Abstract
The isothermal crystallization process, kinetics, melting behavior and equilibrium melting point of bio-based semi-aromatic high-temperature polyamide PA5T/56 were studied in detail by differential scanning calorimetry. The results showed that the crystallization properties of PA5T/56 are strongly dependent on temperature, with increases in isothermal crystallization temperature, the crystallization process becomes longer and the crystallization speed slows down. The isothermal crystallization process and kinetics were analyzed by Avrami equation, the Avrami index (n value) is between 1.84 and 2.41, it is speculated that the growth mode of the crystal is the coexistence of one-dimensional needle growth and two-dimensional sheet growth, which is confirmed by two-dimensional small angle X-ray scattering instrument and field emission scanning electron microscope. At the same time, according to Turnbull–Fisher equation, it is reasonable to use Arvami equation to treat the isothermal crystallization process of PA5T/56. According to Hoffman–Weeks theory, the equilibrium melting point of PA5T/56 raises with the increase in PA5T content. The isothermal crystallization activation energy of PA5T/56 was calculated by Arrhenius equation, and the kg value of PA5T/56 was calculated by Lauritzen–Hoffman equation, both of them increase first and then decrease with the increase in PA5T content. Therefore, the study on the crystallization mechanism and crystal formation process of PA is of great significance for controlling the crystallinity of semi-crystalline polymers to obtain the required performance.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Semi-aromatic polyamide molecular chain contains both flexible aliphatic segment and rigid aromatic structure. Therefore, it has the aliphatic and fully aromatic polyamide characteristics. It not only has excellent heat resistance and mechanical properties, but also has the characteristics of low water absorption and high fluidity. It can be melt extruded and injection molded under conventional conditions, it is widely used in the field of high-temperature resistant resin [1].
At present, PA6T is the main semi-aromatic high-temperature polyamide [2], and its raw materials are from non-renewable resources. In recent years, due to the gradual consumption of non-renewable resources and the gradual deterioration of the environment, people pay more and more attention to bio-based polymer materials, and become a research hotspot [3]. Bio-based semi-aromatic high-temperature polyamide PA5T/56 is a semi-crystalline polymer, its crystalline morphology and degree have a great influence on the mechanical properties, dimensional stability, heat resistance, wear resistance and water absorption of the materials [4]. At present, the research on the crystallization properties of semi-aromatic polyamides mainly focuses on polyhexanediamine terephthalate (PA6T), polynonyldiamine terephthalate (PA9T), polydecanediamine terephthalate (PA10T), polydodecanediamine terephthalate (PA12T) and their composites. Wang et al. [5] studied the isothermal crystallization behavior of melt polymerized high-temperature resistant polyamide 10 T and 10 T/11 resin by DSC, and calculated the Avrami index and equilibrium melting point. Liu et al. [6] successfully prepared PA10T/66/GF composites by blending the synthesized long carbon chain high-temperature resistant copolymer nylon 10 T/66 (PA10T/66) with glass fiber (GF). The non-isothermal crystallization behaviors of PA10T/66 and PA10T/66/GF were analyzed by DSC. Wang et al. [7] studied the non-isothermal crystallization kinetics of PA6T/11 by DSC. The results have shown that Mo method can better describe the non-isothermal crystallization process of PA6T/11. In addition, the non-isothermal crystallization activation energy was obtained by Kissinger method and the crystallization rate coefficient was obtained by means of Khanna, which are 324.836 kJ/mol and 42.1 h−1 for PA6T/11, respectively. Su et al. [8] prepared semi-aromatic polyamide PA10T and its copolymer poly-p-phthaloyl decanediamine/polydodecyldecanediamine (PA10T/1012) by one pot polymerization. The non-isothermal crystallization behaviors of PA10T and PA10T/1012 were studied and compared in detail by DSC. The results showed that the cooling rate of PA10T/1012 needed to be increased to obtain the same relative crystallinity as PA10T per unit time. The above studies showed that the research on the crystallization kinetics of materials will help to improve the service properties of materials and understand the forming mechanism of materials.
