Abstract
Purpose of Review
In this review, we investigate the role of classic and novel adipocytokines in cancer pathogenesis synopsizing the mechanisms underlying the association between adipocytokines and malignancy. Special emphasis is given on novel adipocytokines as new evidence is emerging regarding their entanglement in neoplastic development.
Recent Findings
Recent data have emphasized the role of the triad of overweight/obesity, insulin resistance and adipocytokines in cancer. In the setting of obesity, classic and novel adipocytokines present independent and joint effects on activation of major intracellular signaling pathways implicated in cell proliferation, expansion, survival, adhesion, invasion, and metastasis. Until now, more than 15 adipocytokines have been associated with cancer, and this list continues to expand. While the plethora of circulating pro-inflammatory adipocytokines, such as leptin, resistin, extracellular nicotinamide phosphoribosyl transferase, and chemerin are elevated in malignancies, some adipocytokines such as adiponectin and omentin-1 are generally decreased in cancers and are considered protective against carcinogenesis.
Summary
Elucidating the intertwining of inflammation, cellular bioenergetics, and adiposopathy is significant for the development of preventive, diagnostic, and therapeutic strategies against cancer. Novel more effective and safe adipocytokine-centered therapeutic interventions may pave the way for targeted oncotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide cancer constitutes the second leading cause of death [1]. It is expected that cancer incidence will continue to augment due to the increase in the prevalence of risk factors, mainly obesity and DM. In both developing and industrialized countries, there is a dramatic increase in the prevalence of overweight and obesity, defined as excessive or abnormal fat tissue accumulation and characterized as a BMI between 25 and 29.9 and over 30 kg/m2 respectively [2]. Obesity is highly prevalent in females and in urban areas [3]. Generally, obesity develops when there is an imbalance between exceeding energy consumption from dietary intake and energy expenditure from physical and metabolic activity. Obesity is associated with an increased risk of many chronic comorbidities associated with premature mortality, including DM type 2, hypertension, dyslipidemia, CVD, non-alcoholic fatty liver disease, and cancer [4].
There is sufficient evidence from prospective studies and meta-analyses that elevated body fatness, particularly visceral obesity, is associated with an increased risk for many malignancies including colorectal, postmenopausal breast, endometrial, gallbladder, thyroid, renal cell, ovarian, pancreatic, advanced prostate cancer, esophageal adenocarcinoma [5,6,7,8], and lymphohematopoietic cancer [9,10,11,12,13]. Emerging evidence associates higher body fatness in late adolescence and early adulthood with cancer risk at an older age [14]. The pathological expansion of white adipose tissue in obesity, also described as adiposopathy, is characterized by adipocyte hypertrophy and/or hyperplasia, hypoxia, oxidative stress response, disruption in the protein secretory pathway, and induction of angiogenesis [15]. Adiposopathy may provoke inflammatory, metabolic and immunologic changes affecting cell mutation rate, DNA repair, gene function, and induction of epigenetic changes permitting neoplastic transformation and growth [16••, 17••].
In the current review, we investigate the role of classic and novel adipocytokines in cancer pathogenesis synopsizing the mechanisms underlying the association between adipocytokines and malignancy. Special emphasis is given on novel adipocytokines as new evidence is emerging regarding their entanglement in neoplastic development. Elucidating the intertwining of inflammation, cellular bioenergetics, and adiposopathy is significant for the development of preventive, diagnostic, and therapeutic strategies against cancer.
Adipocytokines at the Intersection of Obesity and Cancer
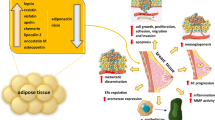
Recent data have emphasized the role of the triad of overweight/obesity, insulin resistance and adipocytokines in cancer. Although the role of obesity in cancer pathogenesis is not fully elucidated, the main pathways connecting obesity and adiposopathy to malignancies comprise (i) hyperinsulinemia and insulin resistance; (ii) abnormalities of the IGF-I system; (iii) chronic low-grade systemic inflammation and oxidative stress; (iv) impaired immune function; (v) the impact of obesity/adiposopathy on sex hormones biosynthesis; and (vi) abnormal variations in the levels of adipocytokines (Fig. 1) [11, 17••, 18,19,20,21].
Besides its mere energy-storage properties, white adipose tissue is a dynamic endocrine organ secreting a constellation of functional heterogeneous adipocytokines, a group of polypeptides that regulate several physiologic and pathologic processes including insulin sensitivity, appetite, inflammation, innate and adaptive immunity, hematopoiesis, and angiogenesis [22, 23]. Until now, more than 15 adipocytokines have been associated with cancer, and this list is still expanding [24]. While the plethora of circulating pro-inflammatory adipocytokines, such as leptin, TNF-α, IL-6, resistin, and extracellular Nampt (eNampt) are elevated in malignancies, some adipocytokines such as adiponectin and omentin-1 are decreased in cancers and are considered protective against carcinogenesis [16••, 17••, 25,26,27]. Classic adipocytokines, formerly discovered adipocytokines such as leptin and adiponectin, have been sufficiently studied in cancer. A well-established connection of classic and novel adipocytokines with cancer risk and progression may include (1) altered plasma or serum concentrations in cancer patients; (2) their differential expression in malignant and benign tissues; (3) their upregulation in tumor tissues; (4) their correlation with advanced stage and poor prognosis as prognostic biomarkers; (5) their association with cancer therapy resistance as predictive biomarkers; (6) their association with in vivo and in vitro models of cancer; and (7) the association of genetic polymorphisms of adipocytokines genes with susceptibility to certain cancer types. Table 1 presents a summary of the mechanism of action of adipocytokines in cancer.
Classic Adipocytokines and Cancer
Adiponectin and Cancer
Adiponectin is a protein composed of 244 amino acids belonging to the C1q/TNF family of proteins [17••]. It was discovered almost simultaneously by four different research groups, in mid-1990s [17••]. Adiponectin is secreted into circulation mainly by adipocytes [28]. Several receptors have been identified as binding sites for adiponectin: AdipoR1 (mainly expressed in skeletal muscles and endothelial cells), AdipoR2 (mainly expressed in liver), and T-cadherin [29, 30]. Adiponectin receptors are expressed in almost any tissue as well as in cancer cells [17••, 28]. Several growth factors such as platelet-derived growth factor, basic fibroblast growth factor, and heparin-binding epidermal growth factor-like growth factor are also bound by adiponectin [31].
A plethora of physiologic effects are exerted by adiponectin [32]. Adiponectin increases sensitivity to insulin and has anti-inflammatory and anti-atherogenic properties [32,33,34]. It also acts as cardioprotectant being involved in lipid metabolism [32,33,34]. Serum adiponectin levels correlate with various disease states [17]. Hypoadiponectinemia, which is the result of both genetic and/or environmental factors, is associated with insulin resistance, DM type 2, gestational DM, hypertension, metabolic syndrome, CVD, liver disease, and several malignancies [33,34,35,36,37,38,39,40,41,42]. On the other hand, increased adiponectin levels have been associated with anorexia nervosa, rheumatoid arthritis, and increased amount of proteinuria in chronic kidney disease [43,44,45,46]. In addition, there is evidence that high adiponectin levels are also associated with low risk for CVD in men, better glucose and lipid control in women [47,48,49], and lower risk for DM type 2 [50].
Besides its other properties, adiponectin exhibits anti-proliferative, anti-migratory, and pro-apoptotic actions [24, 51•]. Recombinant adiponectin has demonstrated anti-tumor effect when used in forms of leukemia, breast adenocarcinoma, and fibrosarcoma [52]. In addition to its direct anti-tumor effect on cells, adiponectin results in lower bioavailability of various growth factors [31]. On the other hand, hypoadiponectinemia correlates with carcinogenesis, both directly and indirectly [17•, 53]. Specifically, low adiponectin levels promote fatty acid and protein synthesis and, thus, cell growth, proliferation, and DNA mutagenesis [54]. Furthermore, hypoadiponectinemia supports tumor proliferation via the increase of anabolic hormones (insulin and IGF-1) and pro-inflammatory cytokines (TNF-a, IL-6) [54]. Recent meta-analyses have shown that decreased serum adiponectin levels are associated with cancers such as breast, prostate, endometrial, colorectal, tongue gastroesophageal, and also multiple myeloma and acute leukemias [55, 56]. In colorectal, gastric, and prostate cancer, low adiponectin levels are associated with cancer grade and stage. Interestingly, hypoadiponectinemia may serve as a useful biomarker for early cancer detection and prognosis [57, 58]. The combination of high BMI and hypoadiponectinemia is associated with more than sixfold the risk for endometrial cancer [59,60,61]. Regarding breast cancer, there is evidence that hypoadiponectinemia is an independent risk factor for disease regardless of age, menopause status, lymph nodes metastases, and hormone receptor status [62]. Adiponectin levels may also give information about the invasiveness of breast cancer [63]. The recent discovery of a novel adiponectin receptor 1 agonist as a therapeutic approach for DM may open analogous therapeutic avenues for anti-cancer treatment [64••]. With a deeper understanding of the role of adiponectin in oncogenesis, safe drugs that modulate its downstream cellular pathways may emerge as an important therapeutic strategy in oncology.
