Abstract
Purpose of Review
Because the fetus expresses paternally-derived foreign antigens, pregnancy poses unique immune challenges. The maternal immune system must balance protecting the semi-allogeneic fetus from immune rejection with defending the mother and fetus from pathogens. Fetally-derived trophoblast cells of the placenta serve as the immunologic interface with soluble and cellular maternal immune effectors and are thereby essential partners in supporting these tightly regulated interactions. While there are multiple ways that the maternal–fetal immune interface is controlled in a healthy pregnancy, this review highlights several of the immune checkpoint regulators thought to be centrally involved in maternal–fetal immunoregulation.
Recent Findings
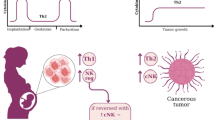
Reproductive immunologists have shown that those fetal trophoblast cells that directly encounter maternal immune cells share many common features with cancer cells, shifting the paradigm of placental immunology away from transplantation biology and toward our extensive understanding of tumorigenesis. Both the post-implantation placenta and the growing neoplasm have many shared goals, including invasion, robust cellular proliferation, angiogenesis, and modulation of host immunity. One way in which both the human placenta and cancer cells protect themselves from immune attack is through the loss of, or neoexpression of, several important cell surface regulators of specific immune interactions known as immune checkpoints. Here, we will discuss several ways that tumors and the placenta utilize immune checkpoint pathways and inhibitors, including the loss of most classical major histocompatibility complex (MHC) molecules and neoexpression of several nonclassical MHC molecules, expression of novel immunosuppressive B7 family members and cell adhesion molecules, such as CD47, and modulation of indoleamine 2,3-dioxygenase (IDO) enzyme activity.
Summary
Finely tuned immune adaptation is fundamental to a successful reproduction. Failure to implement such adaptations can result in a variety of disorders, including pregnancy loss, abnormal placental invasion (e.g., placenta accreta/percreta), preeclampsia, and intrauterine growth restriction. Improved understanding of complex maternal-fetal immune interactions will be crucial to discover mechanisms underpinning these pregnancy complications, which, in turn, will help inform preventative and/or therapeutic clinical interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A successful pregnancy requires tight regulatory control of the maternal immune system. Tasked with tolerance of the semi-allogeneic fetus, the maternal immune system must simultaneously retain the ability to respond to harmful pathogens while allowing for the placental growth and invasion [1]. This balance is especially important in early pregnancy during the placentation process, a series of events with remarkable similarity to tumorigenesis. Both the developing placenta and the invading tumor require robust cell proliferation, establishment of a vasculature via angiogenesis, and, most pertinent to this discussion, invasion of host tissues and modulation of host immune responses. These processes are all tightly linked. Invasion of the maternal decidua basalis by extravillous cytotrophoblasts (EVTs) allows for the remodeling of the uterine spiral arteries to optimize nutrient and oxygen exchange between the mother and her developing fetus [2]. Alterations in this invasion process have been proposed to be etiologic in several pregnancy derangements, including recurrent pregnancy loss, preeclampsia, fetal growth restriction, and preterm labor [3].
Immune checkpoints include enzymatic pathways and cell surface molecules that regulate immune interactions. They have been shown to be central in balancing immune responses in a way that enables robust response to exogenous insults without risking over-response to native antigens or to pregnancy [4, 5]. In this review, we describe the regulation, often inhibitory, of several immune checkpoint mechanisms that are thought to be important to pregnancy maintenance and normal fetal development and make comparisons to their similar role in neoplastic growth.
MHC Molecules
The major histocompatibility complex (MHC), also known in humans as the human leukocyte antigen (HLA) complex, is the most polymorphous set of genes in the human genome [6]. The HLA genes code for surface glycoproteins that function through the presentation of both endogenous and foreign antigens to the host immune system, largely to distinguish between self and non-self or altered-self (e.g., pathogen or neoplasm) antigens [7].
There are 3 main classes of HLA molecules: HLA class I, HLA class II, and HLA class III [8]. HLA class I is expressed on the surface of almost every somatic cell, with differing levels of expression based on tissue type. Playing an essential role in defending against intracellular pathogens, there is further division of MHC class I molecules into Class IA (classical) and Class IB (non-classical). The classical HLA molecules include HLA-A, HLA-B, and HLA-C. These gene products present antigens to cytotoxic CD8 + T cells, and their presence or absence regulates the function of natural killer (NK) cells and natural killer T (NKT) cells. The nonclassical HLA gene products include HLA-E, HLA-F, and HLA-G. These glycoproteins have a more limited variation in the antigens that they can present to leukocytes due to limited gene polymorphism [3, 6]. Their role in true antigen presentation is much more limited than that of HLA-A and -B, and their expression is limited to a much smaller group of cell types.
