Abstract
Senna is a diverse and paraphyletic genus in the subfamily Caesalpinioideae (Fabaceae Lindl.) comprising various species of industrial and medicinal value. To date, the genome-based taxonomic relationship among several Senna species remains enigmatic. Cytogenetic information is invaluable in deciphering phylogenetic relationships and evolutionary history. However, insufficient chromosomal research for many Senna species impedes comparative cytotaxonomic analyses aimed at understanding their genomic evolution. To provide additional Senna-related molecular cytogenetic information, we karyotyped 11 Senna species by employing triple-color fluorescence in situ hybridization using 5S rDNA, 45S rDNA, and Arabidopsis thaliana-type telomeric pre-labeled oligonucleotide probes. Chromosome numbers were predominantly 2n = 28, but 2n = 22 (S. marilandica) and 2n = 24 (S. uniflora) were also observed. While most species revealed only one interstitial 5S rDNA locus, except for S. uniflora which has two loci, a range of one to three 45S rDNA loci were detected at distal chromosomal regions. Additionally, we observed a hemizygous 45S rDNA locus in S. auriculata. In addition to chromosome termini, weak signals for telomeric repeats were found in interstitial regions in S. hirsuta, S. corymbosa, and S. alexandrina. These cytogenetic data can be integrated with molecular phylogenetic data for more comprehensive Senna cytotaxonomic analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Senna Mill., a representative genus from the family Fabaceae Lindl. (Resende et al. 2014), comprises approximately 350 morphologically diverse species of herbs, shrubs, and trees (Cordeiro and Felix 2018). Senna species are distributed throughout circumtropical regions with an extremely wide range of habitats (Marazzi et al. 2006; Pellerin et al. 2019). They are morphologically distinguished based on their androceu, corolla, floral architecture, bracteole, and fruit characteristics (Marazzi et al. 2006). Many Senna species have been recognized for their medicinal and industrial uses such as for treating diverse diseases (e.g., digestive ailments, skin disorders, respiratory illnesses, visual problems, and even heart disease) and producing compounds used in commercial goods, flavoring, perfume, pet food, and coffee (Rahman et al. 2013; Pellerin et al. 2019). Despite their economic and health benefits, a paucity of molecular cytogenetic data has impeded comparative analyses for the evaluation of Senna genome evolution. Although a few comparative cytogenetic studies have been reported, only a few species have data based on fluorescence in situ hybridization (FISH) of 5S and 45S rDNA and telomeric repeats (Rosato et al. 2018; Youn and Kim 2018; Pellerin et al. 2019).
Karyotype data can be used to identify species, reveal past genome rearrangements, and infer taxonomic relationships among related species (Guerra 2008; Jo et al. 2019; Chen et al. 2020). A karyotype, which is a genetically stable characteristic unique to a given species, provides the number, shape, size, and morphology of an organism’s chromosome complement (Pellerin et al. 2019; Zhou et al. 2019a). Chromosomal rearrangements can alter karyotype features resulting in changes in chromosome number (dysploidy) or organization, which often reflect evolutionary events such as speciation (Wölk et al. 2015). Descending dysploidy occurs when chromosomal fusion leads to species with fewer chromosome numbers, whereas ascending dysploidy results from retention of centromere function after a chromosome fission (Winterfeld et al. 2020; Ta et al. 2021; Waminal et al. 2021).
FISH is an invaluable technique in karyotyping (Waminal et al. 2018; Youn and Kim 2018). The 5S rDNA and 45S rDNA sequences are commonly used as FISH probes because they are highly repetitive and widely conserved across taxonomic groups (Pellerin et al. 2018a, b; Waminal et al. 2018; Zhou et al. 2019b). The Arabidopsis thaliana-type telomeric repeat (TTTAGGG)n, the canonical plant telomeric repeat most commonly found at chromosome termini, is also widely conserved across taxonomic groups (Watson and Riha 2010; Peska and Garcia 2020). Interspecies divergence in the chromosomal distribution of rDNA and the telomeric repeat sequences provide phylogenetically useful information for analyzing genome dynamics.
