Abstract
Polylactic acid (PLA)-, polystyrene (PS)-, and polyethylene (PE)-based blends were prepared with Pinus radiata modified/unmodified polyflavonoids and lignin. The modified polyphenols were esterified with maleic anhydride at 20 °C for 24 h to diversify the application potential of the resulting blends. The rheological, morphological, thermal, and mechanical properties of the blends were studied. The type and amount of polyphenols affected the torque values and the time of mixing, as well as thermoplastic chemical structure of the blends. Lignin showed the best miscibility features, while polyflavonoids were less miscible in PE. An increase in the polyphenol amount in the blends led to a decrease in the decomposition temperature (T d) in PLA-based blends. A marginal influence of the polyphenols on the T d values of PS- and PE-based blends was observed, as well. The flexural features were significantly affected by the additive content. An increase in the polyflavonoids load increased the elasticity modulus (E) of the PS- and PLA-based blends. In contrast, unmodified lignin increased the E values of the polymers except PE. Bark polyflavonoids and lignin from radiata pine might be used for the design of thermoplastic blends. The polymer morphology as well as the functional group composition are the key factors to understand the compatibility/miscibility features of the blend components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyphenols are secondary metabolites of high relevance in nature. Among other members of the polyphenol group, polyflavonoids and lignin are highly abundant in vascular plants. Applications of both polyphenolic building-blocks in the areas such as nutrition, polymer engineering, biomedicine, agriculture, and environmental sciences have been discussed recently [1–3]. In fact, several benefits of natural polyphenols as antioxidant, antibacterial/antifungal, or UV-protective agent and as O2-barrier were recognized [4].
In turn, the utilization of polyflavonoids and lignin in material engineering has been limited by their physicochemical properties (e.g., low miscibility in thermoplastics, limited solubility in several organic solvents, and lack of reactive functional groups prone for co-polymerization). Despite limitations, thermoplastic composites based on polyphenols have successfully been prepared [5, 6]. In fact, physicochemical and biological properties of polyphenols might be considered as a crucial point to design functional biomaterials over a wide range of applications.
In view of tailoring properties, as well as for improving miscibility with thermoplastics, chemical modification of certain polyphenols has been reported [7]. In fact, acetylation, hydroxypropylation, and urethanization of polyphenols are recognized pathways to enhance the performance of such building-blocks [4]. Moreover, the esterification of polyflavonoids and lignin with five-membered cyclic anhydrides (Scheme 1) provides several advantages for material design as follows:
-
No side-chain products are obtained during the derivatization reaction,
-
Cyclic anhydrides are more reactive than their linear-chain counterparts, which enables derivatization reactions at room temperature,
-
Unsaturated carboxylic chains from the polyphenol derivatives area desirable moiety for several kinds of polymerization pathways (e.g., radical, ionic, and coordination),
-
α–β Unsaturated polycarboxylic acid derivatives are considered to be biologically active against a wide range of bacteria and fungi, and
-
Unsaturated polycarboxylic moieties might improve the miscibility between polyphenols and selected thermoplastics.
On the other hand, the assessment of Pinus radiata polyphenols is a key task for Chilean researchers, considering that pine species are the most important woody plants in commercial forestry systems. The polyphenol content of P. radiata bark is very high (8–18 wt%). In fact, several innovative technologies are reported to isolate polyphenols from the forestry biomass for industrial applications [8–11]. Among commercial thermoplastics, polylactic acid (PLA), polystyrene (PS), and low-density polyethylene (PE) are high-tonnage polymers in Chile. The main uses comprise the agricultural field, packaging design, insulation, and dumping. However, such polymers have recognized drawbacks such as low UV-resistance for outdoor applications. For instance, limitations might be overcome via thermoplastics melt-blended with natural polyphenols, which exhibit high UV-filter features. Beyond the listed outdoor applications, the compounding between plastics and polyphenols opens new paths for the design of biologically active composites and O2-barrier films.
