Abstract
Purpose
The current review shows evidence for the role of adipokines in breast cancer (BC) pathogenesis summarizing the mechanisms underlying the association between adipokines and breast malignancy. Special emphasis is given also on intriguing insights into the relationship between obesity and BC as well as on the role of novel adipokines in BC development.
Recent Findings
Recent evidence has underscored the role of the triad of obesity, insulin resistance, and adipokines in postmenopausal BC. Adipokines exert independent and joint effects on activation of major intracellular signal networks implicated in BC cell proliferation, growth, survival, invasion, and metastasis, particularly in the context of obesity, considered a systemic endocrine dysfunction characterized by chronic inflammation. To date, more than 10 adipokines have been linked to BC, and this catalog is continuously increasing. The majority of circulating adipokines, such as leptin, resistin, visfatin, apelin, lipocalin 2, osteopontin, and oncostatin M, is elevated in BC, while some adipokines such as adiponectin and irisin (adipo-myokine) are generally decreased in BC and considered protective against breast carcinogenesis.
Summary
Further evidence from basic and translational research is necessary to delineate the ontological role of adipokines and their interplay in BC pathogenesis. More large-scale clinical and longitudinal studies are awaited to assess their clinical utility in BC prognosis and follow-up. Finally, novel more effective and safer adipokine-centered therapeutic strategies could pave the way for targeted oncotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
By 2030 cancer is expected to surpass cardiovascular disease being the prevailing cause of death among all age categories, contributing to a 45% increase in the number of malignancies diagnosis during the next 10 years [1]. This is due to the emergence of the increased prevalence of risk factors, mainly diabesity (diabetes mellitus and obesity) in both developed and developing countries [2].
As a result of the adoption of the Western lifestyle which consists of decreased physical activity and consumption of energy-dense, low-quality foods, the prevalence of excess body weight encompassing overweight and obesity, characterized as a body mass index (BMI) between 25–29.9 and over 30 kg/m2 respectively, has been dramatically increased worldwide with 18% of children and 40% of adults presenting excess body weight [3]. Moreover, obesity is more prevalent in females than males [4].
Based on reports from the International Agency for Research on Cancer (IARC) and the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR), there is a causal association between excess body weight/fatness and the risk of cancers in 15 anatomic positions: endometrium, esophagus (adenocarcinoma), colon and rectum, breast (postmenopausal), ovary, gallbladder, liver, kidney, thyroid, pancreas, stomach (cardia), meningioma, multiple myeloma, prostate (advanced cancer, probable evidence), and oropharyngeal space and larynx (probable evidence) [3, 5].
Across the world, breast cancer (BC) constitutes the most commonly diagnosed malignancy as well as the leading cause of cancer death in women [3, 6]. Furthermore, BC survivors represent the biggest category of women living with cancer in the USA and other developed countries [7]. The evidence for a causal association between excess body weight and postmenopausal BC is sufficient with almost 7% of all postmenopausal BC being attributed to overweight/obesity [8]. The pathological expansion of white adipose tissue in excess body weight, characterized as adiposopathy, provokes fat cell hypertrophy and/or hyperplasia; hypoxia and oxidative stress; perturbation in the protein secretory pathway; metabolic, inflammatory, immunologic, and epigenetic alterations promoting neoplastic transformation and growth [9••, 10••].
The current review examines the role of adipokines in BC pathogenesis summarizing the mechanisms underlying the association between adipokines and malignancy. Special emphasis is given also on intriguing insights into the relationship between obesity and BC as well as on the role of novel adipokines in BC development. Hence, elucidating mechanisms interconnecting excess body fatness with BC risk and mortality is of paramount importance for cancer prevention, diagnostics, and therapeutics.
Intriguing Insights into the Relationship Between Obesity and Breast Cancer
The relationship between obesity and BC is complex depending on histologic subtype, menopausal status, and hormone replacement therapy (HRT) [11]. In postmenopausal women, obesity is associated with BC risk, particularly hormone receptor (HR)–positive tumors in the majority of studies [3, 12, 13••] but not HR-negative or triple negative BC [13••]. However, the association between BC and obesity is attenuated in postmenopausal women taking HRT [14]. Moreover, there is robust epidemiological evidence for a dose-response association between visceral obesity, expressed by the anthropometric indices waist circumference (WC) and waist-to-hip ratio (WHR), and postmenopausal BC [3, 5]. Adult weight gain is also related to an increased risk of postmenopausal BC while, paradoxically, elevated weight in young adulthood (between 18 and 30 ages) is inversely related to postmenopausal BC [3, 15]. Interestingly, women with higher body fat levels, determined by dual-energy x-ray absorptionmetry, despite being within the normal BMI range, are at increased risk for invasive postmenopausal BC, underscoring the role of dysregulated metabolic and inflammatory biomarkers associated with excess body fat, particularly trunk fat [16, 17]. More importantly, obesity-associated metabolic disorders such as metabolic syndrome, diabetes mellitus (DM) type 2, and hypercholesterolemia are associated with increased risk for postmenopausal BC, particularly HR-positive tumors [11, 18].
In contrast to postmenopausal BC, obesity is linked to a decreased risk of premenopausal BC [3, 5]. However, many studies have highlighted that obesity is associated with an elevated risk for HR-negative, basal-like and triple negative BC in premenopausal women [11, 13••, 19]. Overall, regarding histologic subtype, obesity could be a potential risk factor of inflammatory and basal-like BC independently from menopausal status [13••, 20]. There is also preclinical evidence from mouse mammary tumors for connections between diet-induced obesity and both basal-like and luminal BC progression but not human epidermal growth factor receptor (HER)-2 and luminal B BC subtypes, despite the fact that these preclinical models do not fully represent human BC subtypes [11, 21, 22].
In both premenopausal and postmenopausal women, obesity is associated with decreased disease-free survival and increased risk of recurrence and mortality, particularly in HR-positive tumors [23, 24]. Besides, excess weight was correlated with increased tumor size and histopathological grade, and positive lymph nodes [13••, 25••]. Postdiagnosis weight gain is related to dismal prognosis especially in women with increased adiposity and sarcopenia [26, 27]. Moreover, obesity has been related with therapy-associated adverse effects comprising lymphedema, chemotherapy toxicity, and infections [11].
Overall, based on the etiologic diversity of BC, more larger prospective studies with sufficient power are required to explore the association between obesity and BC taking into account the menopausal status and the histologic subtype of BC.
Obesity Fat Tissue Promotes a Pro-inflammatory and Pro-oncogenic Environment
Recent evidence has underscored the contribution of the triad of overweight/obesity, insulin resistance, and adipokines in BC, particularly in postmenopausal women. Although the role of obesity in BC pathogenesis is not fully elucidated, the main mechanisms linking obesity and adiposopathy to BC comprise the following: (i) alterations in hormonal systems including both steroid hormones and their bioavailability as well as peptide metabolic hormones such as insulin and the insulin-like growth factor (IGF)-1 system; (ii) chronic low-grade systemic inflammation and oxidative stress; (iii) abnormal variations in the levels of adipokines; and (iv) intra-breast fat accumulation [3, 9••, 10••, 28].
As an endocrine tissue, adipose tissue regulates the production and bioavailability of sex hormones, which are considered to mediate the association of adiposity with BC risk by the following: (i) expressing aromatase enzymes, which transform androgens to estrogens, and less active (androstenedione, estrone) to more potent hormonal forms (testosterone, estradiol) and (ii) by increasing the bioavailability of free estradiol and testosterone, through hyperinsulinemia, elevated IGF-1 bioavailability, and decreased hepatic secretion of sex hormone–binding globulin (SHBG) [3, 29]. In postmenopausal women, the rate of transformation of androgens to estrogens is higher amid obese women [29].
Besides its energy-storage properties, white adipose tissue represents a metabolically dynamic secretory organ producing by a variety of cells (including adipocytes and macrophages) a wide range of functional heterogeneous adipokines, which regulate numerous physiologic and pathologic pathways comprising insulin sensitivity, appetite, inflammation, innate and adaptive immunity, hematopoiesis, and angiogenesis [10••, 30,31,32]. To date, more than 10 adipokines have been linked to BC, and this catalog is continuously increasing [9••, 33,34,35,36,37]. As fat tissue expands in excess weight, more pre-adipocytes produce leptin. Hypoxia promotes alterations in the gene expression of adipocytes, particularly in pro-inflammatory adipokines, and the immune environment [13••]. Chronic inflammation in obese adipose tissue is stimulated and sustained by the nuclear factor-κΒ (NF-κΒ) [38]. Therefore, obese fat tissue promotes a pro-inflammatory and pro-oncogenic environment. Interestingly, emerging evidence from epidemiologic and translational studies has shown that the local ectopic breast adipose tissue presents deleterious and tumorigenic effects for the development and progression of BC, being associated with a more pronounced hormonal and inflammatory milieu impacting on tumor promotion and progression [10••, 39]. The breast adipose tissue, mostly occupied by adipocytes, represents the breast stroma which secretes adipokines participating in the crosstalk with BC cells and contributing to increased BC cell proliferation, invasion, and resistance to therapy [40]. Cancer-associated adipocytes, which are mainly characterized by their small size and the modification of lipid droplets, are situated in the invasive front of BC cells and represent cardinal mediators of tumor progression via their paracrine and endocrine actions [41].
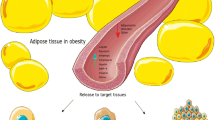
Whilst the constellation of circulating pro-inflammatory adipokines and cytokines, such as leptin, tumor necrosis factor (TNF)-α, interleukin (IL)-6, resistin, and extracellular nicotinamide phosphoribosyl-transferase (eNampt) is increased in BC, few adipokines such as adiponectin are decreased in BC and are considered protective against breast carcinogenesis [42, 43]. Classic adipokines, including leptin and adiponectin, have been sufficiently examined in BC [30, 44, 45]. Figure 1 shows the main variations of plasma adipokine concentrations and implicated mechanisms in BC.
Important variations of plasma adipokine concentrations and implicated mechanisms in breast cancer. BC, breast cancer; ERs, estrogen receptors; MMP, matrix metalloproteinase. (Both images of breast tissue and images of cancerous cell, angiogenesis, metastatic dissemination, and cell invasion are derived from the free medical site http://smart.servier.com/ by Servier licensed under a Creative Commons Attribution 3.0 Unported License)
The connection of adipokines with BC risk and progression is based on the following: (1) altered plasma concentrations in BC patients compared with controls as shown in meta-analyses; (2) their association with advanced stage and dismal prognosis in BC (prognostic biomarkers); (3) their differential expression in malignant and benign breast tissues and their upregulation in breast tumor tissues; (4) their association with cancer therapy resistance (predictive biomarkers); (5) their association with in vivo and in vitro models of BC; (6) the association of genetic polymorphisms of adipokines genes and their receptor genes with BC [9••]. Table 1 depicts meta-analyses examining the association between main adipokines and BC. Table 2 summarizes the main mechanisms of actions of adipokines in BC.
