Abstract
The low survival rates of in vitro-propagated plantlets under ex vitro conditions greatly inhibits the production of virus-free apple rootstock plantlets and necessitates tight control of ex vitro environments during plantlet acclimatization. Accordingly, this study investigated the effects of light intensity on the ex vitro acclimation of apple plantlets. In vitro-propagated ‘M9’ apple plantlets were acclimatized for 6 weeks under different light treatments: 60 μmol m−2 s−1 (L), 100 μmol m−2 s−1 (M), 140 μmol m−2 s−1 (H), 180 μmol m−2 s−1 (VH), 60 → 100 μmol m−2 s−1 at 2 weeks (L2M4) or 4 weeks (L4M2), 60 → 100 → 140 μmol m−2 s−1 (L2M2H2), and 60 → 140 μmol m−2 s−1 at 4 weeks (L4H2). Survival rate, maximum quantum yield of photosystem II (Fv/Fm), growth-related parameters, and photosynthetic rate were measured. The H and VH treatments yielded the lowest survival rates (78 and 71%, respectively), whereas the M treatment yielded the highest (95%). Meanwhile, the Fv/Fm ratio at 6 weeks after transplanting decreased with increasing light intensity at 4 and 5 weeks, whereas photosynthetic rate at 5 weeks after transplanting and stem diameter at 6 weeks after transplanting increased with increasing light intensity. Furthermore, the M treatment yielded greater relative growth rates than the other treatments at 2–4 weeks, and both the M and L2M2H2 treatments yielded significantly greater relative growth rates at 4–6 weeks. These results suggest that the M and L2M2H2 treatments are appropriate for the acclimatization of in vitro-propagated ‘M9’ apple plantlets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Because apples exhibit long juvenile periods and self-incompatibility, apples are generally propagated using vegetative propagation methods (Kim et al. 1998). However, during such propagation, any viruses harbored by the source plant are likely to be transferred to the resulting propagules, and some major viruses of apple crops (e.g., apple mosaic virus, rubbery wood disease agent, apple stem grooving virus, apple stem pitting virus, and apple chlorotic leaf spot virus) have been reported to cause significant reductions in yield and fruit quality (Cembali et al. 2003). Therefore, there is a growing need for new propagation methods for the mass production of virus-free apple seedlings. Virus-free apple seedlings can also be mass propagated using tissue culture methods, including the use of apical meristems, which are generally free of virus infection (Chatenet et al. 2001; Walkey 1968) and the removal of viruses from apple plantlets by heat treatment (i.e., thermotherapy), ribavirin treatment (i.e., chemotherapy), or a combination of both (Campbell 1962; Hansen and Lane 1985; Hu et al. 2015; Paprstein et al. 2008).
Plant tissues are cultivated in vitro using conditions that are favorable to cell division (e.g., low light intensity, high relative humidity, and exogenous sucrose supplementation), which are quite different from the conditions that promote ex vitro growth. Therefore, the plantlets developed under in vitro conditions generally exhibit physiological characteristics, such as thin cuticle layers, underdeveloped stomatal apparatuses, and low chlorophyll contents (Estrada-Luna et al. 2001; Pinto et al. 2011; Pospišilová et al. 1999), which result in severe water loss and photosynthesis inhibition under ex vitro conditions, thereby reducing survival rate and ex vitro acclimatization (Carvalho et al. 2001; Hayashi et al. 1988; Hazarika 2003; Jeon et al. 2006). In previous studies, PEG treatment has been used to reduce water loss from plantlets (Dami and Hughes 1997), and ABA and high-concentration CO2 treatments have been used to improve stomatal conductance and, subsequently, post-transplantation water status (Pospíšilová et al. 1998; Pospošilová et al. 1999). Moreover, the use of hydroponic systems (e.g., the DFT system), which allow the gradual reduction of relative humidity and the consistent provision of sufficient water and nutrients to the underdeveloped roots, are effective in improving the acclimatization and survival rates of in vitro-propagated apple plantlets (Ko et al. 2018a, b).