Important and characteristic MHC molecule expression patterns are essential to immune interactions at the developing maternal fetal interface [9]. Many trophoblast cells in the human placenta have very low to no expression of the classical HLA-A and -B molecules, and no trophoblast cell expresses MHC class II molecules [10]. This loss of expression is also seen in many tumors [11] and is a good example of the loss of a major cell surface immune molecules that may be considered immune checkpoints. In contrast, the classical MHC molecule, HLA-C, and the non-classical MHC products, HLA-E, HLA-G, and possibly HLA-F, are expressed on the surface of the most invasive of the trophoblast cell types, the EVTs [3]. This fairly unusual overall pattern of MHC expression is not uncommon in tumor cell types [12], and the expression of HLA-G, -E, and, to a lesser extent, -F, is quite limited to the placenta and to tumor cells.
HLA-G
HLA-G has seven different transcript isoforms, some coding for membrane-bound proteins (HLA-G1, G2, G3, and G4) and others for soluble proteins (G5, G6, G7; also known as sHLA-G). HLA-G is a ligand for three main receptors: immunoglobulin-like transcript 2/leukocyte immunoglobulin–like receptor subfamily B member 1/CD85 antigen-like family member J (ILT2 /LILRB1/ CD85j) found on T cells, NK cells, and B cells; immunoglobulin-like transcript 4/leukocyte immunoglobulin–like receptor subfamily B member 1/CD85 antigen-like family member B (ILT4/LILRB2/ CD85d) found on myeloid cells; and killer cell immunoglobulin-like receptor 2DL4 (KIR2DL4/CD158d) found on NK cells [13,14,15,16]. The KIR2DL4 receptor is a type of killer cell immunoglobulin–like receptor (KIR) that has both stimulatory and inhibitory functions. Residing primarily within endosomes, this receptor interacts with sHLA-G released by trophoblast cells, downregulating the cytotoxicity of decidual NK cells while stimulating the release of proinflammatory and pro-angiogenic cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), interferon-γ (IFN-γ), and tumor necrosis factor α (TNF-α) [17,18,19]. This proinflammatory response is essential for the promotion of decidual remodeling by invading EVTs. ILT2 and ILT4 are the primary inhibitory receptors for HLA-G. ILT2, in particular, plays a major role in suppressing NK and CD8 + T cell cytotoxicity and induces a more tolerogenic phenotype among dendritic cells and macrophages [20]. These receptors also promote IFN-γ release which, in turn, drives spiral artery remodeling within the basalis decidua [10].
Studies have shown that, in addition to direct inhibition of NK and CD8 + T cell cytotoxic activities, the presence of HLA-G on fetal trophoblast cells indirectly protects them from immune destruction through the actions of regulatory T cells (Tregs). Tregs maintain the pro-inflammatory/anti-inflammatory equilibrium in immune responses. At the maternal fetal interface, these cells are activated by fetal antigens presented by MHC class II molecules [3]. It is hypothesized that the Treg response to pregnancy is largely dependent on the recognition that fetal tissue is allogeneic. To this point, when fetal/maternal HLA mismatching increases, so too does the production and conversion of T cells into Tregs in order to inhibit an alloreactive T cell response [21]. HLA-G has been specifically shown to induce differentiation of CD4 + T cells into Tregs [22]. Clinically, abnormalities in HLA-G expression and activities have been implicated in several pregnancy-related disease processes, including recurrent pregnancy loss and preeclampsia [3].
HLA-F
HLA-F is a Class Ib HLA molecule that can be found on EVT cells. The two main receptors for this ligand are killer cell immunoglobulin–like receptor 3DL1 (KIR3DL1) and killer cell immunoglobulin–like receptor, three Ig domains and long cytoplasmic tail 2 (KIR3DL2), which are both inhibitory receptors expressed on decidual NK cells [5]. HLA-F binds longer peptides for antigen presentation than other MHC class I molecules. In its open conformation form, antigenic peptide and the typical binding partner for MHC class I molecules, beta 2-microglobulin, are absent. This open conformation allows for interactions between HLA-F heavy chains and other HLA molecules, including HLA-E. Such interactions are believed to help to stabilize ligand-receptor pairing between decidual NK cells and EVTs [16, 23], relegating HLA-F to a largely “assistant” role for other placental MHC I interactions.
HLA-E
HLA-E is an MHC class IB protein expressed both in the cytoplasm and on the surface of EVTs [24]. The main receptors for HLA-E are natural killer cell receptor 2C (NKG2C) on decidual NK cells, natural killer cell receptor 2A (NKG2A) on decidual NK cells, and CD8 + on T cells. Cell surface expression of HLA-E helps to protect cells from cytotoxic damage by presenting peptides derived from other HLA class I molecules to NK and CD8 + T cells. In particular, it protects HLA-G-containing cells through the presentation of HLA-G signal peptides to inhibitory receptors on otherwise cytotoxic immune cells, allowing HLA-G to indirectly promote immune tolerance through HLA-E [16].
HLA-C
As the only HLA Class IA protein expressed by fetal EVTs, maternally derived HLA-C plays an important role in alerting the maternal immune system to potential pathogens through typical antigen presentation to maternal CD8 + T cells. However, the polymorphic nature of HLA-C often leads to dissimilarity between the trophoblast-expressed paternally derived HLA-C alleles and the maternal HLA-C phenotype. This allele mismatch has the potential to increase cytotoxic/cytolytic activation of decidual T cells and NK cells toward fetal tissue [25]. Although studies have been mixed regarding the exact role of HLA-C mismatch in pregnancy complications, evidence of HLA-C specific antibodies in patients with recurrent miscarriage highlights the potential role that HLA-C allele mismatch may play in unexplained recurrent pregnancy loss [26, 27].