Adipokines and Breast Cancer
Adiponectin and Breast Cancer
Adiponectin is a polypeptide composed of 244 amino acids belonging to the C1q/TNF family of proteins [30]. It was discovered almost simultaneously by four different research groups in the 1990s [30]. Adiponectin is secreted into the circulation mainly by adipocytes and, to a lesser extent, by the skeletal muscle, heart, liver, bone marrow, and central nervous system [30, 46]. Αdiponectin affects its target tissues through its receptors: AdipoR1 (specific for skeletal muscle and endothelial cells), AdipoR2 (specific for liver), and T-cadherin [47]. Adiponectin receptors are ubiquitously expressed in healthy as well as in cancerous tissue [30]. Other growth factors such as platelet-derived growth factor, basic fibroblast growth factor, and heparin-binding epidermal growth factor-like growth factor, are also bound by adiponectin [48]. The circulating levels of adiponectin exhibit an inverse association with adipose tissue mass and have been shown to exert protective roles against the development of obesity-related disorders, such as metabolic syndrome, diabetes, cardiovascular diseases, and malignancies [30].
Besides its other properties, adiponectin exhibits anti-proliferative, anti-migratory, and pro-apoptotic actions [9••, 49]. A large but heterogeneous body of data has shown that adiponectin negatively influences carcinogenesis [30]. The principal pathway that is activated by adiponectin is the AMPK/LKB1, a pathway involved in the regulation of cell proliferation, apoptosis, angiogenesis, and cellular metabolism. When adiponectin binds to its receptor, it facilitates the translocation of LKB1/STE20-related adaptor protein (STRAD)/scaffolding mouse 25 protein (MO25) from the cell nucleus to the cytoplasm and promotes the phosphorylation of LKB1. Simultaneously, it activates AMPK that, in turn, inhibits MAPK, PI3K/Akt, WNT-β-catenin, NF-κB, and JAK2/STAT3 pathways [50, 51].
Although the effects of adiponectin on carcinogenesis have been extensively studied, the exact mechanism of its action has not been fully elucidated in the context of BC.
Adiponectin’s effects on BC cells depend on their estrogen receptor status. In ER-negative BC cells, it suppresses cell growth and apoptosis, and inhibits proliferation, invasion, and migration [49]. On the other hand, results are contradictory when examining its effects on ER-positive BC cells [52,53,54,55,56]. In ER-positive BC cells, low adiponectin levels permit the interaction of adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 1 (APPL1) with AdipoR1, ERα, insulin-like growth factor (IGF-IR), and c-Src [57]. This complex activates MAPK signaling that promotes BC cell growth [57]. Moreover, adiponectin has a differential influence on cyclin D1 expression and tumor progression depending on ER status [58]. Cyclin is downregulated in ERα-negative cells and upregulated in ERα-positive cells, events that correspond to tumor reduction and growth respectively [58]. Studies have shown that ER status may modulate the effect of adiponectin on cell metabolism. Cancer cells rely mainly on aerobic glycolysis (Warburg effect), a property that is largely sustained by regulators such as fatty acid synthase (FASN) and Acetyl-coA carboxylase (ACC) [59, 60]. LKB1/AMPK is also a crucial pathway in regulating energy homeostasis, such as glucose uptake, glycolysis, fatty acid oxidation, and mitochondrial biogenesis [59, 61, 62]. In ERα-negative BC, adiponectin may inhibit fatty acid synthesis through activation of AMPK/ACC, while in ERα-positive BC, it cannot intervene in this process [58, 63].
Adiponectin has also been found in adipocyte exosomes—the lipid bilayer vesicles secreted by adipocytes [64], which constitute mediators of cell-to-cell signaling in the complex tumor microenvironment. Exosomes from human adipose-derived mesenchymal stem cells (ADSCs) and pre-adipocytes promote proliferation and migration of BC cells and BC stem cells respectively. Exosomes secreted by pre-adipocytes also regulate breast tumor stem cell formation and migration [65, 66]. More research is needed to explore the role of exosomic adiponectin in BC.
The most recent meta-analysis by Gu and colleagues investigated the association of serum adiponectin levels and BC finding that serum adiponectin was lower in BC patients irrespective of menopausal status [67•]. Interestingly, two other meta-analyses have found a significant association between adiponectin levels and postmenopausal BC patients but not premenopausal [68, 69]. Macis et al. compared “high” vs “low” adiponectin groups and found a 34% reduction in BC risk favoring the “high” adiponectin group while a subgroup analysis for menopausal status confirmed the association only for postmenopausal women [70]. Overall, based on meta-analyses examining the relation of serum adiponectin and BC, a common pattern emerges: when all women are included in the analysis, elevated adiponectin is associated with reduced BC risk but this association is more pronounced in postmenopausal women. More larger prospective studies are required to delineate the potentially mediating role of adiponectin in BC.
Leptin and Breast Cancer
Leptin, a 16-kDa polypeptide produced mainly from the adipose tissue, was discovered by Friedman and colleagues in 1994 [71]. It is the product of the Ob gene and after its secretion, it circulates in a free and a bound form [72]. Leptin affects its target tissues through the leptin receptor (LEPR), a single transmembrane protein that is ubiquitously expressed [71]. Leptin secretion is in proportion to the adipose tissue mass and serves as a message of satiety and energy adequacy suppressing appetite [73]. LEPR can affect multiple intracellular pathways including Janus kinase/signal transducer and activator of transcription (JAK/STAT3), mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase/v-Akt murine thymoma viral oncogene homolog (PI3K/Akt), extracellular signal-regulated kinase 1/ 2 (ERK1/2), 5′ AMP-activated protein kinase (AMPK), and insulin receptor substrate (IRS) [73, 74].
In the context of cancer, JAK2 activates STAT3 and 5 promoting the expression of genes crucial to tumorigenesis, affecting cell proliferation, invasion, angiogenesis, and inflammation [46]. In addition, leptin upregulates the expression of anti-apoptotic proteins, inflammatory markers (tumor necrosis factor-α/TNF-a, IL-6), angiogenic factors (VEGF), and the hypoxia-inducible factor-1a (HIF-1a) [75, 76]. The ERK signaling promotes the activation of transcription factors that induce cell division [77]. Moreover, after JAK2 stimulation, PI3K and Akt are activated affecting glucose metabolism, cell growth, proliferation, and apoptosis [78].
The oncogenic mechanism of leptin in breast tissue involves the stimulation of JAK/STAT3 and PI3K pathways [79]. Leptin can inhibit apoptosis of BC cells favoring the expression of anti-apoptotic genes (bcl-xL, bax) and induce angiogenesis by stimulation of VEGF production [80]. Leptin displays an interesting relationship with estrogen signaling. Leptin can potentiate estrogen signaling through three mechanisms: (1) upregulation of aromatase, (2) direct activation of ERα, and (3) suppression of p53 [81,82,83]. In vivo, leptin administration was found to double tumor size after 13 weeks when compared with estradiol treatment [84]. A study that assessed the possible synergistic effect of estrogen and leptin in BC development revealed that ER signaling promotes leptin-induced autophagy that in turn contributes to BC growth [85].
In vivo studies have shown that elimination of peripheral tissue leptin signaling with concurrent preservation of leptin receptor signaling can decrease BC development and progression [86]. When leptin signaling was inhibited in mammary tumor virus-Wnt-1 mice (MMTV-Wnt-1), tumor growth was reduced and BC stem cell (CSC) population was suppressed [87]. Moreover, Western diet–induced obese rats exhibited increased BC incidence and aggressiveness as well as upregulated leptin and LEPR expression and signaling [88].
Interestingly, recent evidence has shown that leptin can render BC cells less susceptible to treatment with tamoxifen, an effect probably mediated by induction of the membrane tyrosine kinase HER2 receptor expression [89]. Moreover, leptin may promote CSC proliferation, migration, and angiogenesis through a complex signaling combination between Notch, IL-1, and leptin, termed NILCO [90, 91]. Chang and colleagues have demonstrated that leptin can further promote the formation of breast CSC by epigenetic downregulation of miR-200c [88]. Additionally, leptin signaling may activate fatty acid b-oxidation (FAO), through upregulation of carnitine palmitoyltransferase 1B (CPT1B), inducing BC stemness and resistance to chemotherapy, phenomena reversed by FAO and/or leptin signaling inhibition [92]. Leptin is considered a mediator molecule between stromal cells and tumor microenvironment.
Clinical data have confirmed the correlation of serum leptin and BC. The most recent meta-analysis investigating the relationship of leptin levels with BC concluded that BC patients exhibited higher serum leptin levels. Subgroup analysis for BMI and menopausal status revealed that the association was significant only in overweight and obese postmenopausal women [93•]. In another meta-analysis, Niu and colleagues have demonstrated that serum leptin levels were escalating from healthy controls to benign breast tumor, local BC, and lymph node–positive BC subgroups [94].
Resistin and Breast Cancer
Resistin is an adipokine of 12.5 kDa that is secreted by mononuclear cells and adipocytes [95]. Discovered in 2001, it was regarded as the mediator between obesity and diabetes (the name “resistin” stems from the property of insulin resistance amplification) [96]. Resistin exerts its effects through binding to Toll-like receptor 4 (TLR4), resulting in activation of the PI3K, p38, MAPK, and NF-kB pathways [97].
High levels of resistin have been associated with many disease states, such as visceral obesity, coronary artery disease, lung disease, various malignancies, and critical illness [9••].
Resistin may trigger tumorigenesis via inflammation (PI3K and NF-kB pathways), immune cell extravasation (MAPK pathway), expression of cardinal molecules for adhesion of cancerous cells (NF-κB pathway), and promotion of survival and invasiveness of tumor cells (PI3K and MAPK pathways) [9••].
In the context of BC, researchers using MCF-7 BC cell lines discovered that resistin enhances the metastatic potential of BC cells by promotion of epithelial-to-mesenchymal transition (EMT) and stemness, and these effects were largely attributed to adenylyl cyclase–associated protein 1 (CAP1) [98]. In line with this, resistin was shown to promote metastasis in MDA-MB-231 human BC cells through phosphorylation of the ezrin, radixin, and moesin (ERM) complex [99].
Interestingly, two groups have indicated that resistin may confer chemoresistance properties to BC cells [100, 101]. One group proposed that the stimulation of AMPK/mTOR/ULK1 and c-Jun N-terminal kinase (JNK) signaling induces autophagy bypassing doxorubicin-induced apoptosis [100], whereas another found that chemoresistance was mediated through STAT3 activation [101].
Clinical data linking resistin to BC have been heterogeneous with some studies highlighting its association with postmenopausal BC [33, 35, 102]. Independent groups have found that elevated resistin expression in BC tissue is associated with adverse clinical and pathological characteristics as well as poor patient survival [35, 103].