Bio-based semi-aromatic high-temperature polyamide PA5T/56, as a newly developed bio-based high-temperature engineering plastic, has high melting point (\(T_{{\text{m}}}\)), glass transition temperature (\(T_{{\text{g}}}\)), thermal deformation temperature (HDT), acid resistance, wear resistance and thermal stability. Its disadvantages are high water absorption and high production cost. At present, the crystallization behavior of bio-based semi-aromatic high-temperature polyamide PA5T/56 is less studied. Therefore, it is of great significance for controlling the crystallinity of semi-crystalline polymers to obtain the required performance by studying the variation of macro-crystalline structure parameters with time under different isothermal conditions and mastering the crystallization mechanism and crystal formation process of such polymers [9]. The isothermal crystallization characteristics of PA5T/56 were studied by DSC, and the isothermal crystallization behavior and kinetics were analyzed, some parameters of isothermal crystallization such as Avrami index, equilibrium melting point and activation energy were obtained, it provides a theoretical basis for the application of bio-based semi-aromatic high-temperature polyamide PA5T/56.
The novelty of this paper is that the selected raw material is bio-based semi-aromatic high-temperature polyamide material, and the variation of macro-crystalline structure parameters with time under different isothermal conditions is studied.
Experimental
Materials
Bio-based semi-aromatic high-temperature polyamide PA5T/56 (the bio-base component is pentanediamine), Shanghai Cathay Biotechnology Co., China. The samples with 50%, 55%, 60% and 65% PA5T were numbered 1, 2, 3 and 4, respectively, and put it in a vacuum oven at 90 °C for 12 h to wait for testing. The number average molecular weights of PA5T/56 samples 1, 2, 3 and 4 ranged from 24,195 to 28,458 g/mol, and the molecular weight distribution width (PDI) ranged from 2.62 to 2.83, the relative viscosity ranged from 2.0 to 2.3. The specific preparation process is as follows:
Weighed appropriate amount of water, antioxidant, defoamer, catalyst and a certain proportion of pentanediamine, adipic acid and terephthalic acid into the reactor simultaneously. Replaced the air in the reactor with nitrogen (N2) for three times, and then started heating. The experiments were carried out in the order of concentration, pressure rise, pressure maintaining, depressurization and vacuumization, finally, when the torque reached the expected value, discharge.
Instruments
Differential scanning calorimeter (DSC): Q2000, TA Company, American; Analytical Balance, XSE205DU, Mettler Toledo Company, Switzerland; Two-dimensional small angle X-ray scattering instrument (2D-SAXS): nanostar, Bruker Company; field emission scanning electron microscope (SEM): Regulus8230, Hitachi Company.
Crystallization kinetics test of PA5T/56
An amount of 5 mg sample is weighed and put into an aluminum crucible. The reference sample is an empty crucible. Under the protection of N2 (flow rate: 50 mL/min), the temperature is raised to 330 °C at the rate of 10 °C/min, and maintaining at 330 °C for 3 min to eliminate the heat history. Then, the temperature is rapidly lowered to the set isothermal crystallization temperature (\(T_{{\text{c}}}\)) at the rate of 100 °C/min. After isothermal crystallization for a certain time, the temperature is raised to 330 °C at the rate of 10 °C/min. The change of heat flow rate is recorded.
Results and discussion
Isothermal crystallization curve of PA5T/56
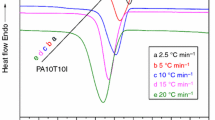
The bulk crystallization phenomenon of semi-crystalline polymer can occur between the \(T_{{\text{g}}}\) and \(T_{{\text{m}}}\). Figure 1 shows the heat flow versus time curves of PA5T/56 at different crystallization temperatures i.e., isothermal crystallization curves. It can be seen from Fig. 1 that the decrease in isothermal crystallization temperature, the range of exothermic peak of PA5T/56 becomes narrower, the crystallization time becomes shorter, and the peak shape becomes sharper. This is mainly because at lower crystallization temperature, due to further undercooling, the nucleation free energy becomes smaller, the nucleation growth rate is faster, the crystallization is relatively easy, and the crystallization process is faster. On the contrary, if the crystallization temperature is higher, the crystal growth rate is faster and faster, but at the same time, the thermal movement of molecules is also more and more intense, the nucleation is not easy to generate, and the nucleation growth rate is smaller and smaller, which leads to the crystallization rate of polymer become smaller with the increase in crystallization temperature, and the crystallization becomes difficult.
