Abstract
A novel type of copper-based regulating agents was first applied in radical polymerization of methyl methacrylate and acrylonitrile. It was shown that systems based on copper complex with redox-active bis(acetonaphthene) ligand (dpp-BIAN↔CuCl)2, carbon tetrachloride and different activating agents such as ascorbic acid or amines (tert-butylamine, diethylamine, triethylamine and pyridine) were capable to initiate polymerization of the above monomers in wide temperature range from 25 to 110 °C. The amine structure and basicity were found to make great impact on polymerization and molecular weight parameters of the obtained products. The systems developed are capable of conducting polymerization up to high monomer conversions in a wide temperature range leading to polymers with rather low molecular weight and moderate polydispersity. The most efficient system in terms of controlling the molecular weight characteristics of poly(methyl methacrylate) among others is the system based on (dpp-BIAN↔CuCl)2 and diethylamine, which makes it possible to obtain polymer with a polydispersity index of 1.36–1.60. In case of acrylonitrile polymerization, the highest polymer yield is achieved using a copper complex and triethylamine. The results of MALDI TOF mass spectroscopy measurements showed the presence of chlorine atoms at the chain ends of macromolecules. The formation of “living” chains during polymerization of methyl methacrylate was confirmed by the synthesis of block-copolymers with styrene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catalysis of polymerization processes by transition metal complexes is one of the most promising and rapidly growing directions of modern polymer science and material sciences [1, 2]. The use of transition metal complexes allows polyolefin synthesis with high degree of stereoregularity at low pressure [1], to conduct ring opening polymerization [2] and atom transfer radical polymerization (ATRP) [3,4,5] as well as to realize many other valuable polymerization techniques [6,7,8]. These methods of polymerization have been demonstrated as an effective route for preparing new polymer structures such as polymer brushes [9], star polymers [10, 11], block copolymers [12] and graphene nanoplatelets [13]. The efficiency of metal-based catalytic systems may be finely tuned by varying catalyst structure, e.g. by changing ligand environment.
From this standpoint, the use of so-called redox-active ligands, which can dramatically change reactivity of metal centers seems very promising to tune catalyst activity as in organic synthesis so in polymerization processes [14,15,16]. For example, complexes of d-metals such as palladium [15,16,17], copper [17], cobalt [18], chromium [19], nickel [20] with diimine ligands including acenaphthene imine derivatives display high catalytic performance in olefin polymerization, exceeding other types of catalysts. Complexes of transition and non-transition metals with redox-active ligands were found to be active regulators of radical polymerization of vinyl monomers [21]. The presence of redox-active ligands in catalyst structure can dramatically change polymerization mechanism. It was shown that bis-(triphenylphosphine)-3,6-di(tert-butyl)benzosemiquinone-1,2-copper(I) revealed a high regulating ability in radical polymerization of various monomers under UV- or radical initiation [22]. A mononuclear copper complex with acenaphthene ligand [CuCl2(2,4,6-Me3C6H2-BIAN)] was successfully applied in reverse ATRP of styrene in the presence of azobisisobutyronitrile (AIBN) [23]. Polymerization was characterized with first-order kinetic plot. The molecular weight linearly increased with conversion, but molecular weight distribution of obtained samples was rather broad (Mw/Mn= 2.2–2.4).
Investigation of methyl methacrylate (MMA) and styrene polymerization in the presence of cobalt complex with redox-active ligand showed its ability to regulate molecular weight parameters of polymers synthesized using alkyl halides or AIBN as a radical source [24, 25]. The presence of iminobenzosemiquinone ligand in complex structure allows minimizing catalytic chain transfer to cobalt center during MMA polymerization [26]. It was shown that chain transfer constant for explored complex was four orders of magnitude lower in comparison with cobalt porphyrins and cobaloxime complexes known as catalytic chain transfer agents in methacrylate polymerization [27].
The presence of sterically hindered redox-active quinone-based ligands in complexes of non-transitional elements of 14th group allowed these compounds to participate in reversible termination of propagating polymer chain [28, 29]. In this case, ligand acts as a redox-active center responsible for the regulation of polymerization contrary to transition metal complexes where regulating ability is determined by metal atom. Mono- and bis-catecholate complexes of non-transition metals are capable of accepting carbon-centered radicals and act as regulators of MMA and styrene polymerization, but polymers formed in these conditions have rather broad molecular weight distributions (Mw/Mn= 2.1–2.6).
Among the recently obtained compounds with redox-active ligands a binuclear (dpp-BIAN↔CuCl)2 has a great appeal to act as efficient catalyst for controlled polymerization. The choice of the given complex is determined by the presence in its structure the redox-active 1,2-bis(o,o′-bis(isopropyl)phenylimino)acenaphthene ligand as well as a copper atom. As the metal atom, so the ligand in this complex seems to be capable of participation in reversible reduction/oxidation processes. This fact allowed us to propose this complex to act as a catalyst for ATRP. Following this proposition, we investigated the peculiarities of polymerization of MMA and acrylonitrile (AN) chosen as model monomers in the presence of (dpp-BIAN↔CuCl)2 and revealed the role of ligand environment of this complex and activating agents in initiation and control of radical polymerizations.
