Abstract
Pregnancy is an immunological paradox, with renowned Nobel Prize winning transplantation biologist Sir Peter Brian Medawar being the first to introduce this concept back in 1953. This concept considers how the maternal immune system can tolerate the developing fetus, which is 50% antigenically foreign to the uterus. There have been significant advances in our understanding of the immune system in regulating fertility, pregnancy and in complications of these, and what was once considered a paradox can be seen as a highly evolved system. Indeed, the complexity of the maternal–fetal interface along with our ever-advancing knowledge of immune cells and mediators means that we have a better understanding of these interactions, with gaps still present. This chapter will summarise the key aspects of the role of the immune system at each stage of pregnancy and highlight the recent advances in our knowledge.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
The female reproductive tract is abundant with immune cells and mediators, and there are many factors which can modulate their production, recruitment and mechanism of action. In the absence of pregnancy, the immune system serves to protect the female reproductive tract from infection. In every region of the female reproductive tract and at every stage of the uterine cycle (Monin et al. 2020), as well as in response to coitus, through pregnancy, to post-partum uterine involution, components of the immune system are present and function to provide an environment suitable for the establishment of a successful pregnancy and ensure the return of the uterus to a pre-pregnancy state.
Recently, the concept of the pregnant uterus as a sterile environment has also been challenged. For decades, it has been assumed that the developing fetus does not have a natural microbiome and the presence of microorganisms within the uterus before birth is associated with disease and creation of a potential threat to the success of pregnancy. In recent times, this has been questioned with the investigation of the presence of some microorganisms within the amniotic fluid, meconium and placenta. Table 1 summarises some of the key studies in this area, highlighting the sample types studied and major organism types identified. Indeed, this is an interesting and developing area of reproductive research, with consideration now being given to the possibility that the microbiome of the uterus can influence fertility, conception and pregnancy success (Bardos et al. 2019; Chen et al. 2017; Moreno et al. 2016). This, however, remains a controversial and ongoing area of study with debate around true detection of species versus contamination of samples (Blaser et al. 2021; Fricke and Ravel 2021; Bushman 2019; Perez-Munoz et al. 2017).
It is therefore widely acknowledged that the female reproductive tract is a unique mucosal environment, but we must also consider the fluctuation in immune function which arises in response to cyclical changes in hormonal levels. In Chap. 2, we learned about the influence of sex hormones in driving differential responses in immunity between females and males. For the first 8 weeks of pregnancy, progesterone, which is often described as the pregnancy hormone, is produced by corpus luteum of the ovary. After this time, progesterone production is taken over by the placenta and its concentration continues to rise dramatically in maternal serum until birth. Estrogens are produced primarily by developing follicles and the corpus luteum. Estradiol is the main estrogen in women of fertile age, estriol is produced by the placenta during pregnancy and estrone is produced in women of menopausal age (Kuijper et al. 2013). Levels of estrogens in maternal serum and secreted in urine increase dramatically during pregnancy, but fall after delivery of the fetus (Kuijper et al. 2013). In this chapter, we will also consider how these powerful systemic increases in estrogens and progesterone, required to sustain pregnancy, can act as a mechanism to modulate the maternal immune system (Oertelt-Prigione 2012; Pennell et al. 2012; Robinson and Klein 2012; Menzies and Henriquez 2009; Druckmann and Druckmann 2005). While these hormonal changes occur to support pregnancy, they can have consequences for the pathogenesis of infections or autoimmune diseases.
2 The Impact of Seminal Fluid on the Uterine Environment
The delivery of spermatozoa to the female reproductive tract is aided by the seminal plasma in which they are found. Seminal plasma is much more than a transport medium, and it makes up 95–98% of semen contents (Rodriguez-Martinez et al. 2011), providing nourishment and protection for sperm, and containing a multitude of peptides and proteins that influence sperm function and prepare the uterine epithelia for pregnancy (Schjenken and Robertson 2020; Rodriguez-Martinez et al. 2011) through inducing changes in gene expression and cellular composition (Robertson 2005). Seminal plasma contains an array of biologically and immunologically active compounds including hormones, such as estrogen and progesterone as well as prostaglandin E2 and an array of cytokines (Hampl et al. 2013; Robertson 2005; Maegawa et al. 2002), including interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNFα), interleukins (IL)-1β, -6, -8, -10, -12 and TGF-β (Bromfield 2014). Most notably, the cytokine TGF-β, which is abundant within seminal plasma of both mice (Tremellen et al. 1998) and humans (Loras et al. 1999), is present in all three isoforms (TGF-β1, TGF-β2, TGF-β3) and can induce proinflammatory cytokine production by cervical cells (Sharkey et al. 2012a). Interestingly, studies have shown that levels of TGF-β in human seminal plasma fluctuate, with sexual abstinence impacting on content of TGF-β1 and TGF-β2 and TGF-β1 correlating inversely with age (Sharkey et al. 2016). These findings, along with the suggestion that other semen contents may also demonstrate patterns of fluctuation, have potential implications for semen analysis and understanding the content and quality of semen for assisted conception practices.
The presence of immunomodulatory molecules within seminal plasma is required for the success of pregnancy, whether it is via natural conception or assisted reproduction. Indeed, the importance of the inflammatory response to mating is demonstrated by studies showing that In Vitro Fertilisation (IVF) is more successful in women who are exposed to semen at the time of embryo transfer (Tremellen et al. 2000). Identifying the role of individual components of seminal plasma, and their role in immunomodulation of the uterus for pregnancy, is an area of fertility research which has gained considerable ground in recent years. For example, recently, it has been shown in an in vitro study, that expression of CD25 and IL-10 by female T cells is modulated by exposure to seminal plasma, with differences observed between seminal plasma donated from males who experience recurrent pregnancy losses and their control counterparts (du Fosse et al. 2022).
In humans, deposition of semen results in the influx of macrophages, dendritic cells (DCs), and T cells into the epithelial and stromal compartments of the ectocervix, accompanied by an increase in gene expression for the cytokines granulocyte macrophage colony stimulating factor (GM-CSF), IL-1α, IL-6 and the chemokine CXCL8 (Sharkey et al. 2012b). Macrophages and DCs remain within the stromal region of the cervix, while increases in T cells are found in both the epithelium and stroma, with a greater proportion of these being CD8+ than CD4+ (Sharkey et al. 2012b).
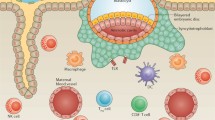
This immunomodulation of the uterine epithelium prepares the site for implantation, but also serves to prime the uterus and create a hospitable environment for the introduction of paternal antigens. After mating, there is a substantial increase in the cellularity of the uterine draining (para-aortic) lymph nodes (dLNs) in mice (Johansson et al. 2004), the main site of cross-presentation of paternal antigen (Moldenhauer et al. 2009). Recent studies have shown that in mice, the DC populations within the uterus and dLNs change significantly in the period between mating and implantation (Yasuda et al. 2020). Interestingly, these studies suggest that most of the DCs found within the uterus migrate to the site just prior to implantation. As summarised in Fig. 1, seminal plasma drives the activation and expansion of specific CD4+ and CD8+ T cells, with maternal Antigen-Presenting Cells (APCs) cross-presenting paternal antigens to CD8+ T cells in a TAP-dependent manner (Moldenhauer et al. 2009). Another T cell population considered to be important for the establishment of tolerance is T regulatory cells (Tregs) (Aluvihare et al. 2004). Studies in mice show that as the female approaches the estrus phase of the cycle, Tregs accumulate in the uterine horn, and this is accompanied by expression of T cell-specific chemokines CCL3, CCL4, CCL22 and CX3CL1 (Kallikourdis and Betz 2007). More specifically, studies have shown that CCL19 is likely to be a key recruiter of Tregs to the uterus (Guerin et al. 2011), and this is driven by seminal fluid (Shima et al. 2015; Kahn and Baltimore 2010; Robertson et al. 2009a) and priming by tolerogenic APCs in the uterus (Shima et al. 2020). While Treg populations increase both systemically and locally during pregnancy in humans, paternal-antigen-specific T cells have not yet been identified (Tsuda et al. 2019). The importance of Treg cells in maintaining an environment suitable for pregnancy, as it progresses, will be discussed in later sections.
Female response to seminal plasma. The uterine epithelium responds to immunomodulatory molecules (e.g. TGF-β, CXCL8, IFN-γ, Prostaglandin-E) within seminal plasma by producing T regulatory-attracting chemokines. Dendritic cells cross present paternal antigens to CD8+ T cells within the para-aortic lymph nodes, which drain the uterus, and are known to increase in cellularity post-mating. These mechanisms serve to prepare the maternal immune system for the development of a semiallogeneic fetus (du Fosse et al. 2022; Guerin et al. 2011; Robertson et al. 2009a, b; Kallikourdis and Betz 2007; Aluvihare et al. 2004)
3 Maternal Rejection of Semen
The importance of the immunomodulation induced by seminal plasma within the female uterus is highlighted by consideration of the consequences of the rejection of semen. Immune infertility is estimated to impact 20% of couples of reproductive age (Brazdova et al. 2016) with many potential underlying causes, affecting both male and female reproductive systems. One such cause impacting fertility is the production of anti-sperm antibodies (ASAs) which can cause infertility (Brazdova et al. 2016). Indeed, fertile women (and men) are also thought to produce ASAs as a normal physiological process; however, this does not necessarily lead to infertility (Vickram et al. 2019). In some women, for unknown reasons, there can be a failure of tolerance to the complex antigenic milieu of the sperm, leading to pathological production of ASAs and therefore fertility issues. Pathological ASA production by women impacts on several aspects of sperm function. Incubation of ASA from infertile women with normal sperm for 1 h in vitro leads to a reduction in sperm motility, viability and membrane integrity (Pujianto et al. 2018). The negative impact of ASA on pregnancy is sufficient that recent studies are now considering the potential of ASAs to create vaccines that can be utilised as a form of contraception (Vickram et al. 2019; Baldeon-Vaca et al. 2021).