Figure 1 shows that the recrystallization of the four samples occurred in different degrees at different crystallization temperatures, and the two crystallization peaks merged gradually with the decrease in crystallization temperature. This is mainly due to the fact that the crystals of the samples which polymerized first and then crystallized are generally highly oriented fibrous crystals, in the process of isothermal crystallization i.e., annealing, the heterogeneous nucleation initiated by impurities and the homogeneous nucleation initiated by molecular chain folding on the side surface of the fibrous crystal will lead to the growth of new lamellae on the side surface of the fibrous crystal, thus recrystallization occurs and shish-kebab is formed with the shape is similar to “mutton kebab”. Moreover, at higher crystallization temperature, the nucleation rate is higher than the crystal growth rate, which leads to the growing slowly of new formed lamellae, results in a wider crystallization peak. However, at lower crystallization temperature, the opposite is true, so a sharp crystallization peak appeared and the two peaks merged gradually with the decrease in crystallization temperature.
By integrating the crystallization curves of polymer, the relative crystallinity, \(X_{{\text{t}}} ,\) at different crystallization times can be obtained, the equation is [10]:
where \(X_{{\text{c}}} \left( t \right)\) and \(X_{{\text{c}}} \left( {t = \infty } \right) \) are the relative crystallinity at time \(t\), and at the completion of crystallization, respectively; \(\frac{{{\text{d}}H_{{\text{c}}} }}{{{\text{d}}t}}\) is the crystallization heat flux at time \(t\). Figure 2 shows the relationship between relative crystallinity and crystallization time of PA5T/56. It can be seen from Fig. 2 that the \(X_{{\text{t}}}\) ~ \(t\) curves of samples 1, 2 and 3 are different from that of sample 4. This phenomenon is mainly related to the molecular chain structure and recrystallization of the samples. Because there will still be a small amount of tiny crystalline particles in the molten sample, it plays the role of nucleating agent during isothermal crystallization. At the same time, an appropriate amount of benzene rings also plays a role of nucleating agent in PA5T/56, and excessive benzene rings will affect the crystallization due to their own steric hindrance effect. Therefore, when isothermal crystallization is started, the tiny crystalline particles in samples 1, 2 and 3 will rapidly recrystallize to form the so-called shish-kebab, which is faster than the growth rate of other crystal forms. However, sample 4 has a high content of benzene ring, which hinders the movement of molecular chain, and the growth rate of all crystal forms is basically the same. Therefore, the phenomenon shown in Fig. 2 occurs. Moreover, at the same crystallization temperature, the relative crystallinity of polymer at the initial stage of crystallization increases slowly with the extension of time. After a period of crystallization, the relative crystallinity increases rapidly until the later stage of crystallization, and finally the relative crystallinity increases slowly again. This is mainly because polymer crystallization needs to go through two stages: nucleation and crystal growth. In the early stage of crystallization, polymer segments must overcome a certain potential barrier and form orderly chain bundles as crystal nuclei through molecular thermal motion, the higher the undercooling, the faster the nucleation rate. When a large number of crystal nuclei are formed, the crystals grow rapidly and the relative crystallinity increases rapidly. In the later period of crystallization, because the relative crystallinity is larger and tended to be perfect, some imperfect or defective crystals need to be further improved, so the relative crystallinity raises slowly again. In the preliminary stage of crystallization, some of the curves will have a platform for the growth of relative crystallinity to slow down, which is mainly due to the slower growth rate of recrystallized crystal at higher crystallization temperature, which prolongs the crystallization time.