This work reports the preparation and characterization of several blends based on commercial thermoplastics (i.e., PLA, PS, and PE), and polyphenols (polyflavonoids and lignin). Rheological, morphological, molecular, thermal, and mechanical analyses were performed. The effect of the chemical modification (esterification with maleic anhydride) of polyphenols was evaluated. The new types of thermoplastic-based materials presented in this study are expected to play an important role beyond the traditional uses.
Experimental
Materials
Partially crystalline polylactic acid (PLA) Ingeo biopolymer 3251 grade supplied by Nature Works Co., LLC, USA was used in this study. Important technical features were: average M w: 90,000–150,000 Da, dispersity (D): 1.6, melting temperature (T m): 170 °C, glass transition temperature (T g): 61 °C, and L-lactide content: 98%.
Polystyrene (PS) Styron 484 supplied by Dow Chemical Co., Europe GmbH (Switzerland) was used. Important technical features were: density: 1.05 g cm−3, flow index: 2.8 g/10 min (190 °C, 2.16 kg), thermal expansion: 9 cm °C, T m: 210–250 °C, and T g: 100–102 °C.
Low-density polyethylene (LDPE) supplied by Hanwha (South Korea) was used. Important technical features were density: 0.921 g cm−3, flow index: 2.0 g/10 min (190 °C, 2.16 kg), T m: 110 °C, and Vicat softening point: 90 °C.
Polyflavonoids (T) as non-water soluble radiata pine bark polyflavonoids (PRI) from a pilot-plant feedstock extracted with a methanol/water solution (1:20, w/v) at 120 °C were used [11]. Briefly, PRI are represented by a broad mixture of polyphenols (D: 1.5–2.8) mostly 4 → 8 linked [9, 10].
Lignin (L) from P. radiata was extracted by the acetosolv process in acid media. The method was comprised of (1) delignification, (2) separation of lignin and hemicellulose, (3) acetic acid removal from the pulp, and (4) solvent separation [10–12]. Delignification was performed in a conical-made stainless steel reactor (volume of 800 L, DIN 1.4571) operated up to 12 bar. The extraction system consisted of a continuous extrusion press with a compression ratio of 1/5 (Vetter brand, type Bv) and a 3000 L dilution tank with stirrer. P. radiata lignin was extracted with acetic acid (87%) at 120 °C. Lignin isolation was carried out by water added to the black liquor, which induced both the lignin precipitation and the hemicellulose solubilization.
Lignin samples from the pilot-plant were exhaustively washed with distilled water to remove the acid traces, oven-dried, and stored in a freezer (5 °C) prior to use.
Methods
Polyphenols modification with maleic anhydride
Esterification of polyphenols with maleic anhydride was described elsewhere [8]. Briefly, polyphenols (25 mmol) were dissolved in 100 mL of DMSO under constant stirring at room temperature (T: 20 °C). After complete dissolution, 600 mmol of maleic anhydride (MAH) was added step-wise within 5 min. The solution was constantly stirred for 24 h and, afterwards, poured into cooled water (T: 5 °C) to isolate the reaction products.
This suspension was centrifuged at 4000 rpm during 10 min, and the solids were washed five times with 50 mL of cool water and then oven-dried (T: 40 °C, t: 48 h). The derivatives were then grinded in a mortar and keep in a desiccator until use. Supernatant was dialysed (Spectrum Lab Inc., Merck, Germany, pore size: 300 Da, Ø: 2 cm) within 72 h. The resulting fraction was subjected to roto-evaporation at 40 °C for 3 h until 2/3th of the initial volume was evaporated. The solid from the supernatant was washed twice with cold water and freeze-dried.
Blends formulation
Blends based on PE, PLA, and PS were prepared at 110 °C, 170 °C, and 240 °C, respectively. The mixing time (t mix) in the rheometer was 15 min, which was defined according to the previous findings [8].