Experimental
Materials
N-Hexane, methylene chloride, benzene, toluene, tetrahydrofuran (THF) and other solvents purchased from Component Reaktiv (Russia) were purified, dried and distilled using standard methods. Dimethylformamide (DMF) was dried over KOH and distilled at 46 °C at 2–4 Torr. Dimethylsulfoxide (DMSO) was dried over calcium hydride and distilled at 50 °C at 2–4 Torr. Tert-butylamine (Aldrich, Japan), diethylamine (Aldrich, USA) and triethylamine (Aldrich, Japan) were distilled over sodium. Pyridine was dried over KOH and distilled. Ascorbic acid (Aldrich, USA) and 2,2′-bipyridyl (Aldrich, USA) were used as received. AIBN (Aldrich, Ireland) was recrystallized from methanol and stored at − 15 °C. Carbon tetrachloride (Component Reaktiv, Russia) was dried over calcium hydride and distilled. A 0.1 mol L− 1 solution of CCl4 in toluene was used as initiator.
MMA (Aldrich, Japan) was washed from inhibitor by 10% aqueous alkali solution and then by distilled water, separated, dried over calcium chloride and calcium hydride and distilled under reduced pressure. AN (Aldrich, The Netherlands) was dried over calcium hydride and distilled under atmospheric pressure.
Copper BIAN complex was synthesized as described in [30].
Polymerization
In a typical polymerization experiment, a portion of copper complex was placed in a glass tube (2.5 × 10− 3 mol L− 1 for MMA or 1.25–5.00 × 10− 3 mol L− 1 for AN) and a required amount of monomer (MMA—9.46 mol L− 1 or AN—5.07 mol L− 1), initiator (0.5–1.0 × 10− 2 AIBN or 1.25–2.5 × 10− 2 mol L− 1 CCl4), activator (1–5×10− 2 mol L− 1) in the case of a halogen-based initiator and solvent (in case of AN) was added. ATRP of MMA was carried out at a molar ratio of components: [monomer]:[(dpp-BIAN↔CuCl)2]:[CCl4]:[activator] = [3800]:[1]:[10]:[20]. The tube was degassed through three freeze–pump–thaw cycles, sealed under vacuum and placed in a thermostatic bath. After a period of time, the tube was frozen in liquid nitrogen to stop polymerization, while opened to atmosphere the reaction mixture was diluted by ethyl acetate and poured into n-hexane (for MMA) or distilled water (for AN). The precipitated polymer was dissolved in methylene chloride (MMA) or DMF (AN) and re-precipitated by n-hexane (MMA) or water (AN) to remove residues of monomer, initiator and copper complex. The prepared polymers were dried under reduced pressure to constant weight. The polymer yield was calculated as ratio of weight of isolated polymer to weight of initial monomer.
Poly(methyl methacrylate)-block-polystyrene copolymers were synthesized using poly(methyl methacrylate) (PMMA) as macroinitiator. Initial PMMA was obtained through polymerization of MMA at 70 °С in the presence of (dpp-BIAN↔CuCl)2 (2.5 × 10− 3 mol L− 1), CCl4 (2.5 × 10− 2 mol L− 1) and activator (Et3N or ascorbic acid, 5 × 10− 2 mol L− 1). After polymerization the excess of monomer was evaporated under reduced pressure and the polymer was dried. A required amount of PMMA was dissolved in styrene–toluene 1:2 (v:v) mixture to obtain 15% weight solutions and placed into glass tubes. New portions of copper complex (2.5 × 10− 3 mol L− 1) and activators (5 × 10− 2 mol L− 1) were added, the tube was degassed, sealed and placed into the thermostatic bath. The polymers formed were precipitated by n-hexane. The PMMA and polystyrene homopolymers were extracted in Soxhlet apparatus using acetonitrile and cyclohexane, respectively. After extraction, polymers were dried up to constant weight to determine composition and molecular weight parameters.