Leptin and Cancer
Leptin, a hormone produced mainly from the adipose tissue, was discovered by Friedman and colleagues in 1994 [65]. Leptin secretion is proportionate to the adipose tissue mass and serves as a message of satiety and energy adequacy inhibiting appetite [66]. Leptin exerts its effects through the LEPR, which is a single transmembrane protein, expressed in most tissues. In turn, the receptor affects multiple intracellular signaling pathways including JAK/STAT3, MAPK, PI3K/Akt, ERK1/2, AMPK, and IRS pathways [66, 67]. In the context of cancer, JAK2 activates STAT3 and 5 that upregulate the transcription of genes crucial to mechanisms of oncogenesis, such as cell proliferation, invasion, angiogenesis, and inflammation [28]. Furthermore, leptin increases the expression of anti-apoptotic proteins, inflammatory markers (TNF-a, IL-6), angiogenic factors (VEGF), and the HIF-1a [68, 69]. The ERK phosphorylation results in downstream activation of transcription factors that activate response elements of the c-fos gene promoting cell division [70]. Also, following JAK2 activation, PI3K and Akt are phosphorylated, and, as a result, glucose utilization, cell growth, cell proliferation, and apoptosis are induced [71]. Leptin has been shown to correlate with breast cancer depending on the menopausal status, showing a positive and negative correlation in postmenopausal and premenopausal women, respectively [72, 73]. The underlying oncogenic mechanism in mammary tissue involves mainly the JAK/STAT3 and PI3K pathways [74]. Moreover, leptin can inhibit apoptosis of BC cells favoring the expression of anti-apoptotic genes (bcl-xL, bak, and bax) and induce angiogenesis by stimulation of VEGF production [75]. Leptin displays an interesting interplay with ERs. Leptin can lead to stimulation of ERa, enhancement of aromatase expression, and suppression of p53 in ER-positive cancer cell lines [76, 77]. In the opposite direction, estradiol enhances the expression of leptin and LEPR in MCF7 BC cell lines [74]. The role of leptin on ER negative BC is not clear. In prostate cancer, leptin has been shown to promote oncogenicity in androgen insensitive prostate cancer cell lines [28] but not in androgen sensitive cell lines [78]. This is in accordance with hyperleptinemia observed in advanced prostate cancer which is more androgen refractory [67]. Also, leptin may promote oncogenesis in the gastrointestinal tract [67]. This has been shown in experimental settings in colon and gastric cancer. In patients with gastric cancer, leptin promoted invasiveness through the Rho/ROCK pathway [79] and correlated with aggressiveness of colorectal cancer [80].
However, in contrast to many in vitro studies where supraphysiologic levels of leptin were used, observational epidemiological studies have reported inconsistent associations between serum leptin levels and risk of several malignancies [26, 27, 81, 82]. Moreover, any associations of leptin with cancers reported in these studies may be due to the uncontrolled confounding via fat mass since all epidemiologic studies adjust for BMI which does not fully account for fat mass as a surrogate marker. Meta-analyses have shown positive associations of leptinemia with breast cancer, especially in overweight and obese women, a higher risk for endometrial cancer and inconclusive results for lung cancer [83, 84, 85•]. Our research group, which has studied extensively adipocytokines in malignancies, has shown that hypoleptinemia and not hyperleptinemia was associated to pancreatic cancer independently from BMI and weight loss [86, 87], to B cell chronic lymphocytic leukemia [88] and to low-risk myelodysplastic syndrome after adjustment for BMI and other risk factors [89,90,91]. Therefore, although basic research studies have shown positive effects of leptin on cancer cells using higher doses of leptin levels, this may not have relevance to humans and cannot be translated into clinical applicability. Overall, the evidence has shown that leptin in normal physiological circulating levels is not associated with cancer risk.
Novel Adipocytokines and Cancer
Resistin and Cancer
Resistin is a small adipocytokine of 12.5 kDa, secreted by mononuclear inflammatory cells and adipose cells [25]. It was discovered in 2001 and was considered to be a linking hormone between obesity and diabetes (the name “resistin” implies resistance to insulin) [92]. Resistin binds TLR4 resulting in activation of the PI3K, p38 MAPK, and NF-kB pathways [26]. The main effect of TLR4 binding is the secretion of pro-inflammatory cytokines such as TNF-a and IL-12 [26]. Additional effects include increased cell proliferation, migration, and adhesion [25].
The most notable physiological effects of resistin include potential pro-diabetic and pro-inflammatory activity [25]. There is evidence that hyperresistinemia is associated with many disease states comprising visceral obesity, coronary artery disease, lung disease, various malignancies, and critical illness [25, 93, 94]. Resistin may trigger tumorigenesis via inflammation (PI3K and NF-kB pathways), immune cell extravasation (MAPK pathway), expression of cardinal molecules for adhesion of cancerous cells (NF-kB pathway), and promotion of survival and invasiveness of tumor cells (PI3K and MAPK pathways) [24].
In a recent meta-analysis, hyperresistinemia was linked to an increased incidence of obesity-related cancers such as breast, endometrial, and colorectal cancer [95]. Some epidemiological studies, mainly case-control studies, have also linked high resistin levels to non-obesity-related cancers (such as esophageal, gastric and lung cancer) [25]. Additionally, increased resistin levels have been associated with lymphohematopoietic cancer [25]. However, despite its strong association to various types of cancers, resistin presents a limited diagnostic or predictive ability as a cancer biomarker [95].
Visfatin/eNampt and Cancer
Visfatin, also known as Nampt or PBEF, is a 52-kDa protein, the product of the NAMPT gene, that acts as an enzyme, adipocytokine, and a growth factor [96,97,98]. Nampt can be found in two forms, the intracellular-iNampt, and the extracellular-eNampt [16••]. iNampt participates in NAD biosynthesis, an important electron carrier, representing thereby a crucial function in cell metabolism. eNampt is excreted by a variety of tissues such as adipose, liver, and heart, and the mechanism that this is accomplished is thought to be cell lysis [97]. eNampt has been associated with various pathologies including diabetes, obesity, aging, atherosclerosis, cardiac hypertrophy, and autoimmune diseases [99,100,101]. Regarding oncogenesis, eNampt displays pro-inflammatory, proliferative, anti-apoptotic, and pro-angiogenic effects [102]. It induces inflammation through the activation of NF-kB and promotes proliferation through the upregulation of Notch-1, cyclin D1, cyclin dependent kinase 2, MAPKs, ERK-1/2, and p38 signaling pathways [7, 103,104,105]. Moreover, eNampt may function as an endocrine mediator in some cancers and can contribute through its immunosuppressive properties to the characteristic immune-evasive properties of malignancies [106]. Serum eNampt is elevated in many cancers and is generally correlated with worse prognosis and advanced stage [102, 107]. Specifically, it has been associated not only with the risk of obesity-associated malignancies, such as breast, endometrial, and colorectal cancer [16••] but also with the risk of male oral squamous cell, gastric and hepatocellular carcinoma, bladder and prostate cancer, non-small cell lung cancer, brain tumors, and hematologic malignancies [16••, 24]. Molecules that inhibit Nampt are being investigated in clinical trials, either as a monotherapy or as a part of combination therapy that enhances chemotherapy effects by triggering depletion of energy in cancer cells [16••].
Chemerin and Cancer
Chemerin is a small protein of 16 kDa, expressed in lung, liver, and white adipose tissue [108]. It is involved in innate and adaptive immunity by binding protein-coupled receptor chemokine-like receptor 1 (CMLR1) [109]. Apart from immunity, chemerin plays a role in adipogenesis and adipocyte metabolism [108, 110].
Chemerin levels are positively correlated with BMI and components of the metabolic syndrome [24, 111, 112]. Increased chemerin levels have been associated with neuroblastoma, NSCLC, and tongue, esophageal, gastric, and colorectal cancer [113, 114]. Interestingly, chemerin levels may serve as an important prognostic factor for gastric cancer patients’ post-operative survival [115]. The mechanisms by which chemerin is promoting tumorigenesis comprise inflammation, angiogenesis, and induction of matrix metalloproteinases [24, 116]. It has recently been suggested that the chemerin/CMLR1 pathway may also serve as a potential therapeutic target for malignancies such as neuroblastoma [117].
On the contrary, a recent study in mice hepatocellular cancer (HCC) showed that chemerin may have tumor-inhibitory effects, by suppressing the inflammatory microenvironment of the tumor [118•]. This fact may underscore a potential therapeutic role of chemerin against inflammation-associated tumors such as HCC [118•]. It also implies that chemerin may present differential effects on different types of cancers.