Among the various ex vitro conditions, light is especially important for ensuring plant growth and development because it is crucial to photosynthesis and photomorphogenesis (Müller et al. 2001). However, it is easy to induce photoinhibition when in vitro-propagated plantlets with undeveloped photosynthetic apparatuses are transplanted ex vitro, owing to high light stress, even when the light intensity is far below the light saturation point of optimal photosynthesis (Ali et al. 2005; Carvalho and Amâncio 2002; Osório et al. 2010). In other words, in vitro-propagated plantlets with undeveloped photosynthetic apparatuses are likely to generate excessive levels of reactive oxygen species (ROS), owing to superfluous light energy (despite the relatively low light intensities of ex vitro conditions during acclimatization), thereby causing oxidative stress (Ali et al. 2005; Baťková et al. 2008; Faisal and Anis 2009, 2010). However, acclimatizing plantlets are able to endure such oxidative stress under ex vitro conditions through the activation of antioxidant enzymes, such as superoxide dismutase (SOD; EC 1.15.1.1), catalase (CAT; EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), and glutathione reductase (GR; EC 1.6.4.2; Noctor et al. 2002; Guan et al. 2008), within a certain threshold of light intensity.
Accordingly, the aim of this study was to investigate the effect of light intensity on the growth and acclimation of in vitro-propagated apple plantlets using precise environmental control in a plant factory.
2 Materials and methods
2.1 Plant materials and acclimation conditions
After primary culture, virus-free apical meristems were collected from a shoot-induced ‘M9’ dwarf apple rootstock and cultured for 8 weeks in Murashige and Skoog (MS) medium that contained 0.5 mg L−1 BA, 30 g L−1 sucrose, and 8.4 g L−1 agar. Thereafter, the apple plantlets were cultivated for 12 weeks in a culture flask with a rubber cap, which was equipped with a ventilating filter for roots and contained MS medium, which was supplemented with 0.5 mg L−1 IBA, 30 g L−1 sucrose, and 8.4 g L−1 agar. Next, preliminary acclimation (PA) was initiated by opening the rubber cap halfway, which made the relative humidity in the flasks 90%. Then, after 1 week, the apple plantlets were taken from the culture flask, washed with distilled water to completely remove the MS medium pieces and callus, and transferred to a deep-flow technique (DFT) hydroponic system, which was previously reported as being effective for the acclimation and growth of in vitro-propagated apple plantlets (Ko et al. 2018a). After transplantation to the DFT system, the plantlets were subject to a 6-week acclimatization period under the following conditions: 25 °C; 16-h photoperiod with a 8:1:1 ratio of red, white, and blue LEDs; and RH that was reduced from 90% to 80% and 60% after 2 and 4 weeks, respectively.
2.2 Light intensity treatments
After a week of preacclimation at 60 µmol m−2 s−1, groups of apple plantlets were subject to a 6-week acclimatization period under one of eight light intensity treatments: light intensity constant group [60 µmol m−2 s−1 (low, L), 100 µmol m−2 s−1 (medium, M), 140 µmol m−2 s−1 (high, H), 180 µmol m−2 s−1 (very high, VH)], and light intensity changed group [60 changed to 100 µmol m−2 s−1 at 2 weeks (L2M4) or 4 weeks (L4M2), 60 changed to 100 µmol m−2 s−1 at 2 weeks changed to 140 μmol m−2 s−1 at 4 weeks (L2M2H2), and 60 changed to 140 μmol m−2 s−1 at 4 weeks (L4H2; Table 1)]. Each plot (80 × 90 cm, L × W) was divided into 12 points, and the light intensity of each point was measured using a photometer (LI-1400; Li-Cor, Lincoln, NE, USA) equipped with a quantum sensor (LI-190R; Li-Cor). Furthermore, during the 6-week acclimatization period, the apple plantlets were rotated daily to ensure uniform light intensity distribution.
2.3 Survival rate
The survival rate of each treatment group was calculated as the percentage of in vitro-propagated apple plantlets that survived the 6-week acclimatization period.