Melting behavior and equilibrium melting point of PA5T/56 after isothermal crystallization
Figure 3 shows the melting curves of the samples after isothermal crystallization, and the melting points of related polymers in Fig. 3 are listed in Table 1. It can be seen from Fig. 3 that with the increase in crystallization temperature, the melting peak of polymer gradually moves towards high temperature, that is, the melting point increases and the melting range is wide, and double melting peaks appear at the same time. This is mainly because the isothermal crystallization of the samples is equivalent to annealing. Generally speaking, annealing at lower temperature is mainly to eliminate stress, expel various defects, perfect crystal lattice and improve crystallinity, but there is no obvious crystal thickening. When annealing is done in the middle temperature, the lamellae can be further thickened into a more thermodynamic stable state, mainly through the diffusion of solid chain. Whereas, when annealing is at a higher temperature, the melting point of lamellae with finite thickness is still far lower than the thermodynamic equilibrium melting point of infinite crystal, and the thicker lamellae still has the opportunity to grow. Figure 4 is a schematic diagram of lamellar thickening and growth, therefore, the lamellar mainly occurs local melting recrystallization mechanism. Therefore, the inner part of the samples after isothermal crystallization is more perfect, and the thickness of the crystal also increases in varying degrees, which makes the melting enthalpy per unit volume increases, \(T_{{\text{m}}} { }\) raises, and the crystal is more stable. Moreover, the isothermal crystallization makes the crystal thicken, but also leads to a wider distribution of crystal thickness. There will be a wider temperature range for polymer crystal melting, which is the melting range. At the same time, after isothermal crystallization treatment, the crystal morphology of the samples changes from fibrous crystal to shish-kebab. Because the complex structure of shish-kebab determines its complex thermal properties, it shows multiple melting peaks on DSC heating curves, and the melting peak at lower temperature corresponds to epiphytic lamellae, while the peak height of these melting peaks first increases and then decreases. With the increase in PA5T content the peak height reaches the highest at sample 2, which just confirms the conclusion analyzed in Fig. 2. It can be seen from Table 1 that the melting point of the polymer increases with the increase in PA5T content at the same crystallization temperature. This is mainly due to the increases in PA5T content, which makes the density of benzene ring in the molecular chain increases, improves the internal rotation barrier of the molecular chain, and improves the rigidity of the molecular chain. Moreover, with the increase in PA5T content, the relative molecular weight of the polymer raises. Consequently, the melting point increases due to the two reasons. For the same PA5T/56 sample, the melting point of PA5T/56 raises with the increase in isothermal crystallization temperature, which is mainly due to the increase in isothermal crystallization temperature, the nucleation rate of polymer increases and the crystal growth rate reduces. When there is enough crystallization time, increasing the crystallization temperature will contribute to the formation of more crystal nuclei, thus the crystallinity of polymer is improved and the melting point is increased.
Under different crystallization conditions, the thickness and the degree of perfection of the crystal formation will be different. The larger the thickness and the more perfect the crystal, the higher would be the melting point of the polymer. When the crystal thickness tends to infinity, the melting point of the polymer will reach a limit value, which is called the equilibrium melting point, \(T_{{\text{m}}}^{0} \) [11]. The \(T_{{\text{m}}}^{0}\) can be determined by Hoffman–Weeks extrapolation, the equation is as follows [12]:
where \(T_{{\text{m}}} \) is the melting point measured experimentally, \(T_{{\text{c}}} \) is the crystallization temperature, \(T_{{\text{m}}}^{0}\) is the equilibrium melting point, \(\gamma \) is the crystal thickness factor, which is the ratio of the initial thickness to the final thickness of polymer molecular crystal.
Figure 5 shows the equilibrium melting point curve of PA5T/56, the crystallization temperature, \(T_{{\text{c}}} \), plotted by melting point, \(T_{{\text{m}}} \). The extrapolation of the obtained straight line intersected with the straight line, \(T_{{\text{m}}} = T_{{\text{c}}}\), and the intersection point is the equilibrium melting point, \(T_{{\text{m}}}^{0}\). Equilibrium melting point, \(T_{{\text{m}}}^{0}\), and crystal thickness factor, \(\gamma ,\) of PA5T/56 are listed in Table 2. It can be seen from Table 2 that with the increase in PA5T content, the \(T_{{\text{m}}}^{0}\) of PA5T/56 is gradually raised, and the degree of crystallization perfection of the polymer increases. The \(\gamma\) decreases gradually and tends to be stable, this may be because with the increase in PA5T content, the content of rigid molecular chain increases and the mobility of molecular chain decreases, which causes reductions in the segment flexibility of polymer molecules and the decreases in crystal growth rate, so the crystal thickness gradually becomes thinner. However, when the content of PA5T continues to increase, the segment flexibility of polymer molecules decreases to the extreme value, and the decreasing trend of crystal growth rate also slows down, so the \(\gamma\) gradually tends to become stable.
