ng/ml for the TCMR and four consecutive weekly doses of intravenous gammaglobulin (IVIG; 0.5 g/kg per dose) were given for ABMR. The transplant team discussed the importance of adherence with the patient and a wrist watch with an alarm was purchased for the patient to set medication reminders.
Progressive chronic graft injury
Over the following 3 years, there was a gradual decline in the patient eGFR (41 ml/min) with proteinuria (>3 g/24 h) and microhematuria; in addition, the patient also developed progressive hypertension and hypercholesterolemia. Tacrolimus levels remained within target when checked, though the patient missed some of her monthly serum creatinine blood draws as well as two of her scheduled out-patient appointments. The patient was re-biopsied to evaluate if the cause of her progressive allograft dysfunction was on-going humoral allograft rejection with continued non-adherence to immunosuppressive treatment, versus a relapse of her primary IgA glomerulonephritis due to the presence of microhematuria. Figure 2 shows images from the third biopsy, which revealed severe general allograft inflammation, with glomerulitis and capillaritis by polymorphonuclear cells, double contours and thickness of the glomerular basement membrane in almost 60 % of the glomeruli, highly suggestive of transplant glomerulopathy. Compared with the previous biopsy, there was progression of IF/TA in up to 50 % of the biopsy specimen. Peritubular capillaries and mesangial cells showed diffuse immunofluorescent staining positivity for C4d and IgA respectively. Two DSAs (DR11 and A2) were identified with high MFI of 8,700 and 10,750 respectively. The biopsy confirmed a diagnosis of chronic ABMR and a relapse of IgA glomerulonephritis. The patient admitted to continued, sporadic medication non-adherence over the previous 2 years and stated that her tacrolimus levels were “normal” as she would take her medications the days before the lab draw to ensure adequate blood–drug levels.
Pictures of the third biopsy in the present case. a Severe general allograft inflammation with notable glomerulitis and capillaritis by Polymorphonuclear (PMN) cells (white arrows). b Immunofluorescent staining studies illustrating diffuse C4d positivity in most peritubular capillaries (PTCs) of the biopsy. c Double contours of the glomerular basement membrane are present in almost 60 % of the glomeruli, with typical patterns of transplant glomerulopathy evident. d Ultrastructural changes showing multi-layering of the PTC basement membrane were also evident under microscopy
Management of the ABMR, recurrent IgA, and non-adherence
Salvage therapy for the ABMR was instituted with 4-weekly intravenous infusions of 375 mg/m2 of rituximab, Solu-Medrol pulses, and an increased dose of mycophenolate mofetil. The patient was referred to the social worker and psychologist for counseling for issues with medication non-adherence. The microhematuria stabilized; however, proteinuria and renal function worsened progressively to an eGFR of 18 ml/min, necessitating hemodialysis for terminal chronic allograft dysfunction for ABMR secondary to non-adherence and recurrent IgA nephropathy.