Characterization
The molecular weight distributions of the obtained polymers were measured by SEC with a chromatographic system (Knauer Smart Line, Germany) equipped with a Knauer model 2300 refractive-index detector. Tetrahydrofuran was used as an eluent in PMMA and PMMA-b-polystyrene polymerization at a flow rate of 1 mL min−1 at 25 °C. Styragel packed columns with pore size 103 and 105 Å (Phenomenex, USA) were employed. In the case of AN, a 0.1 mol L−1 LiBr solution in DMF was used as the eluent at a flow rate of 1 mL min−1 at 40 °C. A linear styragel packed column (Phenomenex linear (2), USA) was employed. The calibration was performed with poly(methyl methacrylate) standards (Polymer Standards Service, Phenomenex, USA) ranging from 2400 to 970,000 g mol−1. In case of AN, the molecular weight was recalculated using Mark–Houwink equation:
The MALDI (matrix-assisted laser desorption/ionization) and TOF (time-of-flight) mass spectra were recorded using Bruker Microflex LT mass spectrometer (Germany) using DCTB as a matrix. A solution of polymer (2 µL, 10 mg mL−1), a matrix (10 µL, 20 mg mL−1) and sodium trifluoroacetate (5 µL, 5 mg mL−1) were mixed in a vial and 2 µL of obtained solution was placed on a stainless steel target plate, dried and analyzed.
A cyclic voltammetry was performed in inert atmosphere using an IPC Pro Potentiostat (Volta, Russia) using a platinum disk working electrode (1 mm), platinum counter electrode and a Ag|AgNO3 pseudo-reference electrode [31]. Tetrabutylammonium tetrafluoroborate (Aldrich, Switzerland) was used as a supporting electrolyte.
IR spectra of thin films of the polymers were recorded on the surface of ZnSe glass plates using an Infralum FT–801 apparatus (Simex Analytical Equipment, Russia) in the 3500–550 cm− 1 wavenumber range. The compositions of the block copolymers were calculated from the calibration of the mixture of homopolymers. The bands of stretching vibrations of the carbonyl group of MMA (1730 cm− 1) and out-of-plane C–H deformation vibrations of the styrene aromatic ring (700 cm− 1) were selected as analytical bands. The error in determining the composition of the copolymer was ± 5%.
The registration of electronic absorption spectra (ESP) for products of model reactions (dpp-BIAN↔CuCl)2 with amines in benzene was carried out with a UV–visible Spectrophotometer Uvmini-1240 with scan range of 200–1100 nm (Shimadzu, Japan) at room temperature.
Results and discussion
Metal complexes bearing redox-active ligands may be considered as compounds having two reaction centers capable of redox processes: a metal and a ligand. So, we decided to investigate electrochemical behavior of (dpp-BIAN↔CuCl)2 in 1,2-dichloroethane solution to estimate oxidative and reduction potentials. The presence of one oxidative peak at a cyclic voltammetry curve (Fig. S1 in Supplementary file) shows that oxidation of this complex proceeds irreversibly. This fact allowed us to make a proposition that as (dpp-BIAN↔CuCl)2 possesses reductive properties it is capable of participating in oxidative addition of atoms or radicals. At the same time, the irreversible oxidation probably reflects in irreversible addition. To test this proposition polymerization of MMA and AN in the presence of explored compound was investigated in detail.
Radical polymerization of MMA in the presence of copper BIAN complex
Polymerization of MMA initiated by AIBN was conducted at 70 or 90 °C in the presence or absence of copper complex. The obtained data (Table S1 in Supplementary file) indicated that the introduction of (dpp-BIAN↔CuCl)2 into the polymerization media had no significant influence on polymerization. Monomer conversion and molecular weight parameters of the obtained samples remained invariable after the addition of complex. These results indicated very low reactivity of the (dpp-BIAN↔CuCl)2 toward propagating polyMMA macroradicals. The sterically hindered carbon-centered radicals reacted neither with the metal center nor with the redox-active ligand.
Experiments on the polymerization of MMA in the presence of (dpp-BIAN↔CuCl)2 with carbon tetrachloride as initiator showed that the proposed mixture was capable of initiating polymerization in wide temperature range from 25 to 110 °C. The data summarized in Table 1 indicated that polymerization proceeded with rather slow rate and monomer conversion does not exceed 20% after 100 h of polymerization. Introduction of aliphatic amines into polymerization mixture resulted in increase of polymerization rate and monomer conversion. At the same time, the polymerization rate increased in the range of used activators: t-BuNH2 < Et2NH < Et3N.
The molecular weights of obtained the polyMMA varied in broad range depending on monomer conversion and nature of initiating system. In spite of the rather high polydispersity of the obtained polymer (Mw/Mn=1.3–2.5) the molecular weight distribution remained unimodal (Fig. 1). The polymers with the lowest Mw/Mn values were obtained when diethylamine was used as activating agent. The increase of the polymerization temperature resulted in higher polymerization rate and formation of polymers with broader molecular weight distribution. The polymerization rate tended to increase with increasing amine basicity.