Isothermal crystallization kinetics analysis of PA5T/56 Avrami [13] equation can be used to analyze the crystallization kinetics of polymer under isothermal conditions:
By taking the logarithm on both sides:
where K is the kinetic rate constant; n is the Avrami index, which is related to the nucleation mechanism and growth mode, and is equal to the sum of the time dimension of nucleation process and the space dimension of growth. Avrami equation can quantitatively describe the pre-crystallization stage (or main crystallization stage) of polymer, however, in the later period of crystallization (or sub crystallization stage), the phenomenon of polymer crystallization deviating from Avrami equation will appear due to the encounter or collision of growing spherulites [14]. Therefore, in the \(\log \left\{ { - \ln \left[ {1 - X_{{\text{t}}} } \right]} \right\}\) ~ \(\log t\) curves of PA5T/56 would be linear fitted in the range of relative crystallinity 20–80%, Avrami index, n, and \(\log K\) can be obtained from the slope and intercept of the straight line, which are listed in Table 3.
Figure 6 shows the crystallization kinetics curves of PA5T/56, it can be seen from Fig. 6 that \(\log \left\{ { - \ln \left[ {1 - X_{{\text{t}}} } \right]} \right\}\) versus \(\log t\) curves gives a good linear relationship, which indicates that Avrami equation is suitable for describing the isothermal crystallization process of PA5T/56.
The time required to complete 50% crystallization is defined as half crystallization period \(t_{1/2}\), and the reciprocal of half crystallization period is usually defined as crystallization rate \(\tau_{1/2 }\) [15]. Another important kinetic parameter of polymer is the maximum crystallization time \(t_{\max }\) [16], it refers to the crystallization time from the beginning of crystallization to the maximum crystallization rate at different isothermal crystallization temperatures. The relative crystallinity corresponding to this time point is the relative crystallinity, \(X_{{\text{t}}}\), at the maximum crystallization rate. The above parameters are listed in Table 3.
It can be seen from Table 3 that the Avrami index, n, of PA5T/56 is ranged between 1.84 and 2.41, while the theoretical value of Avrami index, n, should be an integer, ranging from 1 to 4, but the nucleation mode is divided into homogeneous nucleation and heterogeneous nucleation, in which homogeneous nucleation takes the ordered chain bundles formed by molecular thermal movement are used as crystal nuclei, which is dependent on time and has the characteristics of simultaneous nucleation and growth; Heterogeneous nucleation mainly takes imperfect crystal, defective crystal, foreign impurity, solid small particles as crystal nuclei to grow, and it is independent from time, and has the characteristics of continuous nucleation and growth [4]. Therefore, the heterogeneous nucleation and secondary crystallization in the polymer system lead to the Avrami index, n, not being an integer. From the n value of PA5T/56, it can be inferred that the crystal growth mode of PA5T/56 may be the coexistence of one-dimensional needle growth and two-dimensional sheet growth. In addition, Figs. 7 and 8 are 2D-SAXS image and SEM image of brittle fracture section of sample 4 after its isothermal crystallization treatment, respectively. It can be seen from Figs. 7 and 8 that there is a crystal growth mode dominated by lamellae in PA5T/56 sample, accompanied by a small amount of fibrous crystals and shish-kebab, which confirms the above speculation. At the same crystallization temperature, the n and \(\tau_{1/2}\) of PA5T/56 raise with the increase in PA5T content, which indicates that the increases in PA5T content leads to the growth mode of PA5T/56 gradually tending to two-dimensional sheet growth which accelerates the crystallization rate. This may be because with the increase in benzene ring content, the chain length of the same structural unit is shortened, the entanglement structure of molecular chain is reduced, the symmetry and regularity of polymer structure are increased, the distance between molecules is increased, the intermolecular of hydrogen bonding is reduced, the intermolecular slip would be easier, which increases the rate of nucleation and crystal growth. Thus, the crystallization rate is increased. Due to the double crystal peak in samples 1, 2 and 3, it is impossible to measure \(t_{\max }\) accurately. Therefore, only \(t_{\max }\) and \(X_{{\text{t}}}\) of sample 4 has been listed. It can be seen from the \(t_{\max }\) and \(X_{{\text{t}}}\) values of sample 4 that the crystallization rate is gradually raised with the decrease in crystallization temperature, and a certain degree of crystallization lag phenomenon occurs. The main reason for the crystallization lag phenomenon is that the nucleation rate is faster and the crystal growth rate is slower at a lower temperature, which leads to thin and imperfect crystals, so it needs extra time to perfect and the crystallization lag phenomenon occurs.
