Abstract
The influence of bis[4,6-di-tert-butyl-N-(2,6-dimethylphenyl)-o-iminobenzosemiquinono]cobalt (II) on radical polymerization of methyl methacrylate initiated by AIBN at 70–90 °C was studied. The introduction of cobalt complex bearing redox ligands into reaction media allows to provide polymerization of methyl methacrylate without self-acceleration with linear increase of molecular weight with conversion giving polymers with relatively low molecular weight distribution. The results of IR and NMR spectrometry and MALDI mass spectroscopy measurements show that the possibility of β-hydrogen transfer between complex and propagating radical during polymerization is minimal. The electron donating additives as tert.-butyl amine and pyridine have no activating effect on polymerization. The results of our experiments provided at different concentrations of initiator show that polymerization proceeds via degenerative chain transfer mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The discovery and rapid development of Reversible Deactivated Radical Polymerization (RDRP) revealed a significant breakthrough in synthetic polymer chemistry and resulted in wide scientific interest in this area. The major advantages of RDRP are the possibility of synthesis of well-defined homo- and copolymers with desired structure and topology. The RDRP may be realized via three major ways based on different types of chemical reactions. They are Dissociation-Combination (Scheme 1a), Atom Transfer Radical Polymerization (Scheme 1b); Degenerative Transfer Processes (Scheme 1c) [1–10]:
In spite of evident difference in mechanisms, all three methods are based on one general idea of replacement of irreversible bimolecular termination by reversible interaction of propagating radical with specially introduced species, converting chains into the dormant state. The labile bond [Р – X] at the end of dormant polymer chain is easily breaks under thermal, photolytic activation or by chemical reaction giving back propagating radical. So, a step by step propagation of polymer chain is realized due to repeating deactivating and activating reactions. (Scheme 1).
Different classes of compounds including nitroxides [1, 2], complexes of transition metals [3–8], dithiocarbamates and xantogenates [9] as well as many other compounds [10] were proposed for mediating RDRP. Among other parameters, easy accessibility and high efficiency in polymerization of a wide range of monomers are the most important ones. Complexes of iron, copper, cobalt and some other metals are ones satisfying these criteria [3–8, 11, 12].
Complexes of cobalt are widely used for mediating polymerization of different monomers [7, 8, 13, 14]. It should be mentioned that polymerization in the presence of cobalt complexes may proceed via various mechanisms depending on monomer nature. Basing on the ability to undergo Catalytic Chain Transfer vinyl monomers can be relatively divided into two groups as shown at Scheme 2.
The first group is represented by monomers with α-methyl groups. Such monomers tend to undergo hydrogen transfer reactions. Its polymerization proceeds in accordance with mechanism Catalytic Chain Transfer Polymerization (CCTP) [13–18]. In this case polymerization proceeds in conventional radical manner and cobalt complex acts as an effective chain transfer agent reducing molecular weight of the polymers.
The monomers which have fewer tendencies to catalytic chain transfer reactions form the second group. In case of these compounds a carbon-cobalt bond in the intermediate 1 brakes giving back propagating radicals. Such type of reactions is classified as Cobalt Mediated Radical Polymerization (CMRP) and may be realized either by Dissociation-Combination (DC) [18–33] or by Degenerate Transfer Polymerization (DTP) [22, 26, 29–33] pathways.
Thus, the nature of the monomer should be taken into account for successful synthesis of polymers with desired structure. The polymerization of methyl methacrylate (MMA) [13–15, 17] and other methacrylates [13, 14, 16] in the presence of cobalt porphyrins and cobaloximes complexes proceeds via CCTP mechanism. At the same time polymerization of butyl acrylate using cobalt porphyrin proceeds by way of homolytic cleavage of Co-C bond [19]. The DC mechanism is also realized in case of polymerization of various acrylates [20–22], acrylic acid [23], styrene [24], vinyl acetate [22, 25–27, 30–35] and acrylonitrile [22, 28]. DC and DTP mechanisms have many in common and may be realized simultaneously [29–33]. The realization of polymerization via DTP method allows to decrease concentration of cobalt catalyst and to minimize catalytic chain transfer through hydrogen abstraction.