B7 Immune Checkpoint Molecules
The B7 family of molecules, a group of immune checkpoint proteins, is one of the best-characterized and widely-distributed signaling molecule superfamilies; their primary role is to serve as signaling mechanisms that modulate T cell activation [28,29,30]. The 11 known members of the B7 family are B7-1 (CD80), B7-2 (CD86), B7-H1 (programmed cell death ligand 1, PD-L1, CD274), B7-DC (PD-L2, CD273, PDCD1LG2), B7-H2 (ICOS-L), B7-H3, B7-H4 (VTCN1), B7-H5 (VISTA), BTNL2 (butyrophilin-like 2, BTL-II), B7-H6, and B7-H7 (HHLA2). Members of this superfamily can exert both stimulatory and inhibitory signals that depend on the cell type- and location-specific B7 family member ligand pairing [31].
B7 and Human Trophoblast
B7-H1, B7-H2, B7-DC, B7-H3, and B7-H4 are expressed in the human placenta, and their levels fluctuate during different stages of pregnancy [32, 33]. B7-H1 is highly expressed in the syncytiotrophoblast (STB) in early and term normal human placenta [32], while B7-DC and B7-H4 are prominent on the surface of STB during early pregnancy but are not present in human trophoblast at term [33]. B7-DC is abundant in first trimester STB, but absent in term STB, when localization has largely switched to placental endothelial cells [33]. B7-DC exhibits a higher affinity for programmed cell death protein 1 (PD-1; see below) than B7-H1, but its expression in the placenta is, comparatively, much more limited. Still, its interactions with PD-1 profoundly inhibit B7-CD28 signals at relatively low antigen concentrations and can thereby play an important role in promoting T cell proliferation and inflammatory cytokine production in the placenta [34, 35]. B7-H4 is expressed at high levels on early gestation STB [36], as well as on decidual macrophages [37]. B7-H4 plays an important role in early placental development by inhibiting the expression of classical MHC Class I molecules, shifting the differentiation of cytotrophoblast cells from invasion to syncytialization and inducing changes in peripheral natural killer cells that make them more closely mimic a decidual NK cell phenotype [38].
B7-H2 is expressed on EVT cells, macrophages, and Tregs in the first trimester and term placenta [33]. B7-H2 expression is associated with largely protolerogenic T helper 2 (Th2) cell effector function in terms of cell surface marker expression, differentiation, and cytokine production [39,40,41]. Similar to B7-H2, B7-H3 is narrowly expressed on the surface of EVTs and macrophages in the first trimester and term placenta [33]. Here, it is considered a coinhibitory molecule for T cells [42, 43]. In addition to its role in immunological pathways, B7-H3 has nonimmunologic, protumorigenic activities, including the promotion of cell migration and invasion, inhibition of apoptosis, and enhancement of cell viability, chemoresistance, and endothelial-to-mesenchymal (EMT) transition [44]. B7-H5, also known as VISTA, is well known for immune checkpoint inhibition, regulating host T cell and myeloid functions in the tumor microenvironment [45,46,47]. However, high B7-H5 expression levels have also been detected in trophoblast cells, including STB, EVT, and CTB (cytotrophoblast cells) from term human placentas [48, 49]. Its role in placental function and dysfunction remains to be determined.
B7 and Decidual Immune Cells
The expression of B7-1 and B7-2 is largely restricted to lymphoid cells, mainly class II human leukocyte antigen (HLA) + cells, including decidual dendritic cells (DCs) and macrophages at the maternal–fetal interface [28]. B7-1 and B7-2 assist decidual DCs in maintaining a largely protolerogenic, Th2-dominant state that is thought to be beneficial to gestational outcomes. Engagement of B7-1 or B7-2 with its ligand, cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), leads to the production of indoleamine 2,3-dioxygenase (IDO), an enzyme that is highly expressed at the human maternal–fetal interface and is capable of influencing T cell-related fetal rejection (see below) [50, 51]. Levels of B7-1 and B7-2 expression are higher in decidual macrophages isolated from early/mid-pregnancy than from those isolated at term [52], suggesting their involvement in human placental development and implantation.
The increased surface expression of B7-H1 on healthy, human decidual CD4 + T cell, Treg, NKT-like, and CD56 + NK cell subsets is accompanied by an increase in expression of the PD-1 immune checkpoint molecule in decidual immune cells. This strongly suggests that B7-H1/PD-1 interactions maintain a protolerogenic local immunological environment at the maternal–fetal interface by interacting with a variety of decidual immune cells.
Human BTNL2 has been identified as a B7-like molecule within the butyrophilin (BTN) gene family that is encoded by the MHC locus in humans [53]. BTNL2 may play an important role in inflammation and immune response by controlling T cell responses and promoting the generation of Tregs [54, 55]. Recombinant BTNL-2-Ig is now being studied as a potential treatment for graft-versus-host disease [56], suggesting it could also serve as a modulator of maternal–fetal immune interactions, either endogenously or as an exogenous therapy. To date there are no published reports on BTNL2 in the human placenta.