Omentin-1 and Cancer
Omentin-1, discovered in 2006 and originally recognized as intelectin-1, is secreted from the adipose tissue, mainly from the visceral fat [119]. It has been considered as one of the missing links explaining the higher burden of DM type 2 and cardiovascular disease attributed to visceral obesity compared to subcutaneous obesity [67]. Omentin-1 is produced by stromal vascular cells of the adipose tissue exerting its actions in an endocrine, autocrine, and paracrine manner [119]. Omentin-1 enhances insulin’s effects by stimulating insulin-mediated glucose uptake by subcutaneous and visceral adipocytes in vitro via the Akt signaling [119]. Besides the inverse correlation of omentin-1 levels with obesity, its levels are decreased under the spectrum of altered metabolic parameters including increased waist circumference, dyslipidemia, and hypertension [120]. As a result, omentin-1 may function as a marker of cardiovascular risk [121]. In HEPG2 cell lines, omentin-1 exerted anti-oncogenic effects through promotion of apoptosis via upregulating p21 that, in turn, increased p53, bax/bcl2 ratio and activated caspase 3 system [122]. Elevated levels of omentin-1 were detected in patients with malignant mesothelioma and prostate cancer [123•]. On the contrary, omentin-1 was markedly decreased in renal cancer patients [124]. Omentin-1 was associated with better outcomes in patients with gastric and stage IV colorectal cancer [123•]. Baseline omentin-1 levels have been correlated with colorectal cancer risk, presenting a potential interaction with the adiposity state of the patients [125]. This contradictory finding to the tumor suppressive properties of omentin-1 was attributed to its potential correlation with intestinal inflammation and the enhancement of the Akt pathway [125]. Furthermore, omentin-1 was elevated in stage III colorectal cancer patients in comparison to healthy controls [126]. Pancreatic cancer patients displayed higher levels of omentin-1 as a compensatory response to the inflammation and/or weight loss due to cancer cachexia, a complex metabolic state characterized by loss of muscle and adipose tissue [127]. Omentin-1 correlated with pancreatic tumor size but failed to predict survival [127]. Finally, omentin-1 effectively inhibited the growth, invasion, and metastasis of neuroblastoma cells in vitro and in vivo via stimulation of the N-myc downstream-regulated gene 2 expression [128].
Apelin and Cancer
Apelin is a 9-kDa peptide identified in 1998. It is expressed in various different tissues such as the brain, liver, kidney, heart, lung, gastrointestinal tract, adrenal gland, adipose tissue, and endothelium [24, 129]. Apelin binds a G protein-coupled receptor which results in the activation of the ERK and PI3K/Akt pathways [129].
The physiologic functions of apelin include blood pressure control, insulin and histamine release regulation, angiogenesis, and hypothalamic regulation of fluid and food intake [130]. There is no clear association between BMI and circulating apelin levels [131] while there is a strong correlation of this adipocytokine with hyperinsulinemia and pathogenesis of DM type 2 [132].
Higher apelin levels have been associated with cholangiocarcinoma, prostate, oral, ovarian, colon, endometrial, lung, and gastroesophageal cancers [133], serving as a potential marker for cancer progression [134]. Apelin may promote metastasis through enhanced proliferation, migration, invasion, and resistance to apoptosis [133] .
Retinol-Binding Protein 4 and Cancer
Retinol-binding protein 4 (RBP4), a soluble 21-kDa polypeptide, is mainly produced by the liver [135]. It functions as a vitamin A carrier that transports it to the periphery, throughout the body [136]. Adipose tissue is a secondary site of RBP4 synthesis; hence, RBP4 is classified as an adipocytokine. When it reaches its target tissues, RBP4 possibly acts by binding to cell surface receptors or through retinoic acid and retinoic acid-X receptors [136]. Although RBP-4 has been implicated in insulin resistance, epidemiological evidence has not been conclusive [137, 138]. RBP4 has been positively associated with triglycerides, total cholesterol, LDL-cholesterol, and high blood pressure [135]. Regarding oncogenesis, RBP4 promotes JAK/STAT signaling via its receptor STRA6. In culture with RBP-4, breast and colon carcinoma cells as well as fibroblasts acquired oncogenic properties such as cell proliferation, migration, and invasion [135]. However, proliferation of colon tumor cells was significantly inhibited after knocking down STRA6 receptor [135]. Upregulation of STRA6 and RBP4 has been documented in colorectal and breast cancer [135]. In ovarian cancer cell lines, RBP4 stimulated cancer cell migration and proliferation through the RhoA/Rock1 and ERK pathways, suggesting a potential oncogenic role [139]. Observational studies associating RBP4 and cancer have been inconclusive. In a case-control study, elevated RBP4 levels were found in BC patients, especially those who were PR and ER receptor negative [140]. On the contrary, in a cross-sectional study, lower RBP4 levels were found in patients with CRC [141].
Vaspin and Cancer
Vaspin was first identified in the Otsuka Long-Evans Tokushima fatty rat and is a protease inhibitor [24, 142]. It is mainly secreted by visceral adipose tissue [24]. Other sites of production are the stomach, liver, pancreas, and hypothalamus [24].
Despite its unclear mechanism of action, vaspin binds GRP78 and activates Akt and AMPK which regulate glucose and lipid metabolism [24]. Through these pathways, vaspin may improve obesity-related metabolism dysfunction [24]. Vaspin increases insulin sensitivity and presents anti-inflammatory and apoptotic properties [142, 143]. Vaspin may play a role in various diseases such as diabetes, obesity, metabolic syndrome, PCOS, and coronary artery disease [144, 145]. The potential connection of vaspin with cancer needs further investigation [22]. From observational studies, it has been shown that decreased vaspin levels are associated with endometrial cancer, while increased levels are associated with CRC [146, 147]. In a recent study, it is suggested that vaspin, like leptin, may be a helpful assessment tool for the clinical staging of endometrial cancer [148].
Nesfatin and Cancer
Nesfatin-1, discovered in 2006, was identified as an anorexigenic peptide with a role in appetite control and body weight [149, 150]. It is the N-terminal part of nucleobindin 2 (NUCB2) and is expressed in brain loci pertaining to feeding regulation [149]. Besides its brain expression, nesfatin-1 is mainly produced by the subcutaneous adipose tissue but also the gastric endocrine cells and the pancreatic beta cells [151]. Cytokines that promote inflammation, such as TNF-a and IL-6, induce the secretion of nesfatin [24]. As per its oncogenicity, nesfatin inhibited cell proliferation by altering elements of the cell cycle in HO-8910 ovarian epithelial carcinoma cells, an effect reversed by the RhoA/ROCK signaling pathway [152]. Among other mechanisms, apoptosis is dependent on the GTPase RhoA and its downstream effector, ROCK, a further downstream molecule [153]. Nesfatin-1 increased RhoA activity, as well as the activity of ROCK, thus triggering apoptosis of HO-9010 cells [152]. Moreover, nesfatin-1 induced apoptosis in HO-9010 cells by modulating the mTOR pathway [152]. Serum nesfatin has been found significantly lower in lung cancer patients, mainly attributed to cachexia [154].
Osteopontin and Cancer
Osteopontin is a pro-inflammatory adipocytokine that was first described as a protein of bone calcified matrix [155]. It is expressed in a plethora of different cell types such as adipocytes, immune system cells, hepatocytes, smooth and skeletal muscle cells, endothelial cells, osteoblasts, osteocytes, chondrocytes, and fibroblasts [148]. It is also found in tissues such as the brain, placenta, mammary glands, and kidneys [156]. It induces the activation of MMP-2 and MMP-9 [157, 158]. Osteopontin is involved in biomineralization, inflammation and remodeling [156]. It is linked to disease states such as obesity, DM, non-alcoholic steatohepatitis, and cancer [156, 159]. An overexpression of osteopontin is present in various types of cancer, such as stomach, lung, breast, and ovarian cancer and melanoma [158, 160]. Osteopontin may be used as a biomarker characterizing cancer aggression, especially grade [161]. The mechanisms connecting osteopontin with tumorigenesis include angiogenesis, metastasis, and evasion of apoptosis [159, 162]. Several strategies such as blocking osteopontin activity with antibodies/small molecule inhibitors and osteopontin silencing via the use of RNAi technology, are suggested as therapeutic approaches in cancer [161]. However, further investigation on these novel targeted therapies is needed [161].
Oncostatin and Cancer
Oncostatin M (OSM) was discovered in 1986, as a molecule that can effectively inhibit the proliferation of melanoma cell lines as well as other cancer cell lines, exhibiting oncostatic properties [163]. OSM belongs to the IL-6 family being secreted by activated T cells and macrophages and involved in the inflammatory response [164]. OSM interacts with the cell signaling molecule gp130, requiring a second receptor to join the complex for the signal to be transduced [165]. It binds to the gp130 complex with either LIFRα, termed as OSM receptor type I, or OSMRβ complex termed as the OSM receptor type II [165]. Several signaling pathways including JAK/STAT3, MAPK, and PI3K can be stimulated by gp130 cytokines, although their spectrum depends on the target tissue [165]. OSM stimulates growth of Kaposi sarcoma cells through the ERK-2 and PI3K pathways [166, 167]. Recent evidence has shown that the adipose tissue can secrete OSM promoting BC progression through JAK/STAT3 pathway upregulation [168]. Moreover, OSM may promote cell invasion and angiogenesis in osteosarcoma cell lines facilitating matrix degradation and angiogenesis in prostate cancer cells [169, 170]. In a mouse breast cancer model, OSM promoted metastasis, increased circulating tumor cells, and decreased survival [171•]. Oncostatin levels have been associated with a poor outcome in breast cancer patients, an effect possibly mediated by estrogen receptor downregulation [172].
Preventive and Clinical Implications
Based on current epidemiological evidence, a considerable percentage of cancer cases may be preventable through maintaining a healthy weight, following a diet with fruits, vegetables, and olive oil, increasing physical exercise and reducing alcohol intake [173]. The American Society of Clinical Oncology has underscored that obesity is one of the most important preventable lifestyle risk factor for cancer mortality [174], overtaking smoking.