The proper choice of ligand environment in cobalt complex is also very important factor for realization of controlled polymerization. Polymerization of MMA in the presence of halogenated tris (triphenylphosphine) cobalt or cobalt acetate proceeds via Atom Transfer Radical Polymerization mechanism [36, 37] while the contribution of CCTP mechanism is minimal. At the same time polymerization of MMA may be realized in DC regime using binuclear cobalt complexes [38]. So, changing ligands in cobalt complexes allows to direct polymerization to one or another mechanism.
The recent investigation of styrene and MMA polymerization in the presence of bis[4,6-di-tert-butyl-N-(2,6-dimethylphenyl)-o-iminobenzosemiquinono]cobalt (II) (Co (ISQ-Me)2) and AIBN or alkyl halides as initiators showed that proposed systems allow to conduct polymerization without self-acceleration and control molecular weights of formed polymers [39–41]. The aim of this work is to investigate such process in details and to determine contributions of different mechanisms to polymerization in the presence of Co (ISQ-Me)2 and N-(2,2-dimethylphenyl)-3,5-di-tert-butyl-o-benzoquinonimine (imQ-Me):

Experimental section
Materials
Hexane, ethyl acetate, methylene chloride, benzene, toluene were dried under approptiate agents (calcium hydride or metallic sodium) and distilled. Tetrahydrofuran was dried over sodium hydroxide and distilled over metallic sodium [42]. Methyl methacrylate was purified from inhibitor by 10 % aqueous alkali solution, dried over calcium chloride, and sodium hydride and distilled under reduced pressure. AIBN was recrystallized from methanol. Bis[4,6-di-tert-butyl-N-(2,6-dimethylphenyl)-o-iminobenzosemiquinono]cobalt (II) and N-(2,2-dimethylphenyl)-3,5-di-tert-butyl-o-benzoquinonimine were obtained as described earlier [43, 44] and characterized by NMR, IR- and UV-spectroscopy.
Polymerization
Polymerization of MMA was conducted in bulk or in benzene solution. In a typical polymerization experiment 18.6 mg (1.13 × 10−4 mol, 0.1 mol%) of AIBN, 20.0 mg (2.84 × 10−5 mol, 0.025 mol%) of Co (ISQ-Me)2 were dissolved in 12 ml of freshly distilled MMA. A 1.5 ml of prepared solution was placed in a glass tube. The tube was degassed via three freeze-pump-thaw cycles, sealed under vacuum and placed in thermostat heated at 70 or 90 °C. After period of time the tube was frozen in liquid nitrogen to stop polymerization, opened to atmosphere, the reaction mixture was diluted by ethyl acetate and poured into n-hexane. The precipitated polymer was dissolved in ethyl acetate or THF and reprecipitated by n-hexane to remove residues of monomer, initiator and cobalt complex. The prepared polymers were dried under reduced pressure to constant weight. The polymer yield was calculated as ratio of weight of isolated polymer to weight of initial monomer.
Poly (methyl methacrylate)-block-polystyrene copolymers were synthesized using poly(methyl methacrylate) (PMMA) as macroinitiator. Initial PMMA was obtained via polymerization of MMA at 70°С in the presence of the cobalt complex (0.025 mol%) and the initiator AIBN (0.1 mol%) for 5 h. The PMMA was freeze-dried, and its molecular weight characteristics were determined by size exclusion chromatography (SEC); then, it was dissolved in styrene. (The concentration of the PMMA in the solution was 20 wt%.) The reaction mixture was degassed via three freeze-pump-thaw cycles, and the ampoule was sealed under vacuum; copolymerization was performed at 70°С for 85 h.
Characterization
The molecular weight distributions of the obtained polymers were measured by SEC with a chromatographic system (Knauer Smart Line) equipped with a Knauer model 2300 refractive-index detector. Tetrahydrofurane was used as the eluent at a flow rate of 1 ml/min at 25 °C. Styragel packed columns with pore size 103 and 105 Å (Phenomenex) were employed. The calibration was performed with poly(methyl methacrylate) standards (Polymer Standards Service) ranging from 2400 to 970,000 g/mol.