4 Mechanisms Involved in Preventing Fetal Rejection
4.1 What Is the Implantation Window?
In humans, implantation occurs approximately 8–10 days post-ovulation (Wilcox et al. 1998), with the chance of conception approximately 30% per cycle (Zinaman et al. 1996). With such a low chance of success, it is unsurprising that around 75% of early losses are attributed to implantation failure (Wilcox et al. 1998). Indeed, it can be considered that early pregnancy loss (before 10 weeks’ gestation) is the default consequence of fertilisation (Annual Capri Workshop 2020). As advances in reproductive technology have developed, so too has our understanding of the underlying molecular, biochemical and immunological mechanisms associated with successful pregnancy outcomes and our appreciation that uterine and embryological and factors equally contribute to success.
The uterine endometrium goes through a remodelling process of decidualization to become receptive to the blastocyst, creating a period of 48 h within the luteal phase of the ovarian cycle known as the “implantation window”. What is interesting to note is that decidualization occurs irrespective of the presence of a blastocyst. During decidualization, endometrial fibroblast-like stromal cells change to become larger, more rounded decidual stromal cells (DSCs) (Dunn et al. 2003), which can be characterised by their secretion of prolactin (Telgmann and Gellersen 1998; Wu et al. 1995; Daly et al. 1983), insulin-like growth factor binding protein-1 (Matsumoto et al. 2008) and tissue factor (Lockwood et al. 1993) as well as expression of transcription factors such as Fox01 (Adiguzel and Celik-Ozenci 2021). The timing of the expression of these molecules is also crucial, as exemplified by studies showing that premature expression of prolactin is associated with repeated implantation failure (Berkhout et al. 2020). Adequate decidualization is a critical point for the fate of the pregnancy. Either decidualization continues in preparation for blastocyst implantation, or it breaks down to begin menstruation. Creation of the environment in preparation for the implantation window is dependent on a number of morphological, biochemical and hormonal changes (Aghajanova et al. 2008; Ng et al. 2020), but is also influenced by the immune environment (Murata et al. 2021).
4.2 Implantation as an Immunological Event
Implantation requires inflammation as a normal adaptation, involving various immune cells and mediators to support the preparation of the endometrial lining for receiving the blastocyst (Sehring et al. 2022). Contrary to this, excessive or aberrant inflammation can lead to implantation failure or miscarriage (Pantos et al. 2022). To understand the role of inflammation during implantation, it is first necessary to consider the presence of immune cells in the non-pregnant uterus.
4.3 Macrophages During Implantation
Macrophages are mononuclear phagocytic cells which are key players of the innate immune system. Macrophages constitute approximately 20% of the total number of decidual leukocytes at the maternal–fetal interface (Zhang et al. 2017; Ning et al. 2016), playing a number of key roles at implantation. In addition, these cells are highly responsive to the female sex hormones, adapting their role and distribution accordingly even in the non-pregnant state (De and Wood 1990; Ning et al. 2016). Both progesterone and estrogen module the inflammatory activities of macrophages (Jones et al. 2008; Menzies et al. 2011; Liu and Wang 2013).
At implantation, macrophage numbers increase within the decidua to aid in the clearance of cellular debris (Abrahams et al. 2004) and degradation of artefacts present after mating (De et al. 1991). Murine studies utilising a macrophage depletion model have confirmed the necessity for these cells at implantation through disruption of progesterone production by the corpus luteum (Care et al. 2013). Subsequent studies have shown that during the implantation period, macrophages of the M2 subtype are present in the stromal region and in close proximity to the lumen and glands (Ono et al. 2020).
Beyond implantation, macrophages are also found within the placenta as two distinct populations: decidual macrophages and Hofbauer cells (Mezouar et al. 2021) with the ability to isolate and study these independent cell types only recently becoming a possibility (Lasch et al. 2022). Classification of placental macrophages as M1 or M2 is proving somewhat more difficult for researchers to ascertain; however, studies suggest that first and early second trimester macrophages resemble M1-types macrophages (Zhang et al. 2017) and late second and third trimester macrophages resemble the M2-type (Gustafsson et al. 2008). CD14+ macrophages within the term placenta are of both maternal (30%) and fetal (70%) origin (Mezouar et al. 2019) and exhibit characteristics of M1 macrophages (Mezouar et al. 2021; Gustafsson et al. 2008).
4.4 Tregs as Master Regulators Within the Pregnant Uterus
Tregs have been the subject of much study and review in both humans and mice over the past 15 or so years and are considered to be pregnancy-protective (Muralidhara et al. 2022; Tsuda et al. 2021, 2019; Krop et al. 2020; Shigeta et al. 2020; Zhang and Sun 2020; Jorgensen et al. 2019; La Rocca et al. 2014; Teles et al. 2013; Leber et al. 2010; Zenclussen et al. 2010; Guerin et al. 2009; Aluvihare et al. 2004). As mentioned earlier, Tregs accumulate within the endometrium during the pre-implantation period (Kallikourdis and Betz 2007) and offer a protective role at the implantation site. Murine studies of allogeneic and syngeneic matings demonstrate Tregs which become activated against self-antigens early after embryo implantation to create a tolerant environment (Chen et al. 2013). Fetal-antigen-specific Treg cells are not present at embryo implantation; however, their number increases as pregnancy progresses (Rowe et al. 2012). Decidual Tregs increase with advancing gestation (Somerset et al. 2004), and there is preferential recruitment of fetus-specific Treg cells from the maternal blood to the decidua (Tilburgs et al. 2008). Interestingly, decidual Treg cell numbers are reduced in pregnant women with pre-eclampsia compared to levels found in normal pregnant women (Quinn et al. 2011). This suggests a key role for Treg cells in regulation of the maternal–fetal interface and is now a developing area of research (Robertson et al. 2019).
This leads to the question as to how Tregs cells regulate the immune response during pregnancy? Treg cells modulate the activities of T cells. Culturing T cells in the presence of medium conditioned by placental trophoblasts demonstrated a skewing towards the production of Th2-associated transcription factors and cytokines and inhibition of those associated with Th1 and Th17 cells (Liu et al. 2011). Traditionally, pregnancy was considered a Th2-associated phenomenon, with the placenta and uterine environment skewed towards an abundance of Th2-type cytokines (Lin et al. 1993; Wegmann et al. 1993); however, this well-accepted paradigm has now been amended to include Th17 and Tregs (Saito et al. 2010). Indeed, the balance between Th17 cells and Treg cells is critical for the outcome of pregnancy in humans. During healthy pregnancy, the ratio of circulating Treg cells to Th17 cells is increased significantly compared to non-pregnant controls; however, in pre-eclamptic women, this skewing away from Th17 cells is not observed (Santner-Nanan et al. 2009).
4.5 The Specialities of Uterine Natural Killer (NK) Cells
Natural Killer (NK) cells are a type of innate lymphoid cell (ILC) crucial in the early innate defences against various pathogens, viruses in particular. In humans, two main populations of blood, or peripheral, NK cells can be defined based on expression of the surface molecule CD56. About 90% of peripheral NK cells express relatively low levels of CD56 and are defined as CD56dim, and these cells are characterised by a high level of spontaneous lytic activity (Dosiou and Giudice 2005). Contrary to this, CD56bright NK cells, so called as they express relatively high levels of CD56, have little lytic activity. A third type of NK cells can be identified, unique to the uterus. The phenotype and regulation of uterine NK (uNK) cells within the pregnant uterine environment are crucial for pregnancy success (Faas and de Vos 2017; Giuliani et al. 2014; Manaster and Mandelboim 2010; Vacca et al. 2013, 2011). This is demonstrated by the link between recurrent pregnancy loss and dysregulation between uNK cells and peripheral NK cells (Mahajan et al. 2022; Giuliani et al. 2014; Tang et al. 2011).
uNK cells differ from both of the peripheral NK phenotypes, CD56dim and CD56bright, but have more in common with this latter phenotype, including weak lytic activity and lack of expression of FCγRIII (CD16) (Manaster and Mandelboim 2010). Binding of IgG to CD16 results in activation of antibody-dependent cell-mediated cytotoxicity (ADCC) by NK cells (Bryceson et al. 2006), and so the absence of CD16 on uNK cells demonstrates another tolerogenic feature of these cells. Endometrial uNK cells are fairly inactive in the non-pregnant uterus (Manaster et al. 2008). uNK cells constitute 50–70% of immune cells within first trimester decidua near to the blastocyte implantation site (Manaster and Mandelboim 2010; Bulmer et al. 2010; Mahajan et al. 2022). These decidual uNK cells still express many activating NK receptors, but rather than inducing cytotoxicity and potentially a reaction towards the fetally derived cells, these uNK cells act to support trophoblast invasion and angiogenesis (Hanna et al. 2006) as well as regulation of Th17 cell function at the fetal–maternal interface (Fu et al. 2013).
4.6 Cytokines and Chemokines in the Pregnant Uterus
So far, we have considered some of the key immune cells which are involved in the tolerance of fetally derived cells during pregnancy. The trafficking of cells and their actions are influenced by key chemokines and cytokines which may be produced by these cells or act on these cells. With regard to the uterus during pregnancy, there are a few cytokines, namely LIF and IL-15, which stand out as having key roles in modulating the immune environment.