Pyridine (Py) had less activating influence on MMA polymerization in the presence of the copper complex in contrast to aliphatic amines. With pyridine, the increase of polymerization temperature from 70 to 90 °C resulted in significant drop in polymerization rate. This is probably due to the substitution of BIAN ligand by pyridine resulting in formation of less active copper complexes. The obtained data are in agreement with earlier published results indicating that copper pyridine complexes are characterized by low catalytic activity in ATRP process [5]. Moreover, the introduction of pyridine into reaction media results in the absence of color change during the sample preparation through freeze–pump–thaw cycle in contrast to initial (dpp-BIAN↔CuCl)2 complex. The characteristic feature of the latter one is the change of solution color on freezing due to intermolecular electron transfer between metal and ligand. So, the absence of color change confirms the substitution of the dpp-BIAN ligand by pyridine which reflects on peculiarities of polymerization. The polymers formed in these conditions were characterized by extremely broad and multimodal molecular weight distribution (Fig. 1) which may be caused by the formation of new reaction centers during polymerization.
It is well-known [32] that amines are capable of reacting with organic halides under photoirradiation conditions leading to free radicals through single electron transfer. The radicals formed due to this reaction may further initiate polymerization. In this work, the polymerization of MMA in the presence of 2.5 × 10− 2 mol L− 1 carbon tetrachloride and 5 × 10− 2 mol L− 1 of the explored amines was conducted to estimate the contribution of the reaction in the absence of light. The results of experiments showed that monomer conversion reached only 10–20% in 100 h in using CCl4–amine systems. These values are significantly lower than those obtained in the presence of (dpp-BIAN↔CuCl)2 complex. Moreover, the obtained polymers had very high molecular weights (3000–4000 kDa) which were two orders of magnitude higher than the case of using copper complex. The obtained values clearly indicated that copper complex participated in MMA polymerization process playing a key role in it.
Using ascorbic acid as an activating ligand allowed MMA polymerization process with rather high rate at 25–70 °C (Table 1). The obtained polymers had unimodal molecular weight distribution (Fig. 1) but rather high polydispersity (Mw/Mn ≈ 2.5–2.7) relative to polymers obtained in presence of amines. The molecular weights of the obtained polymers were about 300 kDa and slightly decreased with temperature increase while the polydispersity remained the same. Thus, diethylamine seemed to be the most preferable activating agent for obtaining narrow-dispersed polymers, while triethylamine and ascorbic acid provided the most accelerating effect.
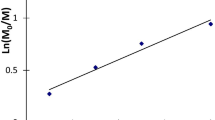
The detailed investigation of MMA polymerization in the presence of [CCl4–(dpp-BIAN↔CuCl)2–amine] systems showed that semi-logarithmic kinetic plots were not linear (Fig. 2a). Polymerizations tended to self-acceleration at conversions over 50%. Amine nature and its basicity had an impact on polymerization rate. Using triethylamine allowed conducting process with high rate up to high monomer conversion at 70 °C. At the same time, monomer conversion was significantly lower in case of diethylamine (Table 1). Polymerization of MMA using ascorbic acid as an activating agent also resulted in uncontrollable polymerization. In this case initial polymerization rate was close to that in case of tertiary amine, but monomer conversion was limited by 60%.
a Semi-logarithmic kinetic plots for MMA polymerization, b the dependence of Mn on conversion for MMA polymerization. Line: theoretical Mn values were calculated using equations: \({M_{{\text{n,th}}}}=\frac{{{{[M]}_0} \times {\text{Conv}}. \times {M_{{\text{monomer}}}}}}{{{{[{\text{Initiator}}]}_0}}}+{M_{{\text{initiator}}}}.\), c The dependence of Mw/Mn on conversion for MMA polymerization, and d MWD curves of PMMA obtained in the presence of Et2NH. The monomer conversion is indicated near the curves. Polymerization conditions: in the presence of Et3N or ascorbic acid at 70 °С; in the presence of Et2NH at 90 °C. Reagent concentrations: 9.46 mol L− 1 [MMA]0, 2.5 × 10− 3 mol L− 1 (dpp-BIAN↔CuCl)2, 2.5 × 10− 2 mol L− 1 CCl4, and 5 × 10− 2 mol L− 1 activator
The dependency of molecular weight on conversion, depicted in Fig. 2b, indicates the absence controlled polymerization when using triethylamine or ascorbic acid as activating agents. On the contrary, some decrease of polymer molecular weight observed is an indication of chains formation with lower molecular weights at later stages of polymerization. This fact can be explained either by an increase of initiation rate throughout the process or by chain transfer processes. The polymers formed under these conditions had rather broad molecular weight distributions: Mw/Mn ∼ 2.0–2.2 for Et3N and Mw/Mn ∼ 2.5–2.7 for ascorbic acid; an independency on conversion (Fig. 2c).
The use of diethylamine instead of tertiary amine, as an activating agent, dramatically changed polymerization process. In such case, increase of monomer conversion results in an increase of polymer molecular weight in good agreement with the theoretical values calculated from assumption that each CCl4 molecule generates one polymer chain (Fig. 2b). Despite that polymerization was not fully controlled as it proceeded with non-linear kinetic plot at 90 °C (Fig. 2a). The increase of polymerization rate at high monomer conversions was caused by higher quantity of propagating species and resulting in an increase of molecular weight distribution at high conversions (Fig. 2c). At the same time, the molecular weight distribution curves remained unimodal and gradually shifted towards the area of high molecular weights with increase of conversion (Fig. 2d).