The theoretical number-average molecular weight Mn,th was calculated using Eqs. 1 and 2:
where [M]0, [Co (ISQ-Me)2]0, and [I]0 are the initial concentrations of MMA, cobalt complex and initiator, respectively, P is the monomer conversion, MM and \( {\mathrm{M}}_{{Co\left(ISQ\hbox{-} \mathrm{Me}\right)}_2} \) are the molar masses of monomer and cobalt complex, respectively, f the initiator efficiency, k d the decomposition rate coefficient of the initiator and f c the coupling factor.
In a DC process Mn,th given by the Eq. 1. In a DTP system Mn,th was calculated using Eq. 2 In this case the term “2” means that 1 molecule of azo initiator gives 2 primary radicals with a certain efficiency f (taken as 0.5 in this study). The term 1 − f c /2 represents the number of chains produced in a radical − radical termination event with f c the coupling factor (f c = 1 means 100 % bimolecular termination by combination, f c = 0 means 100 % bimolecular termination by disproportionation). In this study a value of f c = 0 was used. Decomposition rate coefficient of the initiator was estimated from the Arrhenius eq. as.
k d AIBN(70°C) = 4.36 × 10−5 s−1 and k d AIBN(90°C) = 5.35 × 10−4 s−1 assuming activation energy for the initiator dissociation E a = 130,230 J·mol−1 and Arrhenius frequency factor A = 2.89 × 1015 s−1.
The MALDI TOF mass spectra were recorded using Bruker Microflex LT mass spectrometer using DCTB as a matrix. A solution of polymer (2 μl, 10 mg/ml), a matrix (10 μl, 20 mg/ml) and sodium trifluoroacetate (5 μl, 5 mg/ml) were mixed in a vial and 2 μl of obtained solution was placed on a stainless steel target plate, dried and analyzed.
IR spectra were recorded using Infralum FT-801 spectrometer in solid KBr matrix. UV-Vis spectra were recorded in hexane using KFK-3 spectrophotometer (λ = 400–900 nm). ESR spectra were recorded using Bruker-ER-200D-SRC spectrometer. A solution of initiator, cobalt complex MMA and toluene was placed in a ESR tube and degassed via three freeze-pump-thaw cycles. The ESR spectra were recorded in frozen state at 180 K.
The NMR spectra of PMMA were recorded on Bruker DPX-400 (400 MHz) spectrometer in CDCl3 solution. The triad tacticities were determined from the 1H NMR signals due to the α-methyl protons.
Results and discussion
The coordination number of cobalt atom in Co (ISQ-Me)2 complex is 4. Thus this compound is coordinatinatively unsaturated and may attach small molecules or atoms giving five-coordinated species [43, 45, 46]. The determined possibility of existence of such five-coordinated o-iminobenzoquinone cobalt complexes with carbon-centered radicals bearing Co-C bond [47, 48] allowed us to propose Co (ISQ-Me)2 as controlling agent for polymerization of MMA [39–41].
The influence of o-iminobenzoquinone cobalt complex on MMA polymerization
The results of our experiments on polymerization of MMA in the presence of AIBN and Co(ISQ-Me)2 show that introduction of cobalt complex has effect on polymerization rate and molecular weight parameters of the formed samples (Fig. 1, Table 1). The dependence of ln [M]0/[M] on time (line 2) is linear indicating first-order kinetics of polymerization and stable concentration of propagating species throughout the process. The molecular weight of the samples linearly increases with conversion as it depicted on Fig. 2. Note that the experimental values significantly different from the theoretical values Mn,th calculated from Eq. 1, i.e. assuming of DC process. These values are more close to the Mn,th obtained from Eq. 2, for calculating molecular weight in case of proceeding DTP process. This fact indicates that polymerization of MMA in the presence of Co(ISQ-Me)2 mainly proceeds via DTP or CCTP mechanism while the impact of DC process is wery low. However, this dependence does not pass through the origin. It may be explained as a result of slow formation of five-coordinated cobalt complex 2 at the initial stage of polymerization (see the Scheme 3 below). Furthermore, the values of Mn determined by SEC are higher than the theoretical values calculated from Eq. 2. It points to termination of propagating radicals by disproportionation or recombination.