Isothermal crystallization activation energy of PA5T/56
The isothermal crystallization activation energy is an important parameter to describe the difficulty of polymer crystallization. According to Arrhenius equation, the activation energy can be calculated as follows [17]:
The logarithms on both sides would give:
where K is the kinetic rate constant, \(K_{0} \) is a constant independent of temperature, \(\Delta E \) is the isothermal crystallization activation energy, \(R\) is the gas constant, and \(T_{{\text{c }}}\) is the crystallization temperature. Figure 9 shows the isothermal crystallization activation energy curves of PA5T/56, a straight line can be obtained by plotting \(\ln K/n\) to \(1/T_{{\text{c}}}\), and the isothermal crystallization activation energy, \(\Delta E\), can be calculated from the slope of the straight line. It can be seen from Fig. 9 that the linear relationship of PA5T/56 is relatively good (correlation coefficient, \(r\) > 0.964). The calculations of isothermal crystallization activation energies of samples 1, 2, 3 and 4 give − 45.03 kJ/mol, − 198.99 kJ/mol, − 325.95 kJ/mol and − 187.99 kJ/mol, respectively. According to the change of crystallization rate with temperature the crystallization activation energy is reflected by higher activation energy, and the stronger would be the effect of temperature. Figure 10 shows the molecular structure diagram of PA5T/56, it shows that the increase in benzene ring content causes greater sensitivity of polymer crystallization rate toward temperature, which may be related to the molecular structure formula of PA5T/56. Because the addition of PA5T does not affect the distance between PA56 molecular chains, the plane of amide bond can remain unchanged with only slight transfer in the plane. Therefore, with the increase in PA5T content, the entanglement of molecular chain is reduced, the viscosity of polymer melt is decreased, and the mobility of chain segment increases, so the sensitivity of polymer crystallization rate toward temperature increases. When the content of PA5T increases to a certain value, the content of benzene ring is relatively high, the molecular chain regularity of polymer increases, the melt viscosity raises, and the effect of temperature on the crystallization rate of polymer weakens, so the activation energy is reduced.
Study on crystal growth mode by Turnbull–Fisher equation
The Turnbull–Fisher equation can be used to describe the growth of polymer crystals. The equation is as follows [18]:
where \(G \) is the spherulite growth rate, \(G_{0} \) is the rate of nucleation with critical size, \(K \) is Boltzmann constant, \(\Delta F_{{\text{D}}}^{*} \) is the activation free energy required for the diffusion of the chain segment into the crystal interface, and \(\Delta F^{ \ne } \) is the activation free energy required for the formation of stable nuclei. Therefore, the first term of the index is called the migration term, and the second term is called the nucleation term. Between \( T_{{\text{g}}}\) and \(T_{{\text{m}}}\), the closer \(T_{{\text{c}}} \) is to \(T_{{\text{g}}}\), the faster nucleation and the slower growth, so the nucleation term is large and the migration term is small, and the crystallization rate is determined by the migration term. The closer \(T_{{\text{c}}} \) towards \(T_{{\text{m}}}\), the faster growth and the slower nucleation, so the migration term is large and the nucleation term is small, and the crystallization rate is determined by the nucleation term [19]. The \(T_{{\text{c}}} \) used in this study is very close to \(T_{{\text{m}}}\) of the samples, so the crystallization rate is determined by the nucleation term, therefore, Eq. (7) can be changed into:
The logarithms of both sides would give:
According to Hoffman equation [20], Eq. (9) can be transformed into:
where \(\chi \) is a parameter involving enthalpy and interfacial free energy, and finally, Lin [21] has deduced Eq. (11) as follows:
where \(B\) and \(C \) are constants and \(\Delta T \) is undercooling, \(\Delta T = T_{{\text{m}}}^{0} - T_{{\text{c}}}\). Due to the double crystal peak in samples 1, 2 and 3, it is impossible to measure \(t_{\max }\) accurately, therefore, only sample 4 is processed. Figure 11 is the crystallization growth curve of PA5T/56 (Turnbull–Fisher equation), a straight line can be obtained by plotting \({ }1/(T_{{\text{c}}}^{2} \Delta T)\) with \({\text{lnt}}_{\max }\). It can be seen from Fig. 11 that the linear relationship of sample 4 is good, and the correlation coefficient is 0.9956, which shows that it is reasonable to use Avrami equation to treat the isothermal crystallization process of PA5T/56.