2.4 Chlorophyll fluorescence
The maximum quantum yield of photosystem (PS) II (Fv/Fm) was measured at 4 and 5 weeks after transplantation to assess the effects of light intensity on plant stress levels. For measurement, the plants were subjected to dark conditions for 30 min and then chlorophyll fluorescence was measured using a chlorophyll fluorescence image analyzer (FC-800-O; Photon System Instruments, Ltd., Brno, Czech Republic). All plants were measured using the same set of values (Fluor Cam software v. 2.0; Photon System Instruments), with actinic illumination and super pulse illumination set at 17 and 8%, respectively.
2.5 Growth characteristics
Plant height and stem diameter were measured using a ruler and digital Vernier calipers (NA530-300S; Bluebird, Seoul, Korea), respectively, at 2, 4, and 6 weeks after transplantation. At the end of the experiment, the fresh weights (FWs) of the plantlet shoots and roots were measured using an electronic scale (SI-234; Denver Instrument, Denver, CO, USA), and the dry weights (DWs) of the plantlet shoots and roots were measured after the materials were freeze-dried (Alpha 2-4 lsc plus; Christ, Osterode, Germany) at − 90 °C and 0.5 mbar for 72 h. Finally, the total leaf areas of detached leaves from each plantlet were measured using a leaf area meter (LI-3100C; Li-Cor Inc., Lincoln, NE, USA), and SPAD values, which can be used as an indirect measure of chlorophyll content, were measured three times on the third youngest leaf of each plantlet, using a portable chlorophyll meter (SPAD-502; Konica Minolta, Tokyo, Japan).
2.6 Photosynthetic rate and electron transport rate
The photosynthetic rates of apple plantlets under each light intensity treatment were measured at 5 weeks after transplanting ex vitro using a portable photosynthetic machine (LI-6400; Li-Cor Inc., Lincoln, NE, USA) equipped with a leaf chamber fluorometer (LI-6400-40; Li-Cor Inc., Lincoln, NE, USA). The measurement was conducted at 3 h after the start of the 16-h photoperiod (i.e.,10 a.m.) for 3 h, with measurement conditions that were equivalent to those of the cultivation environment (air flow rate of 300 μmol s−1, CO2 concentration of 500 μmol mol−1, and leaf temperature of 25 °C), and the leaf chamber fluorometer was installed at a height that was the same as the set light intensity to measure photosynthetic rate of the apple plantlet under each of the eight light treatments.
Electron transport rate (ETR) was measured at 6 weeks after transplanting ex vitro. All plants were subjected to dark adaptation for 30 min using leaf clips, and the 4th leaf from the apical meristem was measured using a portable fluorometer (PAM-2000; Heinz Walz GmbH, Effeltrich, Germany). The plants were measured using PamWin software (Walz, Effeltrich, Germany).
2.7 Starch content
The freeze-dried shoot samples were ground at 13,000 rpm using a Tube Mill control (IKA, Wilmington, NC, USA) and stored at 4 °C until analysis. A portion (0.1 g) of each powdered sample was mixed with 10 mL 80% ethanol in a 15-mL conical tube, subjected to ultrasonic extraction (SK5210HP; Hangzhou Nade Scientific Instrument, Zhejiang, China) at 25 °C for 1 h, and then centrifuged (5810R; Eppendorf, Hamburg, Germany) at 4 °C for 10 min at 3250 × g. The resulting pellets were stored at − 80 °C.
The starch contents of the pellets were analyzed using a slightly modified version of the dinitrosalicylic acid method (Miller 1959). Each pellet was dissolved in 2 mL distilled water; autoclaved at 121 °C for 30 min; hydrolyzed by adding 0.2 M Na-acetate buffer (pH 5.5), 1 mL 30 U amyloglucosidase (Sigma-Aldrich), and 1 mL 10 U β-amylase (Sigma-Aldrich); and then centrifuged at 13,000 × g for 10 min. Aliquots (50 μL) of each of the resulting supernatants were mixed with 0.5 mL dinitrosalicylic acid reagent (DNS) and reacted in boiling water for 5 min. After cooling completely, 0.1 mL of each sample was mixed with 0.9 mL distilled water, and the absorbance (525 nm) of each mixture was measured using a spectrophotometer (UV-1800; Shimadzu, Kyoto, Japan). Finally, the starch content of each sample was calculated as milligrams of glucose per shoot DW of in vitro-propagated apple plantlets.