Discussion
This case report illustrates the frequently observed clinical dilemma of managing a patient with a successful transplant, with progressive loss of functioning graft tissue due to deliberate medication and monitoring non-adherence. Subclinical alloimmune activation [7] and chronic ABMR [2, 3] are the primary causes of progressive allograft loss and result from insufficient prescribed maintenance immunosuppression and/or chronic immunosuppression non-adherence. The transplant field lacks sensitive and specific immune-monitoring tools for the accurate evaluation of the donor-specific T- and B-, NK- and monocyte-cell responses to ascertain the extent to which cellular and humoral immunity directly contributes to kidney injury and rejection [8, 9]. Lachmann et al. reported a significant deleterious impact on subsequent mean allograft survival in patients with DSAs and non-DSA anti-HLA alloantibodies compared with kidney transplant recipients without HLA alloantibodies [10]. Anti-HLA antibodies may not be detected in the early stages of humoral injury; they can also appear and disappear post-transplant, which recuses them as diagnostic markers for early alloimmune injury, rather making their persistent detection an associative biomarker of chronic humoral injury [11]. Furthermore, recent reports point to the direction that not all circulating anti-HLA antibodies, but importantly, those with complement-binding capacities, seem to be of more relevance to facilitating allograft rejection and thus allograft loss [12, 13]. Attempts to individualize immunosuppression according to baseline clinical and demographic characteristics, such as race and HLA mismatch, alone, are not sufficient for the stratification of individual alloimmune risk, such as to titrate the immunosuppression load for each patient, to effectively dampen alloimmunity [14]. The variability in the alloimmune risk profiles of patients are evident from the historical observance of many kidney transplant recipients with stable allograft function on minimal/single drug immunosuppression [15], and the observance that deliberate withdrawal of immunosuppression beyond the first year after transplantation is associated with reduced allograft survival [16] in some and stable, excellent allograft function in others [15, 17]. The low-risk patients may also have sufficient graft accommodation to be operationally tolerant to their HLA-matched allografts [18] and work is currently underway to better define biomarkers that can harness this pro-tolerogenic state, such that these patients can safely minimize immunosuppression. In our case study, it is possible that the prescribed dose of immunosuppression was lower than needed to suppress alloimmunity. Studies are ongoing to better define non-invasive biomarkers for detecting alloimmune activation in the blood [9, 19] and urine [20]. Some of these biomarkers can predict the onset of clinical and histological rejection, many months before our current efforts at detecting this injury [19]; access to this type of monitoring would have identified immune activation from non-adherence in our patient, much before substantive histological injury and fall in eGFR, thus offering the scope of early intervention, injury reversal and tissue preservation. It is clear that using drug trough levels as a form of monitoring for non-adherence is flawed in an unsupervised, outpatient setting, where dosing can be deliberately manipulated by the patient to mask non-adherence [21].
Humoral rejection, once established, sets the graft for accelerated allograft loss, as it is generally recalcitrant to the standard approaches used to manage cellular rejection [22, 23]. Several therapeutic strategies, such as plasmapheresis, immunoadsorption, rituximab, bortezomib, and IVIG, have been reported to have variable benefit for the treatment of acute ABMR in both uncontrolled and controlled non-randomized studies (Fig. 3) [24–31].
Proposed strategies to prevent kidney allograft rejection. TX transplantation, DSA donor-specific alloantibodies, IS immunosuppression, Tac tacrolimus, IVIG intravenous Immunoglobulin, mAb monoclonal antibody, ABMR antibody-mediated rejection, IF/TA interstitial fibrosis and tubular atrophy, TG transplant glomerulopathy, PTC peritubular capillaries, ASC antibody-secreting cells, Ag antigen
Non-adherence results in an increase in graft loss due to ABMR [4]. The prevalence of non-adherence in pediatric renal transplant recipients is estimated to be more than 30 %, with a high risk of late acute rejection and graft loss [5], maximally seen at the time of transition to adult care. Although several factors are known to influence the risk of non-adherence, such as the number and frequency of drug administration, the particular period of adolescence, the transition period to adult care, the socio-economic class, education levels, and factors related to the patient, the underlying disease, and healthcare system support [32, 33], non-adherence remains a huge management problem in the young transplant recipient, and requires a multi-disciplinary team approach of prevention, early detection, and behavior modification (Fig. 3). Current approaches to the management of non-adherence lie in regular screening for drug trough levels (with the premise that not all patients try to show normalized drug trough levels even during their routine medical revisions, as was seen in our case), the routine surveillance of allograft biopsies to detect sub-clinical injury [34, 35] and serial screening for de novo anti-donor antibodies [36, 37]; there is substantive support that unrecognized subclinical rejection is associated with subsequent allograft fibrosis [7, 38, 39], and early treatment reduces both the number of subsequent acute rejection episodes and the presence of chronic lesions related to transplant glomerulopathy [40, 41].