While the predominant diploid chromosome number in Senna is 2n = 28 (Rice et al. 2015), descending dysploid karyotypes of 2n = 22–26 are also commonly observed (Cordeiro and Felix 2018; Pellerin et al. 2019). Published data on chromosome number is lacking in a number of Senna species. To broaden the karyotype information in Senna, we performed triple-color FISH using rDNA and telomeric repeat sequence probes in 11 Senna species. To our knowledge, there are currently no reports of FISH karyotyping using rDNA and telomeric probes in these Senna species. This analysis revealed interspecific karyotype variations that provide insight into karyotype dynamics in Senna. These preliminary data will also facilitate cytogenetic mapping of major species-specific repeats, improve our understanding of taxonomic relationships and evolutionary history, and provide useful information for future Senna genomic research and breeding projects.
2 Materials and methods
2.1 Plant materials and chromosome preparation
Seeds of the 11 Senna species were purchased from the National Plant Germplasm System (NPGS, USA) and Rare Palm Seeds (RPS, Germany) (Table 1). Concentrated sulfuric acid (Sigma-Aldrich Co., St. Louis, MO, USA) was used to treat the seeds before germination to break seed dormancy and expedite germination (Baskin et al. 1998). Root tips were collected and pre-treated in 2 mM 8-hydroxyquinoline for 5 h at 18 °C then stored in 70% ethanol at 4 °C until use.
Chromosome preparation was performed according to our published protocol (Waminal and Kim 2012; Peniton et al. 2019) with minor alterations. Briefly, fixed root tips were washed in distilled water and digested in an enzyme solution containing 1% pectolyase Y-23 (Duchefa, Haarlem, The Netherlands) and 2% cellulase R-10 (Phytotechnology Laboratories, USA) for 60–90 min at 37 °C. Chromosomes were then fixed in chilled Carnoy’s solution and centrifuged. Supernatants were aspirated, and the precipitates were resuspended in aceto-ethanol (9:1 v/v) and mounted onto slides in a humid chamber. After air drying, slides were soaked in 2% (v/v) formaldehyde fixative (Merck Schuchardt OHG, Hohenbrunn, Germany) for 5 min to preserve the chromosomes, quickly dipped into distilled water, and finally dehydrated in a series of ethanol concentrations (70, 90, and 100%) (Vrána et al. 2012).
2.2 Fluorescence in situ hybridization (FISH)
FISH was performed according to Waminal and Kim (2012) with some modifications. Pre-labeled oligoprobes (PLOPs) for 5S rDNA, 45S rDNA, and Arabidopsis-type telomeric sequences are described in Waminal et al. (2018). Hybridization solutions consisted of 50% formamide, 10% dextran sulfate, 2 × saline sodium citrate buffer (SSC), 50 ng/µL of each PLOP, and nuclease-free water to a total volume of 40 µL. Slides were denatured at 80 °C for 5 min, then placed in a humid chamber at 37 °C for at least 45 min. Slides were then washed carefully in 2 × SSC and dehydrated in a series of ethanol concentrations (70, 90, and 100%) for 3 min each at room temperature. Finally, chromosomes were counterstained with DAPI premixed in Vectashield antifade solution. Chromosome images were captured using a BX53 fluorescence microscope (Olympus, Tokyo, Japan) equipped with a DFC365 FS CCD camera (Leica Microsystems, Wetzlar, Germany), and analyzed with Cytovision ver. 7.2 software (Leica Microsystems). Images were finalized using Photoshop CS6 (Adobe Inc., San Jose, California, USA).
2.3 Karyotyping
We used at least three metaphase spreads with the best morphology for total chromosome length (TCL, 2n) measurements using Image J software ver. 1.51 k (Schneider et al. 2012). Homologous chromosomes were paired and arranged based on rDNA and telomeric repeat FISH signals, chromosome length, and centromere position. Chromosome type was classified according to the criteria of Levan et al. (2009). Karyograms and idiograms were generated using Adobe Photoshop CS6.