Blends preparation
Solid components (PLA, PRI, and PRI3) were pretreated by oven-drying at 60 °C for 72 h to eliminate possible absorbed water on the surface of the particles. These dry components were added in a specific order to a rheometer. PLA and polyflavonoids were mixed for 2 min in a mixer and then loaded into the rheometer (mixing time, t mix: 0). PEG-400 was loaded after the PLA-melting (t mix: 1.5 min). For each treatment, three blends (50 g) were separately prepared.
Cold-blend processing
Blends were ground in an electric mill (IKA, Basic MF10, China) and 30 g were compression molded on a LabTech LP20-B (Thailand) press (T: 175 °C, t: 5 min, p: 32 bar). Laminates of 100 mm2 (thickness: 1.5 mm) were used for mechanical and microscopy testing.
Characterization
Rheology measurements were carried out in a torque rheometer (Brabender 50 EHT, Germany) with roller blades. The rotor speed was 50 rpm and the free volume of the chamber was 20% of the total volume.
Particle size distribution and morphology analysis of the blends were assessed by a confocal fluorescence microscopy. The fluorescence emission pattern of polyflavonoids and blends was assessed by a systematic analysis with a Zeiss LSM 780 confocal microscope equipped with an EC Plan-Neofluar (40×, 1.3 NA) objective lens at the opposite side of the cover slip. Dry powdered samples were irradiated by laser light (laser I: λ 405nm, laser II: λ 488nm, and laser III: λ 561nm) and fluorescence emissions were recovered. The images were obtained by the average of two scans. In all experiments, at least three sites of the blend were studied and no appreciate variation was observed in the fluorescence properties among or within samples. Considering the numerical aperture and the wavelength of excitation, the spatial resolution was approximately 200 nm. The optical zoom was 40× and a further digital zoom was used.
Image analysis of the maximum intensity projection was used to estimate the particle size distribution of polyflavonoids as a referential parameter of the components miscibility. IMARIS software (version 7.5.2, with Measurement Pro module) allowed to estimate various types of numerical values based on the three-dimensional structures in confocal images.
Volume calculation of polyflavonoid particles was carried out using an automatic segmentation tool (Spots). The particle volume values of each image were plotted in a normalized histogram with logarithmic scale, and the relation between the particle volume and the volume of all particles was calculated. Three readings were averaged to define the trend of particle size distribution.
Thermogravimetric analysis was performed on a Netzsch TGA instrument (TG 209 F3 Tarsus, Germany). Approximately, 6 ± 2 mg of each sample was heated at 10 °C min−1 to 600 °C under 20 mL min−1 N2 flow. The degradation temperature (Td) was the maximum of the 1st derivative.
Mechanical testing
Selected laminate samples were cut into a gauge length of 60 mm and a width of 1.5 ± 0.2 mm and kept in an oven overnight. Afterwards, the Young’s modulus (E) was determined by a standard method (ISO 527-1 5B). A universal tensile testing machine (SmarTens 005, KARG Industrietechnik, Germany) was used, employing a crosshead speed of 10 mm × min−1. Eight laminates were used for each treatment (Table 1).
Results and discussion
Rheology and processability features
Modified (TM) and unmodified polyflavonoids (T) reduced the torque values during the mixing regardless the type of thermoplastic (Table 2). The effect of polyflavonoids on the rheological behavior was expected, considering that such oligomers are highly polar compounds in comparison to the studied polymers (i.e., PE, PS, and PLA).
In contrast, lignin-based composites showed the highest torque values in comparison to polyflavonoids (Table 2), which indicates strong molecular interactions with thermoplastics at the molten state. In fact, the chemical composition of the polyphenolic additives in terms of functional groups, as well as the morphology of the polymer matrix involved in the mixing process are recognized as the key factors to understand the rheological behavior of the polyphenol-based blends.
It is worth to notice that the chemical modification decreased the torque value significantly, which can be interpreted on the basis that the esterification with maleic anhydrides yields highly polar unsaturated carboxylic acid containing moieties that interfere chain–chain interactions between the polyphenol derivatives and the thermoplastic polymers.