Besides expansion of fat mass, obesity is considered a systemic endocrine dysfunction characterized by chronic inflammation. Adipocytes support tumor metabolism while their products, adipocytokines, are cardinal mediators of tumor progression via their paracrine and endocrine actions. In the setting of obesity, classic and novel adipocytokines present independent and joint effects on activation of major intracellular signaling pathways implicated in cell proliferation, expansion, survival, adhesion, invasion, and metastasis.
Plasma levels of classic (adiponectin and leptin) and novel adipocytokines such as resistin, eNampt, and chemerin may be modifiable by weight control, adoption of a balanced diet, and physical activity [16•, 25, 175, 176]. Bariatric surgery is linked to cancer risk reduction through modulation of the adipocytokines profile, particularly elevation of adiponectin and decrease of leptin, resistin, eNampt, and chemerin [177,178,179,180]. Paradoxically, although novel adipocytokines are not only adipose-cell-derived, they are responsive to adiposity alterations. Glycemic control can restore adipocytokine concentrations [181]. Pharmacologic agents such as metformin or PPAR-γ agonists that increase adiponectin and decrease resistin and eNampt levels in both humans and mice could be at the forefront of therapeutic strategies for obesity-related malignancies [16••, 25, 181, 182]. Lipid-lowering drugs, calcium-channel blockers, folic acid, oleic acid, and vitamin C and D supplementation may significantly improve adipocytokine levels [4, 16••, 25]. Some nutraceuticals such as curcumin, a polyphenol derived from turmeric, may modulate mRNA and protein levels of resistin and eNampt [183].
In preclinical studies, pegylated leptin receptor antagonist 2 has been shown to decrease the proliferation and angiogenesis of BC cells [184, 185]. Peptide-based adiponectin receptor agonists such as ADP355, which is an adiponectin mimetic, has been reported to limit the proliferation of adiponectin receptor-positive cancer cell lines [186, 187]. Targeting resistin and eNampt inhibition, either by biochemical or antibody neutralization, by antisense oligonucleotides, or by antagonism of their putative receptors, may be an effective strategy in cancer therapeutics, particularly in depleting the tumor inflammatory microenvironment [16••, 25]. If their receptors and signaling pathways are clearly determined, inhibition of resistin and eNampt downstream targets may be further explored in the cancer therapeutics armamentarium. Combination treatment with Nampt inhibitors and chemotherapeutic drugs or radiation may represent an emerging strategy enhancing the efficacy of existing chemotherapeutic agents [16••]. Hence, continued research is necessary to establish whether novel adipocytokines could be a potential therapeutic target for both cancer and obesity.
Adipocytokines, particularly adiponectin, are potentially useful diagnostic and prognostic biomarkers, reflecting advanced stage, adverse prognosis, and inflammatory state. Hypoadiponectinemia warrants assiduous investigation to rule out cardiometabolic diseases and cancer [188, 189] as well as worsened prognosis in cancer [182]. Recent data suggest that eNampt and resistin may be promising cancer biomarkers reflecting advanced stage and adverse prognosis [16••, 25, 190, 191]. Nevertheless, more large-scale prospective and longitudinal studies are needed to explore the diagnostic, prognostic, and predictive utility of classic and novel adipocytokines as cancer biomarkers and to rule out a potential “epiphenomenon” effect of adipocytokines variation in the context of tumor systemic inflammatory response.
The development of reliable, “user friendly” and practical automated laboratory techniques (enzyme-linked immunosorbent, electro-chemiluminescence immunoassays, etc.) and standardization of immunoassay procedures are needed to investigate the physiologic and pathophysiological relevance of adipocytokines. Also, there is a considerable number of unanswered practical issues in the clinical laboratory setting. What adipocytokine levels should be considered unhealthy and what are their optimal concentrations for cancer prevention? Their reference range should be determined as they may differ by age, gender, race, various preanalytical parameters, and assay methodology. Detection of SNPs of adipocytokines genes and quantification of adipocytokine expression in neoplastic tissues by using molecular techniques could provide additional data for prognosis and therapeutic response [17••, 192, 193]. To investigate the potential association of adipocytokines and cancer risk, adequately powered Mendelian randomization studies employing genetic determinants of adipocytokines derived from genome-wide associations studies are ideal because they circumvent confounding of lifestyle variables and reverse causation [194,195,196].
Conclusion
In conclusion, this review provides evidence for a connection between classic and novel adipocytokines, and cancer. High-throughput technologies such as proteomics and metabolomics will identify novel adipocytokines. Further research in basic and translational research is essential to elucidate the ontological role of novel adipocytokines and their interplay in cancer pathogenesis. Basic research studies are required to investigate the epigenetic regulation of adipocytokines genes and to map out their receptors and critical signaling pathways. More clinical longitudinal studies are expected to determine a wide spectrum of obesity-related biomarkers and assess their clinical utility in cancer prognosis and follow-up. Finally, novel more effective and safe adipocytokine-centered therapeutic interventions may pave the way for targeted oncotherapy.
Abbreviations
- AdipoR1/R2:
-
Adiponectin receptor 1/2
- Akt:
-
v-Akt murine thymoma viral oncogene homolog
- AMPK:
-
5’ AMP-activated protein kinase
- BC:
-
Breast cancer
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- DNA:
-
Deoxyribonucleic acid
- ER:
-
Estrogen receptor
- ERK 1/2:
-
Extracellular signal-regulated kinase 1/2
- GRP78:
-
Glucose-regulated protein 78
- GTP:
-
Guanosine-5′-triphosphate
- HIF-1a:
-
Hypoxia-inducible factor-1a
- IL:
-
Interleukin
- IGF:
-
Insulin-like growth factor
- IRS:
-
Insulin receptor substrate
- JAK:
-
Janus kinase
- JNK:
-
Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- LEPR:
-
Leptin receptor
- LIFRα:
-
Leukemia inhibitory receptor alpha
- MMP:
-
Matrix metalloproteinase
- mTOR:
-
Mammalian target of rapamycin
- NAD:
-
Nicotinamide adenine dinucleotide
- Nampt:
-
Nicotinamide phosphoribosyl transferase
- NF-κB:
-
Nuclear factor-κB
- NSCLC:
-
Non-small cell lung carcinoma
- OSM:
-
Oncostatin M
- PBEF:
-
Pre-B cell colony-enhancing factor
- PCOS:
-
Polycystic ovary syndrome
- PI3K:
-
Phosphatidylinositol 3-kinase
- PPAR:
-
Peroxisome proliferator-activated receptors
- PR:
-
Progesterone receptor
- RBP-4:
-
Retinol-binding protein
- ROCK:
-
Rho-associated coiled coil-containing protein kinase
- SNPs:
-
Single nucleotide polymorphisms
- STAT:
-
Signal transducer and activator of transcription
- STRA6:
-
Stimulated by retinoic acid 6
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular cellular adhesion molecule-1
- VEGF:
-
Vascular endothelial growth factor
- WHR:
-
Waist-to-hip ratio
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jemal A, Miller KD, Ma J, Siegel RL, Fedewa SA, Islami F, et al. Higher lung cancer incidence in young women than young men in the United States. N Engl J Med. 2018;378:1999–2009. https://doi.org/10.1056/NEJMoa1715907.
Pischon T, Nimptsch K. Obesity and risk of cancer: an introductory overview. Recent Results Cancer Res. 2016;208:1–15. https://doi.org/10.1007/978-3-319-42542-9_1.
Kelly T, Yang W, Chen C-S, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–7. https://doi.org/10.1038/ijo.2008.102.
Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017;6:195–203. https://doi.org/10.1007/s13679-017-0263-x.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–8. https://doi.org/10.1056/NEJMsr1606602.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. https://doi.org/10.1056/NEJMoa021423.
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. https://doi.org/10.1016/S0140-6736(08)60269-X.
Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–5. https://doi.org/10.1016/j.jacc.2013.06.027.
Lichtman MA. Obesity and neoplasms of lymphohematopoietic cells. Blood Adv. 2016;1:101–3. https://doi.org/10.1182/bloodadvances.2016001685.
Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47:1606–15. https://doi.org/10.1016/j.ejca.2011.01.020.
Dalamaga M, Christodoulatos GS. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm Mol Biol Clin Investig. 2015;23:5–20. https://doi.org/10.1515/hmbci-2015-0016.
Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122:1418–21. https://doi.org/10.1002/ijc.23176.
Larsson SC, Wolk A. Obesity and risk of non-Hodgkin’s lymphoma: a meta-analysis. Int J Cancer. 2007;121:1564–70. https://doi.org/10.1002/ijc.22762.
Park Y, Colditz GA. Diabetes and adiposity: a heavy load for cancer. Lancet Diabetes Endocrinol. 2018;6:82–3. https://doi.org/10.1016/S2213-8587(17)30396-0.
van Kruijsdijk RCM, van der Wall E, Visseren FLJ. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomark Prev. 2009;18:2569–78. https://doi.org/10.1158/1055-9965.EPI-09-0372.
•• Dalamaga M, Christodoulatos GS, Mantzoros CS. The role of extracellular and intracellular nicotinamide phosphoribosyl-transferase in cancer: diagnostic and therapeutic perspectives and challenges. Metabolism. 2018;82:72–87. https://doi.org/10.1016/j.metabol.2018.01.001. This review explores the role of Nampt in cancer pathophysiology as well as synopsizes the mechanisms underlying the association between extracellular and intracellular Nampt and malignancy.
•• Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–94. https://doi.org/10.1210/er.2011-1015. This review investigates the role of adiponectin in cancer pathophysiology as well as synopsizes the mechanisms underlying the association between adiponectin and cancer.
Dalamaga M. Obesity, insulin resistance, adipocytokines and breast cancer: new biomarkers and attractive therapeutic targets. World J Exp Med. 2013;3:34–42. https://doi.org/10.5493/wjem.v3.i3.34.
Pickens CA, Sordillo LM, Zhang C, Fenton JI. Obesity is positively associated with arachidonic acid-derived 5- and 11-hydroxyeicosatetraenoic acid (HETE). Metabolism. 2017;70:177–91. https://doi.org/10.1016/j.metabol.2017.01.034.
Lee MK, Kim J-Y, Kim D-I, Kang D-W, Park J, Ahn K-Y, et al. Effect of home-based exercise intervention on fasting insulin and adipocytokines in colorectal cancer survivors: a randomized controlled trial. Metabolism. 2017;76:23–31. https://doi.org/10.1016/j.metabol.2017.07.005.
Mendonça FM, de Sousa FR, Barbosa AL, Martins SC, Araújo RL, Soares R, et al. Metabolic syndrome and risk of cancer: which link? Metabolism. 2015;64:182–9. https://doi.org/10.1016/j.metabol.2014.10.008.
Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev. 2016;17:361–76. https://doi.org/10.1111/obr.12377.
Sahin-Efe A, Katsikeris F, Mantzoros CS. Advances in adipokines. Metabolism. 2012;61:1659–65. https://doi.org/10.1016/j.metabol.2012.09.001.
Reizes O, Berger NA. Adipocytokines, energy balance, and cancer. Springer International Publishing Switzerland; 2017. https://doi.org/10.1007/978-3-319-41677-9
Dalamaga M. Resistin as a biomarker linking obesity and inflammation to cancer: potential clinical perspectives. Biomark Med. 2014;8:107–18. https://doi.org/10.2217/bmm.13.99.
Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18:29–42. https://doi.org/10.1016/j.cmet.2013.05.010.
Moon H-S, Dalamaga M, Kim S-Y, Polyzos SA, Hamnvik O-P, Magkos F, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev. 2013;34:377–412. https://doi.org/10.1210/er.2012-1053.
Lee CH, Woo YC, Wang Y, Yeung CY, Xu A, Lam KSL. Obesity, adipokines and cancer: an update. Clin Endocrinol. 2015;83:147–56. https://doi.org/10.1111/cen.12667.
Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. https://doi.org/10.1210/er.2005-0005.
Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin M-F. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. https://doi.org/10.1007/s12020-009-9278-8.
Wang Y, Lam KSL, Xu JY, Lu G, Xu LY, Cooper GJS, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–7. https://doi.org/10.1074/jbc.M501149200.
Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–61S. https://doi.org/10.3945/ajcn.2009.28449C.
Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–66. https://doi.org/10.1093/ajcn/86.3.858S.
Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5. https://doi.org/10.1038/sj.bjc.6603051.
Hivert M-F, Sullivan LM, Fox CS, Nathan DM, D’Agostino RB, Wilson PWF, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–72. https://doi.org/10.1210/jc.2008-0425.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. https://doi.org/10.1210/jcem.86.5.7463.
Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9.
Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–16.
Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiworapongsa T, et al. Maternal serum adiponectin multimers in gestational diabetes. J Perinat Med. 2009;37:637–50. https://doi.org/10.1515/JPM.2009.101.
Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–75. https://doi.org/10.1111/j.1365-2796.2004.01426.x.
Durante-Mangoni E, Zampino R, Marrone A, Tripodi M-F, Rinaldi L, Restivo L, et al. Hepatic steatosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349–57. https://doi.org/10.1111/j.1365-2036.2006.03114.x.
Lee DY, Rhee E-J, Chang Y, Sohn CIL, Shin H-C, Ryu S, et al. Impact of systemic inflammation on the relationship between insulin resistance and all-cause and cancer-related mortality. Metabolism. 2018;81:52–62. https://doi.org/10.1016/j.metabol.2017.11.014.
Delporte ML, Brichard SM, Hermans MP, Beguin C, Lambert M. Hyperadiponectinaemia in anorexia nervosa. Clin Endocrinol. 2003;58:22–9.
Ebina K, Fukuhara A, Ando W, Hirao M, Koga T, Oshima K, et al. Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin Rheumatol. 2009;28:445–51. https://doi.org/10.1007/s10067-008-1074-y.
Senolt L, Pavelka K, Housa D, Haluzík M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35:247–52. https://doi.org/10.1016/j.cyto.2006.09.002.
Zoccali C, Mallamaci F. Adiponectin and leptin in chronic kidney disease: causal factors or mere risk markers? J Ren Nutr. 2011;21:87–91. https://doi.org/10.1053/j.jrn.2010.10.014.
Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–9.
Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4542–8. https://doi.org/10.1210/jc.2005-0372.
Gorgui J, Gasbarrino K, Georgakis MK, Karalexi MA, Nauche B, Petridou ET, et al. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: a systematic review and meta-analyses. Metabolism. 2017;69:51–66. https://doi.org/10.1016/j.metabol.2017.01.002.
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–88. https://doi.org/10.1001/jama.2009.976.
• Boutari C, Mantzoros CS. Inflammation: a key player linking obesity with malignancies. Metabolism. 2018;81:A3–6. https://doi.org/10.1016/j.metabol.2017.12.015. This study reviews the relationship of obesity and cancer under the common theme of inflammation.
Diedrich J, Gusky HC, Podgorski I. Adipose tissue dysfunction and its effects on tumor metabolism. Horm Mol Biol Clin Investig. 2015;21:17–41. https://doi.org/10.1515/hmbci-2014-0045.
Pérez-Hernández AI, Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Mechanisms linking excess adiposity and carcinogenesis promotion. Front Endocrinol (Lausanne). 2014;5:65. https://doi.org/10.3389/fendo.2014.00065.
Wang Y, Lam KSL, Xu A. Adiponectin as a negative regulator in obesity-related mammary carcinogenesis. Cell Res. 2007;17:280–2. https://doi.org/10.1038/cr.2007.14.
Ma J-J, Shang J, Wang H, Sui J-R, Liu K, Du J-X. Serum adiponectin levels are inversely correlated with leukemia: a meta-analysis. J Cancer Res Ther. 2016;12:897–902. https://doi.org/10.4103/0973-1482.186695.
Wei T, Ye P, Peng X, Wu L-L, Yu G-Y. Circulating adiponectin levels in various malignancies: an updated meta-analysis of 107 studies. Oncotarget. 2016;7:48671–91. https://doi.org/10.18632/oncotarget.8932.
Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. https://doi.org/10.1016/j.urology.2004.12.053.
Gialamas SP, Petridou ET, Tseleni-Balafouta S, Spyridopoulos TN, Matsoukis IL, Kondi-Pafiti A, et al. Serum adiponectin levels and tissue expression of adiponectin receptors are associated with risk, stage, and grade of colorectal cancer. Metabolism. 2011;60:1530–8. https://doi.org/10.1016/j.metabol.2011.03.020.
Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–7. https://doi.org/10.1210/jc.2002-021209.
Mantzoros C, Petridou E, Alexe D-M, Skalkidou A, Dessypris N, Papathoma E, et al. Serum adiponectin concentrations in relation to maternal and perinatal characteristics in newborns. Eur J Endocrinol. 2004;151:741–6.
Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–704.
Chen D-C, Chung Y-F, Yeh Y-T, Chaung H-C, Kuo F-C, Fu O-Y, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett. 2006;237:109–14. https://doi.org/10.1016/j.canlet.2005.05.047.
Jeong Y-J, Bong J-G, Park S-H, Choi J-H, Oh H-K. Expression of leptin, leptin receptor, adiponectin, and adiponectin receptor in ductal carcinoma in situ and invasive breast cancer. J Breast Cancer. 2011;14:96–103. https://doi.org/10.4048/jbc.2011.14.2.96.
•• Kim S, Lee Y, Kim JW, Son Y-J, Ma MJ, Um J-H, et al. Discovery of a novel potent peptide agonist to adiponectin receptor 1. PLoS One. 2018;13:e0199256. https://doi.org/10.1371/journal.pone.0199256. This study describes the discovery of an adiponectin agonist peptide that may provide insight in the development of malignancy therapeutics.
Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. https://doi.org/10.1038/27376.
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim S-Y, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–84. https://doi.org/10.1152/ajpendo.00315.2011.
Booth A, Magnuson A, Fouts J, Foster M. Adipose tissue, obesity and adipokines: role in cancer promotion. Horm Mol Biol Clin Investig. 2015;21:57–74. https://doi.org/10.1515/hmbci-2014-0037.
Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer. 2006;13:629–40. https://doi.org/10.1677/erc.1.01169.
Choi J-H, Park S-H, Leung PCK, Choi K-C. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J Clin Endocrinol Metab. 2005;90:207–10. https://doi.org/10.1210/jc.2004-0297.