The non-pregnant human endometrium of non-pregnant humans expresses several chemokine receptors (e.g. CXCR1, CXCR2, CCR5, CXCR4, CCR2, CCR3, CXCR3 and CX3CR1) and produces a number of chemokines during the implantation window (e.g. CCL2, CCL4, CCL5, CCL7, CCL11, CCL14, CCL16, CCL21, CCL22, CXCL8, CX3CL1), all of which have been extensively reviewed within the literature (Ramhorst et al. 2016; Du et al. 2014; Park and Yang 2011; Red-Horse et al. 2004, 2001). Many of these chemokines are involved in the trafficking of immune cells, including macrophages, Tregs and uNK cells to the decidua. It has been suggested that chemokines and their receptors may also play other significant roles during the establishment of pregnancy, with receptors found on cells of the blastocyst and extravillous trophoblasts (Dominguez et al. 2003a, b; Dimitriadis et al. 2005; Hannan and Salamonsen 2008, 2007; Hannan et al. 2006). As will be discussed later, chemokines and their receptors have also been extensively studied for their roles in driving inflammation at the other end of the gestational period—at the point of labour.
One of the most characterised factors involved in implantation is Leukemia Inhibitory Factor (LIF), and its role during pregnancy has been extensively reviewed in the literature (Pantos et al. 2022; Li et al. 2020; Winship et al. 2015; Moberg et al. 2015). LIF is a highly glycosylated 40–50 kDa glycoprotein that functions as a pleiotropic anti-inflammatory cytokine, part of the IL-6 family. Studies using LIF-deficient mice have shown it that is essential for successful implantation (Stewart et al. 1992) through binding the LIF-receptor (LIFR) and inducing STAT3 activation (Suman et al. 2013; Poehlmann et al. 2005) as well as inducing both autocrine and paracrine signalling pathways in the endometrium to facilitate implantation (Cullinan et al. 1996; Dominguez et al. 2002). LIF is expressed during the mid-late phase of the menstrual cycle and early stages of pregnancy, in both the glandular and luminal epitheliums (Pantos et al. 2022; Markert et al. 2011). The importance of LIF in this scenario is demonstrated by studies showing that women diagnosed with recurrent implantation failure have significantly less LIF in their endometrial glandular epithelium than normal pregnant women (Mariee et al. 2012). By contrast, it was found that women with recurrent implantation failure have higher levels of the cytokine IL-15 in the stroma (Mariee et al. 2012) and in the placenta (Toth et al. 2010) than in control women with normal pregnancies. IL-15 is a 14–15 kDa cytokine that is a member of the IL-2 family of cytokines. IL-15 has the ability to influence both innate and adaptive arms of the immune response, by stimulating NK cells, T cells and NKT cells. IL-15 transcripts are strongly expressed during the secretory, or luteal, phase of the cycle in humans a time in which a large number of NK cells are present within the uterus (Gordon 2021; Chegini et al. 2003; Kitaya et al. 2000). IL-15 is important for the differentiation of uNK cells within the endometrium during regular regeneration activities and during pregnancy (Strunz et al. 2021), but the placenta has low expression of IL-15 in the early development stages, but this gradually increases throughout pregnancy, with a peak in levels at the point of labour (Gordon 2021). Furthermore, placental explant studies have shown that IL-15 production is significantly lower in pre-eclamptic placenta samples than healthy term placentas (Agarwal et al. 2001).
5 Altered Human Leukocyte Antigen Expression by Trophoblasts
The placenta is an organ unique to pregnancy and crucial for its success. The process of placentation begins when the blastocyst implants into the decidua, with extravillous trophoblasts (EVT) making their way into the uterine wall. A number of pregnancy complications, such as placenta accrete, as serious condition when the placenta grows too far into the uterine wall, and recurrent spontaneous abortions, have clear links with dysregulation of trophoblast invasion (Illsley et al. 2020; Moser et al. 2018). Given the invasive nature of this process, tight regulation is required in order to prevent attack of the trophoblasts by maternal leukocytes.
What mechanisms are in place to prevent attack of these fetally derived, invasive cells? Interestingly, EVT cells have a unique expression of Human Leukocyte Antigen (HLA). EVTs do not express the polymorphic MHC class I molecules HLA-A and HLA-B or the class II HLA-D molecules, but do display the class I molecules HLA-C, HLA-E, HLA-F and HLA-G (Apps et al. 2009; Hackmon et al. 2017). Expression patterns are summarised in Table 2.
HLA-C, HLA-E and HLA-F expression by EVTs provides a mechanism of protection for the fetus through modulation of activities of maternal NK cells (Papuchova et al. 2019). HLA-C can bind the NK cell inhibitory receptor KIR, thereby acting to “switch off” these cytotoxic cells which may react against fetally derived antigens (Sharkey et al. 2008). HLA-C is thought to play a key role in early implantation through controlling the depth of invasion of trophoblasts (Hackmon et al. 2017). In addition, maternal T cells may specifically recognise fetal HLA-C on trophoblast cells, and that this activation promotes Treg differentiation (Tilburgs et al. 2009). The surface expression of HLA-C is known to be regulated at a transcriptional and post-transcriptional level (Papuchova et al. 2019).
HLA-E is expressed by EVTs and this has been shown to be very much restricted to first trimester (Hackmon et al. 2017). This molecule regularly forms complexes with HLA-G, and together, these mediate the inhibitory actions of uNK cells (King et al. 2000a). HLA-G is the most studied of the placenta-associated HLA molecules, with seven isoforms identified: HLA-G1, HLA-G2, HLA-G3, HLA-G4 being membrane bound and HLA-G5, HLA-G6, HLA-G7 being soluble (Hviid 2006; Hunt and Langat 2009). The trophoblast-bound molecules have roles in binding inhibitory NK cell receptors to limit their action and also to control the degree of trophoblast invasion into the uterine wall. The soluble forms have been associated with modulation of T cell function through impairment of expression chemokine receptors (Morandi et al. 2010). The function of HLA-F has only been more recently explored, and it has been found that it is found on the cell surface of actively migrating EVTs and this supports their specific role in early EVT invasion and interactions with uNK cells (Hackmon et al. 2017).
6 Immunomodulation by Placental Exosomes
Exosomes are defined as membrane-bound extracellular vesicles (EVs) with a size of ~ 40–160 nm in diameter, originating from the endosome of a cell (Kalluri and LeBleu 2020). Interest in the immunomodulatory functions of exosomes has grown significantly in recent years, and studies into the impact of exosomes derived from the placenta have also created much interest. In fact, the identification of placental EVs was reported as far back as 1998 (Knight et al. 1998). Exosomes derived from the placenta have been associated with crucial immunomodulatory functions for the successful maintenance of pregnancy (Bai et al. 2021; Nair and Salomon 2018; Tong et al. 2018).
Throughout pregnancy, the placenta continually sheds EVs into the maternal bloodstream, with the syncytiotrophoblast layer being the main source of these (Salomon et al. 2017; Tannetta et al. 2017; Dragovic et al. 2015). Placental EVs contribute to the modulation of the maternal immune environment for the protection of the fetus, by altering the actions of immune cells. For example, activation of the potentially cytotoxic NK cells through the NKG2D activating receptor is reduced by the binding to NKG2D ligands on placental EVs (Hedlund et al. 2009). More recently, it has been shown that placental EVs can secrete HLA-E, promoting the secretion of IFN-γ and VEGF by decidual NK cells (Jiang et al. 2021) at implantation. Indeed, placental EVs increase significantly through the first trimester (Sarker et al. 2014; Kshirsagar et al. 2012). In addition, placental EVs can modulate the differentiation, activation and subtype polarisation of decidual macrophages to favour pregnancy (Aldo et al. 2014). One way in which this can occur is through driving the induction of the proinflammatory cytokine IL-1β from macrophages (Atay et al. 2011). Studies have shown that placental EVs lead to downmodulation of T cell activity, and thereby protection of pregnancy, through inhibiting T cell proliferation and cytotoxicity, driving Treg differentiation and T cell apoptosis (Stenqvist et al. 2013).
7 The Role of the Maternal Immune System During Parturition and Post-partum Involution
The initiation of labour, or parturition, in humans is complex, and the underlying mechanisms are not yet fully understood; however, many pathways have been considered such as the activation of the hypothalamic–pituitary–adrenal axis, prostaglandin production, an increase in the responsiveness to oxytocin and the functional withdrawal of progesterone (Smith 2007).
In addition to these physiological mechanisms, the infiltration of inflammatory cells and their mediators is now considered key in driving normal labour at term. Indeed, the impact of inflammation within the uterus on labour initiation has been eluded to for some time, with infection being responsible for 30–50% of cases of preterm birth (Goldenberg and Thompson 2003; Goldenberg et al. 2000; Romero et al. 2006). Normal labour is associated with an influx of inflammatory cells into the uterus, driven by an increase in chemokine and proinflammatory cytokine production at this site. Labour is associated with inflammation and this is characterised by an influx of macrophages, neutrophils and T cells to the myometrium and cervix (Osman et al. 2003; Thomson et al. 1999) along with an increase in the production of proinflammatory cytokines, including IL-1β, TNF-α and IL-6 (Osman et al. 2003; Young et al. 2002) and chemokines such as CXCL8 (IL-8), CCL2, CCL3 and CCL5 (El-Azzamy et al. 2017; Gomez-Lopez et al. 2010). Indeed, the utilisation of chemokine inhibitors to halt preterm birth has recently been investigated (Shynlova et al. 2021; Coleman et al. 2020). Until recently, it was considered that inflammation within the myometrium was a driver of labour induction; however, it is now recognised that it is more likely that inflammation is a consequence of labour induction, designed to prepare the uterus for post-partum remodelling (Singh et al. 2021, 2017).