3 Results
3.1 Chromosome counts
The 11 species could be grouped into three groups according to their diploid chromosome number, 2n = 22, 24, and 28 (Table 2). Because 2n = 28 is considered the predominant chromosome number in Senna (Cordeiro and Felix 2018), and was most frequently represented in our data, all species with different 2n numbers were regarded as descending dysploidy karyotypes (Winterfeld et al. 2020; Ta et al. 2021; Waminal et al. 2021).
Chromosome morphology, differential intensities, and chromosomal distribution of rDNA and telomeric repeats enabled the identification of homologous chromosomes in the 11 Senna species. The total chromosome length (TCL) among these 11 species ranged from 54.16 to 133.2 μm (Table 2). S. uniflora had the longest chromosomes, whereas S. notabilis had the shortest chromosomes. The average chromosome length (TCL/2n) also differed among the species, indicating that cyclic changes in genome size may have occurred during genus diversification (Seijo and Fernández 2003).
Homologous chromosome complements of the 11 species included metacentric, submetacentric, and sub-telocentric chromosomes. Only S. alata had all metacentric chromosome pairs. Other species included metacentric and submetacentric chromosomes. In addition, S. siamea, S. corymbosa, and S. lindheimeriana had one pair each of sub-telocentric chromosomes (Table 2, Fig. 3).
3.2 Chromosomal distribution of rDNA and telomeric probes
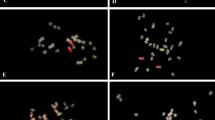
The rDNA probes displayed varied chromosomal distributions and signal intensities across the 11 Senna species (Fig. 1). All species presented a single 5S rDNA locus, except for S. uniflora, which possessed two loci (Fig. 2). The 5S rDNA signals were frequently detected in the penultimate chromosome number and were generally localized to proximal chromosome regions (Figs. 2 and 3). The number and intensity of 45S rDNA signals varied considerably among the species. Three pairs were detected in S. alata, S. alexandrina, S. auriculata, S. notabilis, S. polyphylla, and S. siamea. Two pairs were found in S. uniflora, and one pair each was found in S. corymbosa, S. hirsuta var. leptocarpa, S. lindheimeriana, and S. marilandica. Most of these signals were found in the terminal regions of the short arms of the respective chromosomes. A hemizygous 45S rDNA locus was observed in chromosome 6 of S. auriculata (Fig. 2), and we did not observe any juxtaposition between 5S rDNA and 45S rDNA signals in any species.
The Arabidopsis-type telomeric repeat hybridized to the terminal regions of all chromosomes in all species (Table 2, Figs. 1, 2, and 3). In addition, some chromosomes also displayed weak interstitial telomeric repeat (ITR) signals in peri-centromeric regions in S. alexandrina, S. corymbosa, and S. hirsuta (Fig. 2). A pair of ITR signals was detected on chromosome 1 in all three species. The remaining pairs were localized to chromosome 12 in S. alexandrina, 2 and 6 in S. corymbosa, and 9 and 12 in S. hirsuta (Fig. 2).
4 Discussion
Data on chromosome number and FISH-based karyotype in Senna are relatively scarce (Cordeiro and Felix 2018). In our previous work, we presented FISH karyotypes using 5S and 45S rDNA and telomeric repeats in 12 Senna species, including S. tora whose genome has been recently sequenced (Youn and Kim 2018; Pellerin et al. 2019; Kang et al. 2020; Ta et al. 2021; Waminal et al. 2021). To complement previous data and improve our understanding of karyotype diversity in Senna, we further analyzed the karyotypes of 11 additional Senna species. To our knowledge, this is the first report on the chromosome numbers of S. lindheimeriana, S. marilandica, S. polyphylla, and S. uniflora.