The dependences of Mw on conversion for polymerization of MMA in the presence of 0.1 % mol of AIBN at 70 °C: 1-without additives; 2–0.025 % mol. of Co(ISQ-Me)2; 3–5 in the presence of imQ-Me ( ∆ - 0.025 mol%, ▲ - 0.05 mol%, ♦ - 0.1 mol%) The dotted line represents the theoretical Mn,th via conversion: A - calculated from Eq. 1, B - determined via Eq. 2
The curves of molecular weight distribution for polymers prepared in the presence of Co(ISQ-Me)2 at 70°С are depicted of Fig. 3. Curves are unimodal and modes gradually shift toward higher values of molecular weights with an increase in the conversion of MMA. It should be mentioned that molecular weight distribution of samples formed at high conversions is rather broad, but it is narrower than of PMMA samples obtained via conventional radical polymerization without cobalt complexes. Such high values of Мw/Mn for controlled radical polymerization may be considered as a result of simultaneous propagation of polymerization via several mechanisms as it was described in introduction. Polymerization of MMA in the presence of Co (ISQ-Me)2 may be realized via different mechanisms as it depicted on Scheme 3.
The system based on Co(ISQ-Me)2 and AIBN makes it possible to synthesize block copolymers PMMA-b-PSt. Molecular weight of block copolymers increases relative to that of the initial PMMA (Fig. 4). However, the polydispersity indexes of PMMA-b-PS are significantly higher than Mw/Mn of the initial PMMA. The mechanism of polymerization can’t be determined only on the basis of analysis of kinetical curves of polymerization, as the linear dependence of ln [M]0/[M] on time is typical for all three mentioned mechanisms. The dependence of molecular weight on conversion doesn’t provide complete information about the mechanisms too. So, we performed investigation of this process to understand it more deep.
The influence of N-(2,6-dimethylphenyl)-3,5-di-tert.-butyl-o-benzosemiquinonimine on MMA polymerization
Conducting of polymerization at 90 °C resulted in change of color of polymerization mixture and increase of molecular weight distribution of polymers formed. It indicated that the investigated Co (ISQ-Me)2 decomposes at high temperatures. The products of it decomposition may interact with propagating radicals and have influence on polymerization. So, we decided to investigate the influence of imQ-Me as one of possible products of complex decomposition on polymerization of MMA. It should be mentioned that quinones and iminobenzoquinones tend to undergo reversible redox transitions [45, 49] and participate in radical reactions, for example as inhibitors and regulating agents in polymerization [10].
The kinetic dependences of MMA polymerization in the presence of imQ-Me are presented on Fig.1. The results of our experiments clearly show that introduction of imQ-Me to polymerization mixture has no significant effect on polymerization rate. At the same time increasing of imQ-Me concentration results in decrease of maximal monomer conversion (Tables 1, 2, Fig. 1). The data summarized in Table 2 shows that imQ-Me has no significant influence on molecular-weight parameters of synthesized samples. The MW of samples obtained in the presence of imQ-Me are the same as of samples obtained only in the presence of AIBN. The curves of the molecular weight distribution of the polymers produced in the presence of imQ-Me at 70 °C do not shift towards higher molecular weight with increasing conversion of the MMA, but there is a substantial broadening of the these curves (Fig.1-ESM) The conducted experiments showed that imQ-Me had no significant effect on polymerization of MMA initiated by AIBN. It may be explained by inability of mentioned compound to act as regulating agent of polymerization through reversible interaction with propagating radical.
The results of performed experiments allowed us to exclude imQ-Me from species which may have influence on polymerization when using Co(ISQ-Me)2 as regulating agent. The comparison of polymerization of MMA in the presence of equal amounts of imQ-Me or Сo(ISQ-Me)2 indicated that the presence of metal atom is crucial for regulating of polymerization.
The examination of possibility of β-hydrogen transfer in MMA polymerization in the presence of cobalt complex
CCTP is the most favorable mechanism for polymerization of methacrylates in the presence of cobalt complexes [13–18]. Thus the investigation of β-hydrogen transfer reaction of Co(ISQ-Me)2 seemed us necessary for determination of polymerization mechanism.
The effective chain transfer constants on Co (ISQ-Me)2 determined by Maio or linearization methods [41] are significantly lower than ones for porphyrin or oxime complexes (Table 3). At the same time this value is close to the value determined for mercaptans. At the same time the comparison of MMA polymerization in the presence of Co(ISQ-Me)2 and С12Н25SH shows that the influence of cobalt complex on polymerization is probably govern by its interaction with propagating radical and formation of five-coordinated complex 2 (Scheme 3).