Post-partum uterine repair and involution are a highly efficient process, required for the success of future pregnancies. Studies into the underlying molecular and immunological mechanisms of human post-partum repair have been slow to make it into the scientific literature, mainly due to the practical and ethical issues involved in collecting appropriate tissue samples. Much of what we do know about post-partum involution has come from other models of tissue repair (Paliulyte et al. 2017) use of mouse and rat models. Repair of the uterus requires extracellular matrix remodelling, proliferation, apoptosis and breakdown of collagen (Salamonsen 2003; Hsu et al. 2014).
8 Consequences of Pregnancy for the Immune System
8.1 Impact of Pregnancy Hormones on the Immune System
The female body significantly changes its hormonal balance for pregnancy maintenance. The sex hormones progesterone and estrogens can have many effects on the cells of the immune system (Wira et al. 2015; Menzies and Henriquez 2009; Robinson and Klein 2012) which can have wider implications. For example, pregnancy affects maternal autoimmune diseases and the outcome of infectious diseases.
The number and activation status of circulating leukocytes changes in the mother during normal pregnancy. There is an increase in the total leukocyte count during pregnancy, with an increase in the number of peripheral monoyctes and granulocytes (Belo et al. 2005; Luppi et al. 2002a, b), but a decrease in the number of lymphocytes (Castilla et al. 1989). In pregnant women, monocytes are activated, producing the proinflammatory cytokines IL-1β and IL-12 (Luppi et al. 2002a) but by comparison neutrophils appear to have reduced effector functions, with a reduction in chemotaxis and microbial killing ability (Kindzelskii et al. 2004; Crouch et al. 1995; El-Maallem and Fletcher 1980).
While the role of T cell subsets has been studied for some time and discussed earlier within this chapter, data about the impact of pregnancy on B cells are only just emerging in the literature. While B cells constitute 5–15% of circulating lymphocytes, the number of these changes significantly in response to pregnancy. Numbers of B cells generally fall in late pregnancy and the post-partum period (Lima et al. 2016). B cells are now characterised as different subsets, B1 and B2 B cells. B1 cells produce autoantibodies, which are detrimental to pregnancy, whereas B2 cells are responsible for production of asymmetric antibodies (IgG molecules with a modified Fab region), which can be protective for pregnancy (Muzzio et al. 2013; Gutierrez et al. 2005; Zenclussen et al. 2001; Barrientos et al. 2009). We previously discussed the importance of Tregs for the maintenance of pregnancy, but what about B regulatory cells (Bregs)? Much of what we know so far about Bregs has come from studies into autoimmunity, tolerance and cancer (Guzman-Genuino and Diener 2017) and is considered to be mediators of immunosuppression, so this would suggest a key role in pregnancy protection. Bregs are a major source of the immunosuppressive cytokine IL-10, and murine studies have shown that Bregs are required for pregnancy tolerance, with IL-10 being a main mediator of this (Rolle et al. 2013; Jensen et al. 2013).
8.2 Impact of Pregnancy on the Response to Infection
It is well established that generally, women exhibit much more vigorous humoral responses than men, with higher serum levels of IgG and total IgM (Eidinger and Garrett 1972; Giltay et al. 2000). Sex hormones play a critical role in modulating immune function, and the importance of understanding these influences is highlighted by consideration of the sex bias in the susceptibility to a number of infections. The same can be said for pregnancy, and it is well known that the altered immune environment leads to pregnant women being more susceptible to some infections.
An example of this is the fact that the pregnant women are more susceptible to the impacts of the protozoan parasite Toxoplasma gondii. The change in the uterine immune environment described within this chapter, that is the development of a Th2 dominant milieu, favours the transmission and replication of this parasite, which is normally controlled by a Th1-type response (Roberts et al. 1995). Transmission of the parasite leads to congenital toxoplasmosis (Gomez-Chavez et al. 2019; Singh 2016; Hampton 2015; Torgerson and Mastroiacovo 2013), which can have a range of detrimental outcomes for the fetus, dependent on the stage of pregnancy at which transmission occurs.
Due to the immunological modifications which occur systemically during pregnancy, it is therefore unsurprising that pregnant women are generally more susceptible to respiratory infections (Englund and Chu 2018), including COVID-19 (Forestieri et al. 2022) and influenza (Mertz et al. 2019), leading to a higher risk of hospitalisation.
8.3 Autoimmune Disease During Pregnancy
As well as infections, the sex and pregnancy-associated hormones have been shown to have a significant impact on the incidence and progression of autoimmune disease. With global ageing populations and increasing incidence of autoimmune disease, it may be necessary to explore personalised treatment options.
The significant improvement in symptoms experienced by Rheumatoid Arthritis (RA) patients during pregnancy allows us to hypothesise that treatment options based on pregnancy-associated immune modifications could be useful. Women are three times more likely to develop RA than men; however, approximately 75% of women with RA experience an improvement in their symptoms as a direct consequence of the systemic effects of pregnancy (Forger and Villiger 2020). This effect is not long-lasting, and within 3 months post-partum, typical RA symptoms return (Adams Waldorf and Nelson 2008). While much remains to be learned about the underlying mechanisms for the disease amelioration, recent studies have eluded to the downregulation of effector T cells, upregulation of Tregs and alterations to antibodies as potential reasons. More specifically, RA is associated with changes in the glycosylation of the IgG Fc region; however in pregnancy, it has been found that galactosylation of these immunoglobulins is related to the symptom improvement (Bondt et al. 2013, van de Geijn et al. 2009).
Multiple Sclerosis (MS) is characterised by the immune attack of the myelin sheath surrounding nerves. Generally, women are three times more likely to develop MS as men (Disanto and Ramagopalan 2013) with women more likely to carry the associated HLA DRB1 allele (Bove and Chitnis 2014); however, men are more likely to develop more progressive disease with poorer recovery after attacks. Similar to RA, pregnancy is associated with a reduction in the incidence of relapses, but a return of the condition in the months following delivery (Confavreux et al. 1998). Progesterone, estriol and estradiol may play an important role in the preventing relapses in sufferers of this condition (El-Etr et al. 2005; Kim et al. 1999). More recently, a Danish nationwide cohort study has shown no links between MS and recurrent pregnancy loss (Mikkelsen et al. 2022).
Another autoimmune disease where pregnancy impacts on the disease is systemic lupus erythematosus (SLE); however, unlike with RA and MS, symptoms are not improved with pregnancy, in fact in many cases symptoms worsen. Women with SLE are at a much greater risk of developing pregnancy complications and fetal loss (Moroni and Ponticelli 2016; Foocharoen et al. 2009; Kalok et al. 2019). SLE is defined immunologically by the production of autoantibodies against nuclear antigens (Crispin et al. 2010). More specifically, 45% of SLE patients exhibit autoantibodies to the estrogen receptor, ER-α, which interferes with T cell homeostasis (Colasanti et al. 2012).
9 Conclusion
As we learn more about the human immune system, we have to explore how these immune functions are modulated during pregnancy and in turn how this impacts on the ability to deal with infections and other immune-related diseases. In this chapter, we have explored the key mechanisms involved in protection of the fetus from the maternal immune system and eluded to how this immunomodulation can impact on other aspects of the mother’s health, namely, response to infection and autoimmune disease. Much remains to be determined about how we can apply this increased knowledge of the immune adaptations to pregnancy for clinical use, to find more efficient ways of predicting, diagnosing and treating both fertility issues and pregnancy complications.