Most Senna species investigated in this study were diploid, with a predominant chromosome number of 2n = 28, corresponding with previous reports (Souza and Iseppon 2004; Cordeiro and Felix 2018). However, S. marilandica (2n = 22) and S. uniflora (2n = 24) showed descending dysploid karyotypes, which may have resulted from post-polyploidy dysploidization after a polyploidization of ancient karyotypes with 2n = 14 (Biondo et al. 2012; Shchapova 2013; Winterfeld et al. 2020). Similar processes have also occurred in several other plants such as Brassica, Cucumis, Nothoscordum, Brachyscome, and Senna tora (Maluszynska and Heslop-Harrison 1993; Watanabe et al. 1995; Koo et al. 2010; Pellerin et al. 2019; Waminal et al. 2021). Indeed, changes in chromosome count may have played a role in the occurrence of reproductive isolation and speciation in Senna (Freyman and Höhna 2018).
Using FISH, we observed interspecific differences in the signal patterns of our markers, indicating species specificity and the usefulness of our probes in distinguishing each species. A hemizygous 45S rDNA pattern similar to that observed in the short arm of chromosome 6 in S. corymbosa has been observed in other Senna and non-Senna species (Lan and Albert 2011; Mancia et al. 2015; Waminal et al. 2016; Pellerin et al. 2019). This hemizygous locus may be explained by homology-mediated unequal crossing over between non-allelic homologous repeat units, which significantly shortened one site, making it undetectable by FISH (Pellerin et al. 2019).
Most species displayed a single locus proximal distribution of 5S rDNA, except for S. uniflora, which showed two loci. These results corroborate the reduced copy number and interstitial distribution of 5S rDNA often observed in flowering plants (Roa and Guerra 2012, 2015). Our results also revealed that 5S and 45S rDNA are not linked in the same chromosomal region; thus, genomic rearrangements by conversion and crossing-over should occur with greater frequency (Waminal and Kim 2012). Independent localization suggests that 5S and 45S rDNA experienced distinct evolutionary processes (Mantovani et al. 2005). Variations in the distribution pattern of rDNA repeats in groups of related species have been explained via structural rearrangement events such as translocations, inversions, duplications, and deletions. All of these events commonly result in structural changes in the karyotype (Silvestri et al. 2020).
Although telomeric sequences are normally located at chromosomal termini (Fuchs et al. 1995), some ITRs were detected in either three or two chromosome pairs in S. hirsuta, S. corymbosa, and S. alexandrina (Fig. 2). ITRs have been observed in a few chromosomes in several Senna species, especially in S. tora, where they are extensively amplified in all chromosomes (Pellerin et al. 2019). ITR signals have also been discovered in animals and some other plant species (Uchida et al. 2002; He et al. 2013; Souza et al. 2016). ITR size, number, and distribution could vary inter- or intra-specifically. Although the origin and evolution of ITRs remain largely unexplored in plants, some proposed mechanisms to explain ITR formation include unequal gene conversion, chromosomal fusion, crossing-over, DNA replication, transposition of telomeric repeats by mobile elements, or the translocation of an ITR during genetic recombination (He et al. 2013; Aksenova and Mirkin 2019). The ITRs observed in Senna species suggest that telomere-mediated inter-chromosomal rearrangements are a major pathway in the evolutionary dynamics of most Senna species (Sousa et al. 2014). This observation is supported by the high frequency of descending dysploids in Senna, as dysploidy often arises from inter-chromosomal rearrangements including end-to-end translocations and nested chromosome insertions (Winterfeld et al. 2020; Ta et al. 2021; Waminal et al. 2021). Recent studies have shown that ITRs are dynamic elements that play essential roles in telomere maintenance and the regulation of gene expression through interactions with telomeres (Ruiz-Herrera et al. 2008; Aksenova and Mirkin 2019).
If these ITRs are formed by chromosomal fusion with reciprocal translocation, the product of this translocation would be a submetacentric chromosome with a weakly detectible ITR, plus a single chromosome and a small fragment (Schubert and Lysak 2011). However, we did not find such small chromosomes in Senna. Another mechanism, called a fusion–fission cycle or a Robertsonian rearrangement, has been used to explain ITRs in other plants (Schubert et al. 1995). With these mechanisms, both centromeric and telomeric sequences are retained, although one of the centromeres and the interstitial telomeric sequences must be inactivated for proper mitosis (Sousa et al. 2014).