In general, polyflavonoids decreased the resistance of the rheometer blades. In contrast, lignin-based systems increased the resistance in the first minutes of mixing. The behavior can be explained considering the low polarity of lignin in comparison to flavonoids, which enabled strong molecular interactions regardless of the type of polymer. In addition, the effect of the chemical modification on torque values can be also explained considering that the physicochemical properties of polyphenols dramatically change during O-alkylation reactions [8].
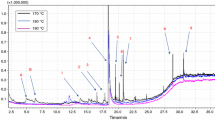
On the other hand, the mixing time (t mix) was strongly dependent on the chemical modification, the polyphenol type, and the chemical structure of the polymer (Fig. 1). Modified polyphenols reduced the t mix on PE-based blends regardless of the additives content. In contrast, modified/unmodified lignin increased the t mix on PS-blends, while polyflavonoids decreased the t mix significantly regardless of the chemical modification. However, modified polyflavonoids and lignin increased the t mix in a high extend in the PLA-based systems.
It is worth to notice that the chemical structure of the grafting had a high impact on the mixing behavior for PE- and PLA-based polymers. In contrast, the aromaticity of the additives may affect the t mix of the PS-based systems. The aromatic moieties of polyphenols might have affected the heat transfer process during the melt blending and in consequence changing the t mix. In fact, the low heat capacity of aromatic compounds in binary and ternary thermodynamic systems in comparison to aliphatic chemicals is a well-known issue [13]. In general, the heat capacity of the additives and the functional group content showed the strongest influences on the t mix at the molten state. However, the interaction between the chemical modification and the polyphenols content affected the rheological features as a function of the polymer type in a different way. Either the torque or t mix values describe how the chemical modification of the additives affects the rheology behavior significantly.
Particle size distribution
Confocal fluorescence microscopy enables polyphenol quantification in a wide range of experimental conditions [14]. In fact, morphological and structural pattern of polyphenolic macromolecules might be assessed in this way. As the technique was applied to polyphenol-based materials, it had to be considered the particle distribution effect on the plastic matrix is hard to access using the conventional techniques [8]. Considering that the polyphenol-based blends are colored composites, the evaluation of the fluorescence emission pattern was performed on the basis that PE, PS, and PLA are not fluorescent active polymers. Such advantageous features were taken in advance to study the morphology of the polyflavonoids and lignin particles dispersed in thermoplastics [3, 8].
Fluorescence emission spectra was assessed with three kinds of lasers (λ 1: 405, λ 2: 488, and λ 3: 561 nm). Maximum fluorescence emissions were observed within a broad range of wavelengths (λ 1 → 480–500 nm, λ 2 → 560–630 nm, and λ 3 → 600–620 nm). In addition, no differences regarding chemical modification and polyphenol type on the fluorescence patterns were detected (data not shown). Considering the results, red light laser (λ 3: 561 nm) was used to gain insight into the polyphenol particle morphology.
Maximum intensity projections of selected blends are shown in Fig. 2. It is worth to notice that a wide range of particle shapes were observed depending on the polyphenol and the thermoplastic types.
Modified polyflavonoid particles showed clear agglomeration on the PE matrix. The results can be apparently explained considering the differences in the polarity between polyphenols and PE. However, polyphenols dispersed/dissolved on PS and PLA polymers showed an apparently good distribution regardless of the polyphenol type.
It is worth to notice that PLA-based blends show high intensity of green and red light probably associated with the decreasing of the particle size as described previously [8]. The results suggested that a longer period of mixing (t mix: >15 min) is required to get a better dispersion of polyflavonoid particles in the PE matrix.
In general, the image processing revealed significant differences in morphological parameters of the polyflavonoids and lignin particles. Modified and unmodified lignin showed green fluorescence on the background as an evidence of the apparent significant dispersion/dissolution into the polymer matrix. In fact, the high miscibility of acetosolv lignin in thermoplastic had been reported previously [15, 16].