In this case report, our patient showed an excellent baseline immune profile for successful kidney transplantation; she was HLA haploidentical with the living-related donor kidney, had short cold ischemia time to minimize the ischemia–reperfusion injury process that could activate innate and adaptive alloimmunity, and she had a negative crossmatch test without evidence of pre-formed circulating anti-HLA alloantibodies. Immunosuppressive therapy was dosed post-transplant by center protocol and resulted in optimal allograft function, absence of early rejection and preservation of the allograft parenchyma at the 6-month biopsy, and the absence of circulating alloantibodies. Stable graft function, without acute rejection, in recipients of living donor, well-matched allografts, can guarantee graft survival of up to 20 years [42]. Unfortunately, non-adherence to immunosuppression, even sporadic, paved the way for donor HLA allorecognition, which continued un-noticed for months/years before the late markers of eGFR decline, proteinuria, and anti-HLA antibodies were detected. By this time, the graft was primed with humoral injury, with staining for C4d and features of chronic ABMR, and thus marked for accelerated loss. Non-adherence to immunosuppression also likely increased the risk of recurrent IgA deposits, which further aggravated tissue injury and rescue immunosuppressive treatment failed. Re-transplantation is a difficult option to prioritize for a patient who has lost a previous transplant because non-adherence. Nevertheless, the young age of the recipient and the known morbidity on dialysis necessitate team management to improve non-adherence such as to qualify as a transplant recipient again. Many programs institute 3- to 6-month periods of probation, whereby a change in behavior needs to be demonstrated after appropriate interventions for evaluating and managing non-adherence [43] have been done; these can involve regular clinic visits, laboratory draws, stable drug trough levels, and an increased demonstration of taking responsibility for individual management. Arguments for avoiding pre-emptive transplantation, to allow a young patient to perceive the morbidity of dialysis as a means of improve non-adherence, are difficult to protocolize and support for ethical reasons given the substantive benefit of quality of life after organ transplantation [44].
Appropriate maintenance immunosuppression is fundamentally based on CNIs, primarily tacrolimus, and anti-metabolites, which help to prevent the activation of direct and indirect antigen presentation pathways [45]. ABMR is difficult to treat, although some benefit has been reported [23] with the approach to clear anti-HLA antibody burden by plasmapheresis, immunoadsorption, rituximab, and bortezomib [27–31, 46, 47]. Interestingly, a C5 complement inhibitor monoclonal antibody (eculizumab) has emerged as a novel potential strategy for ABMR, which does not remove or modulate alloantibodies; instead, it nullifies cell lysis by preventing the membrane attack complex (C5b-9) from forming after antibody deposition [48, 49]. While we await upcoming RCTs, the Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Working Group recommend the use of corticosteroids, plasmapheresis, IVIG, rituximab, and lymphocyte-depleting antibodies alone or in combination when treating acute ABMR [50]. Owing to the scarcity of RCTs, treatment recommendations for chronic ABMR are limited; however, it seems reasonable that therapeutic options for acute ABMR should be equally valuable because the pathophysiology lies on a continuum (Fig. 3). Nevertheless, chronic ABMR is a much more complex biological scenario with severe and irreversible graft lesions, and thus treatment strategies are generally unable to reverse these changes. Minor benefits in temporary stabilization of graft function and DSAs have been seen in chronic ABMR with rituximab with/without IVIG [51–54], suggesting the importance of an effective immunomodulation of the B cell compartment. Ongoing clinical trials may reveal better options for chronic ABMR management; they are testing the efficacy of rituximab with or without IVIG for the treatment of chronic ABMR in the United Kingdom (NCT00476164), United States (NCT00565331), and Spain (NCT201002374667), and eculizumab for chronic ABMR (NCT01327573).
In conclusion, while outstanding progress has been made in our understanding of the pathological conditions underlying allograft rejection, we urgently need precise and prospective immune monitoring that can assess the anti-donor alloimmune status before and after HLA disparate organ transplantation to guide immunosuppression dosing, early alloimmune injury, and to improve decision-making [14, 55, 56]. The onset of ABMR in the context of poor compliance with immunosuppression therapy is a major clinical problem among young kidney transplant recipients that is associated with accelerated graft damage and loss. Despite the development of novel and potent immunosuppressive therapies over recent years, the lack of RCTs assessing both their cost-effectiveness and complication rates, make ABMR a challenging condition to treat. Early identification of non-adherence with effective multidisciplinary interventions and the serial assessment of potential subclinical rejection through non-invasive sensitive and specific biomarkers, independent of drug trough levels, are highly relevant and important in advancing clinical care and improving graft survival.