B7 Family Tumor Markers
Currently, there is not data to support the expression of the well-known tumor markers, B7-H6 and B7-H7, in pregnancy-related tissues [57, 58]. Their roles in tumorigenesis, however, suggest they could exert immunomodulatory activities that could support placental development. B7-H6 is selectively expressed by tumor cells, and its downregulation makes tumor cells vulnerable to NK-mediated tumor lysis [58]. B7-H7 (H long terminal repeat-associating 2, HHLA2) is expressed on monocytes and stimulated B cells and inhibits cytokine production by, and proliferation of, CD4 + and CD8 + T cells [59].
Soluble and Exosomal B7 Molecules
In addition to their cellular expression in trophoblast cell subtypes and decidual immune cells, alterations have been observed in the soluble forms of certain B7 molecules, such as B7-H1 and B7-H4, in pregnant women. Soluble B7-H1 levels were reported to be higher in the sera of pregnant women than in non-pregnant and postpartum women [60]. During early pregnancy, elevated levels of soluble B7-H4 (sB7-H4) are observed in women who experience preterm premature rupture of the amniotic membranes [61, 62], as well as in women at an increased risk for preeclampsia [63], when compared to women with uncomplicated pregnancies. Changes in the soluble forms of B7 molecules have been implicated in immunological alterations associated with maintenance of a healthy pregnancy and the development of preeclampsia [63, 64]. sB7-H1 and sB7-H4 have been detected in the sera of cancer patients, and their expression levels are closely linked to progression and prognosis [65,66,67].
Both early and term placental trophoblast cells have been shown to secrete B7-H1 and B7-H3 via exosomes [68]; these exosomes and similar trophoblast-derived microvesicles can be engulfed by phagocytes and serve as vehicular shuttles for paternally-inherited placental antigens that are ultimately cross-presented to maternal T cells by a variety of antigen-presenting cells [43]. Exosomal B7 molecules are also released from cancer cells. Metastatic melanoma cells release exosomes carrying PD-L1 on their surface that suppress the function of CD8 T cells and facilitate tumor growth [69]. Additionally, a high expression of B7-H3 was observed in urinary exosomes isolated from colorectal cancer patients [70]. Similarities between requirements for growth and immune privilege in cancer and pregnancy could lead to a better understanding of the maternal–fetal immune response.
Other Immune Checkpoint Modulators
Indoleamine 2,3-Dioxygenase
Indoleamine 2,3-dioxygenase (IDO) is the initial and rate-limiting enzyme that breaks down tryptophan into kynurenine [71, 72]. Since tryptophan is necessary for T cell proliferation and activation, IDO-induced tryptophan degradation functions to promote T cell suppression. T cells are further differentiated into immunosuppressive Foxp3+ T regulatory cells via kynurenine-mediated hydrocarbon receptor (AhR) activation [73, 74]. Following stimulation with proinflammatory mediators such as IFN-γ, IDO is widely expressed in various tissues of mammals. IDO exhibits particularly high endogenous activity in cells present at the maternal–fetal interface, including trophoblast cells, decidual stromal cells, decidual immune cells, and vascular endothelial cells of the chorion and decidua [75]. Emerging evidence has shown that the expression and activity of IDO differ not only among non-pregnant, normal pregnant, and pathological pregnancy conditions, but also by gestational age [76,77,78]. In normal pregnancy, IDO is involved in maternal–fetal immune tolerance and protection against pathogens. When pregnant mice carrying syngeneic or allogeneic fetuses were exposed to 1-methyl-tryptophan (1MT), an IDO inhibitor, all of the allogeneic fetuses, but none of the syngeneic concepti, were rejected. This rejection phenomenon was mediated by a single paternally inherited MHC class I loci and was fully dependent on maternal T cell activity [79]. Pregnant mice deficient in recombination activating gene 1 (RAG-1) expression, and thereby unable to develop lymphocytes, were not affected by 1MT treatment. These results strongly suggest that IDO plays a crucial role in defense of the conceptus against allogenic rejection. Subsequent studies have demonstrated that IDO eliminates pathogens through nutrient competition via tryptophan depletion [80,81,82]. IDO could also promote trophoblast invasion into the decidualized endometrium and remodeling of maternal spiral arteries, both essential to placental perfusion and fetal development [83, 84]. A decrease in the expression levels of IDO is associated with pregnancy complications such as preeclampsia and intrauterine growth restriction [77, 85,86,87]. Considering the role of IDO in cancer development, where its high expression and activity support immune escape for tumor survival [88, 89], decreased IDO levels and activity in pregnancy are likely implicated in exaggerated inflammatory responses at the maternal–fetal interface and adverse alterations in placental and fetal growth and function.