Wang Y, Prywes R. Activation of the c-fos enhancer by the erk MAP kinase pathway through two sequence elements: the c-fos AP-1 and p62TCF sites. Oncogene. 2000;19:1379–85. https://doi.org/10.1038/sj.onc.1203443.
Frankenberry KA, Skinner H, Somasundar P, McFadden DW, Vona-Davis LC. Leptin receptor expression and cell signaling in breast cancer. Int J Oncol. 2006;28:985–93.
Harris HR, Tworoger SS, Hankinson SE, Rosner BA, Michels KB. Plasma leptin levels and risk of breast cancer in premenopausal women. Cancer Prev Res. 2011;4:1449–56. https://doi.org/10.1158/1940-6207.CAPR-11-0125.
Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: a meta-analysis. PLoS One. 2013;8:e67349. https://doi.org/10.1371/journal.pone.0067349.
Andò S, Catalano S. The multifactorial role of leptin in driving the breast cancer microenvironment. Nat Rev Endocrinol. 2011;8:263–75. https://doi.org/10.1038/nrendo.2011.184.
García-Robles MJ, Segura-Ortega JE, Fafutis-Morris M. The biology of leptin and its implications in breast cancer: a general view. J Interf Cytokine Res. 2013;33:717–27. https://doi.org/10.1089/jir.2012.0168.
Jardé T, Perrier S, Vasson M-P, Caldefie-Chézet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47:33–43. https://doi.org/10.1016/j.ejca.2010.09.005.
Dubois V, Jardé T, Delort L, Billard H, Bernard-Gallon D, Berger E, et al. Leptin induces a proliferative response in breast cancer cells but not in normal breast cells. Nutr Cancer. 2014;66:645–55. https://doi.org/10.1080/01635581.2014.894104.
Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63:800–9. https://doi.org/10.1016/j.eururo.2012.11.013.
Dong Z, Fu S, Xu X, Yang Y, Du L, Li W, et al. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer. 2014;110:1801–10. https://doi.org/10.1038/bjc.2014.70.
Koda M, Sulkowska M, Kanczuga-Koda L, Surmacz E, Sulkowski S. Overexpression of the obesity hormone leptin in human colorectal cancer. J Clin Pathol. 2006;60:902–6. https://doi.org/10.1136/jcp.2006.041004.
Dalamaga M, Polyzos SA, Karmaniolas K, Chamberland J, Lekka A, Triantafilli M, et al. Fetuin-A levels and free leptin index are reduced in patients with chronic lymphocytic leukemia: a hospital-based case-control study. Leuk Lymphoma. 2016;57:577–84. https://doi.org/10.3109/10428194.2015.1075523.
Dalamaga M, Karmaniolas K, Panagiotou A, Hsi A, Chamberland J, Dimas C, et al. Low circulating adiponectin and resistin, but not leptin, levels are associated with multiple myeloma risk: a case–control study. Cancer Causes Control. 2009;20:193–9. https://doi.org/10.1007/s10552-008-9233-7.
Pan H, Deng L-L, Cui J-Q, Shi L, Yang Y-C, Luo J-H, et al. Association between serum leptin levels and breast cancer risk. Medicine (Baltimore). 2018;97:e11345. https://doi.org/10.1097/MD.0000000000011345.
Wang P-P, He X-Y, Wang R, Wang Z, Wang Y-G. High leptin level is an independent risk factor of endometrial cancer: a meta-analysis. Cell Physiol Biochem. 2014;34:1477–84. https://doi.org/10.1159/000366352.
• Tong X, Ma Y, Zhou Q, He J, Peng B, Liu S, et al. Serum and tissue leptin in lung cancer: a meta-analysis. Oncotarget. 2017;8:19699–711. https://doi.org/10.18632/oncotarget.14963. This meta-analysis investigated the relation of leptin serum and tissue levels with lung cancer.
Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case–control study. Cancer Causes Control. 2009;20:625–33. https://doi.org/10.1007/s10552-008-9273-z.
Dalamaga M, Polyzos SA, Karmaniolas K, Chamberland J, Lekka A, Migdalis I, et al. Circulating fetuin-A in patients with pancreatic cancer: a hospital-based case-control study. Biomarkers. 2014;19:660–6. https://doi.org/10.3109/1354750X.2014.974071.
Dalamaga M, Crotty BH, Fargnoli J, Papadavid E, Lekka A, Triantafilli M, et al. B-cell chronic lymphocytic leukemia risk in association with serum leptin and adiponectin: a case–control study in Greece. Cancer Causes Control. 2010;21:1451–9. https://doi.org/10.1007/s10552-010-9573-y.
Dalamaga M, Karmaniolas K, Chamberland J, Nikolaidou A, Lekka A, Dionyssiou-Asteriou A, et al. Higher fetuin-A, lower adiponectin and free leptin levels mediate effects of excess body weight on insulin resistance and risk for myelodysplastic syndrome. Metabolism. 2013;62:1830–9. https://doi.org/10.1016/j.metabol.2013.09.007.
Dalamaga M, Karmaniolas K, Nikolaidou A, Chamberland J, Hsi A, Dionyssiou-Asteriou A, et al. Adiponectin and resistin are associated with risk for myelodysplastic syndrome, independently from the insulin-like growth factor-I (IGF-I) system. Eur J Cancer. 2008;44:1744–53. https://doi.org/10.1016/j.ejca.2008.04.015.
Dalamaga M, Nikolaidou A, Karmaniolas K, Hsi A, Chamberland J, Dionyssiou-Asteriou A, et al. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: a case-control study. Oncology. 2007;73:26–32. https://doi.org/10.1159/000120995.
Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. https://doi.org/10.1038/35053000.
Krecki R, Drozdz J, Szcześniak P, Orszulak-Michalak D, Krzemińska-Pakuła M. Novel atherogenesis markers for identification of patients with a multivessel coronary artery disease. Kardiol Pol. 2008;66:1173-80-2.
Forrest OA, Chopyk DM, Gernez Y, Brown MR, Conrad CK, Moss RB, et al. Resistin is elevated in cystic fibrosis sputum and correlates negatively with lung function. J Cyst Fibros. 2018; https://doi.org/10.1016/j.jcf.2018.05.018.
Gong W-J, Zheng W, Xiao L, Tan L-M, Song J, Li X-P, et al. Circulating resistin levels and obesity-related cancer risk: a meta-analysis. Oncotarget. 2016;7:57694–704. https://doi.org/10.18632/oncotarget.11034.
Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–7.
Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. https://doi.org/10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L.
Ming G, Li X, Yin J, Ai Y, Xu D, Ma X, et al. JAZF1 regulates visfatin expression in adipocytes via PPARα and PPARβ/δ signaling. Metabolism. 2014;63:1012–21. https://doi.org/10.1016/j.metabol.2014.05.006.
Zhang LQ, Heruth DP, Ye SQ. Nicotinamide phosphoribosyltransferase in human diseases. J Bioanal Biomed. 2011;3:13–25. https://doi.org/10.4172/1948-593X.1000038.
Duarte-Pereira S, Silva SS, Azevedo L, Castro L, Amorim A, Silva RM. NAMPT and NAPRT1: novel polymorphisms and distribution of variants between normal tissues and tumor samples. Sci Rep. 2014;4:6311. https://doi.org/10.1038/srep06311.
Olszanecka-Glinianowicz M, Owczarek A, Bożentowicz-Wikarek M, Brzozowska A, Mossakowska M, Zdrojewski T, et al. Relationship between circulating visfatin/NAMPT, nutritional status and insulin resistance in an elderly population—results from the PolSenior substudy. Metabolism. 2014;63:1409–18. https://doi.org/10.1016/j.metabol.2014.07.013.
Galli M, Van Gool F, Rongvaux A, Andris F, Leo O. The nicotinamide phosphoribosyltransferase: a molecular link between metabolism, inflammation, and cancer. Cancer Res. 2010;70:8–11. https://doi.org/10.1158/0008-5472.CAN-09-2465.
Moschen AR, Gerner RR, Tilg H. Pre-B cell colony enhancing factor/NAMPT/visfatin in inflammation and obesity-related disorders. Curr Pharm Des. 2010;16:1913–20.
Park H-J, Kim S-R, Kim SS, Wee H-J, Bae M-K, Ryu MH, et al. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget. 2014;5:5087–99. https://doi.org/10.18632/oncotarget.2086.
Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58.
Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–23. https://doi.org/10.1182/blood-2014-07-589069.
Reddy PS, Umesh S, Thota B, Tandon A, Pandey P, Hegde AS, et al. PBEF1/NAmPRTase/Visfatin: a potential malignant astrocytoma/glioblastoma serum marker with prognostic value. Cancer Biol Ther. 2008;7:663–8.
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–88. https://doi.org/10.1074/jbc.M700793200.
Wittamer V, Franssen J-D, Vulcano M, Mirjolet J-F, Le Poul E, Migeotte I, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–85. https://doi.org/10.1084/jem.20030382.
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–94. https://doi.org/10.1210/en.2007-0175.
Chakaroun R, Raschpichler M, Klöting N, Oberbach A, Flehmig G, Kern M, et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism. 2012;61:706–14. https://doi.org/10.1016/j.metabol.2011.10.008.
Aronis KN, Sahin-Efe A, Chamberland JP, Spiro A, Vokonas P, Mantzoros CS. Chemerin levels as predictor of acute coronary events: a case–control study nested within the veterans affairs normative aging study. Metabolism. 2014;63:760–6. https://doi.org/10.1016/j.metabol.2014.02.013.