We observed that S. corymbosa and S. hirsuta had similar numbers and distributions of 5S rDNA, 45S rDNA, telomeric repeats, and even ITR signals (Figs. 2 and 3). This similarity was also observed in S. occidentalis (Pellerin et al. 2019; Ta et al. 2021), suggesting a closer relationship between S. hirsuta, S. corymbosa, and S. occidentalis. Based on FISH signal similarity, we speculate that S. alata, S. alexandrina, S. auriculata, S. notabilis, S. polyphylla, and S. siamea are closely related, whereas S. corymbosa is closely related to S. hirsuta and S. lindheimeriana in another clade. Molecular phylogenomic data will further clarify these relationships.
5 Conclusion
FISH karyotypes of 11 Senna species were established using three-color probes targeting 5S rDNA, 45S rDNA, and telomeric repeat sequences. The interspecific karyotypic variation in the species studied constitutes useful data for identifying each species and elucidating interspecific relationships in Senna. FISH karyotype analysis using major species-specific repeats as probes, and phylogenomic analyses using chloroplast genomes may provide a clearer picture of the genome dynamics in Senna. The determination of highly abundant repeats using next-generation sequencing data and application of such markers in more Senna species are essential for further studies.
References
Aksenova AY, Mirkin SM (2019) At the beginning of the end and in the middle of the beginning: structure and maintenance of telomeric DNA repeats and interstitial telomeric sequences. Genes 10:118. https://doi.org/10.3390/genes10020118
Al-Turki TA, Filfilan SA, Mehmood SF (2000) A cytological study of flowering plants from Saudi Arabia. Willdenowia 30:339–358. https://doi.org/10.3372/wi.30.30211
Baskin JM, Nan X, Baskin CC (1998) A comparative study of seed dormancy and germination in an annual and a perennial species of Senna (Fabaceae). Seed Sci Res 8:501–512. https://doi.org/10.1017/s0960258500004475
Biondo E, Miotto S, Schifino-Wittmann M, Castro B (2012) Cytogenetics and cytotaxonomy of Brazilian species of Senna Mill. (Cassieae–Caesalpinioideae–Leguminosae). Caryologia 58:152–163. https://doi.org/10.1080/00087114.2005.10589445
Chen L, Su D, Sun J, Li Z, Han Y (2020) Development of a set of chromosome-specific oligonucleotide markers and karyotype analysis in the Japanese morning glory Ipomoea nil. Sci Hortic 273:109633. https://doi.org/10.1016/j.scienta.2020.109633
Cordeiro JMP, Felix LP (2018) Intra- and interspecific karyotypic variations of the genus Senna Mill. (Fabaceae, Caesalpinioideae). Acta Bot Bras 32:128–134. https://doi.org/10.1590/0102-33062017abb0274
Freyman WA, Höhna S (2018) Cladogenetic and anagenetic models of chromosome number evolution: a Bayesian model averaging approach. Syst Biol 67:195–215. https://doi.org/10.1093/sysbio/syx065
Fuchs J, Brandes A, Schubert I (1995) Telomere sequence localization and karyotype evolution in higher-plants. Oesterr Bot Wochenbl 196:227–241. https://doi.org/10.1007/BF00982962
Guerra M (2008) Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenet Genome Res 120:339–350. https://doi.org/10.1159/000121083
He L, Liu J, Torres GA, Zhang H, Jiang J, Xie C (2013) Interstitial telomeric repeats are enriched in the centromeres of chromosomes in Solanum species. Chromosome Res 21:5–13. https://doi.org/10.1007/s10577-012-9332-x