In a recent meta-analysis of 13 studies, resistin levels were associated with an increased incidence of obesity-related cancers (breast, endometrial, and colorectal cancer) but despite its association with BC, resistin levels were found to be of limited diagnostic and predictive value [104]. Another very recent meta-analysis which evaluated the association of several adipokines with BC has shown significantly higher resistin in BC patients without a significant association between resistin levels and menopausal status [25••].
Visfatin/Nampt and Breast Cancer
Visfatin, also known as Nampt or pre-B cell colony–enhancing factor (PBEF), is a 52-kDa protein, that is produced by the NAMPT gene. It exhibits a multi-faceted role acting concurrently as an enzyme, adipokine, and a growth factor [105, 106]. Nampt exists in two forms, the intracellular-iNampt and the extracellular-eNampt [42]. iNampt participates in NAD biosynthesis that functions as an important electron carrier, and exerts a crucial function in cell metabolism. eNampt is excreted by a multitude of tissues such as adipose, liver, and heart, and the mechanism of excretion is thought to be cell lysis [107]. eNampt has been implicated in several diseases including diabetes, obesity, aging, atherosclerosis, cardiac hypertrophy, and autoimmune diseases [9••].
With respect to cancer development, visfatin displays pro-inflammatory, proliferative, anti-apoptotic, and pro-angiogenic effects [108]. It has been shown to promote inflammatory processes through the activation of NF-kB and induce cell proliferation through the upregulation of Notch-1, cyclin D1, cyclin-dependent kinase 2, MAPK, ERK-1/2, and p38 signaling pathways [109,110,111]. eNampt may also function in an endocrine manner contributing through its immunosuppressive properties to the surviving strategies of cancer that take advantage of immune evasion [112]. Serum eNampt is elevated in many cancers, and is generally correlated with worse prognosis and aggressive behavior [9••].
Preclinical and clinical studies have implicated visfatin/Nampt in BC pathogenesis. In MCF-7 BC cells, eNampt mediated the upregulation of SIRT1 and p53 deacetylation, contributing to BC progression [113]. These mechanisms were confirmed in BC cell lines where eNampt induced BC cell proliferation and suppressed apoptosis through AKT/PI3K and ERK/MAPK activation [114].
Higher visfatin expression in BC tissue correlated with more malignant tumor behavior as well as poor patient survival [115], tumor size, ER negativity, progesterone receptor (PR) negativity, and decreased recurrence rate after hormone therapy [115]. Visfatin expression alone was associated with poor disease-free and overall survival, and this association was more pronounced in combination with ER- and PR-negative status [115]. Another group has confirmed the association of visfatin with tumor aggressiveness, but also tried to elucidate the underlying mechanism. They found that phosphorylation of c-Abl and STAT3 in breast tumor tissues was associated with high serum visfatin levels. Inhibiting c-Abl and STAT3 reversed eNampt-induced cell viability and metastatic potential [116].
Several studies have indicated that serum visfatin levels are elevated in BC [34, 36, 37]. Interestingly, serum visfatin levels when integrated in a multi-factorial ROC analysis may predict BC progression [117]. A recent meta-analysis has shown that higher visfatin levels were associated with cancer risk [118] while another meta-analysis of BC patients revealed that mean concentration of visfatin was higher in BC patients than controls without taking into account menopausal status [25••].
Novel Adipokines and Breast Cancer
Apelin, a 9-kDa peptide identified in 1998 and encoded by the APLN gene, is the endogenous ligand of the G-protein-coupled receptor APJ and exerts its action through the activation of the ERK and PI3K/Akt pathways [119]. Increased apelin levels are found in mammary gland and its secretion in the milk is abundant. Apelin possesses various metabolic functions, such as regulation of insulin secretion and sensitivity, blood pressure, and fluid homeostasis while it plays a role in lymphangiogenesis and neoangiogenesis [120,121,122]. Notably, in MCF-7 BC cells, apelin induced cell proliferation and invasion via the ERK1/2 pathway [123], whereas it activated tumor neoangiogenesis in TS/A mammary carcinoma cells [124], demonstrating potent angiogenic properties. Several immunohistochemical studies have shown higher apelin expression in human BC [125, 126], while Salman et al. found increased circulating serum levels of apelin in postmenopausal BC patients compared with controls and a significant reduction after treatment with an aromatase inhibitor [127]. Moreover, recent data have revealed a strong association of apelin with lymph node metastasis and TNM staging in BC showing that this adipokine can be used as an independent prognostic factor for BC [125].
Chemerin is a 14-kDa protein which acts through binding to G-protein-coupled receptors and plays a multifunctional role in adipogenesis, immunity, and metabolic activity [128, 129]. Its implication in cancer is conflicting as it can trigger tumorigenesis by promoting angiogenesis, inflammation, and matrix metalloproteinase (MMP) activity, whilst it also exhibits antitumor properties depending on its concentration [130, 131]. Chemerin’s main receptor, ChemR23, is found in breast tissue. Chemerin expression is downregulated in BC samples compared with normal controls and seems to be correlated with poor survival outcome [132]. However, it has been shown that induction of chemerin overexpression in the EMT6 BC model suppressed tumor growth by recruiting immune cells into the tumor microenvironment [133•]. There is conflicting evidence regarding the clinical utility of chemerin as a prognostic factor in BC. El-Sagheer et al. showed that chemerin expression in breast tissue correlated with poor prognosis and unfavorable clinical and pathological parameters [132]. However, in another study, serum chemerin levels were not associated with BC stage, as there was no difference in patients with metastatic and non-metastatic BC [134].
Encoded by the FNDC5 gene, irisin is a newly discovered adipo-myokine that is involved in the browning of white adipose tissue regulating energy expenditure and systemic metabolism [135,136,137]. This 12-kDa protein is predominantly secreted from skeletal muscle but immunohistochemical studies have also revealed local production in various central and peripheral tissues [138, 139]. The antitumor effect of irisin has been shown in a recent in vitro study where a considerable tumor suppressive result was noted on the number, migration, and viability of malignant BC cell lines, with the induction of cell apoptosis and the suppression of NF-κB activity [140]. Few clinicoepidemiologic studies have examined circulating irisin in BC. Serum concentration of irisin in patients with BC was significantly lower than in healthy participants and was correlated with tumor stage [141]. In line with the previous findings, lower serum levels of irisin were observed in BC patients with spinal metastasis than BC patients without spinal metastasis, where irisin emerged as an independent prognostic factor in BC after adjustment for age and BMI [142].
Lipocalin 2 (Lcn2), also known as neutrophil gelatinase–associated lipocalin (NGAL), is a 25-kDa secretory peptide, member of the lipocalin family which is involved in transportation of small hydrophobic molecules and immune response [143]. Lcn2 was recently recognized as an adipokine that is also secreted from adipose tissue of both mice and humans. Upregulated expression levels of Lcn2 have been reported in tissue, serum, and urine of BC patients [144••]. A growing body of evidence from in vitro and in vivo studies have shown that Lcn2-tumorigenic and metastatic potential is induced via the promotion of EMT, cell migration and invasion, VEGF production, and angiogenesis [145,146,147,148]. The mechanisms explaining and supporting the Lcn2-oncogenic and metastatic potential comprise the activation of multiple signal pathways, including PI3K/Akt/NF-κB, HIF-1a/ERK, and the protective formation of the MMP-9/Lcn2 complex [144••]. Of note, Lcn2 silencing or inhibition in BC cells or mouse models destabilizes MMP-9/Lcn2 complex, reduces MMP-9 activity, cell migration, and invasion, decreases VEGF and angiogenesis, and may lessen tumor progression [144••]. A meta-analysis of four single-centered trials has highlighted the diagnostic potential of Lcn2 in BC [149]. Additionally, various studies have shown that Lcn2 correlated with histological grade, BC relapse, metastasis, and poor prognosis, including estrogen receptor (ER)– negative status [147, 150,151,152,153,154], suggesting that Lcn2 may serve as a promising noninvasive diagnostic and prognostic biomarker in BC.
Oncostatin M (OSM), a 24-kDa protein identified in 1986 and encoded by the OSM gene, is a pleiotropic cytokine that belongs to the IL-6 family being involved in inflammation, hematopoiesis, and bone formation [155, 156]. OSM interacts with the gp130 complex with either OSM receptor type I (known as LIFR) or OSM receptor II (known as OSMR), stimulating several signaling pathways such as JAK/STAT3 and PI3K [157, 158]. In vitro studies have shown that OSM expression correlated not only with tumor progression in BC cell lines via a JAK/STAT3-dependent mechanism [159], but also with phenotypic changes associated with mesenchymal and stem cell–like differentiation via the PI3K pathway upregulation [160]. The ability of OSM to facilitate metastasis in BC has also been shown in a mouse BC model where OSM potentiated pre-intravasation events, increased circulating tumor cells, and promoted lung metastasis [161]. Moreover, recent accumulating clinical and experimental evidence have associated elevated expression of OSM with decreased BC survival and a worse clinical outcome mediated by estrogen receptor downregulation [162,163,164], suggesting a potential role of OSM in BC prognosis.
Osteopontin (OPN), also called bone sialoprotein 1, is a 44-kDa cytokine-like, calcium binding, and multifunctional protein involved in biomineralization, inflammation, and tissue remodeling [165, 166]. OPN interacts with a plethora of cell surface receptors, including several integrins and CD44 [167]. Particularly in BC, OPN has been shown to preferentially bind to specific integrins, such as ανβ1, ανβ3, ανβe, and ανβ5 receptors which are associated with different signaling pathways, resulting in an increase in cell adhesion, migration, and invasion [168,169,170]. Elevated OPN expression in tissue, plasma, or serum in BC has been found in many studies, with higher OPN concentrations being associated with higher tumor grade [171,172,173,174], while a number of studies have demonstrated that OPN may be correlated with BC progression and metastasis [175,176,177]. Interestingly, OPN downregulation via the miR-181c inhibited cell proliferation and enhanced chemosensitivity in resistant BC cells [178•]. A growing body of evidence has highlighted the prognostic value of OPN in BC, as shown in a recent meta-analysis where high OPN and particularly OPN splice variant-c levels were correlated with poor survival [179]. Moreover, in another meta-analysis, OPN overexpression was positively associated with lymph node metastasis as well as overall and disease-free survival in BC [180].
Implications in Public Health and Therapeutics
A small but considerable percentage of BC cases could be preventable through maintaining a healthy weight, adopting a diet with fruits, nuts, vegetables, whole grains, and olive oil, reducing unhealthy diet (consumption of sugar, trans-fats and saturated fats, refined grains, red and processed meat), increasing physical exercise, and decreasing alcohol intake [3, 181, 182]. Figure 2 presents potential therapeutic strategies in postmenopausal BC, which is associated with obesity. The American Society of Clinical Oncology has highlighted that obesity is one of the most cardinal preventable lifestyle risk factor for cancer mortality [183]. Based on IARC and WCRF/AICR reports, physical activity may decrease both postmenopausal and premenopausal (vigorous activity) BC risk and BC mortality [13••, 182, 184] through modulation of insulin resistance, chronic inflammation, and circulation of sex steroid hormones and adipokines. In the SHAPE study of postmenopausal women with BC, a significant reduction of circulating leptin was observed with a physical activity program yielding a weight decrease of more than 5% [185].