The low M w value of pine acetosolv lignin (1000–1500 g mol−1) was the main reason for its good miscibility behavior. However, the dispersion trend of the polyflavonoids particles in the thermoplastic was highly affected by the intrinsic polarity of the polyphenol and the chemical modification degree, as well. The methodology allowed a high count of particles dispersed in the thermoplastics.
Particle volumes were determined based on a selective channel (red, λ 3: 561 nm). As previously described by the maximum intensity projection, the normalized histograms show how thermoplastics and polyphenol types affect the miscibility to a high extent (Fig. 3a–f). However, a differentiated behavior of every polyphenol/thermoplastic system was observed.
Morphological features of polyphenol particles dispersed in thermoplastics (assessed by emission fluorescence microscopy). (single asterisk) highest abundant polyflavonoids particle volume (reference), (double asterisk) highest abundant lignin particle volume (reference): a normalized histogram, illustrating particles volume in PE-based blends, b volume distribution of polyphenol particles in PE-based blends, c normalized histogram, illustrating particle volume of PS-based blends, d volume distribution of polyphenol particles in PS-based blends, e normalized histogram illustrating particle volume of PLA-based blends, f volume distribution of polyphenol particles in PLA-based blends (TP thermoplastic, T polyflavonoids (tannin), TM modified polyflavonoids, L unmodified lignin, LM modified lignin, PE polyethylene, PS polystyrene, and PLA polylactic acid)
Lignin in PE-based blends exhibited the highest counting of fine particles, which described the better miscibility of such polyphenols in the olefin polymer (PE). In contrast, the particle size distribution of modified polyflavonoids in PE showed the higher fraction of large particles. The aromatic content of the polyphenol apparently inhibited the miscibility in the thermoplastic matrix. Differences in the miscibility between polyflavonoids and lignin can be explained considering the aromatic/aliphatic carbon ratio of the monomer unit (polyflavonoids: C12/C3 vs lignin: C6/C3). Therefore, the significant aromatic content of the polyflavonoids affected the miscibility with olefinic polymers, in comparison to the aromatic polar thermoplastic matrix. In contrast, polyphenol particles volume distribution showed significant differences in the PS-based blends.
Modified lignin exhibited significant abundance of the large particles in comparison to the unmodified lignin and polyflavonoids. The differentiated behavior of polyphenols dispersed in PS in comparison to PE can be explained considering that PS is an aromatic polymer. The similar chemical backbone of lignin (monomer unit: C6–C3) and PS (C6–C2) apparently enhanced the compatibility features.
The results highlighted the significant influence of the grafted moieties (α-β-unsaturated carboxylic acid) on the molecular interaction with thermoplastics. On the other hand, PLA-based blends showed pronounced differences in their miscibility depending on the polyphenol type. Lignin exhibited the best miscibility features, while unmodified polyflavonoids showed the worst compatibility trend. The relatively high polarity of PLA, in comparison with PE and PS polymers, favored a better interaction with the highest polar polyphenols. The chemical modification affected the particles volume in the thermoplastic matrix. The volume ranges of the polyphenols dispersed in PLA were rather similar to those observed in PS-based blends. However, modified polyphenols (TM and LM) exhibited the highest volume distribution regardless of the type of thermoplastic.
The results pointed out that the influence of the grafting becomes critical for low polarity polyphenolic building-blocks. However, the morphology of the polyphenols in terms of the conformational features was the most important factor affecting the miscibility of such additives in PLA. Based on such findings, lignin at additive content between 1 and 5 wt% might be used as a functional additive for any studied commercial polymers. However, polyflavonoids are highly recommended for PS- and PLA-based blend formulations.
Furthermore, it is worth to highlight that confocal microscopy coupling to image- processing algorithms provides valuable insights regarding the particles distribution and the morphological features of the natural polyphenols dispersed in the thermoplastic polymers. The particle size determination by using confocal microscopy seems to be a sensitive and robust method for the study of the miscibility patterns of self-fluoresce polyphenolic components dispersed in the thermoplastic polymers. Further investigations regarding the effect of the chemical modification-, and the polyphenol type on the fluorescence emission patterns should be performed.