Irwin HS, Turner BL (1960) Chromosomal relationships and taxonomic considerations in the genus Cassia. Am J Bot 47:309–318. https://doi.org/10.1002/j.1537-2197.1960.tb07130.x
Jo YK, Mazharul IMD, Kim CK, Kim HY, Lim KB (2019) Morphological characteristics and FISH analysis of Hibiscus F1 hybrids and parental lines. Hortic Sci Technol 37:630–639. https://doi.org/10.7235/HORT.20190063
Kang SH, Pandey RP, Lee CM, Sim JS, Jeong JT, Choi BS, Jung M, Ginzburg D, Zhao K, Won SY et al (2020) Genome-enabled discovery of anthraquinone biosynthesis in Senna tora. Nat Commun 11:5875. https://doi.org/10.1038/s41467-020-19681-1
Koo DH, Nam YW, Choi D, Bang JW, De Jong H, Hur Y (2010) Molecular cytogenetic mapping of Cucumis sativus and C. melo using highly repetitive DNA sequences. Chromosome Res 18:325–336. https://doi.org/10.1007/s10577-010-9116-0
Lan T, Albert VA (2011) Dynamic distribution patterns of ribosomal DNA and chromosomal evolution in Paphiopedilum, a lady’s slipper orchid. BMC Plant Biol 11:126. https://doi.org/10.1186/1471-2229-11-126
Levan A, Fredga K, Sandberg AA (2009) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
Maluszynska J, Heslop-Harrison JS (1993) Physical mapping of rDNA loci in Brassica species. Genome 36:774–781. https://doi.org/10.1139/g93-102
Mancia FH, Sohn S-H, Ahn YK, Kim D-S, Kim JS, Kwon Y-S, Kim C-W, Lee T-H, Hwang Y (2015) Distribution of various types of repetitive DNAs in Allium cepa L. based on dual color FISH. Hortic Environ Biotechnol 56:793–799. https://doi.org/10.1007/s13580-015-1100-3
Mantovani M, Abel LD, Moreira-Filho O (2005) Conserved 5S and variable 45S rDNA chromosomal localisation revealed by FISH in Astyanax scabripinnis (Pisces, Characidae). Genetica 123:211–216. https://doi.org/10.1007/s10709-004-2281-3
Marazzi B, Endress PK, De Queiroz LP, Conti E (2006) Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: patterns in the evolution of floral symmetry and extrafloral nectaries. Am J Bot 93:288–303. https://doi.org/10.3732/ajb.93.2.288
Ohri D, Kumar A, Pal M (1986) Correlations between 2C DNA values and habit in Cassia (Leguminosae:Caesalpinioideae). Plant Syst Evol 153:223–227. https://doi.org/10.1007/BF00983689
Pellerin RJ, Waminal NE, Belandres HR, Kim HH (2018a) Karyotypes of three exotic Cucurbit species based on triple-color FISH analysis. Hortic Sci Technol 36:417–425. https://doi.org/10.12972/kjhst.20180041
Pellerin RJ, Waminal NE, Kim HH (2018b) Triple-color FISH karyotype analysis of four Korean wild Cucurbitaceae species. Hortic Sci Technol 36:98–107. https://doi.org/10.12972/kjhst.20180011
Pellerin RJ, Waminal NE, Kim HH (2019) FISH mapping of rDNA and telomeric repeats in 10 Senna species. Hortic Environ Biotechnol 60:253–260. https://doi.org/10.1007/s13580-018-0115-y
Peniton E, Waminal NE, Kim T-H, Kim HH (2019) FISH karyotype comparison between wild and cultivated perilla species using 5S and 45S rDNA probes. Plant Breed Biotechnol 7:237–244. https://doi.org/10.9787/PBB.2019.7.3.237
Peska V, Garcia S (2020) Origin, diversity, and evolution of telomere sequences in plants. Front Plant Sci 11:117. https://doi.org/10.3389/fpls.2020.00117
Rahman MO, Rahman MZ, Begum A (2013) Numerical taxonomy of the genus Senna Mill. from Bangladesh. Bangladesh J Plant Taxon 20:77–83. https://doi.org/10.3329/bjpt.v20i1.15467
Randell B (1970) Adaptations in the genetic system of Australian arid zone Cassia species (Leguminosae, Caesalpinioideae). Aust J Bot 18:77–97. https://doi.org/10.1071/BT9700077