2.8 Statistical analysis
Means of each measurement parameter were calculated from the means of eight replicates per treatment, and analysis of variance (ANOVA) and Duncan’s multiple range test (DMRT) were performed in SAS (SAS 9.4; SAS Institute, Cary, NC, USA).
3 Results
3.1 Survival rate
The VH and H groups exhibited lower survival rates than the other treatments at 1 week after transplantation, and the survival rate of the VH group was only 72% at 3 weeks after transplantation, whereas that of the H group decreased gradually and fell below 80% at 5 weeks after transplantation. The H and VH groups also exhibited the lowest survival rates at 6 weeks after transplantation, with rates of only 78 and 71%, respectively. Meanwhile, the survival rates of the other treatment groups remained at 85% or greater, and the M group exhibited the greatest survival rate (~ 95%) (Fig. 1a).
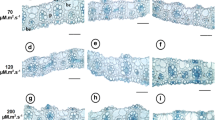
a Effect of acclimatization period on the survival of in vitro-propagated ‘M9’ apple plantlets. b Effect of acclimatization light intensity on the Fv/Fm ratios of in vitro-propagated ‘M9’ apple plantlets at 4 (black symbols) and 5 weeks (white symbols). c In vitro-propagated apple plantlets acclimated under various light intensities for 2 weeks. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level
3.2 Chlorophyll fluorescence
The Fv/Fm values decreased with increasing light intensity at 4 and 5 weeks after transplantation (Fig. 1b). Therefore, the H and VH treatments yielded relatively low Fv/Fm values. The light intensity change treatments resulted in Fv/Fm ratios of 0.75 and 0.71 at 4 and 5 weeks after transplantation, respectively, but these were not significantly different from other treatments (data not shown). At 2 weeks after transplantation, H and VH treated with high light intensity treatment induced damage (Fig. 1c).
3.3 Growth characteristics
The stem diameter of the apple plantlets at 6 weeks after transplantation increased significantly with increasing light intensity, whereas no differences were observed in the increasing-intensity groups (Fig. 2), and the VH and H groups produced stems that were 1.2–1.3 times thicker than those produced by the L group.
Effect of acclimatization light intensity on the stem diameter of in vitro-propagated apple plantlets at 6 weeks after transplanting. Stem diameter was measured as the diameter of the stem of the third leaf from the apical meristem. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level. Different lowercase letters indicate significant differences at p < 0.001 (n = 8)
Figure 3 presents the effect of light intensity at 2–4 weeks and 4–6 weeks after transplantation on the relative growth rates (total leaf area and plant height) of the apple plantlets. The relative growth rates of total leaf area and plant height were similar (Fig. 3a). At 2–4 weeks after transplantation, the L and M groups exhibited higher relative growth rates, and at 4–6 weeks after transplantation, the growth rates were observed to increase gradually with increasing light intensity (L → VH). Meanwhile, at 2–4 weeks after transplantation, the relative growth rates of the increasing-intensity groups were lower than the other groups, and at 4–6 weeks after transplantation, the relative growth rate of the L2M2H2 group was higher than those of the other treatment groups. Furthermore, the M and L2M2H2 groups exhibited the greatest total leaf area at 6 weeks after transplantation, whereas the L and L4H2 groups exhibited the lowest.
Effect of acclimatization light intensity on the growth of in vitro-propagated ‘M9’ apple plantlets. a Relative growth rate and accumulation of total leaf area. b Relative growth rate and accumulation of plant height. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level. Symbols and bars indicate the average values and standard errors of each growth parameter at 6 weeks after transplanting to ex vitro conditions, respectively. Different lowercase letters indicate a significant difference at p < 0.01 (n = 8)
As shown in Fig. 3b, the L group exhibited the greatest relative growth rate for plant height than other treatments, and all light intensity change treatments had similar values as the L treatment at 2–4 weeks after transplantation. However, the relative growth rates of the increasing-intensity groups were all similar to that of the L group, and at 4–6 weeks after transplantation, the relative growth rate of the L group had decreased and was actually lower than the rates exhibited by the other treatment groups. Meanwhile, the relative growth rates of the L2M4 and L2M2H2 groups were higher than that of the M group at 2–4 weeks after transplantation. However, the relative growth rates of all of the increasing-intensity treatments were lower at 4–6 weeks after transplantation than at 2–4 weeks after transplantation. At 6 weeks after transplantation, the height of the M group was significantly greater than that of the other groups and was 30–40% greater than that of the L and L4H2 groups (Figs. 3b, 4).