To confirm this assumption we examined the reaction of Co(ISQ-Me)2 with AIBN and tried to isolate or fix the formation of mentioned five-coordinated complex. The reaction proceeded in benzene solution at 70 °C for 85 h at Co(ISQ-Me)2 : AIBN =1 : 1 ratio. The investigation of isolated products via IR, UV-VIS spectroscopy and MALDI TOF mass spectrometry did not show presence of any five-coordinated cobalt complex. The corresponding spectra are placed in electronic supplementary materials (Fig. 2–ESM, 3–ESM, 4–ESM). We also tried to initiate MMA polymerization by isolated product, but this experiment was also unsuccessful. No polymer was formed for 82 h at 70 °C.
The formation of five-coordinated cobalt complex was not observed by EPR study of Co(ISQ-Me)2 – AIBN solution in toluene. The spectra of toluene solution of 0.025 mol% of Co(ISQ-Me)2 from one side and 0.025 mol% Co(ISQ-Me)2 with 0.1 mol% AIBN from another had the same intensity. This fact clearly indicated the absence of interaction between complex and radicals formed from AIBN dissociation. Thus, the performed experiments showed that reaction of cyanisopropyl radical with Co(ISQ-Me)2 did not give any five-coordinated cobalt complexes with alkyl or hydride ligand at metal center.
At the same time such five-coordinated complexes may form as the result of interaction Co(ISQ-Me) with propagating PMMA radical. The performed EPR study of 0.025 mol% Co(ISQ-Me)2 with 0.1 mol% AIBN in MMA : toluene =1 : 1 solution showed the decrease of signal intensity after 1 h of heating at 70 °C (Fig.5). This fact is evidence of acceptance of propagating radical by four-coordinated cobalt complex leading to five-coordinated one (2) or unstable cobalt hydride complexes in accordance with Scheme 3 . The formed species 2 may decompose through three distinct pathways in accordance with Scheme 3: a) to give back propagating radical in accordance with DC mechanism; b) to enter DTP reaction with other propagating radical; c) to undergo β-hydrogen transfer (CCTP) with formation of hydride cobalt complex and dead polymer chain with double bond at ω-end (3).
The possibility of the CCTP mechanism realization is very low due to the instability of cobalt hydride complexes with redox-active ligands. Such complexes tend to undergo transformation to corresponding aminophenolate 4 reflecting in change of color of reaction mixture from deep blue to red in various protic solvents (alcohols, acids). At the same time such changing was not observed in the case of MMA polymerization. The formation of 4 may be detected by changing of IR spectra by appearance of valence vibration of >N-H bond at 3000–3200 cm−1. At the same time the analysis of recorded IR spectra for product obtained after polymerization of MMA in the presence of Co(ISQ-Me)2 (electronic supplementary materials, Fig.5–ESM), showed absence of valence vibrations of >N–H bond in the mentioned area. This fact confirms the absence of β-hydrogen transfer reactions on cobalt complex during polymerization.
The polymers forming during polymerization proceeding via CCTP mechanism have double bond at ω-end and hydrogen atom at α-end as it depicted at Scheme 4. We performed end-group analysis of polymers by means of 1H NMR and MALDI TOF MS methods.
The recorded 1H NMR spectra of polymers obtained in the presence of Co(ISQ-Me)2 (0.2 mol%) and AIBN (0.2 мол.%) and only by AIBN are presented on Fig. 6. Low-intensive signals at 5.5 and 6.2 ppm corresponding to vinylidene protons indicate the presence of double bonds in polymer macromolecules. The presence of the same signals in NMR spectra recorded for MMA obtained in the presence of AIBN shows that these signals can’t be considered as evidence for realization of CCTP mechanism, but due to the partial termination of the propagating radical [53]. It also should be noted that that NMR spectra of polymers synthesized by CCTP mechanism using cobalt porphyrin complexes as regulating agents characterized by lower values of molecular weight and higher intensity of signals of vinylidene protons at 5.0–6.5 ppm [54, 55].