CD47
CD47 (also known as integrin-associated protein, IAP) is a ubiquitously expressed membrane glycoprotein belonging to the immunoglobulin superfamily. CD47 serves as a ligand for signal regulatory protein alpha (SIRPα, also known as CD172a), a membrane glycoprotein present mainly on macrophages [90]. CD47 − SIRPα interactions induce downstream signal activation that inhibits target cell phagocytosis. To this point, CD47 has been called a “don’t eat me” signaling molecule to characterize its interactions with SIRPα. CD47 has received significant attention in cancer research as a means for tumor cell immune evasion and is also regarded as a key determinant in transplantation success [91,92,93]. Although only a few studies on CD47 have been reported in the reproductive immunology literature, these reports suggest that CD47 may play an important role in maternal–fetal immune interactions. Notably, CD47 has been documented in extracellular vesicles (EVs) extruded from the STB of the placenta into the maternal blood [94, 95]. Since placentally-derived EVs are important mediators of intercellular communication, the presence of CD47 in these EVs could promote tolerance to the STB from which they originated and may prevent the clearance of these vesicles from maternal blood. In addition to inhibition of macrophage phagocytosis, CD47 − SIRPα interactions are also involved in suppression of adaptive immune responses. In fact, blockade of CD47 − SIRPα interactions induces dendritic cell activation and T cell priming, suggesting these interactions could help to bridge innate and adaptive immunity during pregnancy [96, 97]. Further research into the role of CD47 in immune regulation and balance at the maternal–fetal interface is certainly warranted.
Conclusion
Immune checkpoints are essential to tumorigenesis and important cancer therapeutics have been introduced that specifically target these checkpoints. Many of these same immune checkpoints appear to be involved in the immunologic interactions occurring at the human maternal–fetal interface. Alterations in the proper functioning of these immune checkpoints have been linked to early pregnancy loss, disorders of abnormal placentation such as impaired fetal growth, and hypertensive disorders of pregnancy (Table 1). Although there has been significant interest in these molecules and their interactions in human pregnancy, much remains to be learned. Their important and expanding role in cancer therapy, however, suggests that an improved understanding of their function and dysfunction during placental growth and development may offer breakthrough opportunities in the preventative and therapeutic care of women and their babies during pregnancy.
References
Fuhler GM. The immune system and microbiome in pregnancy. Best Pract Res Clin Gastroenterol. 2020;101671:44–5. https://doi.org/10.1016/j.bpg.2020.101671.
Lyall F, Bulmer JN, Duffie E, Cousins F, Theriault A, Robson SC. Human trophoblast invasion and spiral artery transformation: the role of PECAM-1 in normal pregnancy, preeclampsia, and fetal growth restriction. Am J Pathol. 2001;158:1713–21. https://doi.org/10.1016/S0002-9440(10)64127-2.
Aisagbonhi O, Morris GP. Human leukocyte antigens in pregnancy and preeclampsia. Front Genet. 2022;13:884275. https://doi.org/10.3389/fgene.2022.884275.
Abril-Rodriguez G, Ribas A. SnapShot: immune checkpoint inhibitors. Cancer Cell. 2017;31(848–848).
Persson G, Jorgensen N, Nilsson LL, Andersen LHJ, Hviid TVF. A role for both HLA-F and HLA-G in reproduction and during pregnancy? Hum Immunol. 2020;81:127–33. https://doi.org/10.1016/j.humimm.2019.09.006.
Klein J, Sato A. The HLA system First of two parts. N Engl J Med. 2000;343:702–9. https://doi.org/10.1056/NEJM200009073431006.
Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol. 2018;18:325–39. https://doi.org/10.1038/nri.2017.143.
Cruz-Tapias P, Castiblanco, J, Anaya, J. Chapter 10: Major histocompatibility complex: Antigen processing and presentation. In: Anaya, JM, Shoenfeld, Y, Rojas-Villarraga, A, Levy, RA, & Cervera, R, editors. Autoimmunity: From Bench to Bedside (Internet); 2013.
Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA-G: at the interface of maternal-fetal tolerance. Trends Immunol. 2017;38:272–86. https://doi.org/10.1016/j.it.2017.01.009.
Lin XX, Xie YM, Zhao SJ, Liu CY, Mor G, Liao AH. Human leukocyte antigens: the unique expression in trophoblasts and their crosstalk with local immune cells. Int J Biol Sci. 2022;18:4043–52. https://doi.org/10.7150/ijbs.73616.
Aptsiauri N, Garrido F. The challenges of HLA class I loss in cancer immunotherapy: facts and hopes. Clin Cancer Res. 2022;28:5021–9. https://doi.org/10.1158/1078-0432.CCR-21-3501.
Arnaiz-Villena A, et al. HLA-G: function, polymorphisms and pathology. Int J Immunogenet. 2021;48:172–92. https://doi.org/10.1111/iji.12513.
Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 1999;189:1093–100. https://doi.org/10.1084/jem.189.7.1093.
Gonzalez A, Rebmann V, LeMaoult J, Horn PA, Carosella ED, Alegre E. The immunosuppressive molecule HLA-G and its clinical implications. Crit Rev Clin Lab Sci. 2012;49:63–84. https://doi.org/10.3109/10408363.2012.677947.
Xu X, Zhou Y, Wei H. Roles of HLA-G in the maternal-fetal immune microenvironment. Front Immunol. 2020;11:592010. https://doi.org/10.3389/fimmu.2020.592010.