References
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J (2014) The placenta harbors a unique microbiome. Sci Transl Med 6:237ra65
Abrahams VM, Kim YM, Straszewski SL, Romero R, Mor G (2004) Macrophages and apoptotic cell clearance during pregnancy. Am J Reprod Immunol 51:275–282
Adams Waldorf KM, Nelson JL (2008) Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest 37:631–644
Adiguzel D, Celik-Ozenci C (2021) FoxO1 is a cell-specific core transcription factor for endometrial remodeling and homeostasis during menstrual cycle and early pregnancy. Hum Reprod Update 27:570–583
Agarwal R, Loganath A, Roy AC, Wong YC, Ng SC (2001) Expression profiles of interleukin-15 in early and late gestational human placenta and in pre-eclamptic placenta. Mol Hum Reprod 7:97–101
Aghajanova L, Hamilton AE, Giudice LC (2008) Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol 19:204–211
Aldo PB, Racicot K, Craviero V, Guller S, Romero R, Mor G (2014) Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages. Am J Reprod Immunol 72:270–284
Aluvihare VR, Kallikourdis M, Betz AG (2004) Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5:266–271
Annual Capri Workshop G (2020) Correction to: early pregnancy loss: the default outcome for fertilized human oocytes. J Assist Reprod Genet 37:1065
Apps R, Gardner L, Hiby SE, Sharkey AM, Moffett A (2008) Conformation of human leucocyte antigen-C molecules at the surface of human trophoblast cells. Immunology 124:322–328
Apps R, Murphy SP, Fernando R, Gardner L, Ahad T, Moffett A (2009) Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127:26–39
Atay S, Gercel-Taylor C, Taylor DD (2011) Human trophoblast-derived exosomal fibronectin induces pro-inflammatory IL-1beta production by macrophages. Am J Reprod Immunol 66:259–269
Bai K, Li X, Zhong J, Ng EHY, Yeung WSB, Lee CL, Chiu PCN (2021) Placenta-derived exosomes as a modulator in maternal immune tolerance during pregnancy. Front Immunol 12:671093
Baldeon-Vaca G, Marathe JG, Politch JA, Mausser E, Pudney J, Doud J, Nador E, Zeitlin L, Pauly M, Moench TR, Brennan M, Whaley KJ, Anderson DJ (2021) Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine 69:103478
Bardos J, Fiorentino D, Longman RE, Paidas M (2019) Immunological role of the maternal uterine microbiome in pregnancy: pregnancies pathologies and alterated microbiota. Front Immunol 10:2823
Barrientos G, Fuchs D, Schrocksnadel K, Ruecke M, Garcia MG, Klapp BF, Raghupathy R, Miranda S, Arck PC, Blois SM (2009) Low levels of serum asymmetric antibodies as a marker of threatened pregnancy. J Reprod Immunol 79:201–210
Belo L, Santos-Silva A, Rocha S, Caslake M, Cooney J, Pereira-Leite L, Quintanilha A, Rebelo I (2005) Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur J Obstet Gynecol Reprod Biol 123:46–51
Berkhout RP, Lambalk CB, Repping S, Hamer G, Mastenbroek S (2020) Premature expression of the decidualization marker prolactin is associated with repeated implantation failure. Gynecol Endocrinol 36:360–364
Blaschitz A, Lenfant F, Mallet V, Hartmann M, Bensussan A, Geraghty DE, le Bouteiller P, Dohr G (1997) Endothelial cells in chorionic fetal vessels of first trimester placenta express HLA-G. Eur J Immunol 27:3380–3388
Blaser MJ, Devkota S, McCoy KD, Relman DA, Yassour M, Young VB (2021) Lessons learned from the prenatal microbiome controversy. Microbiome 9:8
Bondt A, Selman MH, Deelder AM, Hazes JM, Willemsen SP, Wuhrer M, Dolhain RJ (2013) Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res 12:4522–4531
Bove R, Chitnis T (2014) The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler 20:520–526
Brazdova A, Senechal H, Peltre G, Poncet P (2016) Immune aspects of female infertility. Int J Fertil Steril 10:1–10
Bromfield JJ (2014) Seminal fluid and reproduction: much more than previously thought. J Assist Reprod Genet 31:627–636
Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107:159–166
Bulmer JN, Williams PJ, Lash GE (2010) Immune cells in the placental bed. Int J Dev Biol 54:281–294
Bushman FD (2019) De-discovery of the placenta microbiome. Am J Obstet Gynecol 220:213–214
Cai YJ, Huang L, Leung TY, Burd A (2014) A study of the immune properties of human umbilical cord lining epithelial cells. Cytotherapy 16:631–639
Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA (2013) Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest 123:3472–3487
Castilla JA, Rueda R, Vargas ML, Gonzalez-Gomez F, Garcia-Olivares E (1989) Decreased levels of circulating CD4+ T lymphocytes during normal human pregnancy. J Reprod Immunol 15:103–111
Chegini N, Roberts M, Ripps B (2003) Differential expression of interleukins (IL)-13 and IL-15 in ectopic and eutopic endometrium of women with endometriosis and normal fertile women. Am J Reprod Immunol 49:75–83
Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, Xie H, Chen X, Zeng C, Wen B, Zeng L, Du H, Tang H, Xu C, Xia Y, Xia H, Yang H, Wang J, Wang J, Madsen L, Brix S, Kristiansen K, Xu X, Li J, Wu R, Jia H (2017) The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 8:875
Chen T, Darrasse-Jèze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, Klatzmann D (2013) Self-Specific Memory Regulatory T Cells Protect Embryos at Implantation in Mice. J Immunol 191(5):2273–2281
Colasanti T, Maselli A, Conti F, Sanchez M, Alessandri C, Barbati C, Vacirca D, Tinari A, Chiarotti F, Giovannetti A, Franconi F, Valesini G, Malorni W, Pierdominici M, Ortona E (2012) Autoantibodies to estrogen receptor alpha interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus. Arthritis Rheum 64:778–787
Coleman M, Orvis A, Wu TY, Dacanay M, Merillat S, Ogle J, Baldessari A, Kretzer NM, Munson J, Boros-Rausch AJ, Shynlova O, Lye S, Rajagopal L, Adams Waldorf KM (2020) A broad spectrum chemokine inhibitor prevents preterm labor but not microbial invasion of the amniotic cavity or neonatal morbidity in a non-human primate model. Front Immunol 11:770
Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S (2016) Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 6:23129
Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T (1998) Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in multiple sclerosis group. N Engl J Med 339:285–291
Crispin JC, Liossis SN, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang YT, Tsokos GC (2010) Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med 16:47–57
Crouch SP, Crocker IP, Fletcher J (1995) The effect of pregnancy on polymorphonuclear leukocyte function. J Immunol 155:5436–5443
Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL (1996) Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci U S A 93:3115–3120
Daly DC, Maslar IA, Riddick DH (1983) Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol 145:672–678
De M, Wood GW (1990) Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol 126:417–424
De M, Choudhuri R, Wood GW (1991) Determination of the number and distribution of macrophages, lymphocytes, and granulocytes in the mouse uterus from mating through implantation. J Leukoc Biol 50:252–262
de Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, Smith GCS (2019) Human placenta has no microbiome but can contain potential pathogens. Nature 572:329–334
Dimitriadis E, White CA, Jones RL, Salamonsen LA (2005) Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 11:613–630
Disanto G, Ramagopalan SV (2013) On the sex ratio of multiple sclerosis. Mult Scler 19:3–4
Dominguez F, Remohi J, Pellicer A, Simon C (2002) Paracrine interactions during human implantation. Rev Endocr Metab Disord 3:97–105
Dominguez F, Galan A, Martin JJ, Remohi J, Pellicer A, Simon C (2003a) Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Mol Hum Reprod 9:189–198
Dominguez F, Pellicer A, Simon C (2003b) The chemokine connection: hormonal and embryonic regulation at the human maternal-embryonic interface—a review. Placenta 24(Suppl B):S48–S55
Dosiou C, Giudice LC (2005) Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocr Rev 26:44–62
Dragovic RA, Collett GP, Hole P, Ferguson DJ, Redman CW, Sargent IL, Tannetta DS (2015) Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence nanoparticle tracking analysis. Methods 87:64–74
Druckmann R, Druckmann MA (2005) Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol 97:389–396
Du M-R, Wang S-C, Li D-J (2014) The integrative roles of chemokines at the maternal–fetal interface in early pregnancy. Cell Mol Immunol 11:438–448
du Fosse NA, Lashley E, Anholts JDH, van Beelen E, le Cessie S, van Lith JMM, Eikmans M, van der Hoorn MLP (2022) Impaired immunomodulatory effects of seminal plasma may play a role in unexplained recurrent pregnancy loss: results of an in vitro study. J Reprod Immunol 151:103500
Dunn CL, Kelly RW, Critchley HO (2003) Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod Biomed Online 7:151–161
Eidinger D, Garrett TJ (1972) Studies of the regulatory effects of the sex hormones on antibody formation and stem cell differentiation. J Exp Med 136:1098–1116
El-Azzamy H, Balogh A, Romero R, Xu Y, Lajeunesse C, Plazyo O, Xu Z, Price TG, Dong Z, Tarca AL, Papp Z, Hassan SS, Chaiworapongsa T, Kim CJ, Gomez-Lopez N, Than NG (2017) Characteristic changes in decidual gene expression signature in spontaneous term parturition. J Pathol Transl Med 51:264–283
El-Etr M, Vukusic S, Gignoux L, Durand-Dubief F, Achiti I, Baulieu EE, Confavreux C (2005) Steroid hormones in multiple sclerosis. J Neurol Sci 233:49–54
El-Maallem H, Fletcher J (1980) Impaired neutrophil function and myeloperoxidase deficiency in pregnancy. Br J Haematol 44:375–381
Englund JA, Chu HY (2018) Respiratory virus infection during pregnancy: does it matter? J Infect Dis 218:512–515
Faas MM, de Vos P (2017) Uterine NK cells and macrophages in pregnancy. Placenta 56:44–52