a 1H NMR spectrum of PMMA (Mn = 20,000 Mw/Mn = 1.89), obtained in the presence of 0.2 mol% of Co(ISQ-Me)2 and 0.2 mol% of AIBN in benzene : MMA = 1 : 1 vol mixture at Т = 70°С b 1H NMR spectrum of PMMA (Mn = 18,100 Mw/Mn = 2.10), obtained in the presence of 2 mol% of AIBN in benzene : MMA = 1 : 1 vol. mixture at Т = 70 °C
The performed NMR studies showed that Co(ISQ-Me)2 has no influence on stereoregularity of the formed samples. The polymers thus obtained were predominantly syndiotactic (rr:rm.:mm = 53:34:13), very similar in steric structure to poly(MMA) (e.g., rr:rm.:mm = 54:32:14) radically prepared with AIBN in benzene.
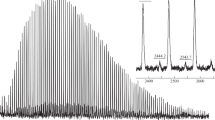
The recorded MALDI TOF mass-spectra of obtained samples are represented by one series of lines separated by 100.1 Da corresponding to MMA unit (Fig. 7). The absolute mass of each peak equals the molecular weight expected for the PMMA with one initiator fragment at α-end and double bond or methyl group at ω-end and sodium ion from the salt used for the MS analysis in accordance with structure 5: Mn = n(100.12) + 68 + 23.

This fact clearly indicates the absence of hydrogen atom at α-end of polymer molecules corroborating lack of catalytic chain transfer during polymerization. In the latter case the polymers formed should have hydrogen atom at the beginning (as on Scheme 4) of the chain and another absolute values of molecular weights.
Thus, the results of our investigations clearly show that catalytic β-hydrogen transfer processes are negligible during polymerization of MMA in the presence by Co(ISQ-Me)2 complex in contrast to polymerization conducted by porphyrin or cobaloxime derivatives. The linear increase of molecular weights on conversion as well as rather low polydispersity of formed samples may be considered as a result of realization of DC or DTP processes.
The ratio between reversible coupling and degenerative chain transfer during polymerization of MMA in the presence of cobalt iminobenzoquinone complex
The apparent rate constant of polymerization defining as a slope of a linear ln[M]0/[M] dependence on time in case of realization DC and DTP mechanism may be expressed by Eqs. 3 and 4 respectively:
where k p app – propagation rate apparent constant, k p – propagation rate constant, k a – activation rate constant, k d – deactivation rate constant, f – efficiency of activation, k i – initiation rate constant.
So, it will depend on initiator concentration in case of realization DTP mechanism. In case of proceeding of polymerization via DC mechanism changing the initiator concentration will not affect on it.
The dependences of ln[M]0/[M] on monomer conversion for polymerization of MMA at different concentrations of AIBN are depicted on Fig. 8. The introduction of Co(ISQ-Me)2 into polymerization mixture results in retardation of polymerization. The increase of AIBN concentration results in increase of polymerization rate and decrease of time necessary for full monomer conversion. The dependences of ln[M]0/[M] on time are linear in case of addition of cobalt complex indicating constant concentration of propagating radicals throughout polymerization. Apparent rate propagation constants were calculated according the slope of linear dependences. The ratio between constants determined at different AIBN concentrations are presented in Table 4. For example, for polymerizations conducted under the presence of 0.025 % of Co(ISQ-Me)2 and AIBN taken in concentration of 0.1 mol% or 0.05 mol% of AIBN respectively this ratio is calculated as 0.4 × 10−4 s−1/0.2 × 10−4 s−1 = 2. This value should be unity in case of realization of DC mechanism and a square root from initiator concentrations ratio (\( \sqrt{1\times {10}^{-2}/5\times {10}^{-3}}=1.41 \)) in case of DTP mechanism in accordance with Eqs. 3 and 4. The comparison of theoretically calculated and measured values allow us to consider DTP mechanism as more favorable. It should be noted that addition of amines into polymerization mixture has no influence on calculated ratio of apparent propagation constants.