Nilsson LL, Hviid TVF. HLA class Ib-receptor interactions during embryo implantation and early pregnancy. Hum Reprod Update. 2022;28:435–54. https://doi.org/10.1093/humupd/dmac007.
Rajagopalan S, Long EO. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front Immunol. 2012;3:258. https://doi.org/10.3389/fimmu.2012.00258.
Rajagopalan S, et al. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. https://doi.org/10.1371/journal.pbio.0040009.
Zhuang B, Shang J, Yao Y. HLA-G: an important mediator of maternal-fetal immune-tolerance. Front Immunol. 2021;12:744324. https://doi.org/10.3389/fimmu.2021.744324.
Krop J, Heidt S, Claas FHJ, Eikmans M. Regulatory T cells in pregnancy: it is not all about FOXP3. Front Immunol. 2020;11:1182. https://doi.org/10.3389/fimmu.2020.01182.
Tilburgs T, et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009;82:148–57. https://doi.org/10.1016/j.jri.2009.05.003.
Svensson-Arvelund J, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194:1534–44. https://doi.org/10.4049/jimmunol.1401536.
Goodridge JP, Burian A, Lee N, Geraghty DE. HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. J Immunol. 2013;191:3553–62. https://doi.org/10.4049/jimmunol.1300081.
Jiang L, et al. Extracellular vesicle-mediated secretion of HLA-E by trophoblasts maintains pregnancy by regulating the metabolism of decidual NK cells. Int J Biol Sci. 2021;17:4377–95. https://doi.org/10.7150/ijbs.63390.
Papuchova H, Meissner TB, Li Q, Strominger JL, Tilburgs T. The dual role of HLA-C in tolerance and immunity at the maternal-fetal interface. Front Immunol. 2019;10:2730. https://doi.org/10.3389/fimmu.2019.02730.
Lashley LE, Haasnoot GW, Spruyt-Gerritse M, Claas FH. Selective advantage of HLA matching in successful uncomplicated oocyte donation pregnancies. J Reprod Immunol. 2015;112:29–33. https://doi.org/10.1016/j.jri.2015.05.006.
Meuleman T, Haasnoot GW, van Lith JMM, Verduijn W, Bloemenkamp KWM, Claas FHJ. Paternal HLA-C is a risk factor in unexplained recurrent miscarriage. Am J Reprod Immunol. 2018;79(2). https://doi.org/10.1111/aji.12797.
Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. https://doi.org/10.1186/gb-2005-6-6-223.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. https://doi.org/10.1146/annurev.immunol.23.021704.115611.
Zhao Y, Zheng Q, Jin L. The role of B7 family molecules in maternal-fetal immunity. Front Immunol. 2020;11:458. https://doi.org/10.3389/fimmu.2020.00458.
Pulanco MC, Madsen AT, Tanwar A, Corrigan DT, Zang X. Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies. Cell Mol Immunol. 2023;20(7):694–713. https://doi.org/10.1038/s41423-023-01019-8.
Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. 2017;36:146–53. https://doi.org/10.1097/pgp.0000000000000305.
Petroff MG, Kharatyan E, Torry DS, Holets L. The immunomodulatory proteins B7-DC, B7–H2, and B7–H3 are differentially expressed across gestation in the human placenta. Am J Pathol. 2005;167:465–73. https://doi.org/10.1016/s0002-9440(10)62990-2.
Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. https://doi.org/10.1038/85330.
Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7:550. https://doi.org/10.3389/fimmu.2016.00550.
Karvas RM, et al. Use of a human embryonic stem cell model to discover GABRP, WFDC2, VTCN1 and ACTC1 as markers of early first trimester human trophoblast. Mol Hum Reprod. 2020;26:425–40. https://doi.org/10.1093/molehr/gaaa029.
Galazka K, et al. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7–H4 macrophages–a preliminary report. Am J Reprod Immunol. 2009;61:136–46. https://doi.org/10.1111/j.1600-0897.2008.00674.x.
Zhou J, et al. The immune checkpoint molecule, VTCN1/B7-H4, guides differentiation and suppresses proinflammatory responses and MHC class I expression in an embryonic stem cell-derived model of human trophoblast. Front Endocrinol (Lausanne). 2023;14:1069395. https://doi.org/10.3389/fendo.2023.1069395.
Löhning M, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197:181–93. https://doi.org/10.1084/jem.20020632.
McAdam AJ, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–40. https://doi.org/10.4049/jimmunol.165.9.5035.
Wang W, Sung N, Gilman-Sachs A, Kwak-Kim J. T Helper (Th) Cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front Immunol. 2020;11:2025. https://doi.org/10.3389/fimmu.2020.02025.
Chapoval AI, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. https://doi.org/10.1038/85339.
Chen C, Shen Y, Qu QX, Chen XQ, Zhang XG, Huang JA. Induced expression of B7–H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp Cell Res. 2013;319:96–102. https://doi.org/10.1016/j.yexcr.2012.09.006.
Li Y, et al. B7–H3 promotes gastric cancer cell migration and invasion. Oncotarget. 2017;8:71725–35. https://doi.org/10.18632/oncotarget.17847.
Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med. 2011;208:577–92. https://doi.org/10.1084/jem.20100619.
Villarroel-Espindola F, et al. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24:1562–73. https://doi.org/10.1158/1078-0432.Ccr-17-2542.
Deng J, et al. Hypoxia-induced VISTA promotes the suppressive function of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Immunol Res. 2019;7:1079–90. https://doi.org/10.1158/2326-6066.Cir-18-0507.
Zong L, Zhang M, Wang W, Wan X, Yang J, Xiang Y. PD-L1, B7–H3 and VISTA are highly expressed in gestational trophoblastic neoplasia. Histopathology. 2019;75:421–30. https://doi.org/10.1111/his.13882.
Zhao SJ, Muyayalo KP, Luo J, Huang D, Mor G, Liao AH. Next generation of immune checkpoint molecules in maternal-fetal immunity. Immunol Rev. 2022;308:40–54. https://doi.org/10.1111/imr.13073.
Mellor AL, Munn DH. Extinguishing maternal immune responses during pregnancy: implications for immunosuppression. Semin Immunol. 2001;13:213–8. https://doi.org/10.1006/smim.2000.0317.
Mackler AM, Barber EM, Takikawa O, Pollard JW. Indoleamine 2,3-dioxygenase is regulated by IFN-gamma in the mouse placenta during Listeria monocytogenes infection. J Immunol. 2003;170:823–30. https://doi.org/10.4049/jimmunol.170.2.823.
Repnik U, et al. Comparison of macrophage phenotype between decidua basalis and decidua parietalis by flow cytometry. Placenta. 2008;29:405–12. https://doi.org/10.1016/j.placenta.2008.02.004.
Stammers M, Rowen L, Rhodes D, Trowsdale J, Beck S. BTL-II: a polymorphic locus with homology to the butyrophilin gene family, located at the border of the major histocompatibility complex class II and class III regions in human and mouse. Immunogenetics. 2000;51:373–82. https://doi.org/10.1007/s002510050633.
Nguyen T, Liu XK, Zhang Y, Dong C. BTNL2, a butyrophilin-like molecule that functions to inhibit T cell activation. J Immunol. 2006;176:7354–60. https://doi.org/10.4049/jimmunol.176.12.7354.
Swanson RM, et al. Butyrophilin-like 2 modulates B7 costimulation to induce Foxp3 expression and regulatory T cell development in mature T cells. J Immunol. 2013;190:2027–35. https://doi.org/10.4049/jimmunol.1201760.
Cui C, et al. In vivo administration of recombinant BTNL2-Fc fusion protein ameliorates graft-versus-host disease in mice. Cell Immunol. 2019;335:22–9. https://doi.org/10.1016/j.cellimm.2018.10.008.
Rieder SA, et al. B7–H7 (HHLA2) inhibits T-cell activation and proliferation in the presence of TCR and CD28 signaling. Cell Mol Immunol. 2021;18:1503–11. https://doi.org/10.1038/s41423-020-0361-7.
Zhu Z, et al. B7H6 serves as a negative prognostic marker and an immune modulator in human pancreatic cancer. Front Oncol. 2022;12:814312. https://doi.org/10.3389/fonc.2022.814312.
Zhao R, et al. HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function. Proc Natl Acad Sci USA. 2013;110:9879–84. https://doi.org/10.1073/pnas.1303524110.
Enninga EAL, et al. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol. 2018;79: e12795.
Mach P, et al. Changes in the blood serum levels of the costimulatory soluble B7–H4 molecule in pregnant women during the peripartal phase. Am J Reprod Immunol. 2015;74:209–15. https://doi.org/10.1111/aji.12392.
Mach P, et al. Serum concentrations of soluble B7–H4 in early pregnancy are elevated in women with preterm premature rupture of fetal membranes. Am J Reprod Immunol. 2016;76:149–54. https://doi.org/10.1111/aji.12527.
Mach P, et al. Soluble B7–H4 blood serum levels are elevated in women at high risk for preeclampsia in the first trimester, as well as in patients with confirmed preeclampsia. Am J Reprod Immunol. 2018;80:e12988. https://doi.org/10.1111/aji.12988.
Enninga EAL, et al. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol. 2018;79(2):e12795. https://doi.org/10.1111/aji.12795
Mach P, Kimmig R, Kasimir-Bauer S, Buderath P. Association of soluble B7–H4 and circulating tumor cells in blood of advanced epithelial ovarian cancer patients. Front Oncol. 2021;11:721067. https://doi.org/10.3389/fonc.2021.721067.
Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8:97671. https://doi.org/10.18632/oncotarget.18311.
Frigola X, et al. Identification of a soluble form of B7–H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–23. https://doi.org/10.1158/1078-0432.CCR-10-0250.
Vargas A, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. Faseb j. 2014;28:3703–19. https://doi.org/10.1096/fj.13-239053.
Chen G, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–6. https://doi.org/10.1038/s41586-018-0392-8.