In vitro-propagated apple plantlets acclimated under various light intensities for 6 weeks. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level
At 2–4 weeks after transplantation, the relative growth rate of shoot FW was greatest in the M group, among the fixed-intensity groups, and among the increasing-intensity groups, the relative growth rates of the L2M4 and L2M2H2 groups were greater than those of the L4M2 and L4H2 groups. At 4–6 weeks after transplantation, the relative growth rate of shoot FW tended to increase with increased light intensity (L → VH). The relative growth rate of shoot FW at 4–6 weeks after transplantation was also similar to that observed for total leaf area (Figs. 3b, 5a). At 6 weeks after transplantation, the H and VH groups exhibited significantly greater shoot FW than the other treatments. However, because the H and VH groups exhibited lower survival rates, owing to initial damages during the acclimation process, the M and L2M2H2 treatments yielded the most promising results overall (Fig. 5a).
Effect of acclimatization to light intensity on the mass accumulation of in vitro-propagated ‘M9’ apple plantlets. a Relative growth rate and accumulation of shoot FW. b Relative growth rate and accumulation of root FW. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level. Symbols and bars indicate the average values and standard errors of each growth parameter at 6 weeks after transplantation to ex vitro conditions, respectively. Different lowercase letters indicate a significant difference at p < 0.01 (n = 8)
Meanwhile, the relative growth rate of root FW at 4–6 weeks after transplantation was 1.5 times greater than that at 2–4 weeks after transplantation. In addition, at 2–4 weeks after transplantation, the relative growth rate of the M group was ~ 3 times greater than that of the other groups, and at 4–6 weeks after transplantation, the relative growth rate of the L4M2 group was the greatest. However, at 6 weeks after transplantation, the L and L4H2 groups exhibited the lowest root FW, whereas the VH group exhibited the greatest root FW (Fig. 5b).
3.4 Photosynthetic rate
The SPAD values of the M and L2M2H2 groups were significantly greater than those of the other six groups at 6 weeks after transplantation (Fig. 6a). In addition, the photosynthetic rate per unit leaf area at 5 weeks after transplantation increased gradually as light intensity increased from L to VH, and the rates of the H, VH, L2M2H2, and L4H2 groups were significantly greater than that of the other group (Fig. 6b). Meanwhile, the electron transport rate (ETR) at 5 weeks after transplantation tended to increase with increasing light intensity; however, no significant differences were observed (Fig. 6c).
a SPAD values of plantlet leaves. b Photosynthetic rate per unit leaf area at 5 weeks. c Electron transport rate of photosystem II at 6 weeks. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level. Different lowercase letters indicate significant differences at p < 0.01 (n = 5)
3.5 Starch content
The starch contents of the M, H, VH, and L2M2H2 groups at 6 weeks after transplantation were significantly greater than those of the other groups (Fig. 7).
Effect of acclimatization to light intensity on the starch contents of in vitro-propagated apple plantlets. L, M, H, and VH indicate low, medium, high, and very high light levels, respectively, as mentioned in Table 1. The subscript numbers indicate the period (week) under each light level. Symbols and bars indicate the average values and standard errors of each growth parameter at 6 weeks after transplantation to ex vitro conditions, respectively. Different lowercase letters indicate a significant difference at p < 0.001 (n = 8)
4 Discussion
4.1 Survival rate and chlorophyll fluorescence
The low light intensity of in vitro culture is considered a limiting factor for photosynthesis under ex vitro conditions, and in vitro-propagated plantlets are vulnerable to photoinhibition under the high light conditions, owing to the limited activation of nonphotochemical quenching (NPQ) (Saez et al. 2012; Van Huylenbroeck et al. 1998). In this study, in vitro-propagated apple plantlets that were preacclimatized at 60 µmol m−2 s−1 failed to exhibit any disorder when transplanted to 60 or 100 µmol m−2 s−1 ex vitro conditions but exhibited damage and low survival lights when transplanted to 140 or 180 µmol m−2 s−1 ex vitro conditions (Fig. 1). Accordingly, light intensities greater than 140 µmol m−2 s−1 appear to cause photoinhibition in in vitro-propagated ‘M9’ apple plantlets.