Wang C, Wu WKK, Liu X, To K-F, Chen GG, Yu J, et al. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: a clinical and experimental study. Peptides. 2014;51:131–8. https://doi.org/10.1016/j.peptides.2013.10.009.
Erdogan S, Yilmaz FM, Yazici O, Yozgat A, Sezer S, Ozdemir N, et al. Inflammation and chemerin in colorectal cancer. Tumour Biol. 2016;37:6337–42. https://doi.org/10.1007/s13277-015-4483-y.
Zhang J, Jin H-C, Zhu A-K, Ying R-C, Wei W, Zhang F-J. Prognostic significance of plasma chemerin levels in patients with gastric cancer. Peptides. 2014;61:7–11. https://doi.org/10.1016/j.peptides.2014.08.007.
Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391:1762–8. https://doi.org/10.1016/j.bbrc.2009.12.150.
Tümmler C, Snapkov I, Wickström M, Moens U, Ljungblad L, Maria Elfman LH, et al. Inhibition of chemerin/CMKLR1 axis in neuroblastoma cells reduces clonogenicity and cell viability in vitro and impairs tumor growth in vivo. Oncotarget. 2017;8:95135–51. https://doi.org/10.18632/oncotarget.19619.
• Lin Y, Yang X, Liu W, Li B, Yin W, Shi Y, et al. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene. 2017;36:3599–608. https://doi.org/10.1038/onc.2016.516. This study displayed the tumor-suppressive effect of chemerin mediated by the inhibition of the tumor microenvironment inflammation.
Yang R-Z, Lee M-J, Hu H, Pray J, Wu H-B, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–61. https://doi.org/10.1152/ajpendo.00572.2004.
Shibata R, Ouchi N, Takahashi R, Terakura Y, Ohashi K, Ikeda N, et al. Omentin as a novel biomarker of metabolic risk factors. Diabetol Metab Syndr. 2012;4:37. https://doi.org/10.1186/1758-5996-4-37.
Panagiotou G, Mu L, Na B, Mukamal KJ, Mantzoros CS. Circulating irisin, omentin-1, and lipoprotein subparticles in adults at higher cardiovascular risk. Metabolism. 2014;63:1265–71. https://doi.org/10.1016/j.metabol.2014.06.001.
Zhang Y-Y, Zhou L-M. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol. 2013;698:137–44. https://doi.org/10.1016/j.ejphar.2012.11.016.
• Kawashima K, Maeda K, Saigo C, Kito Y, Yoshida K, Takeuchi T. Adiponectin and intelectin-1: important adipokine players in obesity-related colorectal carcinogenesis. Int J Mol Sci 2017;18. doi:https://doi.org/10.3390/ijms18040866. This review analyses the complex relation of the tumor suppresive adipokines, adiponectin and intelectin-1 with colorectal cancer.
Shen X-D, Zhang L, Che H, Zhang Y-Y, Yang C, Zhou J, et al. Circulating levels of adipocytokine omentin-1 in patients with renal cell cancer. Cytokine. 2016;77:50–5. https://doi.org/10.1016/j.cyto.2015.09.004.
Aleksandrova K, di Giuseppe R, Isermann B, Biemann R, Schulze M, Wittenbecher C, et al. Circulating omentin as a novel biomarker for colorectal cancer risk: data from the EPIC-Potsdam Cohort Study. Cancer Res. 2016;76:3862–71. https://doi.org/10.1158/0008-5472.CAN-15-3464.
Uyeturk U, Alcelik A, Aktas G, Tekce BK. Post-treatment plasma omentin levels in patients with stage III colon carcinoma. J BUON. 2014;19:681–5.
Karabulut S, Afsar CU, Karabulut M, Alis H, Bozkurt MA, Aydogan F, et al. Clinical significance of serum omentin-1 levels in patients with pancreatic adenocarcinoma. BBA Clin. 2016;6:138–42. https://doi.org/10.1016/j.bbacli.2016.10.002.
Li D, Mei H, Pu J, Xiang X, Zhao X, Qu H, et al. Intelectin 1 suppresses the growth, invasion and metastasis of neuroblastoma cells through up-regulation of N-myc downstream regulated gene 2. Mol Cancer. 2015;14:47. https://doi.org/10.1186/s12943-015-0320-6.
Carpéné C, Dray C, Attané C, Valet P, Portillo MP, Churruca I, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–73.
Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71. https://doi.org/10.1210/en.2004-1427.
Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9. https://doi.org/10.1007/s12020-011-9507-9.
Cavallo MG, Sentinelli F, Barchetta I, Costantino C, Incani M, Perra L, et al. Altered glucose homeostasis is associated with increased serum apelin levels in type 2 diabetes mellitus. PLoS One. 2012;7:e51236. https://doi.org/10.1371/journal.pone.0051236.
Wysocka MB, Pietraszek-Gremplewicz K, Nowak D. The role of apelin in cardiovascular diseases, obesity and cancer. Front Physiol. 2018;9:557. https://doi.org/10.3389/fphys.2018.00557.
Lacquaniti A, Altavilla G, Picone A, Donato V, Chirico V, Mondello P, et al. Apelin beyond kidney failure and hyponatremia: a useful biomarker for cancer disease progression evaluation. Clin Exp Med. 2015;15:97–105. https://doi.org/10.1007/s10238-014-0272-y.
Noy N, Li L, Abola MV, Berger NA. Is retinol binding protein 4 a link between adiposity and cancer? Horm Mol Biol Clin Investig. 2015;23:39–46. https://doi.org/10.1515/hmbci-2015-0019.
Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–11. https://doi.org/10.1530/EJE-11-0431.
Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. https://doi.org/10.1038/nature03711.
Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. https://doi.org/10.1530/JOE-13-0339.
Wang Y, Wang Y, Zhang Z. Adipokine RBP4 drives ovarian cancer cell migration. J Ovarian Res. 2018;11:29. https://doi.org/10.1186/s13048-018-0397-9.
Jiao C, Cui L, Ma A, Li N, Si H. Elevated serum levels of retinol-binding protein 4 are associated with breast cancer risk: a case-control study. PLoS One. 2016;11:e0167498. https://doi.org/10.1371/journal.pone.0167498.
Fei W, Chen L, Chen J, Shi Q, Zhang L, Liu S, et al. RBP4 and THBS2 are serum biomarkers for diagnosis of colorectal cancer. Oncotarget. 2017;8:92254–64. https://doi.org/10.18632/oncotarget.21173.
Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005;102:10610–5. https://doi.org/10.1073/pnas.0504703102.
Heiker JT. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J Pept Sci. 2014;20:299–306. https://doi.org/10.1002/psc.2621.
Wada J. Vaspin: a novel serpin with insulin-sensitizing effects. Expert Opin Investig Drugs. 2008;17:327–33. https://doi.org/10.1517/13543784.17.3.327.
El-Mesallamy HO, Kassem DH, El-Demerdash E, Amin AI. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism. 2011;60:63–70. https://doi.org/10.1016/j.metabol.2010.04.008.
Erdogan S, Sezer S, Baser E, Gun-Eryilmaz O, Gungor T, Uysal S, et al. Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr Relat Cancer. 2013;20:669–75. https://doi.org/10.1530/ERC-13-0280.
Fazeli MS, Dashti H, Akbarzadeh S, Assadi M, Aminian A, Keramati MR, et al. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine. 2013;62:81–5. https://doi.org/10.1016/j.cyto.2013.02.012.
Cymbaluk-Płoska A, Chudecka-Głaz A, Jagodzińska A, Pius-Sadowska E, Sompolska-Rzechuła A, Machaliński B, et al. Evaluation of biologically active substances promoting the development of or protecting against endometrial cancer. Onco Targets Ther. 2018;11:1363–72. https://doi.org/10.2147/OTT.S155942.
Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, Patel S, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–80. https://doi.org/10.1210/en.2009-1358.
Xu L, Wang H, Gong Y, Pang M, Sun X, Guo F, et al. Nesfatin-1 regulates the lateral hypothalamic area melanin-concentrating hormone-responsive gastric distension-sensitive neurons and gastric function via arcuate nucleus innervation. Metabolism. 2017;67:14–25. https://doi.org/10.1016/j.metabol.2016.10.010.
Riva M, Nitert MD, Voss U, Sathanoori R, Lindqvist A, Ling C, et al. Nesfatin-1 stimulates glucagon and insulin secretion and beta cell NUCB2 is reduced in human type 2 diabetic subjects. Cell Tissue Res. 2011;346:393–405. https://doi.org/10.1007/s00441-011-1268-5.
Xu Y, Pang X, Dong M, Wen F, Zhang Y. Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation in vitro. Biochem Biophys Res Commun. 2013;440:467–72. https://doi.org/10.1016/j.bbrc.2013.06.001.
Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, et al. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 2012;81:1075–85. https://doi.org/10.1038/ki.2011.472.
Cetinkaya H, Karagöz B, Bilgi O, Ozgün A, Tunçel T, Emirzeoğlu L, et al. Nesfatin-1 in advanced lung cancer patients with weight loss. Regul Pept. 2013;181:1–3. https://doi.org/10.1016/j.regpep.2012.11.005.
Franzén A, Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985;232:715–24.