Thermal stability
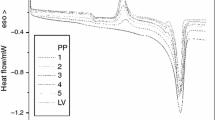
DTG analysis revealed the significant effects of the polyphenol and thermoplastic type on the thermal behavior (Fig. 4). Decomposition temperature (T d) of the blends oscillated between 340 and 500 °C and was strongly associated with the thermoplastic type. Blends prepared with PE showed the highest thermal stability between 450 and 500 °C. In contrast, PLA derived blends showed the lowest thermal resistance regardless of the polyphenol type and blend composition.
DTG curves (first derivate from the TGA trace) of the selected blends. Inserted decomposition temperature (T d) of the PLA-based blend as a function of the polyphenol content, N = 3 (TP thermoplastic, T polyflavonoids (tannin), TM modified polyflavonoids, L unmodified lignin, LM modified lignin, PE polyethylene, PS: polystyrene, and PLA polylactic acid)
It is seems that at low polyphenols content (1–5 wt%), the decomposition pattern of the blends is conditioned by the thermal properties of the polymer matrix. The detrimental effect of lignin on the T d value of the blends was remarkable in comparison with the polyflavonoids. In addition, modified polyphenols (TM and LM) provided the lowest thermal resistance. The results were unexpected considering that polyflavonoids have a low thermal stability in comparison to lignin [2, 4]. However, the behavior might be explained considering that acetosolv lignin from P. radiata is a highly thermally unstable biopolymer in comparison to lignin obtained by alternative isolation techniques.
In addition, the esterification of lignin with maleic anhydride under acid conditions (pH 2–3) seems to affect the thermal stability of such polyphenol to a high extent (unpublished data). The low thermal resistance of the modified polyphenol-based blends is also expected. The α-β unsaturated carboxylic moiety in derivatives enabled early thermal decomposition in consequence of a decarboxylation reaction (T d: 170–180 °C), as well as an ester cleavage [17–19].
In general, the results established that chemical modification of polyphenols affected significantly the thermal resistance of high polarity blends regardless of the miscibility/compatibility trends. From the practical point of view, the thermal resistance of the polyphenols-based blends was relatively high (T d > 200 °C), considering that the main envisaged uses are focus on outdoor applications in agricultural environments (PE-, PS-, and PLA-based bioactive films/UV-filters).
Mechanical testing
The effect of the polyphenol type on the elasticity modulus (E) was investigated. E-values of thermoplastic-based blends seem to be highly dependent on the polymer composition (Table 3). The highest elastic modulus were achieved for blends containing 5 wt% of the unmodified lignin regardless of the thermoplastic type. However, the highest differences in the E-values were achieved for PLA-based blends prepared with 5 wt% modified polyphenols. In any case, variations in the elastic modulus as a function of the polyphenol content can be regarded insignificant compared to the decrease of the E-values due to the polyphenols addition to the neat polymer. However, an increase in the amount of polyphenol additives affected the elastic modulus in a different way depending on the type of thermoplastic polymer.
Modified/unmodified polyflavonoids decreased the elastic modulus to a high extent, mainly in PS-, and PLA-based blends. This result was expected considering that polyphenol and its derivatives exhibit similar polarity. However, the modified polyphenols possess the highest content of polar functionalities. In contrast, L and LM increased the E values significantly regardless of the thermoplastic type. The results can be explained on the basis that lignin has lower polarity than polyflavonoids and the chemical modification slightly influences the polar character of the lignin in comparison with polyflavonoids. Another factor that should be considered is the degree of chemical modification (DS). A higher value of DS in polyflavonoids promoted high impact on the blend properties. In contrast, the low degree of modification in lignin favored the interaction with the polymer matrix in a narrow range of loading.