Foocharoen C, Nanagara R, Salang L, Suwannaroj S, Mahakkanukrauh A (2009) Pregnancy and disease outcome in patients with systemic lupus erythematosus (SLE): a study at Srinagarind Hospital. J Med Assoc Thai 92:167–174
Forestieri S, Marcialis MA, Migliore L, Panisi C, Fanos V (2022) Relationship between pregnancy and coronavirus: what we know. J Matern Fetal Neonatal Med 35:1997–2008
Forger F, Villiger PM (2020) Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat Rev Rheumatol 16:113–122
Fricke WF, Ravel J (2021) Microbiome or no microbiome: are we looking at the prenatal environment through the right lens? Microbiome 9:9
Fu B, Li X, Sun R, Tong X, Ling B, Tian Z, Wei H (2013) Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc Natl Acad Sci U S A 110:E231–E240
Giltay EJ, Fonk JC, von Blomberg BM, Drexhage HA, Schalkwijk C, Gooren LJ (2000) In vivo effects of sex steroids on lymphocyte responsiveness and immunoglobulin levels in humans. J Clin Endocrinol Metab 85:1648–1657
Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT (2014) Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 72:262–269
Goldenberg RL, Thompson C (2003) The infectious origins of stillbirth. Am J Obstet Gynecol 189:861–873
Goldenberg RL, Hauth JC, Andrews WW (2000) Intrauterine infection and preterm delivery. N Engl J Med 342:1500–1507
Gomez-Chavez F, Canedo-Solares I, Ortiz-Alegria LB, Flores-Garcia Y, Luna-Pasten H, Figueroa-Damian R, Mora-Gonzalez JC, Correa D (2019) Maternal immune response during pregnancy and vertical transmission in human toxoplasmosis. Front Immunol 10:285
Gomez-Lopez N, Laresgoiti-Servitje E, Olson DM, Estrada-Gutierrez G, Vadillo-Ortega F (2010) The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod 82:809–814
Gordon SM (2021). Interleukin-15 in outcomes of pregnancy. Int J Mol Sci 22
Guerin LR, Prins JR, Robertson SA (2009) Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update 15:517–535
Guerin LR, Moldenhauer LM, Prins JR, Bromfield JJ, Hayball JD, Robertson SA (2011) Seminal fluid regulates accumulation of FOXP3+ regulatory T cells in the preimplantation mouse uterus through expanding the FOXP3+ cell pool and CCL19-mediated recruitment. Biol Reprod 85:397–408
Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G, Sharma S, Buer J, Ernerudh J (2008) Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE 3:e2078
Gutierrez G, Gentile T, Miranda S, Margni RA (2005) Asymmetric antibodies: a protective arm in pregnancy. Chem Immunol Allergy 89:158–168
Guzman-Genuino RM, Diener KR (2017) Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol 8:172
Hackmon R, Pinnaduwage L, Zhang J, Lye SJ, Geraghty DE, Dunk CE (2017) Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am J Reprod Immunol 77
Hammer A, Hutter H, Blaschitz A, Mahnert W, Hartmann M, Uchanska-Ziegler B, Ziegler A, Dohr G (1997) Amnion epithelial cells, in contrast to trophoblast cells, express all classical HLA class I molecules together with HLA-G. Am J Reprod Immunol 37:161–171
Hampl R, Kubatova J, Heracek J, Sobotka V, Starka L (2013) Hormones and endocrine disruptors in human seminal plasma. Endocr Regul 47:149–158
Hampton MM (2015) Congenital toxoplasmosis: a review. Neonatal Netw 34:274–278
Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12:1065–1074
Hannan NJ, Salamonsen LA (2007) Role of chemokines in the endometrium and in embryo implantation. Curr Opin Obstet Gynecol 19:266–272
Hannan NJ, Salamonsen LA (2008) CX3CL1 and CCL14 regulate extracellular matrix and adhesion molecules in the trophoblast: potential roles in human embryo implantation. Biol Reprod 79:58–65
Hannan NJ, Jones RL, White CA, Salamonsen LA (2006) The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod 74:896–904
Hedlund M, Stenqvist AC, Nagaeva O, Kjellberg L, Wulff M, Baranov V, Mincheva-Nilsson L (2009) Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 183:340–351
Hsu KF, Pan HA, Hsu YY, Wu CM, Chung WJ, Huang SC (2014) Enhanced myometrial autophagy in postpartum uterine involution. Taiwan J Obstet Gynecol 53:293–302
Hunt JS, Langat DL (2009) HLA-G: a human pregnancy-related immunomodulator. Curr Opin Pharmacol 9:462–469
Hviid TV (2006) HLA-G in human reproduction: aspects of genetics, function and pregnancy complications. Hum Reprod Update 12:209–232
Illsley NP, Dasilva-Arnold SC, Zamudio S, Alvarez M, Al-Khan A (2020) Trophoblast invasion: lessons from abnormally invasive placenta (placenta accreta). Placenta 102:61–66
Ishitani A, Sageshima N, Lee N, Dorofeeva N, Hatake K, Marquardt H, Geraghty DE (2003) Protein expression and peptide binding suggest unique and interacting functional roles for HLA-E, F, and G in maternal-placental immune recognition. J Immunol 171:1376–1384
Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC (2013) Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol Reprod 89:90
Jiang L, Fei H, Jin X, Liu X, Yang C, Li C, Chen J, Yang A, Zhu J, Wang H, Fei X, Zhang S (2021) Extracellular vesicle-mediated secretion of HLA-E by trophoblasts maintains pregnancy by regulating the metabolism of decidual NK cells. Int J Biol Sci 17:4377–4395
Johansson M, Bromfield JJ, Jasper MJ, Robertson SA (2004) Semen activates the female immune response during early pregnancy in mice. Immunology 112:290–300
Jones LA, Anthony JP, Henriquez FL, Lyons RE, Nickdel MB, Carter KC, Alexander J, Roberts CW (2008) Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology 125:59–69
Jorgensen N, Persson G, Hviid TVF (2019) The tolerogenic function of regulatory T cells in pregnancy and cancer. Front Immunol 10:911
Kahn DA, Baltimore D (2010) Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A 107:9299–9304
Kallikourdis M, Betz AG (2007) Periodic accumulation of regulatory T cells in the uterus: preparation for the implantation of a semi-allogeneic fetus? PLoS ONE 2:e382
Kalluri R, Lebleu VS (2020) The biology, function, and biomedical applications of exosomes. Science 367
Kalok A, Abdul Cader R, Indirayani I, Abdul Karim AK, Shah SA, Mohamed Ismail NA, Omar MH, Shafiee MN (2019) Pregnancy outcomes in systemic lupus erythematosus (SLE) women. Horm Mol Biol Clin Investig 40
Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR (1999) Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology 52:1230–1238
Kindzelskii AL, Ueki T, Michibata H, Chaiworapongsa T, Romero R, Petty HR (2004) 6-Phosphogluconate dehydrogenase and glucose-6-phosphate dehydrogenase form a supramolecular complex in human neutrophils that undergoes retrograde trafficking during pregnancy. J Immunol 172:6373–6381
King A, Allan DS, Bowen M, Powis SJ, Joseph S, Verma S, Hiby SE, Mcmichael AJ, Loke YW, Braud VM (2000a) HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur J Immunol 30:1623–1631
King A, Burrows TD, Hiby SE, Bowen JM, Joseph S, Verma S, Lim PB, Gardner L, Le Bouteiller P, Ziegler A, Uchanska-Ziegler B, Loke YW (2000b) Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta 21:376–387
Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H (2000) IL-15 expression at human endometrium and decidua. Biol Reprod 63:683–687
Knight M, Redman CW, Linton EA, Sargent IL (1998) Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol 105:632–640
Krop J, Heidt S, Claas FHJ, Eikmans M (2020) Regulatory T cells in pregnancy: it is not all about FoxP3. Front Immunol 11:1182
Kshirsagar SK, Alam SM, Jasti S, Hodes H, Nauser T, Gilliam M, Billstrand C, Hunt JS, Petroff MG (2012) Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 33:982–990
Kuijper EA, Ket JC, Caanen MR, Lambalk CB (2013) Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 27:33–63
La Rocca C, Carbone F, Longobardi S, Matarese G (2014) The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett 162:41–48
Lasch M, Sudan K, Paul C, Schulz C, Kolben T, Dorp JV, Eren S, Beyer S, Siniscalchi L, Mahner S, Jeschke U, Meister S (2022) Isolation of decidual macrophages and Hofbauer cells from term placenta-comparison of the expression of CD163 and CD80. Int J Mol Sci 23
Lauder AP, Roche AM, Sherrill-Mix S, Bailey A, Laughlin AL, Bittinger K, Leite R, Elovitz MA, Parry S, Bushman FD (2016) Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4:29
Leber A, Teles A, Zenclussen AC (2010) Regulatory T cells and their role in pregnancy. Am J Reprod Immunol 63:445–459
Leiby JS, McCormick K, Sherrill-Mix S, Clarke EL, Kessler LR, Taylor LJ, Hofstaedter CE, Roche AM, Mattei LM, Bittinger K, Elovitz MA, Leite R, Parry S, Bushman FD (2018) Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6:196
Li Z, Li R, Li X, Dai H, Han X, Wang X, Yang A (2020) LIF in embryo culture medium is a predictive marker for clinical pregnancy following IVF-ET of patients with fallopian tube obstruction. J Reprod Immunol 141:103164
Lim ES, Rodriguez C, Holtz LR (2018) Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome 6:87
Lima J, Martins C, Leandro MJ, Nunes G, Sousa MJ, Branco JC, Borrego LM (2016) Characterization of B cells in healthy pregnant women from late pregnancy to post-partum: a prospective observational study. BMC Pregnancy Childbirth 16:139
Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG (1993) Synthesis of T-helper 2-type cytokines at the maternal-fetal interface. J Immunol 151:4562–4573
Liu L, Wang Z (2013) Estrogen attenuates lipopolysaccharide-induced nitric oxide production in macrophages partially via the nongenomic pathway. Cell Immunol 286:53–58
Liu F, Guo J, Tian T, Wang H, Dong F, Huang H, Dong M (2011) Placental trophoblasts shifted Th1/Th2 balance toward Th2 and inhibited Th17 immunity at fetomaternal interface. APMIS 119:597–604
Lockwood CJ, Nemerson Y, Guller S, Krikun G, Alvarez M, Hausknecht V, Gurpide E, Schatz F (1993) Progestational regulation of human endometrial stromal cell tissue factor expression during decidualization. J Clin Endocrinol Metab 76:231–236