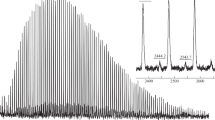
The end-group analysis of polymers formed during polymerization in the presence of diethylamine was performed using MALDI-TOF MS. The spectrum depicted in Fig. 3 represented three series of signals separated by 100 Da corresponding to MMA unit (Table 2). A major one corresponds to macromolecules containing trichloromethyl group in one end of the molecule and terminated either by a hydrogen atom or by a double bond formed during disproportionation. A second one corresponds to polymer molecules with trichloromethyl group at α-end and chlorine atom at ω-end. In both cases, the presence of a sodium cation coordinated to macromolecule was observed. The third less intensive series of signals may be referred to macrocations formed from polymer molecules during the addition of diethylamine moiety at ω-end. Formation of similar structures was observed during copper-catalyzed ATRP in the presence of nitrogen-based ligands [33]. The chlorine-ended macromolecules give the opportunity for re-initiation of polymerization and increase of molecular weight with conversion. The presence of a large amount of “dead” chains determines high polydispersity of the obtained samples.
MALDI-TOF mass spectra recorded for PMMA formed in the presence of (dpp-BIAN↔CuCl)2 (2.5 × 10− 3 mol L− 1), CCl4 (2.5 × 10− 2 mol L− 1) and diethylamine (5 × 10− 2 mol L− 1) (Entry 7 in Table 1)
Thus, the use of secondary amine instead of tertiary amine resulted in higher control over polymerization process. It should be mentioned that the increase of polymerization temperature up to 110 °C led to higher polymerization rate, though it was accompanied by the loss of control over the process (Table 1).
The polymers obtained in the presence of triethylamine or ascorbic acid as activating agents were high molecular weights based on MALDI-TOF MS analyses. To estimate the ratio of “dead” and “living” chains formed during polymerization with these activators an attempt was made to obtain block-copolymers with styrene. The obtained data summarized in Table 3 indicated that about 50% of the initial macroinitiator entered into block copolymer independent of the nature of activating agent. These results indicated that the polyMMA formed in the presence of copper BIAN complex contained, as in chlorine-capped “living” chains, “dead” chains incapable of further propagation. The molecular weight of the copolymer formation relatively increased the molecular weight of the initial polyMMA.
The molecular weight distribution curves obtained using refractive index and UV detectors were uniform corroborating the homogeneity of styrene distribution in macromolecules with different molecular weights (Fig. 4). Using triethylamine as activator resulted in the formation of block-copolymers with slight higher molecular weight distribution relative to macroinitiator, while using ascorbic acid led to slight decrease of polydispersity (Table 3).
MWD curves of PMMA macroinitiators and PMMA-b-polystyrene obtained at 70 °C. (Polymerization conditions provided in Table 3)
The obtained data on polymerization of MMA in the presence of copper complex with BIAN ligand and different activating agents indicate that the proposed system is not suitable for achieving high degree of control over polymerization contrary to conventional copper-based ATRP systems [34,35,36] and reversible-deactivation radical polymerization [37]. This may be determined by different factors such as low activity of complex with redox-active ligand in reversible activation/deactivation of polymer chain as well as by its decomposition during polymerization at high temperature leading to products which can participate in chain termination and transfer reactions.
Polymerization of acrylonitrile in the presence of copper BIAN complex
It is well-known that monomer structure has a significant impact on polymerization conducted in the presence of metal complexes [3,4,5]. So, we decided to investigate the influence of copper complex on polymerization of acrylonitrile, which is characterized by more active propagating radicals.
The introduction of (dpp-BIAN↔CuCl)2 into polymerization of acrylonitrile performed in DMF or DMSO and initiated by AIBN resulted in lower yield as well as in the molecular weight of the obtained polymers (Table S1 in Supplementary file). The molecular weight distribution slightly decreased independent of solvent nature. It should be mentioned that using DMSO as a solvent resulted in the formation of PAN with higher yield and molecular weights. So, copper complex acts as a poor inhibitor for AN polymerization.
We have also performed the polymerization of AN initiated by carbon tetrachloride, (dpp-BIAN↔CuCl)2 in conjunction with different activating agents: amines or ascorbic acid in various solvents. We first tried to use benzene as a solvent for polymerization as (dpp-BIAN↔CuCl)2 complex has a very good solubility in benzene. The results of experiments summarized in Table 4 indicated that moderate polymer yield was observed only when triethylamine was used as an activator. Even in this case the formation of samples with low molecular weight and high polydispersity was observed. The poor efficiency of the proposed system may be determined by low solubility of acrylonitrile in benzene resulting in precipitation of the polymer obtained.