In general, the influence of the polyphenol loading on the mechanical behavior (flexural) may also be explained based on the aromatic content and polar characteristic of the resulting blends. Both factors should be considered to understand the macroscopic properties of the polyphenols-based blends. Polyflavonoids as well as modified lignin showed a poor flexural impact in PE-based blends. This could be explained considering the high content of polar functional groups in both polyphenols [20–22].
In addition, the results pointed out that TM and LM seemed to be highly desirable additives for PLA-based material designs, as esterified derivatives apparently exhibited a better miscibility with the COOH-containing polymer (PLA). From the practical point of view, the modified and unmodified polyflavonoid building-blocks (loading: 1–5 wt%) are highly recommended for the design of PLA- and PS-based composites. In contrast, lignin can be used for all three studied thermoplastics. The chemical modification of lignin seems to have less influence on the performance of thermoplastic blends in comparison to polyflavonoids.
Conclusion
Polyethylene-, polystyrene-, and poly(lactic)-based blends were successfully prepared with modified/unmodified P. radiata polyflavonoids and lignin (1–5 wt%). Chemical modification of radiata pine polyphenols was a viable strategy to improve the performance on the polystyrene- and poly(lactic)-based blends as a consequence of better processability features. However, polyflavonoid derivatives strongly affected the PE-based blend performance. The chemical structure of the polyphenol grafting influences: (i) the processability during the melt blending, (ii) the miscibility of the components, (iii) the thermal resistance, and (iv) the flexural performance. The image processing data based on confocal fluorescence microscopy were successfully utilized to estimate the polyphenol particle volume and in consequence enabling miscibility predictions between the polyphenol additives and thermoplastic polymers. The torque value and the mixing time seemed to be affected by the functional group composition at the molten state. The modified polyphenols showed higher compatibility features in consequence of a dramatic reduction of particle volumes on PS- and PLA-based blends. Despite that, polyphenolic additives affected the decomposition temperature (ΔT d: <15 °C) regardless of the polyphenol type. Blends can be used at temperature below 200 °C. Modified polyphenols, which are esterified with maleic anhydride, might be preferably used on PS- and PLA-based blends, while lignin is recommended for the three thermoplastics used. The interaction between the functional groups and the thermoplastics matrix moieties dictates the behavior of the blends.
References
Pizzi A (2008) In: Belgacem N, Gandini A (eds) Monomers, polymers and composites from renewable resources. Elsevier, New York
García DE (2014) Pinus pinaster (Ait.) bark polyflavonoid and its hydroxypropyl derivatives as building-blocks for bio-material design. PhD Thesis, Freiburg University, Freiburg
Grigsby WF, Kadla JF (2014) Evaluating poly(lactic acid) fiber reinforcement with modified polyflavonoids. Macromol Mater Eng 299:368–378
García DE, Glasser WG, Pizzi A, Paczkowski S, Laborie MP (2016) Modification of condensed tannins: from polyphenol chemistry to materials engineering. New J Chem 40:36–49
Anwer MAS, Naguib HE, Celzard A, Fierro V (2015) Comparison of the thermal, dynamic mechanical and morphological properties of PLA-Lignin & PLA-Polyflavonoid particulate green composites. Compos Part B Eng 82:92–99
García DE, Carrasco JC, Salazar JP, Pérez MA, Cancino RA, Riquelme S (2015) Bark polyflavonoids from Pinus radiata as functional building-blocks for polylactic acid (PLA)-based composites, 3er Congreso Iberoamericano sobre Biorrefinerías (CIAB), 4to Congreso Latinoamericano sobre Biorrefinerías y 2do Simposio Internacional sobre Materiales Lignocelulósicos. Concepción, Chile