Potential therapeutic strategies in postmenopausal breast cancer. Nampt, nicotinamide phosphoribosyl-transferase; PPAR-γ, peroxisome proliferator–activated receptors-γ. (Image of breast cancer tissue is derived from the free medical site http://smart.servier.com/ by Servier licensed under a Creative Commons Attribution 3.0 Unported License)
Intentional weight loss is related with a significant decrease in the risk of postmenopausal BC [186] contributing to a better life expectancy [187]. Ongoing, large, weight loss intervention randomized trials will explore the effects on BC outcomes [13••]. However, the current state of knowledge corroborates the daily incorporation of weight loss intervention in the management of BC. Regarding all-cause mortality in BC patients, a beneficial effect of the Mediterranean diet was observed but no positive effect from other diets such as low-carbohydrate, ketogenic, or vegetarian/vegan diets was found [188]. Based on two very recent meta-analyses, bariatric surgery for morbidly obese women has been shown to reduce the incidence of BC [189, 190]. Due to underpowered and heterogenous studies, limited follow-up, and difficulty in identifying proper controls, larger RCTs are needed to explore the effect of bariatric surgery on BC incidence and outcomes.
In the setting of obesity, which is considered a systemic endocrine dysfunction characterized by chronic inflammation, adipokines exert independent and joint effects on activation of major intracellular signal networks implicated in cell proliferation, growth, survival, invasion, and metastasis [9••]. Circulating levels of adipokines could be modifiable by weight loss, adoption of a balanced diet, and physical activity [30, 42, 95]. Αlthough many adipokines are not only adipocyte-derived, they are responsive to adiposity alterations. Bariatric surgery, which is related to BC risk reduction via regulation of the adipokine profile, may increase levels of adiponectin and decrease levels of leptin, resistin, visfatin/eNampt, and chemerin [191,192,193].
Glycemic control may restore adipokine levels [194]. Anti-diabetic drugs such as metformin or PPAR-γ agonists that elevate adiponectin and decrease resistin and visfatin concentrations in both humans and mice may be at the forefront of therapeutic strategies for BC [195]. Besides its role as an activator of AMP-kinase, metformin potentiates non-AMPK-dependent protective networks such as decreases in leptin, insulin signaling, IGF-1, and inflammatory pathways and increases in adiponectin [195]. Although data regarding metformin and BC incidence and mortality are inconclusive showing, however, a tendency of protective effects on BC particularly in HR-positive and diabetic patients [40, 195,196,197], recent meta-analyses have indicated significant reductions in leptin and other metabolic parameters (hsCRP, glucose, insulin, BMI) in BC patients receiving metformin [196, 198]. More RCTs are awaited to determine the role of metformin in BC risk decrease and prognosis in diabetic and non-diabetic patients as well as an adjuvant therapy in BC reversing chemotherapy resistance [199]. On the other hand, in vitro and in vivo studies have shown that PPAR-γ agonists as well as high-affinity PPAR-γ agonists, which upregulate adiponectin expression and decrease inflammatory cytokines, have the potential to suppress the proliferation and invasion of BC cells through the inhibition of leptin signaling [40, 200]. Nevertheless, PPAR-γ agonists do not seem to affect BC risk when employed as a single agent or in combination with hormone therapy or chemotherapy [40, 201].
Whilst some preclinical and epidemiologic studies have suggested a protective role for statins, aspirin, and other non-steroidal anti-inflammatory drugs in BC (particularly postmenopausal and HR-positive tumors) risk and mortality, other studies did not support these findings, and further large-scale evidence from RCTs is required [202,203,204,205,206,207]. Although calcium channel blockers, folic acid, oleic acid, and vitamin C and D supplementation could significantly restore adipokine levels [9••, 42, 208], controversy exists between the potential association of those agents with BC risk and progression [209,210,211,212,213]. Some phytochemicals such as curcumin and resveratrol as well as dietary flavonoids such as catechin and genistein, which may regulate mRNA and protein levels of adiponectin, resistin, and visfatin [42], have been reported to present anti-neoplastic and chemoprevention effects on BC in experimental studies [214, 215].
Several adipokine-oriented therapeutic approaches have been developed and used in preclinical studies for BC with promising results. Antagonists of the leptin receptor that can suppress leptin signaling as well as adiponectin agonists mimicking adiponectin action have been shown to inhibit the proliferation of BC cells [216, 217]. Indeed, pegylated leptin receptor antagonist 2 as well as other leptin receptor antagonists based on mutants of the full leptin protein or leptin peptide fragments have decreased the proliferation and angiogenesis of ER-positive or ER-negative BC cells in xenograft mice models [216,217,218]. Peptide-based adiponectin receptor agonists such as ADP-355, which is an adiponectin mimetic binding to both AdipoR1 and AdipoR2, have been reported to suppress the growth of BC cell lines and orthotopic xenograft BC models [219]. Nampt inhibitors, which limit NAD production in BC cells, have demonstrated significant in vitro and in vivo antitumor efficacy in an orthotopic MDA-MB-231 triple negative BC xenograft tumor model [220]. Therefore, continued research is necessary to explore whether adipokines may be potential therapeutic targets for both BC and obesity. The research of the role of novel adipokines merits further attention in future studies in obesity and BC.
Adipokines could be useful diagnostic, prognostic, and predictive biomarkers, reflecting BC advanced stage, adverse prognosis, and inflammatory state. However, large-scale prospective and longitudinal studies are required to investigate the diagnostic, prognostic, and predictive utility of adipokines as BC biomarkers and to exclude a potential “epiphenomenon” effect of adipokines variation in the context of BC systemic inflammatory response [9••]. Additional challenges encompass the lack of standardization of adipokine immunoassay procedures and the development of reliable, “user friendly,” and practical automated laboratory technique such as multiplexing technology to explore the physiologic and pathophysiological relevance of adipokines in BC and their clinical utility. To investigate the potential relationship of adipokines and BC risk, adequately powered Mendelian randomization studies using genetic determinants of adipokines derived from genome-wide associations studies are necessary because they circumvent confounding of lifestyle variables and reverse causation improving causal inference in the association of obesity-related biomarkers with cancer risk [221].
Conclusions
In summary, this review shows evidence for an association between adipokines and BC. High throughput technologies such as proteomics and metabolomics will discover novel adipokines. Further evidence from basic and translational research is necessary to delineate the ontological role of adipokines and their interplay in BC pathogenesis. More studies are needed to explore the epigenetic regulation of adipokine genes and to map out their receptors and critical signaling pathways. More large-scale clinical and longitudinal studies are awaited to assess their clinical utility in BC prognosis and follow-up. Finally, novel more effective and safer adipokine-centered therapeutic strategies could pave the way for targeted oncotherapy.
Abbreviations
- ACC:
-
Acetyl-coA carboxylase
- ADSCs:
-
Adipose-derived mesenchymal stem cells
- AdipoR1/R2:
-
adiponectin receptor 1/ 2
- Akt: v-:
-
Akt murine thymoma viral oncogene homolog
- AMPK:
-
5′ AMP-activated protein kinase
- APPL1:
-
Adaptor protein phosphotyrosine interacting with PH domain and leucine zipper 1
- bax:
-
Bcl-2 associated X protein
- BC:
-
breast cancer
- bcl-xL:
-
B cell lymphoma-extra large
- BMI:
-
Body mass index
- CAP1:
-
Adenylyl cyclase–associated protein 1
- CPT1B:
-
Carnitine palmitoyltransferase 1B
- CSC:
-
Cancer stem cell
- c-Src:
-
Proto-oncogene tyrosine-proteine kinase Src
- DM:
-
Diabetes mellitus
- EMT:
-
Epithelial-mesenchymal transition
- eNampt::
-
Extracellular nicotinamide phosphoribosyl-transferase (eNampt)
- ER:
-
Estrogen receptor
- ERK 1/2:
-
Extracellular signal-regulated kinase 1/2
- FAO:
-
Fatty acid b-oxidation
- FASN:
-
Fatty acid synthase
- GRP78:
-
Glucose-regulated protein 78
- GTP:
-
Guanosine-5′-triphosphate
- HER:
-
Human epidermal growth factor receptor
- HIF-1a:
-
Hypoxia-inducible factor-1a
- HR:
-
Hormone receptor
- HRT:
-
Hormone replacement therapy
- hsCRP:
-
High-sensitive C-reactive protein
- IL:
-
Interleukin
- IARC:
-
International Agency for Research on Cancer
- IGF:
-
Insulin-like growth factor
- IRS:
-
Insulin receptor substrate
- JAK:
-
Janus kinase
- JNK:
-
Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- MMTV:
-
Mammary tumor virus
- Lcn2:
-
Lipocalin 2
- LEPR:
-
Leptin receptor
- LIFR:
-
Leukemia inhibitory receptor
- LKB1:
-
else known as STK11 (serine/threonine kinase 11)
- MCF-7:
-
Michigan Cancer Foundation 7
- miR:
-
Micro-RNA
- MMP:
-
Matrix metalloproteinase
- mTOR:
-
Mammalian target of rapamycin
- MO25:
-
Scaffolding mouse 25 protein
- NAD:
-
Nicotinamide adenine dinucleotide
- Nampt:
-
Nicotinamide phosphoribosyl-transferase
- NF-κB:
-
nuclear factor-κB
- NGAL:
-
Neutrophil gelatinase–associated lipocalin
- NILCO:
-
Notch, IL-1, and leptin
- OPN:
-
Osteopontin
- OSM:
-
Oncostatin M
- OSMR:
-
OSM receptor II
- OR:
-
Odds ratio
- PBEF:
-
pre-B cell colony–enhancing factor
- PI3K:
-
Phosphatidylinositol 3-kinase
- PPAR:
-
Peroxisome proliferator–activated receptors
- PR:
-
Progesterone receptor
- RBP-4:
-
Retinol-binding protein
- ROCK:
-
Rho-associated coiled coil-containing protein kinase
- SHBG:
-
Sex hormone–binding globulin
- SIRT1:
-
Sirtuin 1
- SMD:
-
Standardized mean difference
- STAT:
-
Signal transducer and activator of transcription
- STRA6:
-
Stimulated by retinoic acid 6
- STRAD:
-
STE20-related adaptor protein
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular cellular adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- Wnt:
-
Wingless-related integration site
- WC:
-
Waist circumference
- WHR:
-
Waist-to hip ratio
- WCRF/AICR:
-
World Cancer Research Fund/American Institute for Cancer Research
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
The state of cancer care in America. 2014: a report by the American Society of Clinical Oncology. J Oncol Pract. 2014;10:119–42. https://doi.org/10.1200/jop.2014.001386.