Study on crystal growth mode by Lauritzen–Hoffman equation
The Lauritzen–Hoffman equation describes the relationship between the radial growth rate, \(G\), of polymer spherulites and the isothermal crystallization temperature, \(T_{{\text{c}}}\), the equation is [20]:
The logarithms of both sides are:
where \(G\) is the radial growth rate of polymer spherulites, which can be replaced by the crystallization rate \(\tau_{1/2}\) [21], \(G_{0 }\) is a constant, \(U^{*} \) is the migration activation energy of polymer molecular segments moving to crystal growth, generally \(U^{*}\) = 6280 J/mol [22], \( R \) is the gas constant, \( T_{\infty }\) is the freezing temperature of polymer molecular segment, generally \( T_{\infty } = T_{g} - 30 K\), in which \(T_{{\text{g}}}\) of samples 1, 2, 3 and 4 are 375.67 K, 377.63 K, 380.31 K and 382.52 K, respectively. \(K_{{\text{g }}}\) is the nucleation parameter related to energy, and also related to the side surface free energy per unit area, folding surface free energy per unit area and \(T_{{\text{m}}}^{0}\) during nucleation growth [23], \(\Delta T\) is undercooling, \({\text{and}} f = 2T_{{\text{c}}} /\left( {T_{{\text{m}}}^{0} + T_{{\text{c}}} } \right) \) is the temperature correction factor.
Figure 12 is the crystallization growth curve of PA5T/56 (Lauritzen–Hoffman equation), the slope of the straight line can be obtained by linear fitting, that is, the \(K_{{\text{g}}}\) value of the samples, which are listed in Table 4. This shows that with the increase in PA5T content, the \(K_{{\text{g}}}\) value of PA5T/56 first increases and then decreases, that is, the nucleation rate of PA5T/56 first increases and then decreases, which also confirms the results analyzed in Fig. 2.
Conclusion
The isothermal crystallization process, kinetics, melting behavior and \(T_{{\text{m}}}^{0}\) of bio-based semi-aromatic high-temperature polyamide PA5T/56 were studied by DSC. The results showed that the crystallization properties of PA5T/56 have strong dependence on temperature, with the increase in \( T_{{\text{c}}}\), the crystallization process becomes longer and the crystallization speed slows down. The Avrami index, n, was between 1.84 and 2.41, which indicated that the growth mode of crystal was the coexistence of one-dimensional needle growth and two-dimensional sheet growth, which was confirmed by 2D-SAXS and SEM. At the same time, according to Turnbull–Fisher equation, it was reasonable to use Arvami equation to treat the isothermal crystallization process of PA5T/56. According to Hoffman–Weeks theory, \(T_{{\text{m}}}^{0}\) of PA5T/56 increases with the increase in PA5T content. The isothermal crystallization activation energy of PA5T/56 has been calculated by Arrhenius equation, and the \(K_{{\text{g}}}\) value of PA5T/56 was calculated by Lauritzen–Hoffman equation. Both increased and then decreased with the increase in PA5T content.