García DE, Glasser WG, Pizzi A, Paczkowski S, Laborie MP (2014) Substitution pattern elucidation of hydroxypropyl Pinus pinaster (Ait.) bark polyflavonoids derivatives by ESI(-)-MS/MS. J Mass Spectrom 49:1050–1058
García DE (2016) Polycarboxylated flavonoid oligomers as functional additives for polylactic acid-, polystyrene-, and polyethylene-based composites. In: Proceeding of The 13th Pacific Rim bio-based composite symposium, November 13–15th, Concepcion, Chile, pp 112
Berg A, Pozo LO, Fuentes PN (2009) Process for obtaining low and medium molecular weight polyphenols and standardized solid fuel from tree wood or bark. US Patent 20090077871 A1
Berg A, Fuentealba C, Salazar JP (2013) Separation of lignocellulosic components in acetic acid media and evaluation of applications. J-FOR 3:27–32
Bocalandro C, Sanhueza V, Gómez-Caravaca AM, González-Álvarez J, Fernández K, Roeckel M, Rodríguez-Estrada MT (2012) Comparison of the composition of Pinus radiata bark extracts obtained at bench- and pilot-scales. Ind Crop Prod 38:21–26
Jeréz M, Selga A, Sineiro J, Torres JL, Núnez MJ (2007) A comparison between bark extracts from Pinus pinaster and Pinus radiata: antioxidant activity and procyanidin composition. Food Chem 100:439–444
Rihani DN, Doraiswamy LK (1965) Estimation of heat capacity of organic compounds from group contributions. Ind Eng Chem Fundam 4:17–21
Scogings P, Siko S, Taylor R (2014) Calibration of a hand-held instrument for measuring condensed tannin concentration based on UV-and red-excited fluorescence. Afr J Range For Sci 31:55–58
Kubo S, Kadla JF (2004) Poly(ethylene oxide)/organosolv lignin blends: relationship between thermal properties, chemical structure, and blend behavior. Macromolecules 37:6904–6911
Ding R, Wu H, Hunga MT, Bowler N, Kessler MR (2016) Processing and characterization of low-cost electrospun carbon fibers from organosolv lignin/polyacrylonitrile blends. Carbon 100:126–136
Lisperguer J, Nuñez C, Pérez-Guerrero P (2013) Structure and thermal properties of maleated lignin-recycled polystyrene composites. J Chil Chem Soc 58:1937–1940
Teacă CA, Bodîrlău R, Spiridon I (2014) Maleic anhydride treatment of softwood—effect on wood structure and properties. Cell Chem Technol 48:863–868
De Melo JCP, Da Silva Filho EC, Santana SAA, Airoldi C (2009) Maleic anhydride incorporated onto cellulose and thermodynamics of cation-exchange process at the solid/liquid interface. Colloid Surface A 346:138–145
García DE, Glasser WG, Pizzi A, Paczkowski S, Laborie M-P (2015) Hydroxypropyl tannin from Pinus pinaster bark as polyol source in urethane chemistry. Eur Polym J 67:152–165
Luo C, Grigsby WJ, Edmonds NE, Easteal AJ, Al-Hakkak J (2010) Synthesis, characterisation and thermal behaviours of tannin stearates prepared from quebracho and pine bark extracts. J Appl Polym Sci 117:352–360
Grigsby WJ, Bridson JH, Lomas C, Elliot JA (2013) Esterification of condensed tannins and their impact on the properties of poly(lactic acid). Polymers 5:344–360
Acknowledgements
D.E. García likes to thanks Comisión Nacional de Investigación Científica y Tecnológica (CONICYT, Chile, Project FONDECYT 11150056), for the financial support. The authors would like to acknowledge to the Basal Project (PFB-27) from Universidad de Concepción. The technical supports of Mrs. C. Silva, and Mrs. C. Pradenas are recognized with gratitude. In addition, authors also like to thank G. Osorio, and MSc. J. Tereszczuk (Center for Advanced Microscopy, CMA Biobío, Chile). The international academic cooperation of Dr. S. P. Paczkowski (Freiburg University, Germany) made in the framework of the FONDECYT Project is recognized with gratitude.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García, D.E., Gavino, J., Escobar, D. et al. Maleinated polyflavonoids and lignin as functional additives for three kinds of thermoplastics. Iran Polym J 26, 295–304 (2017). https://doi.org/10.1007/s13726-017-0519-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-017-0519-z