Pischon T, Nimptsch K. Obesity and risk of cancer: an introductory overview. Recent Results Cancer Res. 2016;208:1–15. https://doi.org/10.1007/978-3-319-42542-9_1.
Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69:88–112. https://doi.org/10.3322/caac.21499.
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–7. https://doi.org/10.1038/ijo.2008.102.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. https://doi.org/10.1056/NEJMsr1606602.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. https://doi.org/10.3322/caac.21551.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–89. https://doi.org/10.3322/caac.21349.
Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6:e6–e15. https://doi.org/10.1016/s2213-8587(18)30150-5.
•• Spyrou N, Avgerinos KI, Mantzoros CS, Dalamaga M. Classic and novel adipocytokines at the intersection of obesity and cancer: diagnostic and therapeutic strategies. Curr Obes Rep. 2018;7:260–75. https://doi.org/10.1007/s13679-018-0318-7This review summarizes the association of classic and novel adipokines with cancer giving special emphasis on mechanisms of action and clinical studies.
•• Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35. https://doi.org/10.1016/j.metabol.2018.11.001This review shows evidence for the association between obesity and cancer underscoring the role of emerging biological mechanisms.
Allott EH, Hursting SD. Obesity and cancer: mechanistic insights from transdisciplinary studies. Endocr Relat Cancer. 2015;22:R365–86. https://doi.org/10.1530/erc-15-0400.
Canchola AJ, Anton-Culver H, Bernstein L, Clarke CA, Henderson K, Ma H, et al. Body size and the risk of postmenopausal breast cancer subtypes in the California Teachers Study cohort. Cancer Causes Control. 2012. https://doi.org/10.1007/s10552-012-9897-x.
•• Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–97. https://doi.org/10.3322/caac.21405This review summarizes the relationships between obesity and breast cancer development and addresses implicated molecular mechanistic insights and strategies for intervention.
Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–36. https://doi.org/10.1093/epirev/mxt010.
Rosner B, Eliassen AH, Toriola AT, Chen WY, Hankinson SE, Willett WC, et al. Weight and weight changes in early adulthood and later breast cancer risk. Int J Cancer. 2017;140:2003–14. https://doi.org/10.1002/ijc.30627.
Iyengar NM, Arthur R, Manson JE, Chlebowski RT, Kroenke CH, Peterson L, et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a Randomized Clinical Trial and Observational Study. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.5327.
Namazi N, Irandoost P, Heshmati J, Larijani B, Azadbakht L. The association between fat mass and the risk of breast cancer: a systematic review and meta-analysis. Clin Nutr. 2019;38:1496–503. https://doi.org/10.1016/j.clnu.2018.09.013.
Fagherazzi G, Fabre A, Boutron-Ruault MC, Clavel-Chapelon F. Serum cholesterol level, use of a cholesterol-lowering drug, and breast cancer: results from the prospective E3N cohort. Eur J Cancer Prev. 2010;19:120–5. https://doi.org/10.1097/CEJ.0b013e3283354918.
Gaudet MM, Press MF, Haile RW, Lynch CF, Glaser SL, Schildkraut J, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130:587–97. https://doi.org/10.1007/s10549-011-1616-x.
Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–39. https://doi.org/10.1007/s10549-007-9632-6.
Ford NA, Nunez NP, Holcomb VB, Hursting SD. IGF1 dependence of dietary energy balance effects on murine Met1 mammary tumor progression, epithelial-to-mesenchymal transition, and chemokine expression. Endocr Relat Cancer. 2013;20:39–51. https://doi.org/10.1530/erc-12-0329.
Dunlap SM, Chiao LJ, Nogueira L, Usary J, Perou CM, Varticovski L, et al. Dietary energy balance modulates epithelial-to-mesenchymal transition and tumor progression in murine claudin-low and basal-like mammary tumor models. Cancer Prev Res (Phila). 2012;5:930–42. https://doi.org/10.1158/1940-6207.capr-12-0034.
Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–14. https://doi.org/10.1093/annonc/mdu042.
Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34:4203–16. https://doi.org/10.1200/jco.2016.68.4480.
•• Gui Y, Pan Q, Chen X, Xu S, Luo X, Chen L. The association between obesity related adipokines and risk of breast cancer: a meta-analysis. Oncotarget. 2017;8:75389–99. https://doi.org/10.18632/oncotarget.17853This meta-analysis examines the association of the serum levels of several adipokines and the risk of breast cancer.
Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4:798–804. https://doi.org/10.1001/jamaoncol.2018.0137.
Bradshaw PT, Cespedes Feliciano EM, Prado CM, Alexeeff S, Albers KB, Chen WY, et al. Adipose tissue distribution and survival among women with nonmetastatic breast cancer. Obesity (Silver Spring). 2019;27:997–1004. https://doi.org/10.1002/oby.22458.
Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: new biomarkers and attractive therapeutic targets. World J Exp Med. 2013;3:34–42. https://doi.org/10.5493/wjem.v3.i3.34.
Bulun SE, Chen D, Moy I, Brooks DC, Zhao H. Aromatase, breast cancer and obesity: a complex interaction. Trends Endocrinol Metab. 2012;23:83–9. https://doi.org/10.1016/j.tem.2011.10.003.
Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94. https://doi.org/10.1210/er.2011-1015.
Kassi E, Dalamaga M, Hroussalas G, Kazanis K, Merantzi G, Zachari A, et al. Adipocyte factors, high-sensitive C-reactive protein levels and lipoxidative stress products in overweight postmenopausal women with normal and impaired OGTT. Maturitas. 2010;67:72–7. https://doi.org/10.1016/j.maturitas.2010.05.004.
Christodoulatos GS, Dalamaga M. Micro-RNAs as clinical biomarkers and therapeutic targets in breast cancer: Quo vadis? World J Clin Oncol. 2014;5:71–81. https://doi.org/10.5306/wjco.v5.i2.71.
Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause. 2013;20:845–51. https://doi.org/10.1097/GME.0b013e31827f06dc.
Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Elevated serum visfatin/nicotinamide phosphoribosyl-transferase levels are associated with risk of postmenopausal breast cancer independently from adiponectin, leptin, and anthropometric and metabolic parameters. Menopause. 2011;18:1198–204. https://doi.org/10.1097/gme.0b013e31821e21f5.
Dalamaga M, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, Lekka A. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem. 2013;46:584–90. https://doi.org/10.1016/j.clinbiochem.2013.01.001.
Dalamaga M. Nicotinamide phosphoribosyl-transferase/visfatin: a missing link between overweight/obesity and postmenopausal breast cancer? Potential preventive and therapeutic perspectives and challenges. Med Hypotheses. 2012;79:617–21. https://doi.org/10.1016/j.mehy.2012.07.036.
Dalamaga M, Archondakis S, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, et al. Could serum visfatin be a potential biomarker for postmenopausal breast cancer? Maturitas. 2012;71:301–8. https://doi.org/10.1016/j.maturitas.2011.12.013.
Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22:557–66. https://doi.org/10.1016/j.tcb.2012.08.001.
Mullooly M, Yang HP, Falk RT, Nyante SJ, Cora R, Pfeiffer RM, et al. Relationship between crown-like structures and sex-steroid hormones in breast adipose tissue and serum among postmenopausal breast cancer patients. Breast Cancer Res. 2017;19:8. https://doi.org/10.1186/s13058-016-0791-4.
Samuel SM, Varghese E, Varghese S, Busselberg D. Challenges and perspectives in the treatment of diabetes associated breast cancer. Cancer Treat Rev. 2018;70:98–111. https://doi.org/10.1016/j.ctrv.2018.08.004.
Cha YJ, Koo JS. Adipokines as therapeutic targets in breast cancer treatment. Expert Opin Ther Targets. 2018;22:941–53. https://doi.org/10.1080/14728222.2018.1538356.
Dalamaga M, Christodoulatos GS, Mantzoros CS. The role of extracellular and intracellular Nicotinamide phosphoribosyl-transferase in cancer: diagnostic and therapeutic perspectives and challenges. Metabolism. 2018;82:72–87. https://doi.org/10.1016/j.metabol.2018.01.001.
Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. https://doi.org/10.1210/jc.2003-031804.
Moon HS, Dalamaga M, Kim SY, Polyzos SA, Hamnvik OP, Magkos F, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34:377–412. https://doi.org/10.1210/er.2012-1053.
Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18:29–42. https://doi.org/10.1016/j.cmet.2013.05.010.
Lee CH, Woo YC, Wang Y, Yeung CY, Xu A, Lam KS. Obesity, adipokines and cancer: an update. Clin Endocrinol. 2015;83:147–56. https://doi.org/10.1111/cen.12667.
Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. https://doi.org/10.1016/j.febslet.2007.11.070.
Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–7. https://doi.org/10.1074/jbc.M501149200.
Ando S, Gelsomino L, Panza S, Giordano C, Bonofiglio D, Barone I, et al. Obesity, Leptin and breast cancer: epidemiological evidence and proposed mechanisms. Cancers (Basel). 2019;11. https://doi.org/10.3390/cancers11010062.
Boudeau J, Baas AF, Deak M, Morrice NA, Kieloch A, Schutkowski M, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–14. https://doi.org/10.1093/emboj/cdg490.
Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–63. https://doi.org/10.1146/annurev.biochem.75.103004.142702.
Panno ML, Naimo GD, Spina E, Ando S, Mauro L. Different molecular signaling sustaining adiponectin action in breast cancer. Curr Opin Pharmacol. 2016;31:1–7. https://doi.org/10.1016/j.coph.2016.08.001.
Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK, et al. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–9.
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3beta/beta-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006;66:11462–70. https://doi.org/10.1158/0008-5472.can-06-1969.
Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br J Cancer. 2008;98:370–9. https://doi.org/10.1038/sj.bjc.6604166.
Nakayama S, Miyoshi Y, Ishihara H, Noguchi S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res Treat. 2008;112:405–10. https://doi.org/10.1007/s10549-007-9874-3.
Mauro L, Pellegrino M, De Amicis F, Ricchio E, Giordano F, Rizza P, et al. Evidences that estrogen receptor alpha interferes with adiponectin effects on breast cancer cell growth. Cell Cycle. 2014;13:553–64. https://doi.org/10.4161/cc.27455.
Mauro L, Pellegrino M, Giordano F, Ricchio E, Rizza P, De Amicis F, et al. Estrogen receptor-alpha drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015;29:2150–60. https://doi.org/10.1096/fj.14-262808.
Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. https://doi.org/10.1016/j.ccr.2012.02.014.
Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–70. https://doi.org/10.2217/fon.09.174.
Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. https://doi.org/10.1038/ncb2329.
Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–75. https://doi.org/10.1038/nrc2676.