DMF is known to be a good solvent for AN polymerization and is often used in PAN preparation in industry [38]. We have performed some experiments using triethylamine as activating agent and DMF as a solvent. The solvent change did not result in a significant increase in monomer conversion (entries 6 and 9 in Table 4). The increase of amine concentration had no influence on polymer yield but resulted in slight decrease of polydispersity index. Conduction of AN polymerization at higher catalyst concentration resulted in narrowing of molecular weight distribution (Table 4).
The use of DMSO as a solvent resulted in higher monomer conversion (Table 4) due to the better solubility of the polymer product in this solvent. The increase of amine concentration led to slight reduction of polydispersity but had no influence on polymer yield. At the same time a double increase of (dpp-BIAN↔CuCl)2 concentration resulted in a significant increase of monomer conversion. The obtained data indicated that the polymer formed in DMSO solution had higher MW in comparison with polymers obtained in DMF. This fact may be explained either by non-covalent interactions of propagating polymer chains with DMF for example by hydrogen bonding or by difference in the chain transfer constants for DMSO (Cs60 °С = 5.9 × 10− 5) and DMF (Cs60°С = 5.0 × 10− 4) [39]. The molecular distribution curves were unimodal despite of solvent nature (Fig. S2 in Supplementary file).
The MALDI mass spectrum of PAN samples obtained in the presence of triethylamine is represented by two main series of peaks separated by 53 Da corresponding to the mass of the PAN unit (Fig. S3 in Supplementary file). The analysis of absolute m/z values indicates that the major series of peaks correspond to polymer molecules with trichloromethyl group at α-end and chlorine atom at ω-end. The second one corresponds to chains bearing trichloromethyl group at the start and double bond or hydrogen atom at the end (Table 2). In case of PAN samples the amount of polymer chains capped by amine fragment is negligible.
It should be mentioned that as in the case of MMA polymerization almost no polymer was formed when we tried to initiate polymerization using binary CCl4–amine system without copper complex. Polymerization of AN conducted in DMF or DMSO resulted in negligible polymer yield in comparison with experiments with metal complex.
The results obtained in the polymerization of MMA and AN in the presence of proposed system based on copper BIAN complex indicated that addition of aliphatic amines increases the efficiency of initiation. We suppose that splitting of binuclear copper complex by amine forms more active species which further interact with carbon tetrachloride by producing active radicals to initiate polymerization (Scheme 1). This proposition was unambiguously confirmed using UV–Vis spectrometry (Supporting information). Introduction of amines to the benzene solution of (dpp-BIAN↔CuCl)2 results in dramatic change in its UV absorption spectrum. The initial (dpp-BIAN↔CuCl)2 complex is characterized by three absorption maxima at 275, 326 and 437 nm. Introduction of the amines results in hypochromic effect at 326 and 437 nm up to full end of absorption. At the same time, the significant increase of absorption accompanied by bathochromic effect is observed for the short-wave band (275 nm) (Fig. S4 and Table S2 in Supplementary file).
The nature of the amine has a great influence on activity of initiating system. The latter depends, as its basicity on amine structure as well. For example, a primary amine is less effective as an activator. At the same time the use of more basic amines such as pyridine or 2,2′-bipyridyl results in the decay of copper complex with the loss of BIAN ligand leading to a less active species. This fact is clearly seen at 90 °C for MMA or AN polymerization. It should be mentioned that the effectiveness of a catalytic system depends not only on amine basicity but is also governed by steric hindrance and peculiarity of its coordination with copper atom in complex structure [5].
Conclusion
Thus, we can conclude that the explored systems based on (dpp-BIAN↔CuCl)2, alkyl halide and amines as activators are capable of initiating polymerization of monomers with various structures: MMA and AN. In spite of the presence of copper atom and redox-active ligand the proposed systems do not act as ATRP catalysts and do not show a good control over polymerization. It was shown that the introduction of diethylamine into polymerization media results in significant increase of control over process. The developed compositions allow conducting polymerization of MMA and AN up to high conversions in wide temperature range giving polymers with high yield and moderate polydispersity at 1.6–2.0 level.