Kahles F, Findeisen HM, Bruemmer D. Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–93. https://doi.org/10.1016/j.molmet.2014.03.004.
Lai C-F, Seshadri V, Huang K, Shao J-S, Cai J, Vattikuti R, et al. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–89. https://doi.org/10.1161/01.RES.0000227550.00426.60.
Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. https://doi.org/10.1016/j.tcb.2005.12.005.
Johnston NIF, Gunasekharan VK, Ravindranath A, O’Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361–72.
Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mänsson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomark Prev. 2007;16:1087–97. https://doi.org/10.1158/1055-9965.EPI-06-1008.
Bandopadhyay M, Bulbule A, Butti R, Chakraborty G, Ghorpade P, Ghosh P, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014;18:883–95. https://doi.org/10.1517/14728222.2014.925447.
Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–81. https://doi.org/10.1038/sj.bjc.6601839.
Zarling JM, Shoyab M, Marquardt H, Hanson MB, Lioubin MN, Todaro GJ. Oncostatin M: a growth regulator produced by differentiated histiocytic lymphoma cells. Proc Natl Acad Sci U S A. 1986;83:9739–43.
Sanchez-Infantes D, White UA, Elks CM, Morrison RF, Gimble JM, Considine RV, et al. Oncostatin m is produced in adipose tissue and is regulated in conditions of obesity and type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E217–25. https://doi.org/10.1210/jc.2013-3555.
Richards CD. The enigmatic cytokine oncostatin M and roles in disease. ISRN Inflamm. 2013;2013:1–23. https://doi.org/10.1155/2013/512103.
Miles SA, Martínez-Maza O, Rezai A, Magpantay L, Kishimoto T, Nakamura S, et al. Oncostatin M as a potent mitogen for AIDS-Kaposi’s sarcoma-derived cells. Science. 1992;255:1432–4.
Amaral MC, Miles S, Kumar G, Nel AE. Oncostatin-M stimulates tyrosine protein phosphorylation in parallel with the activation of p42MAPK/ERK-2 in Kaposi’s cells. Evidence that this pathway is important in Kaposi cell growth. J Clin Invest. 1993;92:848–57. https://doi.org/10.1172/JCI116659.
Lapeire L, Hendrix A, Lambein K, Van Bockstal M, Braems G, Van Den Broecke R, et al. Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res. 2014;74:6806–19. https://doi.org/10.1158/0008-5472.CAN-14-0160.
Fossey SL, Bear MD, Kisseberth WC, Pennell M, London CA. Oncostatin M promotes STAT3 activation, VEGF production, and invasion in osteosarcoma cell lines. BMC Cancer. 2011;11:125. https://doi.org/10.1186/1471-2407-11-125.
Weiss TW, Simak R, Kaun C, Rega G, Pflüger H, Maurer G, et al. Oncostatin M and IL-6 induce u-PA and VEGF in prostate cancer cells and correlate in vivo. Anticancer Res. 2011;31:3273–8.
• Tawara K, Bolin C, Koncinsky J, Kadaba S, Covert H, Sutherland C, et al. OSM potentiates preintravasation events, increases CTC counts, and promotes breast cancer metastasis to the lung. Breast Cancer Res. 2018;20:–53. https://doi.org/10.1186/s13058-018-0971-5. This study describes the ability of oncostatin-M to facilitate metastasis of breast cancer.
West NR, Murphy LC, Watson PH. Oncostatin M suppresses oestrogen receptor- expression and is associated with poor outcome in human breast cancer. Endocr Relat Cancer. 2012;19:181–95. https://doi.org/10.1530/ERC-11-0326.
Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. https://doi.org/10.1111/j.1749-6632.2012.06750.x.
Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–74. https://doi.org/10.1200/JCO.2014.58.4680.
Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, et al. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765–77. https://doi.org/10.1016/j.jhep.2009.06.016.
Venojärvi M, Wasenius N, Manderoos S, Heinonen OJ, Hernelahti M, Lindholm H, et al. Nordic walking decreased circulating chemerin and leptin concentrations in middle-aged men with impaired glucose regulation. Ann Med. 2013;45:162–70. https://doi.org/10.3109/07853890.2012.727020.
Tee MC, Cao Y, Warnock GL, Hu FB, Chavarro JE. Effect of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surg Endosc. 2013;27:4449–56. https://doi.org/10.1007/s00464-013-3127-9.
Ashrafian H, Ahmed K, Rowland SP, Patel VM, Gooderham NJ, Holmes E, et al. Metabolic surgery and cancer: protective effects of bariatric procedures. Cancer. 2011;117:1788–99. https://doi.org/10.1002/cncr.25738.
Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–81. https://doi.org/10.1210/jc.2005-2248.
Terra X, Auguet T, Guiu-Jurado E, Berlanga A, Orellana-Gavaldà JM, Hernández M, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013;23:1790–8. https://doi.org/10.1007/s11695-013-1033-9.
Zhu J, Schott M, Liu R, Liu C, Shen B, Wang Q, et al. Intensive glycemic control lowers plasma visfatin levels in patients with type 2 diabetes. Horm Metab Res. 2008;40:801–5. https://doi.org/10.1055/s-0028-1082040.
Mallik R, Chowdhury TA. Metformin in cancer. Diabetes Res Clin Pract. 2018; https://doi.org/10.1016/j.diabres.2018.05.023.
Hajavi J, Momtazi AA, Johnston TP, Banach M, Majeed M, Sahebkar A. Curcumin: a naturally occurring modulator of adipokines in diabetes. J Cell Biochem. 2017;118:4170–82. https://doi.org/10.1002/jcb.26121.
Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Res. 2009;11:R36. https://doi.org/10.1186/bcr2321.
Lanier V, Gillespie C, Leffers M, Daley-Brown D, Milner J, Lipsey C, et al. Leptin-induced transphosphorylation of vascular endothelial growth factor receptor increases Notch and stimulates endothelial cell angiogenic transformation. Int J Biochem Cell Biol. 2016;79:139–50. https://doi.org/10.1016/j.biocel.2016.08.023.
Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–13. https://doi.org/10.1016/j.cytogfr.2013.10.001.
Otvos L, Kovalszky I, Olah J, Coroniti R, Knappe D, Nollmann FI, et al. Optimization of adiponectin-derived peptides for inhibition of cancer cell growth and signaling. Biopolymers. 2015;104:156–66. https://doi.org/10.1002/bip.22627.
Tworoger SS, Eliassen AH, Kelesidis T, Colditz GA, Willett WC, Mantzoros CS, et al. Plasma adiponectin concentrations and risk of incident breast cancer. J Clin Endocrinol Metab. 2007;92:1510–6. https://doi.org/10.1210/jc.2006-1975.
Chong DQ, Mehta RS, Song M, Kedrin D, Meyerhardt JA, Ng K, et al. Prediagnostic plasma adiponectin and survival among patients with colorectal cancer. Cancer Prev Res (Phila). 2015;8:1138–45. https://doi.org/10.1158/1940-6207.CAPR-15-0175.
Dalamaga M, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, Lekka A. Serum resistin: a biomarker of breast cancer in postmenopausal women? Association with clinicopathological characteristics, tumor markers, inflammatory and metabolic parameters. Clin Biochem. 2013;46:584–90. https://doi.org/10.1016/j.clinbiochem.2013.01.001.
Dalamaga M, Archondakis S, Sotiropoulos G, Karmaniolas K, Pelekanos N, Papadavid E, et al. Could serum visfatin be a potential biomarker for postmenopausal breast cancer? Maturitas. 2012;71:301–8. https://doi.org/10.1016/j.maturitas.2011.12.013.
Zhang K, Zhou B, Zhang P, Zhang Z, Chen P, Pu Y, et al. Genetic variants in NAMPT predict bladder cancer risk and prognosis in individuals from southwest Chinese Han group. Tumour Biol. 2014;35:4031–40. https://doi.org/10.1007/s13277-013-1527-z.
Kalia M. Biomarkers for personalized oncology: recent advances and future challenges. Metabolism. 2015;64:S16–21. https://doi.org/10.1016/j.metabol.2014.10.027.
Burgess S, Timpson NJ, Ebrahim S, Davey Smith G. Mendelian randomization: where are we now and where are we going? Int J Epidemiol. 2015;44:379–88. https://doi.org/10.1093/ije/dyv108.
Ebrahim S, Davey SG. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet. 2008;123:15–33. https://doi.org/10.1007/s00439-007-0448-6.
Nimptsch K, Song M, Aleksandrova K, Katsoulis M, Freisling H, Jenab M, et al. Genetic variation in the ADIPOQ gene, adiponectin concentrations and risk of colorectal cancer: a Mendelian randomization analysis using data from three large cohort studies. Eur J Epidemiol. 2017;32:419–30. https://doi.org/10.1007/s10654-017-0262-y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Nikolaos Spyrou, Konstantinos I. Avgerinos, Christos S Mantzoros, and Maria Dalamaga declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Metabolism
Rights and permissions
About this article
Cite this article
Spyrou, N., Avgerinos, K.I., Mantzoros, C.S. et al. Classic and Novel Adipocytokines at the Intersection of Obesity and Cancer: Diagnostic and Therapeutic Strategies. Curr Obes Rep 7, 260–275 (2018). https://doi.org/10.1007/s13679-018-0318-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-018-0318-7