Resende K, Prado C, Davide L, Torres G (2014) Polyploidy and apomixis in accessions of Senna rugosa (G.Don) H.S.Irwin & Barneby. Turk J Biol 38:510–515. https://doi.org/10.3906/biy-1312-66
Rice A, Glick L, Abadi S, Einhorn M, Kopelman NM, Salman-Minkov A, Mayzel J, Chay O, Mayrose I (2015) The chromosome counts database (CCDB) - a community resource of plant chromosome numbers. New Phytol 206:19–26. https://doi.org/10.1111/nph.13191
Roa F, Guerra M (2012) Distribution of 45S rDNA sites in chromosomes of plants: structural and evolutionary implications. BMC Evol Biol 12:225. https://doi.org/10.1186/1471-2148-12-225
Roa F, Guerra M (2015) Non-random distribution of 5S rDNA sites and its association with 45S rDNA in plant chromosomes. Cytogenet Genome Res 146:243–249. https://doi.org/10.1159/000440930
Rosato M, Álvarez I, Feliner GN, Rosselló JA (2018) Inter- and intraspecific hypervariability in interstitial telomeric-like repeats (TTTAGGG)n in Anacyclus (Asteraceae). Ann Bot 122:387–395. https://doi.org/10.1093/aob/mcy079
Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E (2008) Telomeric repeats far from the ends: mechanisms of origin and role in evolution. Cytogenet Genome Res 122:219–228. https://doi.org/10.1159/000167807
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Schubert I, Lysak MA (2011) Interpretation of karyotype evolution should consider chromosome structural constraints. Trends Genet 27:207–216. https://doi.org/10.1016/j.tig.2011.03.004
Schubert I, Rieger R, Fuchs J (1995) Alteration of basic chromosome numberby fusion–fission cycles. Genome 38:1289–1292. https://doi.org/10.1139/g95-170
Seijo JG, Fernández A (2003) Karyotype analysis and chromosome evolution in South American species of Lathyrus (Leguminosae). Am J Bot 90:980–987. https://doi.org/10.3732/ajb.90.7.980
Shchapova AI (2013) The diversity of lifecycles and their role in the evolution of basic chromosome numbers in various living species. Russ J Genet Appl Res 3:239–245. https://doi.org/10.1134/S2079059713040102
Silvestri MC, Ortiz AM, Robledo GA, Lavia GI (2020) Chromosome diversity in species of the genus Arachis, revealed by FISH and CMA/DAPI banding, and inferences about their karyotype differentiation. An Acad Bras Cienc 92:e20191364. https://doi.org/10.1590/0001-3765202020191364
Sousa A, Cusimano N, Renner SS (2014) Combining FISH and model-based predictions to understand chromosome evolution in Typhonium (Araceae). Ann Bot 113:669–680. https://doi.org/10.1093/aob/mct302
Souza M, Iseppon A (2004) Cytogenetics and chromosome banding patterns in Caesalpinioideae and Papilionioideae species of Pará, Amazonas, Brazil. Bot J Linn Soc 144:181–191. https://doi.org/10.1111/j.1095-8339.2003.00230.x
Souza G, Vanzela ALL, Crosa O, Guerra M (2016) Interstitial telomeric sites and Robertsonian translocations in species of Ipheion and Nothoscordum (Amaryllidaceae). Genetica 144:157–166. https://doi.org/10.1007/s10709-016-9886-1
Ta TD, Waminal NE, Nguyen TH, Pellerin RJ, Kim HH (2021) Comparative FISH analysis of Senna tora tandem repeats revealed insights into the chromosome dynamics in Senna. Genes Genom. https://doi.org/10.1007/s13258-021-01051-w
Uchida W, Matsunaga S, Sugiyama R, Kawano S (2002) Interstitial telomere-like repeats in the Arabidopsis thaliana genome. Genes Genet Syst 77:63–67. https://doi.org/10.1266/ggs.77.63