References
Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB (2002) Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 346(8):580–590
Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF (2009) Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9:2520–2531
Terasaki PI (2003) Humoral theory of transplantation. Am J Transplant 3:665–673
Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF (2012) Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12:388–399
Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN (2010) Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant 14:603–613
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff Meeting Report Writing Committee (2014) Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14:272–283
Chaudhuri A, Ozawa M, Everly MJ, Ettenger R, Dharnidharka V, Benfield M, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Li L, Sigdel TK, Hsieh SC, Dai H, Naesens M, Waskerwitz J, Salvatierra O Jr, Terasaki PI, Sarwal MM (2013) The clinical impact of humoral immunity in pediatric renal transplantation. J Am Soc Nephrol 24(4):655–664
Bestard O, Nickel P, Cruzado JM, Schoenemann C, Boenisch O, Sefrin A, Grinyó JM, Volk HD, Reinke P (2008) Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol 19(7):1419–1429
Bestard O, Cruzado JM, Lucia M, Bestard O, Cruzado JM, Lucia M (2013) Prospective assessment of antidonor cellular alloreactivity is a tool for guidance of immunosuppression in kidney transplantation. Kidney Int 84(6):1226–1236
Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, Pratschke J, Rudolph B, Schmidt D, Salama A, Schönemann C (2009) Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 87:1505–1513
Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X (2013) Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet 381(9863):313–319
Loupy A, Lefaucheur C, Vernerey D, Rugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X (2013) Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369(13):1215–1226
Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, Scoazec JY, Bachelet T, Lepreux S, Visentin J, Merville P, Fremeaux-Bacchi V, Morelon E, Taupin JL, Dubois V, Thaunat O (2014) Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol. doi:10.1681/ASN.2013101144
Lebranchu Y, Baan C, Biancone L, Legendre C, Morales JM, Naesens M, Thomusch O, Friend P (2014) Pretransplant identification of acute rejection risk following kidney transplantation. Transpl Int 7(2):129–138
Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF, ELITE-Symphony Study (2007) Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357(25):2562–2575
Opelz G, Döhler B (2008) Effect on kidney graft survival of reducing or discontinuing maintenance immunosuppression after the first year post-transplant. Transplantation 86:371–376
Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk HD, Soulillou JP, Hernandez-Fuentes MP, Lechler RI (2010) Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120(6):1848–1861
Brouard S, Mansfield E, Braud C, Li L, Giral M, Hsieh SC, Baeten D, Zhang M, Ashton-Chess J, Braudeau C, Hsieh F, Dupont A, Pallier A, Moreau A, Louis S, Ruiz C, Salvatierra O, Soulillou JP, Sarwal M (2007) Identification of a peripheral blood transcriptional biomarker panel associated with operational renal allograft tolerance. Proc Natl Acad Sci U S A 104(39):15448–15453
Li L, Khatri P, Sigdel TK, Tran T, Ying L, Vitalone MJ, Chen A, Hsieh S, Dai H, Zhang M, Naesens M, Zarkhin V, Sansanwal P, Chen R, Mindrinos M, Xiao W, Benfield M, Ettenger RB, Dharnidharka V, Mathias R, Portale A, McDonald R, Harmon W, Kershaw D, Vehaskari VM, Kamil E, Baluarte HJ, Warady B, Davis R, Butte AJ, Salvatierra O, Sarwal MM (2012) A peripheral blood diagnostic test for acute rejection in renal transplantation. Am J Transplant 12(10):2710–2718