Loras B, Vetele F, el Malki A, Rollet J, Soufir JC, Benahmed M (1999) Seminal transforming growth factor-beta in normal and infertile men. Hum Reprod 14:1534–1539
Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, Deloia JA (2002a) Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol 72:874–884
Luppi P, Haluszczak C, Trucco M, Deloia JA (2002b) Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol 47:72–81
Maegawa M, Kamada M, Irahara M, Yamamoto S, Yoshikawa S, Kasai Y, Ohmoto Y, Gima H, Thaler CJ, Aono T (2002) A repertoire of cytokines in human seminal plasma. J Reprod Immunol 54:33–42
Mahajan D, Sharma NR, Kancharla S, Kolli P, Tripathy A, Sharma AK, Singh S, Kumar S, Mohanty AK Jena MK (2022) Role of Natural Killer cells during pregnancy and related complications. Biomolecules 12
Manaster I, Mandelboim O (2010) The unique properties of uterine NK cells. Am J Reprod Immunol 63:434–444
Manaster I, Mizrahi S, Goldman-Wohl D, Sela HY, Stern-Ginossar N, Lankry D, Gruda R, Hurwitz A, Bdolah Y, Haimov-Kochman R, Yagel S, Mandelboim O (2008) Endometrial NK cells are special immature cells that await pregnancy. J Immunol 181:1869–1876
Mariee N, Li TC, Laird SM (2012) Expression of leukaemia inhibitory factor and interleukin 15 in endometrium of women with recurrent implantation failure after IVF; correlation with the number of endometrial natural killer cells. Hum Reprod 27:1946–1954
Markert UR, Morales-Prieto DM, Fitzgerald JS (2011) Understanding the link between the IL-6 cytokine family and pregnancy: implications for future therapeutics. Expert Rev Clin Immunol 7:603–609
Matsumoto H, Sakai K, Iwashita M (2008) Insulin-like growth factor binding protein-1 induces decidualization of human endometrial stromal cells via alpha5beta1 integrin. Mol Hum Reprod 14:485–489
Menzies FM, Henriquez FL (2009) Immunomodulation by the female sex hormones. Open Infect Dis J 3:61–72
Menzies FM, Henriquez FL, Alexander J, Roberts CW (2011) Selective inhibition and augmentation of alternative macrophage activation by progesterone. Immunology 134:281–291
Mertz D, Lo CK, Lytvyn L, Ortiz JR, Loeb M, Flurisk I (2019) Pregnancy as a risk factor for severe influenza infection: an individual participant data meta-analysis. BMC Infect Dis 19:683
Mezouar S, Ben Amara A, Chartier C, Gorvel L, Mege JL (2019) A fast and reliable method to isolate human placental macrophages. Curr Protoc Immunol 125:e77
Mezouar S, Katsogiannou M, Ben Amara A, Bretelle F, Mege JL (2021) Placental macrophages: origin, heterogeneity, function and role in pregnancy-associated infections. Placenta 103:94–103
Mikkelsen AP, Egerup P, Kolte AM, Westergaard D, Nielsen HS, Lidegaard O (2022) Pregnancy loss and risk of multiple sclerosis and autoimmune neurological disorder: a nationwide cohort study. PLoS ONE 17:e0266203
Moberg C, Bourlev V, Ilyasova N, Olovsson M (2015) Endometrial expression of LIF and its receptor and peritoneal fluid levels of IL-1alpha and IL-6 in women with endometriosis are associated with the probability of pregnancy. Arch Gynecol Obstet 292:429–437
Moldenhauer LM, Diener KR, Thring DM, Brown MP, Hayball JD, Robertson SA (2009) Cross-presentation of male seminal fluid antigens elicits T cell activation to initiate the female immune response to pregnancy. J Immunol 182:8080–8093
Monin L, Whettlock EM, Male V (2020) Immune responses in the human female reproductive tract. Immunology 160:106–115
Morandi F, Ferretti E, Bocca P, Prigione I, Raffaghello L, Pistoia V (2010) A novel mechanism of soluble HLA-G mediated immune modulation: downregulation of T cell chemokine receptor expression and impairment of chemotaxis. PLoS ONE 5:e11763
Moreno I, Codoner FM, Vilella F, Valbuena D, Martinez-Blanch JF, Jimenez-Almazan J, Alonso R, Alama P, Remohi J, Pellicer A, Ramon D, Simon C (2016) Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 215:684–703
Moroni G, Ponticelli C (2016) Pregnancy in women with systemic lupus erythematosus (SLE). Eur J Intern Med 32:7–12
Moser G, Windsperger K, Pollheimer J, De Sousa Lopes SC, Huppertz B (2018) Human trophoblast invasion: new and unexpected routes and functions. Histochem Cell Biol 150:361–370
Muralidhara P, Sood V, Vinayak Ashok V, Bansal K (2022) Pregnancy and tumour: the parallels and differences in regulatory T cells. Front Immunol 13:866937
Murata H, Tanaka S, Okada H (2021) Immune tolerance of the human decidua. J Clin Med 10
Muzzio D, Zenclussen AC, Jensen F (2013) The role of B cells in pregnancy: the good and the bad. Am J Reprod Immunol 69:408–412
Nair S, Salomon C (2018) Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin Immunopathol 40:425–437
Ng SW, Norwitz GA, Pavlicev M, Tilburgs T, Simon C, Norwitz ER (2020) Endometrial decidualization: the primary driver of pregnancy health. Int J Mol Sci 21
Ning F, Liu H, Lash GE (2016) The role of decidual macrophages during normal and pathological pregnancy. Am J Reprod Immunol 75:298–309
Oertelt-Prigione S (2012) The influence of sex and gender on the immune response. Autoimmun Rev 11:A479–A485
Ono Y, Yoshino O, Hiraoka T, Sato E, Fukui Y, Ushijima A, Nawaz A, Hirota Y, Wada S, Tobe K, Nakashima A, Osuga Y, Saito S (2020) CD206+ M2-like macrophages are essential for successful implantation. Front Immunol 11:557184
Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE (2003) Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9:41–45
Paliulyte V, Drasutiene GS, Ramasauskaite D, Bartkeviciene D, Zakareviciene J, Kurmanavicius J (2017) Physiological uterine involution in primiparous and multiparous women: ultrasound study. Obstet Gynecol Int 2017:6739345
Pantos K, Grigoriadis S, Maziotis E, Pistola K, Xystra P, Pantou A, Kokkali G, Pappas A, Lambropoulou M, Sfakianoudis K, Simopoulou M (2022) The role of interleukins in recurrent implantation failure: a comprehensive review of the literature. Int J Mol Sci 23
Papuchova H, Meissner TB, Li Q, Strominger JL, Tilburgs T (2019) The dual role of HLA-C in tolerance and immunity at the maternal-fetal interface. Front Immunol 10:2730
Park DW, Yang KM (2011) Hormonal regulation of uterine chemokines and immune cells. Clin Exp Reprod Med 38:179–185
Pennell LM, Galligan CL, Fish EN (2012) Sex affects immunity. J Autoimmun 38:J282–J291
Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J (2017) A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5:48
Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, Markert UR (2005) Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta 26(Suppl A):S37–S41
Prince AL, Ma J, Kannan PS, Alvarez M, Gisslen T, Harris RA, Sweeney EL, Knox CL, Lambers DS, Jobe AH, Chougnet CA, Kallapur SG, Aagaard KM (2016) The placental membrane microbiome is altered among subjects with spontaneous preterm birth with and without chorioamnionitis. Am J Obstet Gynecol, 214:627e1–627e16
Pujianto D, Hajizah H, Mansur I, Amarudin A (2018) Antisperm antibodies disrupt plasma membrane integrity and inhibit tyrosine phosphorylation in human spermatozoa. Med J Indonesia 27:3–11
Quinn KH, Lacoursiere DY, Cui L, Bui J, Parast MM (2011) The unique pathophysiology of early-onset severe preeclampsia: role of decidual T regulatory cells. J Reprod Immunol 91:76–82
Ramhorst R, Grasso E, Paparini D, Hauk V, Gallino L, Calo G, Vota D, Perez Leiros C (2016) Decoding the chemokine network that links leukocytes with decidual cells and the trophoblast during early implantation. Cell Adh Migr 10:197–207
Red-Horse K, Drake PM, Gunn MD, Fisher SJ (2001) Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol 159:2199–2213
Red-Horse K, Drake PM, Fisher SJ (2004) Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med 6:1–14
Roberts CW, Cruickshank SM, Alexander J (1995) Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun 63:2549–2555
Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS (2009a) Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 80:1036–1045
Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD (2009b) Activating T regulatory cells for tolerance in early pregnancy—the contribution of seminal fluid. J Reprod Immunol 83:109–116
Robertson SA, Green ES, Care AS, Moldenhauer LM, Prins JR, Hull ML, Barry SC, Dekker G (2019) Therapeutic potential of regulatory T cells in preeclampsia-opportunities and challenges. Front Immunol 10:478
Robertson SA (2005) Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 322:43–52
Robinson DP, Klein SL (2012) Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62:263–271
Rodriguez-Martinez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ (2011) Seminal plasma proteins: what role do they play? Am J Reprod Immunol 66(Suppl 1):11–22
Rolle L, Memarzadeh Tehran M, Morell-Garcia A, Raeva Y, Schumacher A, Hartig R, Costa SD, Jensen F, Zenclussen AC (2013) Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol 70:448–453
Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK (2006) Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 11:317–326
Rowe JH, Ertelt JM, Xin L, Way SS. (2012) Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nat 490(7418):102–106
Saito S, Nakashima A, Shima T, Ito M (2010) Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol 63:601–610
Salamonsen LA (2003) Tissue injury and repair in the female human reproductive tract. Reproduction 125:301–311
Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE (2017) Placental exosomes as early biomarker of preeclampsia: potential role of exosomal MicroRNAs across gestation. J Clin Endocrinol Metab 102:3182–3194
Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas De St Groth B, Nanan R (2009) Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 183:7023–7030
Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, Salomon C (2014) Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 12:204
Schjenken JE, Robertson SA (2020) The female response to seminal fluid. Physiol Rev 100:1077–1117