Vrána J, Simková H, Kubaláková M, Cíhalíková J, Doležel J (2012) Flow cytometric chromosome sorting in plants: the next generation. Methods 57:331–337. https://doi.org/10.1016/j.ymeth.2012.03.006
Waminal NE, Kim HH (2012) Dual-color FISH karyotype and rDNA distribution analyses on four Cucurbitaceae species. Hortic Environ Biotechnol 53:49–56. https://doi.org/10.1007/s13580-012-0105-4
Waminal NE, Perumal S, Lee J, Kim HH, Yang T-J (2016) Repeat evolution in Brassica rapa (AA), B. oleracea (CC), and B. napus (AACC) genomes. Plant Breed Biotech 4:107–122. https://doi.org/10.9787/PBB.2016.4.2.107
Waminal NE, Pellerin RJ, Kim N, Jayakodi M, Park JY, Yang TJ, Kim HH (2018) Rapid and efficient FISH using pre-labeled oligomer probes. Sci Rep 8:1–10. https://doi.org/10.1038/s41598-018-26667-z
Waminal NE, Pellerin RJ, Kang S-H, Kim HH (2021) Chromosomal mapping of tandem repeats revealed massive chromosomal rearrangements and insights into Senna tora dysploidy. Front Plant Sci. https://doi.org/10.3389/fpls.2021.629898
Watanabe K, King RM, Yahara T, Ito M, Yokoyama J, Suzuki T, Crawford DJ (1995) Chromosomal cytology and evolution in Eupatorieae (Asteraceae). Ann Missouri Bot Gard 82:581–592. https://doi.org/10.2307/2399838
Watson J, Riha K (2010) Comparative biology of telomeres: where plants stand. FEBS Lett 584:3752–3759. https://doi.org/10.1016/j.febslet.2010.06.017
Winterfeld G, Ley A, Hoffmann MH, Paule J, Röser M (2020) Dysploidy and polyploidy trigger strong variation of chromosome numbers in the prayer-plant family (Marantaceae). Plant Syst Evol. https://doi.org/10.1007/s00606-020-01663-x
Wölk A, Winterfeld G, Röser M (2015) Genome evolution in a Mediterranean species complex: phylogeny and cytogenetics of Helictotrichon (Poaceae) allopolyploids based on nuclear DNA sequences (rDNA, topoisomerase gene) and FISH. Syst Biodivers 13:326–345. https://doi.org/10.1080/14772000.2015.1023867
Youn SM, Kim HH (2018) Chromosome karyotyping of Senna covesii and S. floribunda based on triple-color FISH mapping of rDNAs and telomeric repeats. Plant Breed Biotech 6:51–56. https://doi.org/10.9787/PBB.2018.6.1.51
Zhou HC, Pellerin RJ, Waminal NE, Yang T-J, Kim HH (2019a) Pre-labelled oligo probe-FISH karyotype analyses of four Araliaceae species using rDNA and telomeric repeat. Genes Genom 41:839–847. https://doi.org/10.1007/s13258-019-00786-x
Zhou HC, Waminal NE, Kim HH (2019b) In silico mining and FISH mapping of a chromosome-specific satellite DNA in Capsicum annuum L. Genes Genom 41:1001–1006. https://doi.org/10.1007/s13258-019-00832-8
Acknowledgements
This study was funded by projects (NRF 2017R1A2B2004778 and NRF 2020K1A3A1A39112373) from grants of the National Research Foundation of Korea.
Author information
Authors and Affiliations
Contributions
HHK is the supervisor and project administrator. THN and NEW carried out the experiments, analyzed the data, and wrote the original draft, reviewed, and edited the manuscript. DSL and RJP carried out the experiments, and reviewed and edited the manuscript. TDT, NBC, and BYK reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Cecile Segonzac.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, T.H., Waminal, N.E., Lee, D.S. et al. Comparative triple-color FISH mapping in eleven Senna species using rDNA and telomeric repeat probes. Hortic. Environ. Biotechnol. 62, 927–935 (2021). https://doi.org/10.1007/s13580-021-00364-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00364-9