References
Rwei S-P, Ranganathan P, Chiang W-Y, Lee Y-H (2018) Synthesis of low melting temperature aliphatic-aromatic copolyamides derived from novel bio-based semi aromatic monomer. Polymers 10:793–798
Gao J, Huang W, He W, Long L, Qin S (2021) Superior flame retardancy of glass fiber-reinforced polyamide 6T composites by synergism between DOPO-based derivative and carbon nanotube. J Thermal Anal Calorim 9:121–130
Watt E, Abdelwahab MA, Mohanty AK, Misra M (2021) Biocomposites from biobased polyamide 4,10 and waste corn cob based biocarbon. Compos Part A Appl Sci Manuf 145:106340
Zhang XK, Xie TX, Yang GS (2006) Isothermal crystallization and melting behaviors of nylon 11/nylon 66 alloys by in situ polymerization. Polymer 47:2116–2126
Liu BX, Hu GS, Zhang JT, Yan W (2019) Study on non-isothermal crystallization kinetics of high temperature resistant PA10T/66 and PA10T/66/GF. Appl Eng Plast 47:105–109
Wang ZQ, Hu GS, Zhang JT (2017) Isothermal crystallization kinetics of melt polymerization of high temperature resistant polyamide. Mater Guide 031:137–144152
Wang J, Hu G, Zhang J, Zhang B, Wang Z (2014) Study on non-isothermal crystallization kinetics of heat-resistant PA6T/11 copolyamide and its preparation. Plast Sci Technol 11:34–38
Su CX, Wang DB, Zhang CH, Zhou ZX, Cao M, Jiang SJ, Huang XB (2019) Non-isothermal crystallization kinetics of pa10t and PA10T/1012. Appl Eng Plast 47:105–116
Hu GS, Ding ZY, Li YC, Wang BB (2009) Crystalline morphology and melting behavior of nylon11/ethylene-vinyl alcohol/dicumyl peroxide blends. J Polym Res 16:263–269
Liu HX, Huang YY, Yuan L, He P, Cai Y, Xu Y, Yu Y, Xiong H (2010) Isothermal crystallization kinetics of modified bamboo cellulose/PCL composites. Carbohydr Polym 79:513–519
Zou P, Tang S, Fu Z, Xiong H (2009) Isothermal and non-isothermal crystallization kinetics of modified rape straw flour/high-density polyethylene composites. Int J Therm Sci 48:837–846
Liu P, Xue Y, Men Y (2019) Melt memory effect beyond equilibrium melting point in commercial isotactic polybutene-1. Ind Eng Chem Res 58:44–57
Run M, Song H, Wang S, Bai L, Jia Y (2010) Crystal morphology, melting behaviors and isothermal crystallization kinetics of SCF/PTT composites. Polym Compos 30:87–94
Zhao H, Zhang D, Li Y (2020) Morphology and crystallization kinetics of rubber-modified nylon 6 prepared by anionic in-situ polymerization. Sci Eng Compos Mater 27:204–215
Liu S, Yu Y, Cui Y, Zhang HF, Mo ZS (1998) Isothermal and nonisothermal crystallization kinetics of nylon-11. J Appl Polym Sci 70:2371–2380
Lin CC (2010) The rate of crystallization of poly(ethylene terephthalate) by differential scanning calorimetry. Polym Eng Sci 23:113–116
Tjong SC, Chen SX, Li R (2015) Crystallization kinetics of compatibilized blends of a liquid crystalline polymer with polypropylene. J Appl Polym Sci 64:707–715
Biber E, Gündüz G, Mavis B, Colak U (2010) Compatibility analysis of Nylon 6 and poly(ethylene-n-butyl acrylate-maleic anhydride) elastomer blends using isothermal crystallization kinetics. Mater Chem Phys 122:93–101
Wang ZQ, Hu GS, Zhang JT, Xu JS, Shi WB (2018) Isothermal crystallization kinetics of Nylon 10T and Nylon 10T/1010 copolymers: effect of Sebacic acid as a third comonomer. J Wuhan Univ Technol (Mater Sci) 33:237–245
Neugebauer F, Ploshikhin V, Ambrosy J, Witt G (2016) Isothermal and non-isothermal crystallization kinetics of polyamide 12 used in laser sintering. J Therm Anal Calorim 124:925–933
Liu TX, Mo ZS, Wang SE, Hong F (1997) Isothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone) (PEEKK). Eur Polym J 33:1405–1414
Cai J, MiaoL LW, Yao K, Xiong H (2011) Isothermal crystallization kinetics of thermoplastic starch/poly(lactic acid) composites. Carbohydr Polym 86:941–947
Mondal A, Sohel MA, Arif PM, Thomas S, Sengupta A (2021) Effect of ABS on non-isothermal crystallization kinetics of polyamide 6. J Therm Anal Calorim 20:443–456
Acknowledgements
We thank all those who participated in the study. This research was supported by Shanghai Cathay Biotechnology Co., China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, C., Liu, X. Isothermal crystallization kinetics of bio-based semi-aromatic high-temperature polyamide PA5T/56. Iran Polym J 31, 605–617 (2022). https://doi.org/10.1007/s13726-021-01012-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-021-01012-w