References
Hoff R, Mathers RT (eds) (2010) Handbook of transition metal polymerization catalysts. Wiley, Hoboken
Bielawski CW, Grubbs RH (2007) Living ring-opening metathesis polymerization. Prog Polym Sci 32:1–29
Grishin DF (2011) Synthesis of functional polymers under conditions of controlled atom-transfer radical polymerization. Polym Sci Ser C 53:3–13
Boyer C, Corrigan NA, Jung K, Nguyen D, Nguyen T-K, Adnan NNM, Oliver S, Shanmugam S, Yeow J (2016) Copper-mediated living radical polymerization (atom transfer radical polymerization and Copper(0) mediated polymerization): from fundamentals to bioapplications. Chem Rev 116:1803–1949
Matyjaszewski K, Tsarevsky NV (2014) Macromolecular engineering by atom transfer radical polymerization. J Am Chem Soc 136:6513–6533
Mousawi AA, Kermagoret A, Versace DL, Toufaily J, Hamieh T, Graff B, Dumur F, Gigmes D, Fouassier JP, Lalevée J (2017) Copper photoredox catalysts for polymerization upon near UV or visible light: structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym Chem 8:568–580
Garra P, Kermagoret A, Al Mousawi A, Dumur F, Gigmes D, Morlet-Savary F, Dietlin C, Fouassiera JP, Lalevée J (2017) New copper (I) complex based initiating systems in redox polymerization and comparison with the amine/benzoyl peroxide reference. Polym Chem 8:4088–4097
Mohamadnia Z, Ahmadi E, Haghighi MN, Farandpour A, Rezazadeh Z, Fallahi M (2015) Preparation of LLDPE through tandem ethylene polymerization using chromium and zirconium catalysts. Iran Polym J 24:621–628
Liu B, Yang D, Chen H, Xu H, Wang W, Bai L (2017) Synthesis of novel polymer brushes of poly(acrylonitrile-g-N,N’-dimethylaminoethyl methacrylate) by nitrile modification. Iran Polym J 26:355–364
Xue H, Peng L, Dong Y, Zheng Y, Luan Y, Hu X, Chen G, Chen H (2017) Synthesis of star-glycopolymers by Cu(0)-mediated radical polymerisation in the absence and presence of oxygen. RSC Adv 7:8484–8490
Heshmat-Azad S, Abdolmaleki A (2014) A new star polymethylmethacrylates by atom transfer radical polymerization. Organic Chem Curr Res 3:127
Chuang Y, Wenn B, Gielen S, Ethirajan A, Junkers T (2015) Ligand switch in photoinduced copper-mediated polymerization: synthesis of methacrylate-acrylate block copolymers. Polym Chem 6:6488–6497
Roghani-Mamaqani H, Haddadi-Asl V, Khezri K, Salami-Kalajahi M, Najafi M, Sobani M, Mirshafiei-Langari SA (2015) Confinement effect of graphene nanoplatelets on atom transfer radical polymerization of styrene: grafting through hydroxyl groups. Iran Polym J 24:51–62
Fedushkin IL, Moskalev MV, Lukoyanov AN, Tishkina AN, Baranov EV, Abakumov GA (2012) Dialan with a redox-active bis-amido ligands: unique reactivity toward alkyne. Chem Eur J 18:11264–11276
Mecking S, Johnson LK, Wang L, Brookhart M (1998) Mechanistic studies of the palladium-catalyzed copolymerization of ethylene and α-olefins with methyl acrylate. J Am Chem Soc 120:888–899
Liu W, Brookhart M (2004) Mechanistic studies of palladium(II)-α-diimine-catalyzed polymerizations of cis- and trans-2-butenes. Organometallics 23:6099–6107
Tian J, He X, Liu J, Deng X, Chen D (2016) Palladium(II) and copper(II) chloride complexes bearing bulky α-diimine ligands as catalysts for norbornene vinyl-addition (co)polymerization. RSC Adv 6:22908–22916
Rosa V, Carabineiro SA, Aviles T, Gomes PT, Welter R, Campos JM, Ribeiro MR (2008) Synthesis, characterization and solid state structures of α-diimine cobalt(II) complexes: ethylene polymerization tests. J Organomet Chem 693:769–775
Gao B, Gao W, Wu Q, Luo X, Zhang J, Su Q, Mu Y (2011) Chromium complexes with acenaphthene imine derivative ligands synthesis and catalysis on diene polymerization. Organometallics 30:5480–5486
Pourtaghi-Zahed H, Zohuri GH (2013) Polymerization of propylene catalyzed by α-diimine nickel complexes/methylaluminoxane: catalytic behavior and polymer properties. Polym Bull 70:1769–1780
Kolyakina EV, Grishin DF (2011) From phenol-type inhibitors to agents of the controlled synthesis of macromolecules. Russ Chem Rev 80:683–704
Shamenkova OA, Kopylova NA, Semchikov YD, Kurskii YA, Cherkasov VK, Abakumov GA (2005) Photo(co)polymerization of vinyl monomers in the presence of [bis(triphenylphosphino)(3,6-di-tert-butylbenzosemiquinone-1,2) copper(I)]. Polym Sci Ser B 47:314–318