Mauro L, Naimo GD, Gelsomino L, Malivindi R, Bruno L, Pellegrino M, et al. Uncoupling effects of estrogen receptor alpha on LKB1/AMPK interaction upon adiponectin exposure in breast cancer. FASEB J. 2018;32:4343–55. https://doi.org/10.1096/fj.201701315R.
Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49:3–13. https://doi.org/10.1111/cpr.12233.
Gernapudi R, Yao Y, Zhang Y, Wolfson B, Roy S, Duru N, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat. 2015;150:685–95. https://doi.org/10.1007/s10549-015-3326-2.
Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383:13–20. https://doi.org/10.1007/s11010-013-1746-z.
• Gu L, Cao C, Fu J, Li Q, Li DH, Chen MY. Serum adiponectin in breast cancer: a meta-analysis. Medicine (Baltimore). 2018;97:e11433. https://doi.org/10.1097/md.0000000000011433This very recent meta-analysis demonstrates the association between low adiponectin levels and breast cancer in both premenopausal and postmenopausal women.
Liu LY, Wang M, Ma ZB, Yu LX, Zhang Q, Gao DZ, et al. The role of adiponectin in breast cancer: a meta-analysis. PLoS One. 2013;8:e73183. https://doi.org/10.1371/journal.pone.0073183.
Ye J, Jia J, Dong S, Zhang C, Yu S, Li L, et al. Circulating adiponectin levels and the risk of breast cancer: a meta-analysis. Eur J Cancer Prev. 2014;23:158–65. https://doi.org/10.1097/CEJ.0b013e328364f293.
Macis D, Guerrieri-Gonzaga A, Gandini S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:1226–36. https://doi.org/10.1093/ije/dyu088.
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–84. https://doi.org/10.1152/ajpendo.00315.2011.
Friedman JM, Mantzoros CS. 20 years of leptin: from the discovery of the leptin gene to leptin in our therapeutic armamentarium. Metabolism. 2015;64:1–4. https://doi.org/10.1016/j.metabol.2014.10.023.
Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Invest. 2015;21:57–74. https://doi.org/10.1515/hmbci-2014-0037.
Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. https://doi.org/10.1677/erc.1.01169.
Choi JH, Park SH, Leung PC, Choi KC. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J Clin Endocrinol Metab. 2005;90:207–10. https://doi.org/10.1210/jc.2004-0297.
Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene. 2000;19:1379–85. https://doi.org/10.1038/sj.onc.1203443.
Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–93.
Harris HR, Tworoger SS, Hankinson SE, Rosner BA, Michels KB. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res (Phila). 2011;4:1449–56. https://doi.org/10.1158/1940-6207.capr-11-0125.
Garcia-Robles MJ, Segura-Ortega JE, Fafutis-Morris M. The biology of leptin and its implications in breast cancer: a general view. J Interf Cytokine Res. 2013;33:717–27. https://doi.org/10.1089/jir.2012.0168.
Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43. https://doi.org/10.1016/j.ejca.2010.09.005.
Catalano S, Marsico S, Giordano C, Mauro L, Rizza P, Panno ML, et al. Leptin enhances, via AP-1, expression of aromatase in the MCF-7 cell line. J Biol Chem. 2003;278:28668–76. https://doi.org/10.1074/jbc.M301695200.
Catalano S, Mauro L, Marsico S, Giordano C, Rizza P, Rago V, et al. Leptin induces, via ERK1/ERK2 signal, functional activation of estrogen receptor alpha in MCF-7 cells. J Biol Chem. 2004;279:19908–15. https://doi.org/10.1074/jbc.M313191200.
Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. https://doi.org/10.1016/j.eururo.2012.11.013.
Mauro L, Catalano S, Bossi G, Pellegrino M, Barone I, Morales S, et al. Evidences that leptin up-regulates E-cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res. 2007;67:3412–21. https://doi.org/10.1158/0008-5472.can-06-2890.
Raut PK, Choi DY, Kim SH, Hong JT, Kwon TK, Jeong JH, et al. Estrogen receptor signaling mediates leptin-induced growth of breast cancer cells via autophagy induction. Oncotarget. 2017;8:109417–35. https://doi.org/10.18632/oncotarget.22684.
Park J, Kusminski CM, Chua SC, Scherer PE. Leptin receptor signaling supports cancer cell metabolism through suppression of mitochondrial respiration in vivo. Am J Pathol. 2010;177:3133–44. https://doi.org/10.2353/ajpath.2010.100595.
Zheng Q, Dunlap SM, Zhu J, Downs-Kelly E, Rich J, Hursting SD, et al. Leptin deficiency suppresses MMTV-Wnt-1 mammary tumor growth in obese mice and abrogates tumor initiating cell survival. Endocr Relat Cancer. 2011;18:491–503. https://doi.org/10.1530/erc-11-0102.
Chang CC, Wu MJ, Yang JY, Camarillo IG, Chang CJ. Leptin-STAT3-G9a signaling promotes obesity-mediated breast cancer progression. Cancer Res. 2015;75:2375–86. https://doi.org/10.1158/0008-5472.can-14-3076.
Giordano C, Vizza D, Panza S, Barone I, Bonofiglio D, Lanzino M, et al. Leptin increases HER2 protein levels through a STAT3-mediated up-regulation of Hsp90 in breast cancer cells. Mol Oncol. 2013;7:379–91. https://doi.org/10.1016/j.molonc.2012.11.002.
Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer. PLoS One. 2011;6:e21467. https://doi.org/10.1371/journal.pone.0021467.
Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J Methodol. 2016;6:43–55. https://doi.org/10.5662/wjm.v6.i1.43.
Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018;27:1357. https://doi.org/10.1016/j.cmet.2018.04.018.
• Pan H, Deng LL, Cui JQ, Shi L, Yang YC, Luo JH, et al. Association between serum leptin levels and breast cancer risk: an updated systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11345. https://doi.org/10.1097/md.0000000000011345This recent meta-analysis has shown that higher leptin levels are associated with increased risk for breast cancer, especially for overweight/obese and postmenopausal women.
Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: a meta-analysis. PLoS One. 2013;8:e67349. https://doi.org/10.1371/journal.pone.0067349.
Dalamaga M. Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives. Biomark Med. 2014;8:107–18. https://doi.org/10.2217/bmm.13.99.
Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165:622–32. https://doi.org/10.1111/j.1476-5381.2011.01369.x.
Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC, et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2018;37:589–600. https://doi.org/10.1038/onc.2017.357.
Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, et al. Resistin induces breast cancer cells epithelial to mesenchymal transition (EMT) and stemness through both adenylyl cyclase-associated protein 1 (CAP1)-dependent and CAP1-independent mechanisms. Cytokine. 2019;120:155–64. https://doi.org/10.1016/j.cyto.2019.04.016.
Lee JO, Kim N, Lee HJ, Lee YW, Kim SJ, Park SH, et al. Resistin, a fat-derived secretory factor, promotes metastasis of MDA-MB-231 human breast cancer cells through ERM activation. Sci Rep. 2016;6:18923. https://doi.org/10.1038/srep18923.
Liu Z, Shi A, Song D, Han B, Zhang Z, Ma L, et al. Resistin confers resistance to doxorubicin-induced apoptosis in human breast cancer cells through autophagy induction. Am J Cancer Res. 2017;7:574–83.
Deshmukh SK, Srivastava SK, Zubair H, Bhardwaj A, Tyagi N, Al-Ghadhban A, et al. Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes. Cancer Lett. 2017;396:21–9. https://doi.org/10.1016/j.canlet.2017.03.010.
Georgiou GP, Provatopoulou X, Kalogera E, Siasos G, Menenakos E, Zografos GC, et al. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast. 2016;29:163–9. https://doi.org/10.1016/j.breast.2016.07.025.
Lee YC, Chen YJ, Wu CC, Lo S, Hou MF, Yuan SS. Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol Oncol. 2012;125:742–50. https://doi.org/10.1016/j.ygyno.2012.02.032.
Gong WJ, Zheng W, Xiao L, Tan LM, Song J, Li XP, et al. Circulating resistin levels and obesity-related cancer risk: a meta-analysis. Oncotarget. 2016;7:57694–704. https://doi.org/10.18632/oncotarget.11034.
Zhang LQ, Heruth DP, Ye SQ. Nicotinamide Phosphoribosyltransferase in human diseases. J Bioanal Biomed. 2011;3:13–25. https://doi.org/10.4172/1948-593x.1000038.
Duarte-Pereira S, Silva SS, Azevedo L, Castro L, Amorim A, Silva RM. NAMPT and NAPRT1: novel polymorphisms and distribution of variants between normal tissues and tumor samples. Sci Rep. 2014;4:6311. https://doi.org/10.1038/srep06311.
Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. https://doi.org/10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L.
Galli M, Van Gool F, Rongvaux A, Andris F, Leo O. The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res. 2010;70:8–11. https://doi.org/10.1158/0008-5472.can-09-2465.
Yu-Duan T, Chao-Ping W, Chih-Yu C, Li-Wen L, Tsun-Mei L, Chia-Chang H, et al. Elevated plasma level of visfatin/pre-b cell colony-enhancing factor in male oral squamous cell carcinoma patients. Med Oral Patol Oral Cir Bucal. 2013;18:e180–6. https://doi.org/10.4317/medoral.18574.
Park HJ, Kim SR, Kim SS, Wee HJ, Bae MK, Ryu MH, et al. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget. 2014;5:5087–99. https://doi.org/10.18632/oncotarget.2086.
Gabow PA. Autosomal dominant polycystic kidney disease--more than a renal disease. Am J Kidney Dis. 1990;16:403–13.
Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–23. https://doi.org/10.1182/blood-2014-07-589069.
Behrouzfar K, Alaee M, Nourbakhsh M, Gholinejad Z, Golestani A. Extracellular NAMPT/visfatin causes p53 deacetylation via NAD production and SIRT1 activation in breast cancer cells. Cell Biochem Funct. 2017;35:327–33. https://doi.org/10.1002/cbf.3279.
Gholinejad Z, Kheiripour N, Nourbakhsh M, Ilbeigi D, Behroozfar K, Hesari Z, et al. Extracellular NAMPT/Visfatin induces proliferation through ERK1/2 and AKT and inhibits apoptosis in breast cancer cells. Peptides. 2017;92:9–15. https://doi.org/10.1016/j.peptides.2017.04.007.
Lee YC, Yang YH, Su JH, Chang HL, Hou MF, Yuan SS. High visfatin expression in breast cancer tissue is associated with poor survival. Cancer Epidemiol Biomark Prev. 2011;20:1892–901. https://doi.org/10.1158/1055-9965.epi-11-0399.
Hung AC, Lo S, Hou MF, Lee YC, Tsai CH, Chen YY, et al. Extracellular visfatin-promoted malignant behavior in breast cancer is mediated through c-Abl and STAT3 activation. Clin Cancer Res. 2016;22:4478–90. https://doi.org/10.1158/1078-0432.ccr-15-2704.