Conclusions
The obtained data on polymerization of MMA in the presence of Co(ISQ-Me)2 complex allow us to conclude that realization of DTP mechanism is more favorable in comparison with other pathways. Thus introduction of ortho-iminobenzosemiquinolate ligands capable to reversible one-electron transitions governs the possibility of realizing DTP mechanism for polymerization of MMA in contrast to porphyrin or oxime cobalt complexes which tend to conduct polymerization via CCTP mechanism. Such dramatical change of polymerization mechanism when changing coordinating ligand should be considered while choosing proper chain regulator agent for polymerization.
References
Nicolas J, Guillaneuf Y, Lefay C, Bertin D, Gigmes D, Charleux B (2013) Prog Polym Sci 38(1):63–235
Kolyakina EV, Grishin DF (2009) Russ Chem Rev 78(6):535–568
di Lena F, Matyjaszewski K (2010) Prog Polym Sci 35(8):959–1021
Grishin DF (2011) Polym Sci Ser C 53(1):3–13
Satoh K, Kamigaito M, Sawamoto M (2012) In: Matyjaszewski K, Möller M (eds) Polymer Science: A Comprehensive Reference, vol 3. Elsevier, Amsterdam, pp 429–461
Grishin DF, Grishin ID (2011) Russ J Appl Chem 84(12):2021–2028
Debuigne A, Poli R, Jérôme C, Jérôme R, Detrembleur C (2009) Prog Polym Sci 34(3):211–239
Peng CH, Yang TY, Zhao Y, Fu X (2014) Org Biomol Chem 12(43):8580–8587
Moad G, Rizzardo E, Thang SH (2008) Polymer 49(5):1079–1131
Kolyakina EV, Grishin DF (2011) Russ Chem Rev 80(7):683–704
Matyjaszewski K, Tsarevsky NV (2014) J Am Chem Soc 136(18):6513–6533
Grishin DF, Grishin ID (2015) Russ Chem Rev 84(7):712–736
Gridnev AA, Ittel SD (2001) Chem Rev 101(12):3611–3660
Slavin S, McEwan KA, Haddleton DM (2012) In: Matyjaszewski K, Möller M (eds) Polymer Science: A Comprehensive Reference, vol 3. Elsevier, Amsterdam, pp 249–275
Smirnov BR, Morozova IS, Pushchaeva LM, Marchenko AP, Enikolopyan NS (1980) Dokl Akad Nauk SSSR 255(3):609–612
Mironychev VY, Mogilevich MM, Smirnov BR, Shapiro YY, Golikov IV (1986) Polym Sci USSR 28(9):2103–2107
Burczyk AF, O’Driscoll KF, Rempel GL (1984) J Polym Sci, Polym Chem Ed 22(11):3255–3262
Davis TP, Kukulj D, Haddleton DM, Maloney DR (1995) Trends Polym Sci 3:365–373
Oganova AG, Smirnov BR, Ioffe NG, Enikopyan NS (1983) Dokl Akad Nauk SSSR 268(4):917– 920
Wayland BB, Poszmik G, Mukerjee SL, Fryd M (1994) J Am Chem Soc 116(17):7943–7944
Wayland BB, Basickes L, Mukerjee S, Wei M, Fryd M (1997) Macromolecules 30(26):8109–8112
Lin YC, Hsieh YL, Lin YD, Peng CH (2014) Macromolecules 47(21):7362–7369
Peng CH, Fryd M, Wayland BB (2007) Macromolecules 40(19):6814–6819
Heuts JPA, Forster DJ, Davis TP, Yamada B, Yamazoe H, Azukizawa M (1999) Macromolecules 32(8):2511–2519
Debuigne A, Caille JR, Jerome R (2005) Angew Chem Int Ed 44(7):1101–1104
Chiang L, Allan LN, Alcantara J, Wang MCP, Storr T, Shaver MP (2014) Dalton Trans 43(11):4295–4304
Kaneyoshi H, Matyjaszewski K (2005) Macromolecules 38(20):8163–8169
Debuigne A, Michaux C, Jerome C, Jerome R, Poli R, Detrembleur C (2008) Chem Eur J 14(25):7623–7637
Wayland BB, Peng CH, Fu X, Lu Z, Fryd M (2006) Macromolecules 39(24):8219–8222
Maria S, Kaneyoshi H, Matyjaszewski K, Poli R (2007) Chem Eur J 13(9):2480–2492