Sehring J, Beltsos A, Jeelani R (2022) Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 117:179–186
Sharkey AM, Gardner L, Hiby S, Farrell L, Apps R, Masters L, Goodridge J, Lathbury L, Stewart CA, Verma S, Moffett A (2008) Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol 181:39–46
Sharkey DJ, Macpherson AM, Tremellen KP, Mottershead DG, Gilchrist RB, Robertson SA (2012a) TGF-beta mediates proinflammatory seminal fluid signaling in human cervical epithelial cells. J Immunol 189:1024–1035
Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA (2012b) Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol 188:2445–2454
Sharkey DJ, Tremellen KP, Briggs NE, Dekker GA, Robertson SA (2016) Seminal plasma transforming growth factor-beta, activin A and follistatin fluctuate within men over time. Hum Reprod 31:2183–2191
Shigeta N, Kumasawa K, Tanaka A, Badger wing J, Nakamura H, Sakaguchi S, Kimura T (2020) Dynamics of effector and naive regulatory T cells throughout pregnancy. J Reprod Immunol 140:103135
Shima T, Inada K, Nakashima A, Ushijima A, Ito M, Yoshino O, Saito S (2015) Paternal antigen-specific proliferating regulatory T cells are increased in uterine-draining lymph nodes just before implantation and in pregnant uterus just after implantation by seminal plasma-priming in allogeneic mouse pregnancy. J Reprod Immunol 108:72–82
Shima T, Nakashima A, Yasuda I, Ushijima A, Inada K, Tsuda S, Yoshino O, Tomura M, Saito S (2020) Uterine CD11c+ cells induce the development of paternal antigen-specific Tregs via seminal plasma priming. J Reprod Immunol 141:103165
Shobu T, Sageshima N, Tokui H, Omura M, Saito K, Nagatsuka Y, Nakanishi M, Hayashi Y, Hatake K, Ishitani A (2006) The surface expression of HLA-F on decidual trophoblasts increases from mid to term gestation. J Reprod Immunol 72:18–32
Shynlova O, Boros-Rausch A, Farine T, Adams Waldorf KM, Dunk C, Lye SJ (2021) Decidual inflammation drives chemokine-mediated immune infiltration contributing to term labor. J Immunol 207:2015–2026
Singh N, Herbert B, Sooranna GR, Orsi NM, Edey L, Dasgupta T, Sooranna SR, Yellon SM, Johnson MR (2017) Is myometrial inflammation a cause or a consequence of term human labour? J Endocrinol 235:69–83
Singh N, Herbert B, Sooranna G, Shah NM, Das A, Sooranna SR, Johnson MR (2021) Is there an inflammatory stimulus to human term labour? PLoS ONE 16:e0256545
Singh S (2016) Congenital toxoplasmosis: clinical features, outcomes, treatment, and prevention. Trop Parasitol 6:113–122
Smith R (2007) Parturition. N Engl J Med 356:271–283
Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT (2004) Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112:38–43
Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L (2013) Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol 191:5515–5523
Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359:76–79
Stinson LF, Boyce MC, Payne MS, Keelan JA (2019) The not-so-sterile womb: evidence that the human fetus is exposed to bacteria prior to birth. Front Microbiol 10:1124
Strunz B, Bister J, Jonsson H, Filipovic I, Crona-Guterstam Y, Kvedaraite E, Sleiers N, Dumitrescu B, Brannstrom M, Lentini A, Reinius B, Cornillet M, Willinger T, Gidlof S, Hamilton RS, Ivarsson MA, Bjorkstrom NK (2021) Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci Immunol 6
Suman P, Malhotra SS, Gupta SK (2013) LIF-STAT signaling and trophoblast biology. JAKSTAT 2:e25155
Tang AW, Alfirevic Z, Quenby S (2011) Natural killer cells and pregnancy outcomes in women with recurrent miscarriage and infertility: a systematic review. Hum Reprod 26:1971–1980
Tannetta D, Collett G, Vatish M, Redman C, Sargent I (2017) Syncytiotrophoblast extracellular vesicles—circulating biopsies reflecting placental health. Placenta 52:134–138
Teles A, Zenclussen AC, Schumacher A (2013) Regulatory T cells are baby′s best friends. Am J Reprod Immunol 69:331–339
Telgmann R, Gellersen B (1998) Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update 4:472–479
Theis KR, Romero R, Winters AD, Greenberg JM, Gomez-Lopez N, Alhousseini A, Bieda J, Maymon E, Pacora P, Fettweis JM, Buck GA, Jefferson KK, Strauss JF 3rd, Erez O, Hassan SS (2019) Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol 220:267e1–267e39
Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE (1999) Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 14:229–236
Tilburgs T, Roelen DL, van der Mast BJ, De Groot-Swings GM, Kleijburg C, Scherjon SA, Claas FH (2008) Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol 180:5737–5745
Tilburgs T, Scherjon SA, Van Der Mast BJ, Haasnoot GW, Versteeg VDV-MM, Roelen DL, Van Rood JJ, Claas FH (2009) Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol 82:148–157
Tilburgs T, Meissner TB, Ferreira LMR, Mulder A, Musunuru K, Ye J, Strominger JL (2017) NLRP2 is a suppressor of NF-kB signaling and HLA-C expression in human trophoblastsdagger, double dagger. Biol Reprod 96:831–842
Tong M, Abrahams VM, Chamley LW (2018) Immunological effects of placental extracellular vesicles. Immunol Cell Biol
Torgerson PR, Mastroiacovo P (2013) The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 91:501–508
Toth B, Haufe T, Scholz C, Kuhn C, Friese K, Karamouti M, Makrigiannakis A, Jeschke U (2010) Placental interleukin-15 expression in recurrent miscarriage. Am J Reprod Immunol 64:402–410
Tremellen KP, Seamark RF, Robertson SA (1998) Seminal transforming growth factor beta1 stimulates granulocyte-macrophage colony-stimulating factor production and inflammatory cell recruitment in the murine uterus. Biol Reprod 58:1217–1225
Tremellen KP, Valbuena D, Landeras J, Ballesteros A, Martinez J, Mendoza S, Norman RJ, Robertson SA, Simon C (2000) The effect of intercourse on pregnancy rates during assisted human reproduction. Hum Reprod 15:2653–2658
Tsuda S, Nakashima A, Shima T, Saito S (2019) New paradigm in the role of regulatory T cells during pregnancy. Front Immunol 10:573
Tsuda S, Nakashima A, Morita K, Shima T, Yoneda S, Kishi H, Saito S (2021) The role of decidual regulatory T cells in the induction and maintenance of fetal antigen-specific tolerance: Imbalance between regulatory and cytotoxic T cells in pregnancy complications. Hum Immunol 82:346–352
Vacca P, Moretta L, Moretta A, Mingari MC (2011) Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol 32:517–523
Vacca P, Mingari MC, Moretta L (2013) Natural killer cells in human pregnancy. J Reprod Immunol 97:14–19
Van De Geijn FE, Wuhrer M, Selman MH, Willemsen SP, De Man YA, Deelder AM, Hazes JM, Dolhain RJ (2009) Immunoglobulin G galactosylation and sialylation are associated with pregnancy-induced improvement of rheumatoid arthritis and the postpartum flare: results from a large prospective cohort study. Arthritis Res Ther 11:R193
Vickram AS, Dhama K, Chakraborty S, Samad HA, Latheef SK, Sharun K, Khurana SK, K, A, Tiwari R, Bhatt P, K, V, Chaicumpa W (2019) Role of antisperm antibodies in infertility, pregnancy, and potential forcontraceptive and antifertility vaccine designs: research progress and pioneering vision. Vaccines 7:116
Wegmann TG, Lin H, Guilbert L, Mosmann TR (1993) Bidirectional cytokine interactions in the maternal-fetal relationship—is successful pregnancy a Th2 phenomenon. Immunol Today 14:353–356
Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC (1988) Incidence of early loss of pregnancy. N Engl J Med 319(4):189–194
Winship A, Correia J, Zhang JG, Nicola NA, Dimitriadis E (2015) Leukemia inhibitory factor (LIF) inhibition during mid-gestation impairs trophoblast invasion and spiral artery remodelling during pregnancy in mice. PLoS ONE 10:e0129110
Wira CR, Rodriguez-Garcia M, Patel MV (2015) The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol 15:217–230
Wu WX, Brooks J, Glasier AF, Mcneilly AS (1995) The relationship between decidualization and prolactin mRNA and production at different stages of human pregnancy. J Mol Endocrinol 14:255–261
Yang Y, Chu W, Geraghty DE, Hunt JS (1996) Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol 156:4224–4231
Yasuda I, Shima T, Moriya T, Ikebuchi R, Kusumoto Y, Ushijima A, Nakashima A, Tomura M, Saito S (2020) Dynamic changes in the phenotype of dendritic cells in the uterus and uterine draining lymph nodes after coitus. Front Immunol 11:557720
Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE (2002) Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 66:445–449
Zenclussen AC, Gentile T, Kortebani G, Mazzolli A, Margni R (2001) Asymmetric antibodies and pregnancy. Am J Reprod Immunol 45:289–294
Zenclussen ML, Thuere C, Ahmad N, Wafula PO, Fest S, Teles A, Leber A, Casalis PA, Bechmann I, Priller J, Volk HD, Zenclussen AC (2010) The persistence of paternal antigens in the maternal body is involved in regulatory T-cell expansion and fetal-maternal tolerance in murine pregnancy. Am J Reprod Immunol 63:200–208
Zhang YH, Sun HX (2020) Immune checkpoint molecules in pregnancy: focus on regulatory T cells. Eur J Immunol 50:160–169
Zhang YH, He M, Wang Y, Liao AH (2017) Modulators of the balance between M1 and M2 macrophages during pregnancy. Front Immunol 8:120
Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG (1996) Estimates of human fertility and pregnancy loss. Fertil Steril 65(3):503–509
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Menzies, F.M. (2023). Immunology of Pregnancy and Systemic Consequences. In: Klein, S.L., Roberts, C.W. (eds) Sex and Gender Differences in Infection and Treatments for Infectious Diseases. Current Topics in Microbiology and Immunology, vol 441. Springer, Cham. https://doi.org/10.1007/978-3-031-35139-6_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-35139-6_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35138-9
Online ISBN: 978-3-031-35139-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)