Moi SH, Lee YC, Chuang LY, Yuan SF, Ou-Yang F, Hou MF, et al. Cumulative receiver operating characteristics for analyzing interaction between tissue visfatin and clinicopathologic factors in breast cancer progression. Cancer Cell Int. 2018;18:19. https://doi.org/10.1186/s12935-018-0517-z.
Yoon YS, Kwon AR, Lee YK, Oh SW. Circulating adipokines and risk of obesity related cancers: a systematic review and meta-analysis. Obes Res Clin Pract. 2019. https://doi.org/10.1016/j.orcp.2019.03.006.
Carpene C, Dray C, Attane C, Valet P, Portillo MP, Churruca I, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–73.
Castan-Laurell I, Dray C, Attane C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9. https://doi.org/10.1007/s12020-011-9507-9.
Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71. https://doi.org/10.1210/en.2004-1427.
Berta J, Hoda MA, Laszlo V, Rozsas A, Garay T, Torok S, et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget. 2014;5:4426–37. https://doi.org/10.18632/oncotarget.2032.
Peng X, Li F, Wang P, Jia S, Sun L, Huo H. Apelin-13 induces MCF-7 cell proliferation and invasion via phosphorylation of ERK1/2. Int J Mol Med. 2015;36:733–8. https://doi.org/10.3892/ijmm.2015.2265.
Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. 2007;26:7692–9. https://doi.org/10.1038/sj.onc.1210573.
Hu D, Zhu WF, Shen WC, Xia Y, Wu XF, Zhang HJ, et al. Expression of Apelin and Snail protein in breast cancer and their prognostic significance. Zhonghua Bing Li Xue Za Zhi. 2018;47:743–6. https://doi.org/10.3760/cma.j.issn.0529-5807.2018.10.002.
Wang Z, Greeley GH Jr, Qiu S. Immunohistochemical localization of apelin in human normal breast and breast carcinoma. J Mol Histol. 2008;39:121–4. https://doi.org/10.1007/s10735-007-9135-0.
Salman T, Demir L, Varol U, Akyol M, Oflazoglu U, Yildiz Y, et al. Serum apelin levels and body composition changes in breast cancer patients treated with an aromatase inhibitor. J buon. 2016;21:1419–24.
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–88. https://doi.org/10.1074/jbc.M700793200.
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–94. https://doi.org/10.1210/en.2007-0175.
Shin WJ, Zabel BA, Pachynski RK. Mechanisms and functions of chemerin in cancer: potential roles in therapeutic intervention. Front Immunol. 2018;9:2772. https://doi.org/10.3389/fimmu.2018.02772.
Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev. 2016;17:361–76. https://doi.org/10.1111/obr.12377.
El-Sagheer G, Gayyed M, Ahmad A, Abd El-Fattah A, Mohamed M. Expression of chemerin correlates with a poor prognosis in female breast cancer patients. Breast Cancer (Dove Med Press). 2018;10:169–76. https://doi.org/10.2147/bctt.s178181.
• Pachynski RK, Wang P, Salazar N, Zheng Y, Nease L, Rosalez J, et al. Chemerin suppresses breast cancer growth by recruiting immune effector cells into the tumor microenvironment. Front Immunol. 2019;10:983. https://doi.org/10.3389/fimmu.2019.00983This study has shown for the first time that increased chemerin expression into the breast cancer milieu can suppress tumor growth by recruiting NK and T cells, thus supporting this approach as a promising immunotherapeutic strategy.
Akin S. Serum chemerin level in breast cancer. Int J Hematol Oncol. 2017;27:127–32. https://doi.org/10.4999/uhod.171847.
Hofmann T, Elbelt U, Stengel A. Irisin as a muscle-derived hormone stimulating thermogenesis--a critical update. Peptides. 2014;54:89–100. https://doi.org/10.1016/j.peptides.2014.01.016.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. https://doi.org/10.1038/nature10777.
Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–37. https://doi.org/10.1038/nrendo.2016.221.
Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Cakmak T, et al. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides. 2014;61:130–6. https://doi.org/10.1016/j.peptides.2014.09.014.
Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. https://doi.org/10.1371/journal.pone.0060563.
Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136:E197–202. https://doi.org/10.1002/ijc.29142.
Provatopoulou X, Georgiou GP, Kalogera E, Kalles V, Matiatou MA, Papapanagiotou I, et al. Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC Cancer. 2015;15:898. https://doi.org/10.1186/s12885-015-1898-1.
Zhang ZP, Zhang XF, Li H, Liu TJ, Zhao QP, Huang LH, et al. Serum irisin associates with breast cancer to spinal metastasis. Medicine (Baltimore). 2018;97:e0524. https://doi.org/10.1097/md.0000000000010524.
Flower DR. The lipocalin protein family: structure and function. Biochem J. 1996;318(Pt 1):1–14. https://doi.org/10.1042/bj3180001.
•• Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018;11:8099–106. https://doi.org/10.2147/ott.s181223This review summarizes evidence on the abnormal expression of lipocalin 2 in breast cancer progression, highlights the latest developments of potential lipocalin 2-targeting agents and proposed lipocalin 2-associated molecular mechanisms implicated in breast cancer invasion and metastasis.
Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:3913–8. https://doi.org/10.1073/pnas.0810617106.
Oren B, Urosevic J, Mertens C, Mora J, Guiu M, Gomis RR, et al. Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J Pathol. 2016;239:274–85. https://doi.org/10.1002/path.4724.
Leng X, Wu Y, Arlinghaus RB. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J Cell Physiol. 2011;226:309–14. https://doi.org/10.1002/jcp.22403.
Yang J, McNeish B, Butterfield C, Moses MA. Lipocalin 2 is a novel regulator of angiogenesis in human breast cancer. FASEB J. 2013;27:45–50. https://doi.org/10.1096/fj.12-211730.
Wang Y, Zeng T. Neutrophil gelatinase-associated lipocalin protein as a biomarker in the diagnosis of breast cancer: a meta-analysis. Biomed Rep. 2013;1:479–83. https://doi.org/10.3892/br.2013.89.
Sung H, Choi JY, Lee SA, Lee KM, Han S, Jeon S, et al. The association between the preoperative serum levels of lipocalin-2 and matrix metalloproteinase-9 (MMP-9) and prognosis of breast cancer. BMC Cancer. 2012;12:193. https://doi.org/10.1186/1471-2407-12-193.
Wenners AS, Mehta K, Loibl S, Park H, Mueller B, Arnold N, et al. Neutrophil gelatinase-associated lipocalin (NGAL) predicts response to neoadjuvant chemotherapy and clinical outcome in primary human breast cancer. PLoS One. 2012;7:e45826. https://doi.org/10.1371/journal.pone.0045826.
Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108:389–97. https://doi.org/10.1007/s10549-007-9619-3.
Provatopoulou X, Gounaris A, Kalogera E, Zagouri F, Flessas I, Goussetis E, et al. Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer. 2009;9:390. https://doi.org/10.1186/1471-2407-9-390.
Cheng G, Sun X, Wang J, Xiao G, Wang X, Fan X, et al. HIC1 silencing in triple-negative breast cancer drives progression through misregulation of LCN2. Cancer Res. 2014;74:862–72. https://doi.org/10.1158/0008-5472.can-13-2420.
Tanaka M, Miyajima A, Oncostatin M. A multifunctional cytokine. Rev Physiol Biochem Pharmacol. 2003;149:39–52. https://doi.org/10.1007/s10254-003-0013-1.
Sanchez-Infantes D, White UA, Elks CM, Morrison RF, Gimble JM, Considine RV, et al. Oncostatin m is produced in adipose tissue and is regulated in conditions of obesity and type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E217–25. https://doi.org/10.1210/jc.2013-3555.
Richards CD. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm. 2013;2013:512103. https://doi.org/10.1155/2013/512103.
Gomez-Lechon MJ. Oncostatin M: signal transduction and biological activity. Life Sci. 1999;65:2019–30.
Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, et al. Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res. 2014;74:6806–19. https://doi.org/10.1158/0008-5472.can-14-0160.
West NR, Murray JI, Watson PH. Oncostatin-M promotes phenotypic changes associated with mesenchymal and stem cell-like differentiation in breast cancer. Oncogene. 2014;33:1485–94. https://doi.org/10.1038/onc.2013.105.
Tawara K, Bolin C, Koncinsky J, Kadaba S, Covert H, Sutherland C, et al. OSM potentiates preintravasation events, increases CTC counts, and promotes breast cancer metastasis to the lung. Breast Cancer Res. 2018;20:53. https://doi.org/10.1186/s13058-018-0971-5.
Tawara K, Scott H, Emathinger J, Wolf C, LaJoie D, Hedeen D, et al. HIGH expression of OSM and IL-6 are associated with decreased breast cancer survival: synergistic induction of IL-6 secretion by OSM and IL-1beta. Oncotarget. 2019;10:2068–85. https://doi.org/10.18632/oncotarget.26699.
West NR, Murphy LC, Watson PH. Oncostatin M suppresses oestrogen receptor-alpha expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer. 2012;19:181–95. https://doi.org/10.1530/erc-11-0326.
Doherty MR, Parvani JG, Tamagno I, Junk DJ, Bryson BL, Cheon HJ, et al. The opposing effects of interferon-beta and oncostatin-M as regulators of cancer stem cell plasticity in triple-negative breast cancer. Breast Cancer Res. 2019;21:54. https://doi.org/10.1186/s13058-019-1136-x.
Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303.
Kahles F, Findeisen HM, Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–93. https://doi.org/10.1016/j.molmet.2014.03.004.
Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomark Prev. 2007;16:1087–97. https://doi.org/10.1158/1055-9965.epi-06-1008.
Zhao H, Chen Q, Alam A, Cui J, Suen KC, Soo AP, et al. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:356. https://doi.org/10.1038/s41419-018-0391-6.
Tuck AB, Elliott BE, Hota C, Tremblay E, Chambers AF. Osteopontin-induced, integrin-dependent migration of human mammary epithelial cells involves activation of the hepatocyte growth factor receptor (Met). J Cell Biochem. 2000;78:465–75.
Noti JD. Adherence to osteopontin via alphavbeta3 suppresses phorbol ester-mediated apoptosis in MCF-7 breast cancer cells that overexpress protein kinase C-alpha. Int J Oncol. 2000;17:1237–43. https://doi.org/10.3892/ijo.17.6.1237.
Singhal H, Bautista DS, Tonkin KS, O’Malley FP, Tuck AB, Chambers AF, et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3:605–11.
Rudland PS, Platt-Higgins A, El-Tanani M, De Silva RS, Barraclough R, Winstanley JH, et al. Prognostic significance of the metastasis-associated protein osteopontin in human breast cancer. Cancer Res. 2002;62:3417–27.
Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, et al. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145:610–23.