Debuigne A, Champouret Y, Jerome R, Poli R, Detrembleur C (2008) Chem Eur J 14(13):4046–4059
Santhosh Kumar KS, Gnanou Y, Champouret Y, Daran JC, Poli R (2009) Chem Eur J 15(19):4874–4885
Santhosh Kumar KS, Li Y, Gnanou Y, Baisch U, Champouret Y, Poli R, Robson K, McNeil W (2009) Chem Asian J 4(8):1257–1265
Semsarzadeh MA, Amiri S (2013) J Polym Res 20:139. doi:10.1007/s10965-013-0139-z
Semsarzadeh MA, Amiri S (2012) J Polym Res 19:9891. doi:10.1007/s10965-012-9891-8
Li ZH, Zhang YM, Xue MZ, Zhou L, Liu YG (2005) J Polym Sci Part A: Polym Chem 43(21):5207–5216
Huang ZH, Zhang YM, Li H, Liu YG (2008) Macromol Chem Phys 209(8):825–831
Bao F, Feng L, Gao J, Tan Z, Xing B, Ma R, Yan C (2010) PLoS One 5(10): e13629, www.plosone.org
Kolyakina EV, Poddel'skii AI, Grishin DF (2014) Russ Chem Bull 63(4):987–996
Kolyakina EV, Poddel'sky AI, Grishin DF (2014) Polymer science. Ser B 56(5):566–576
Kolyakina EV, Ovchinnikova Y, Grishin ID, Poddel'skii AI, Grishin DF (2015) Kinet Catal 56(3):267–275
Weissberger A, Proskauer E, Riddick J, Toops E (1955) Organic Solvents. Interscience Publishers, INC, New York
Poddel’sky AI, Cherkasov VK, Fukin GK, Bubnov MP, Abakumova LG, Abakumov GA (2004) Inorg Chim Acta 357(12):3632–3640
Abakumov GA, Druzhkov NO, Kurskii YA, Shavyrin AS (2003) Russ Chem Bull 52(3):712–717
Smolyaninov IV, Okhlobystin AO, Berberova NT, Poddel'sky AI, Eremenko IL (2011) Russ J Coord Chem 37(1):12–25
Abakumov GA, Cherkasov VK, Bubnov MP, Abakumova LG, Ikorskii VN, Romanenko GV, Poddelґsky AI (2006) Russ Chem Bull 55(1):44–52
Bill E, Bothe E, Chaudhuri P, Chlopek K, Herebian D, Kokatam S, Ray K, Weyhermüller T, Neese F, Wieghardt K (2005) Chem Eur J 11(1):204–224
Smith AL, Hardcastle KI, Soper JD (2010) J Am Chem Soc 132(41):14358–14360
Smolyaninov IV, Letichevskaya NN, Kulakov AV, Aref'ev YB, Pashchenko KP, Berberova NT (2007) Russ J Electrochem 43(10):1187–1199
Polymer Handbook (1999) Brandrup J, Immergut EH, Grulke EA (eds), Abe A, Bloch DR (ass. eds), John Wiley and Sons, New York, Chichester, Weinheim, Brisbane, Singapore, Toronto
Vollmerhaus R, Pierik S, Herk A (2001) Macromol Symp 165(1):123–131
De la Fuente JL, Madruga EL (2000) J Polym Sci: A. Polym Chem 38(1):170–178
Odian G (1970) Princeples of polymerization. McGraw-Hill Book Company, New York
Wang W, Stenson PA, Marin-Becerra A, McMaster J, Schrolder M, Irvine DJ, Freeman D, Howdle SM (2004) Macromolecules 37(18):6667–6669
Hatada K, Kitayama T, Ute K, Terewaki Y, Yanagida T (1997) Macromolecules 30(22):6754–6759
Acknowledgments
The work was done under the task of Ministry of Education and Science of Russian Federation (proj. 736) and supported by Russian Foundation for Basic Researches (proj 15-33-20640).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 167 kb)
Rights and permissions
About this article
Cite this article
Kolyakina, E.V., Grishin, I.D., Poddel’sky, A.I. et al. Mechanistic studies of methyl methacrylate polymerization in the presence of cobalt complex with sterically-hindered redox-active ligand. J Polym Res 23, 222 (2016). https://doi.org/10.1007/s10965-016-1114-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-1114-2