Fliedel C, Rosa V, Santos C, Gonzalez PJ, Almeida RM, Gomes C, Gomes PT, Lemos MA, Aullón G, Weltere R, Avilés T (2014) Copper(II) complexes of bis(aryl-imino)-acenaphthene ligands: synthesis, structure, DFT studies and evaluation in reverse ATRP of styrene. Dalton Trans 43:13041–13054
Kolyakina ЕV, Poddel’sky AI, Grishin DF (2014) Radical polymerization of methyl methacrylate in the presence of bis[4,6-di-tert-butyl-N-(2,6-dimethylphenyl)-o-iminobenzosemiquinono]cobalt(II). Russ Chem Bull 63:987–996
Kolyakina EV, Poddel’sky AI, Grishin DF (2014) A sterically hindered cobalt o-iminobenzosemiquinone complex in the polymerization of vinyl monomers. Polym Sci Ser B 56:566–576
Kolyakina EV, Grishin ID, Poddel’sky AI, Grishin DF (2016) Mechanistic studies of methyl methacrylate polymerization in the presence of cobalt complex with sterically-hindered redox-active ligand. J Polym Res 23:222
Kolyakina EV, Ovchinnikova YE, Grishin ID, Poddel’sky AI, Grishin DF (2015) Methyl methacrylate polymerization involving a cobalt ortho-iminobenzosemiquinone complex: determination of the chain transfer constant. Kinet Catal 56:267–275
Kolyakina EV, Vaganova LB, Lado AV, Piskunov AV, Cherkasov VK, Grishin DF (2007) Bis(3,6-di-tert-butylcatecholato)tin(IV) ditetrahydrofuranate in radical polymerization of methyl methacrylate. Russ Chem Bull 56:1363–1368
Vaganova LB, Kolyakina EV, Lado AV, Piskunov AV, Cherkasov VK, Grishin DF (2008) Synthesis of homopolymers and block copolymers of methyl methacrylate and styrene in the presence of bis(3,6-di-tert-butylcatecholato)tin(IV) ditetrahydrofuranate. Polym Sci Ser A 50:153–159
Kern T, Monkowius U, Zabel M, Knör G (2011) Synthesis, crystal structure and charge transfer spectra of dinuclear copper(I) complexes bearing 1,2-bis(arylimino)acenaphthene acceptor ligands. Inorg Chim Acta 374:632–636
Vorotyntsev MA, Casalta M, Pousson E, Roullier L, Boni G, Moise C (2001) Redox properties of titanocene-pyrrole derivative and its electropolymerization. Electrochim Acta 46:4017–4033
Miller JB, Salvador JR (2002) Photoinduced electron-transfer substitution reactions via unusual charge-transfer intermediates. J Org Chem 67:435–442
Couthouis J, Keul H, Moller M (2015) MALDI-TOF analysis of halogen telechelic poly(methyl methacrylate)s and poly(methyl acrylate)s prepared by atom transfer radical polymerization (ATRP) or single electron transfer-living radical polymerization (SET-LRP). Macromol Chem Phys 216:1791–1800
Wang XY, Sun XL, Wang F, Tang Y (2017) SaBOX/copper catalysts for highly syndio-specific atom transfer radical polymerization of methyl methacrylate. ACS Catal 7:4692–4696
Arslan H, Avcı C, Tutkun B, Şengül A (2017) 2,6-Bis-benzimidazolylpyridines as new catalyst in copper-based ATRP. Polym Bull 74:931–948
Wang GX, Lu M, Zhou MJ, Liang E, He B (2018) Photo-induced ATRP of MMA under blue light irradiation in the presence of 3,4,9,10-tetra-(12-alkoxycarbonyl)-perylene as a photocatalyst. Iran Polym J 27:43–48
Zhang Y, Schröder K, Kwak Y, Krys P, Morin AN, Pintauer T, Poli R, Matyjaszewski K (2013) Reversible-deactivation radical polymerization of methyl methacrylate and styrene mediated by alkyl dithiocarbamates and copper acetylacetonates. Macromolecules 46:5512–5519
Grishin DF, Grishin ID (2015) Radical-initiated controlled synthesis of homo- and copolymers based on acrylonitrile. Russ Chem Rev 84:712–736
Ueda A, Nagai S (1999) Transfer constants to monomers, polymers, catalysts and initiators, solvents and additives, and sulfur compounds in free radical polymerization. In: Brandrup J, Immergut EH, Grulke EA, Abe A, Bloch DR (eds) Polymer Handbook. Wiley, New York
Acknowledgements
The work was supported by Russian Foundation for Basic Researches (Proj. 17-03-00498) and by Grant of President of Russian Federation (MK-1142.2017.3). The authors express their gratitude to the corresponding member of RAS, Prof. Fedyushkin IL for the given complex.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kolyakina, E.V., Grishin, I.D., Gruzdeva, L.N. et al. Polymerization of methyl methacrylate and acrylonitrile in the presence of copper BIAN complex. Iran Polym J 27, 599–609 (2018). https://doi.org/10.1007/s13726-018-0636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-018-0636-3