Tuck AB, Chambers AF. The role of osteopontin in breast cancer: clinical and experimental studies. J Mammary Gland Biol Neoplasia. 2001;6:419–29.
Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1:621–32.
Xu K, Tian X, Oh SY, Movassaghi M, Naber SP, Kuperwasser C, et al. The fibroblast Tiam1-osteopontin pathway modulates breast cancer invasion and metastasis. Breast Cancer Res. 2016;18:14. https://doi.org/10.1186/s13058-016-0674-8.
Sharon Y, Raz Y, Cohen N, Ben-Shmuel A, Schwartz H, Geiger T, et al. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–73. https://doi.org/10.1158/0008-5472.can-14-1990.
• Han B, Huang J, Han Y, Hao J, Wu X, Song H, et al. The microRNA miR-181c enhances chemosensitivity and reduces chemoresistance in breast cancer cells via down-regulating osteopontin. Int J Biol Macromol. 2019;125:544–56. https://doi.org/10.1016/j.ijbiomac.2018.12.075This study provides new insights in developing effective interventions for breast cancer patients with acquired resistance to chemotherapy suggesting that the miR-181c/osteopontin axis might be a promising prognostic index and a potential therapeutic target.
Hao C, Wang Z, Gu Y, Jiang WG, Cheng S. prognostic value of osteopontin splice variant-c expression in breast cancers: a meta-analysis. Biomed Res Int. 2016;2016:7310694. https://doi.org/10.1155/2016/7310694.
Xu YY, Zhang YY, Lu WF, Mi YJ, Chen YQ. Prognostic value of osteopontin expression in breast cancer: a meta-analysis. Mol Clin Oncol. 2015;3:357–62. https://doi.org/10.3892/mco.2014.480.
Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. https://doi.org/10.1111/j.1749-6632.2012.06750.x.
Gapstur SM, Drope JM, Jacobs EJ, Teras LR, McCullough ML, Douglas CE, et al. A blueprint for the primary prevention of cancer: targeting established, modifiable risk factors. CA Cancer J Clin. 2018;68:446–70. https://doi.org/10.3322/caac.21496.
Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–74. https://doi.org/10.1200/jco.2014.58.4680.
World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR). Diet, nutrition, physical activity and cancer: a global perspective. In: Continuous Update Project Expert Report 2018. London: WCRF International; 2018. wcrf.org/dietand-cancer/contents. Accessed May 24, 2019.
Rock CL, Pande C, Flatt SW, Ying C, Pakiz B, Parker BA, et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin Breast Cancer. 2013;13:188–95. https://doi.org/10.1016/j.clbc.2012.12.002.
Birks S, Peeters A, Backholer K, O’Brien P, Brown W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes Rev. 2012;13:868–91. https://doi.org/10.1111/j.1467-789X.2012.01010.x.
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. https://doi.org/10.1056/NEJMoa1614362.
Weigl J, Hauner H, Hauner D. Can nutrition lower the risk of recurrence in breast cancer? Breast Care (Basel). 2018;13:86–91. https://doi.org/10.1159/000488718.
Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29:1031–9. https://doi.org/10.1007/s11695-018-3501-8.
Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of breast cancer development? a systematic review. Obes Surg. 2017;27:3014–20. https://doi.org/10.1007/s11695-017-2901-5.
Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–81. https://doi.org/10.1210/jc.2005-2248.
Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavalda JM, Hernandez M, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790–8. https://doi.org/10.1007/s11695-013-1033-9.
Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62. https://doi.org/10.1016/s1470-2045(09)70159-7.
Zhu J, Schott M, Liu R, Liu C, Shen B, Wang Q, et al. Intensive glycemic control lowers plasma visfatin levels in patients with type 2 diabetes. Horm Metab Res. 2008;40:801–5. https://doi.org/10.1055/s-0028-1082040.
Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018;143:409–19. https://doi.org/10.1016/j.diabres.2018.05.023.
Rahmani J, Manzari N, Thompson J, Gudi SK, Chhabra M, Naik G, et al. The effect of metformin on biomarkers associated with breast cancer outcomes: a systematic review, meta-analysis, and dose-response of randomized clinical trials. Clin Transl Oncol. 2019. https://doi.org/10.1007/s12094-019-02108-9.
Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schunemann HJ, et al. Association of metformin with breast cancer incidence and mortality in patients with type II diabetes: A GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2018;27:627–35. https://doi.org/10.1158/1055-9965.epi-17-0936.
Zhang ZJ, Yuan J, Bi Y, Wang C, Liu Y. The effect of metformin on biomarkers and survivals for breast cancer- a systematic review and meta-analysis of randomized clinical trials. Pharmacol Res. 2019;141:551–5. https://doi.org/10.1016/j.phrs.2019.01.036.
Shafiei-Irannejad V, Samadi N, Yousefi B, Salehi R, Velaei K, Zarghami N. Metformin enhances doxorubicin sensitivity via inhibition of doxorubicin efflux in P-gp-overexpressing MCF-7 cells. Chem Biol Drug Des. 2018;91:269–76. https://doi.org/10.1111/cbdd.13078.
Catalano S, Mauro L, Bonofiglio D, Pellegrino M, Qi H, Rizza P, et al. In vivo and in vitro evidence that PPARgamma ligands are antagonists of leptin signaling in breast cancer. Am J Pathol. 2011;179:1030–40. https://doi.org/10.1016/j.ajpath.2011.04.026.
Du R, Lin L, Cheng D, Xu Y, Xu M, Chen Y et al. Thiazolidinedione therapy and breast cancer risk in diabetic women: a systematic review and meta-analysis. Diabetes Metab Res Rev 2018;34. https://doi.org/10.1002/dmrr.2961.
Islam MM, Yang HC, Nguyen PA, Poly TN, Huang CW, Kekade S, et al. Exploring association between statin use and breast cancer risk: an updated meta-analysis. Arch Gynecol Obstet. 2017;296:1043–53. https://doi.org/10.1007/s00404-017-4533-3.
Mansourian M, Haghjooy-Javanmard S, Eshraghi A, Vaseghi G, Hayatshahi A, Thomas J. Statins Use and risk of breast cancer recurrence and death: a systematic review and meta-analysis of observational studies. J Pharm Pharm Sci. 2016;19:72–81. https://doi.org/10.18433/j3202b.
Wu QJ, Tu C, Li YY, Zhu J, Qian KQ, Li WJ, et al. Statin use and breast cancer survival and risk: a systematic review and meta-analysis. Oncotarget. 2015;6:42988–3004. https://doi.org/10.18632/oncotarget.5557.
Lu L, Shi L, Zeng J, Wen Z. Aspirin as a potential modality for the chemoprevention of breast cancer: a dose-response meta-analysis of cohort studies from 857,831 participants. Oncotarget. 2017;8:40389–401. https://doi.org/10.18632/oncotarget.16315.
Zhong S, Chen L, Zhang X, Yu D, Tang J, Zhao J. Aspirin use and risk of breast cancer: systematic review and meta-analysis of observational studies. Cancer Epidemiol Biomark Prev. 2015;24:1645–55. https://doi.org/10.1158/1055-9965.epi-15-0452.
Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–50. https://doi.org/10.1007/s10549-008-0228-6.
Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017;6:195–203. https://doi.org/10.1007/s13679-017-0263-x.
Wright CM, Moorin RE, Chowdhury EK, Stricker BH, Reid CM, Saunders CM, et al. Calcium channel blockers and breast cancer incidence: an updated systematic review and meta-analysis of the evidence. Cancer Epidemiol. 2017;50:113–24. https://doi.org/10.1016/j.canep.2017.08.012.
Sperati F, Vici P, Maugeri-Sacca M, Stranges S, Santesso N, Mariani L, et al. Vitamin D supplementation and breast cancer prevention: a systematic review and meta-analysis of randomized clinical trials. PLoS One. 2013;8:e69269. https://doi.org/10.1371/journal.pone.0069269.
Lazzeroni M, Gandini S, Puntoni M, Bonanni B, Gennari A, DeCensi A. The science behind vitamins and natural compounds for breast cancer prevention. Getting the most prevention out of it. Breast. 2011;20(Suppl 3):S36–41. https://doi.org/10.1016/s0960-9776(11)70292-2.
Zhang YF, Shi WW, Gao HF, Zhou L, Hou AJ, Zhou YH. Folate intake and the risk of breast cancer: a dose-response meta-analysis of prospective studies. PLoS One. 2014;9:e100044. https://doi.org/10.1371/journal.pone.0100044.
Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. Bmj. 2017;359:j4761. https://doi.org/10.1136/bmj.j4761.
Gianfredi V, Vannini S, Moretti M, Villarini M, Bragazzi NL, Izzotti A, et al. Sulforaphane and epigallocatechin gallate restore estrogen receptor expression by modulating epigenetic events in the breast cancer cell line MDA-MB-231: a systematic review and meta-analysis. J Nutrigenet Nutrigenomics. 2017;10:126–35. https://doi.org/10.1159/000480636.
Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–13. https://doi.org/10.1016/j.cytogfr.2013.10.001.
Catalano S, Leggio A, Barone I, De Marco R, Gelsomino L, Campana A, et al. A novel leptin antagonist peptide inhibits breast cancer growth in vitro and in vivo. J Cell Mol Med. 2015;19:1122–32. https://doi.org/10.1111/jcmm.12517.
Leggio A, Catalano S, De Marco R, Barone I, Ando S, Liguori A. Therapeutic potential of leptin receptor modulators. Eur J Med Chem. 2014;78:97–105. https://doi.org/10.1016/j.ejmech.2014.03.048.
Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. https://doi.org/10.1186/bcr2321.
Otvos L Jr, Kovalszky I, Olah J, Coroniti R, Knappe D, Nollmann FI, et al. Optimization of adiponectin-derived peptides for inhibition of cancer cell growth and signaling. Biopolymers. 2015;104:156–66. https://doi.org/10.1002/bip.22627.
Bai J, Liao C, Liu Y, Qin X, Chen J, Qiu Y, et al. Structure-based design of potent nicotinamide phosphoribosyltransferase inhibitors with promising in vitro and in vivo antitumor activities. J Med Chem. 2016;59:5766–79. https://doi.org/10.1021/acs.jmedchem.6b00324.
Nimptsch K, Pischon T. Obesity Biomarkers, metabolism and risk of cancer: an epidemiological perspective. Recent Results Cancer Res. 2016;208:199–217. https://doi.org/10.1007/978-3-319-42542-9_11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gerasimos Socrates Christodoulatos, Nikolaos Spyrou, Jona Kadillari, Sotiria Psallida, and Maria Dalamaga declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Metabolism
GS Christodoulatos and N Spyrou have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Christodoulatos, G.S., Spyrou, N., Kadillari, J. et al. The Role of Adipokines in Breast Cancer: Current Evidence and Perspectives. Curr Obes Rep 8, 413–433 (2019). https://doi.org/10.1007/s13679-019-00364-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-019-00364-y