Wu R, et al. Exosomal B7–H3 facilitates colorectal cancer angiogenesis and metastasis through AKT1/mTOR/VEGFA pathway. Cell Signal. 2023;109:110737. https://doi.org/10.1016/j.cellsig.2023.110737.
Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–20. https://doi.org/10.1038/nrd870.
Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–43. https://doi.org/10.1016/j.it.2012.10.001.
Fallarino F, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–77. https://doi.org/10.1038/sj.cdd.4401073.
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–8. https://doi.org/10.4049/jimmunol.0903670.
Kudo Y, et al. Indoleamine 2,3-dioxygenase: distribution and function in the developing human placenta. J Reprod Immunol. 2004;61:87–98. https://doi.org/10.1016/j.jri.2003.11.004.
Kudo Y, et al. Mechanisms regulating the expression of indoleamine 2,3-dioxygenase during decidualization of human endometrium. Hum Reprod. 2004;19:1222–30. https://doi.org/10.1093/humrep/deh218.
Wei H, et al. Abnormal expression of indoleamine 2, 3-dioxygenase in human recurrent miscarriage. Reprod Sci. 2019 Mar 4;1933719119833788. https://doi.org/10.1177/1933719119833788
Iwahashi N, Yamamoto M, Nanjo S, Toujima S, Minami S, Ino K. Downregulation of indoleamine 2, 3-dioxygenase expression in the villous stromal endothelial cells of placentas with preeclampsia. J Reprod Immunol. 2017;119:54–60. https://doi.org/10.1016/j.jri.2017.01.003.
Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. https://doi.org/10.1126/science.281.5380.1191.
Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78:2632–6. https://doi.org/10.1128/jvi.78.5.2632-2636.2004.
Popov A, et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J Clin Invest. 2006;116:3160–70. https://doi.org/10.1172/JCI28996.
Knubel CP, et al. Indoleamine 2,3-dioxigenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J. 2010;24:2689–701. https://doi.org/10.1096/fj.09-150920.
Kamimura S, Eguchi K, Yonezawa M, Sekiba K. Localization and developmental change of indoleamine 2 3-dioxygenase activity in the human placenta. Acta Med Okayama. 1991;45:135–9. https://doi.org/10.18926/AMO/32206.
Sedlmayr P. Indoleamine 2,3-dioxygenase in materno-fetal interaction. Curr Drug Metab. 2007;8:205–8. https://doi.org/10.2174/138920007780362491.
Kudo Y. The role of placental indoleamine 2,3-dioxygenase in human pregnancy. Obstet Gynecol Sci. 2013;56:209–16. https://doi.org/10.5468/ogs.2013.56.4.209.
Chang RQ, Li DJ, Li MQ. The role of indoleamine-2,3-dioxygenase in normal and pathological pregnancies. Am J Reprod Immunol. 2018;79:e12786. https://doi.org/10.1111/aji.12786.
Zardoya-Laguardia P, et al. Endothelial indoleamine 2,3-dioxygenase-1 regulates the placental vascular tone and is deficient in intrauterine growth restriction and pre-eclampsia. Sci Rep. 2018;8:5488. https://doi.org/10.1038/s41598-018-23896-0.
Moon YW, Hajjar J, Hwu P, Naing A. Targeting the indoleamine 2,3-dioxygenase pathway in cancer. J Immunother Cancer. 2015;3:51. https://doi.org/10.1186/s40425-015-0094-9.
Tang K, Wu YH, Song Y, Yu B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. 2021;14:68. https://doi.org/10.1186/s13045-021-01080-8.
Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol. 2014;32:25–50. https://doi.org/10.1146/annurev-immunol-032713-120142.
Takizawa H, Manz MG. Macrophage tolerance: CD47-SIRP-alpha-mediated signals matter. Nat Immunol. 2007;8:1287–9. https://doi.org/10.1038/ni1207-1287.
Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–32. https://doi.org/10.1016/j.coi.2012.01.010.
Ide K, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–6. https://doi.org/10.1073/pnas.0609661104.
Kovacs AF, et al. The impact of circulating preeclampsia-associated extracellular vesicles on the migratory activity and phenotype of THP-1 monocytic cells. Sci Rep. 2018;8:5426. https://doi.org/10.1038/s41598-018-23706-7.
Tong M, et al. Proteomic characterization of macro-, micro- and nano-extracellular vesicles derived from the same first trimester placenta: relevance for feto-maternal communication. Hum Reprod. 2016;31:687–99. https://doi.org/10.1093/humrep/dew004.
Liu X, et al. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–15. https://doi.org/10.1038/nm.3931.
Vonderheide RH. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21:1122–3. https://doi.org/10.1038/nm.3965.
Funding
The authors’ research is supported by grants 1RO1HD094937 from the National Institutes of Health (DJS), R21A1145071 from the National Institutes of Health (DJS, JZ), and pilot grant from the University of Missouri (SC).
Author information
Authors and Affiliations
Contributions
S.M., J.Z., and S.C. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mebane, S., Zhou, J., Choi, S. et al. Immune Checkpoint Molecules and Maternal–Fetal Immunity. Curr Obstet Gynecol Rep 13, 37–45 (2024). https://doi.org/10.1007/s13669-024-00372-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-024-00372-3