Sigdel TK, Salomonis N, Nicora CD, Ryu S, He J, Dinh V, Orton DJ, Moore RJ, Hsieh SC, Dai H, Thien-Vu M, Xiao W, Smith RD, Qian WJ, Camp DG 2nd, Sarwal MM (2014) The identification of novel potential injury mechanisms and candidate biomarkers in renal allograft rejection by quantitative proteomics. Mol Cell Proteomics 13(2):621–631
Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D (2010) Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant 14:968–975
Lucas JG, Co JP, Nwaogwugwu UT, Dosani I, Sureshkumar KK (2011) Antibody-mediated rejection in kidney transplantation: an update. Expert Opin Pharmacother 12:579–592
Roberts DM, Jiang SH, Chadban SJ (2012) The treatment of acute antibody-mediated rejection in kidney transplant recipients: a systematic review. Transplantation 94:775–783
Ibernón M, Gil-Vernet S, Carrera M, Serón D, Moreso F, Bestard O, Cruzado JM, Grinyó JM (2005) Therapy with plasmapheresis and intravenous immunoglobulin for acute humoral rejection in kidney transplantation. Transplant Proc 37(9):3743–3745
Lefaucheur C, Nochy D, Andrade J, Verine J, Gautreau C, Charron D, Hill GS, Glotz D, Suberbielle-Boissel C (2009) Comparison of combination plasmapheresis/IVIg/anti-CD20 versus high-dose IVIg in the treatment of antibody-mediated rejection. Am J Transplant 9:1099–1107
Bonomini V, Vangelista A, Frasca GM, Frascà GM, Di Felice A, Liviano D'Arcangelo G (1985) Effects of plasmapheresis in renal transplant rejection: a controlled study. Trans Am Soc Artif Intern Organs 31:698–703
Kirubakaran MG, Disney AP, Norman J, Pugsley DJ, Mathew TH (1981) A controlled trial of plasmapheresis in the treatment of renal allograft rejection. Transplantation 32:164–165
Allen NH, Dyer P, Geoghegan T, Harris K, Lee HA, Slapak M (1983) Plasma exchange in acute renal allograft rejection: a controlled trial. Transplantation 35:425–428
Kaposztas Z, Podder H, Mauiyyedi S, Illoh O, Kerman R, Reyes M, Pollard V, Kahan BD (2009) Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transpl 23:63–73
Vangelista A, Frasca GM, Nanni Costa A, Stefoni S, Bonomini V (1982) Value of plasma exchange in renal transplant rejection induced by specific anti-HLA antibodies. Trans Am Soc Artif Intern Organs 28:599–603
Macaluso J, Killackey M, Paramesh A, Zhang R, McGee J, Slakey D, Brock G, Vehaskari M, Cannon R, Buell J (2011) Comparative study of bortezomib therapy for antibody mediated rejection. Am J Transplant 11 [Suppl 2]:160. Abstract #431
Takemoto SK, Pinsky BW, Schnitzler MA, Lentine KL, Willoughby LM, Burroughs TE, Bunnapradist S (2007) A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. Am J Transplant 7:2704–2711
Denhaerynck K, Dobbels F, Cleemput I, Desmyttere A, Schäfer-Keller P, Schaub S, De Geest S (2005) Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int 18:1121–1133
Hymes LC, Greenbaum L, Amaral SG, Warshaw BL (2007) Surveillance renal transplant biopsies and subclinical rejection at three months post-transplant in pediatric patients. Pediatr Transplant 11:536–539
Mengel M, Chapman JR, Cosio FG, Cavaillé-Coll MW, Haller H, Halloran PF, Kirk AD, Mihatsch MJ, Nankivell BJ, Racusen LC, Roberts IS, Rush DN, Schwarz A, Serón D, Stegall MD, Colvin RB (2007) Protocol biopsies in renal transplantation: insights into patient management and pathogenesis. Am J Transplant 7:512–517
Morath C, Opelz G, Zeier M, Susal C (2012) Prevention of antibody-mediated kidney transplant rejection. Transpl Int 25:633–645
Denhaerynck K, Steiger J, Bock A, Schäfer-Keller P, Köfer S, Thannberger N, De Geest S (2007) Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant 7(1):108–116
Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR (2004) Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 78:242–249
Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, Gil-Vernet S, Cruzado JM, Torras J, Grinyó JM, Serón D (2006) Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant 6:747–752
Rush D, Nickerson P, Gough J, McKenna R, Grimm P (1998) Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol 9:2129–2134
Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Méjean A, Charron D, Duong van Huyen JP, Bruneval P, Legendre C, Nochy D (2009) Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant 9:2561–2570
Shapiro R, Sarwal MM (2010) Pediatric kidney transplantation. Pediatr Clin N Am 57(2):393–400
Shaw RJ, Palmer L, Blasey C, Sarwal M (2003) A typology of non-adherence in pediatric renal transplant recipients. Pediatr Transplant 7(6):489–493
Sarwal MM, Bagga A (2013) Quality of life after organ transplantation in children. Curr Opin Organ Transplant. doi:10.1097/MOT.0b013e3283653550
Cruzado JM, Bestard O, Grinyó JM (2009) Control of anti-donor antibody production post-transplantation: conventional and novel immunosuppressive therapies. Contrib Nephrol 162:117–128
Waiser J, Schutz M, Liefeldt L, Schonemann C, Neumayer HH, Klemens B (2010) Treatment of antibody-mediated renal allograft rejection with bortezomib or rituximab. Am J Transplant 10 [Suppl 4] :466. Abstract #1504
Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, Ducloux D, Hurault de Ligny B, Moulin B, Le Gouge A, Lebranchu Y (2013) One year results of the effects of rituximab on acute humoral rejection in renal transplantation: RITUX ERAH, a multicenter randomized placebo controlled trial. Am J Transplant 13 [Suppl 5]:112. Abstract #266
Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM (2011) Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11:2405–2413
Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK, Stewart ZA, Warren DS, Zachary AA, Montgomery RA (2009) The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant 9:231–235
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9 [Suppl 3]:S1–S155
Smith RN, Malik F, Goes N, Farris AB, Zorn E, Saidman S, Tolkoff-Rubin N, Puri S, Wong W (2012) Partial therapeutic response to rituximab for the treatment of chronic alloantibody mediated rejection of kidney allografts. Transpl Immunol 27:107–113
Billing H, Rieger S, Süsal C, Waldherr R, Opelz G, Wühl E, Tönshoff B (2012) IVIG and rituximab for treatment of chronic antibody-mediated rejection: a prospective study in paediatric renal transplantation with a 2-year follow-up. Transpl Int 25(11):1165–1173
Billing H, Rieger S, Ovens J, Süsal C, Melk A, Waldherr R, Opelz G, Tönshoff B (2008) Successful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation 86:1214–1241
Fehr T, Rusi B, Fischer A, Hopfer H, Wüthrich RP, Gaspert A (2009) Rituximab and intravenous immunoglobulin treatment of chronic antibody-mediated kidney allograft rejection. Transplantation 87:1837–1841
Poggio ED, Augustine JJ (2013) Alloreactive T cells and ‘the persistence of memory’. Kidney Int 84:1074–1076
Li L, Khush K, Hsieh SC, Ying L, Luikart H, Sigdel T, Roedder S, Yang A, Valantine H, Sarwal MM (2013) Identification of common blood gene signatures for the diagnosis of renal and cardiac acute allograft rejection. PLoS One 8:e82153
Acknowledgements
This work was supported in part by national Spanish grant (PI13/01263) and the Spanish Red de Investigación Renal (REDinREN, RD12/0021). We would like to thank Dr Montse Gomà from the Pathology Department at Bellvitge University Hospital for providing the histopathology pictures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bestard, O., Sarwal, M.M. Antibody-mediated rejection in young kidney transplant recipients: the dilemma of noncompliance and insufficient immunosuppression. Pediatr Nephrol 30, 397–403 (2015). https://doi.org/10.1007/s00467-014-3020-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-014-3020-3