The reduction of the maximum quantum yield of PS II (Fv/Fm) by light irradiation is sometimes used as an indicator of plant stress (Kumar and Kumar 2003). In this study, Fv/Fm ratios tended to decrease with increasing light intensity, and the H and VH treatments yielded mean values of 0.68 and 0.66, respectively, at 5 weeks after transplantation (Fig. 1b), which suggests that the light levels provided by the H and VH treatments are not appropriate for the acclimation of apple plantlets. Meanwhile, in the increasing-intensity treatments, light intensity failed to affect the Fv/Fm ratios, regardless of growth state, which indicates that gradually increasing light intensity is an effective strategy for reducing plant stress during the acclimatization of in vitro-propagated apple plantlets.
4.2 Growth characteristics
The stem diameter of in vitro-propagated ‘M9’ apple plantlets increased with increasing light intensity under the fixed-intensity treatments but not under the increasing-intensity treatments (Fig. 2). Similarly, continuously low light levels (10% sunlight) have been reported to yield significantly lower stem diameters during the acclimatization of coffee seedlings, and a similar trend was also reported for seedlings grown under changing-intensity treatments (Rodríguez-López et al. 2014). The in vitro-propagated ‘M9’ apple plantlets used in this study are typically used as rootstocks, and rootstock stem diameter is one of the most important factors when scions are grafted onto ‘M9’ rootstocks. Thick and hardened stems are an important requirement for high-quality apple plantlets because it is easy to develop a cambium layer that contains meristematic cells of the graft union. In this study, the H and VH treatments (140 and 180 µmol m−2 s−1) induced stem thickening but also yielded low survival rates, and survival rate is generally considered a priority for the mass production of virus-free apple plantlets. Therefore, stem diameter can only be considered after securing stable survival rates.
The relative growth rate of total leaf area at 2–4 weeks after transplanting decreased with increasing light intensity under the fixed-intensity treatments (Fig. 3a). During the early stages of acclimation, the in vitro-propagated apple plantlets acclimatized under the VH treatment (180 µmol m−2 s−1) exhibited both low levels of photosynthesis and high stress levels, as indicated by Fv/Fm ratios (Fig. 1b). Meanwhile, the relative growth rate of plant height at 4–6 weeks after transplantation tended to increase with increasing light intensity. Indeed, Ko et al. (2018a) reported that greater light intensities were needed to increase active photosynthesis at 4 weeks after the acclimation of ‘M9’ apple plantlets because active growth occurs during this period. This was also supported by the observation that the L2M2H2 treatment yielded high relative growth rates of total leaf area during this period, and the relative growth rate of plant height also exhibited a similar tendency. Rodríguez-López et al. (2014) reported that high levels of photosynthetically active radiation yield increases in both the biomass accumulation and leaf area of greenhouse-grown coffee seedlings.
The relative growth rate of shoot FW was greatest in the M group, and this parameter was associated with high survival rates, by successful acclimation during the initial stage (2 weeks of transplanting) of light treatment (Fig. 1c), as indicated by the high relative growth rates of total leaf area. The relative growth rate of shoot FW at 4–6 weeks after transplantation also increased with increasing light intensity under the fixed-intensity treatments, and shoot FW at 6 weeks after transplantation was significantly greater in the high-light intensity groups (Fig. 4a). These observations could be related to the relative growth rates of total leaf area. When also considering survival rate, it can be concluded that the M and L2M2H2 treatments yielded the best shoot FW results at 6 weeks after acclimatization because the H and VH groups, which exhibited the greatest shoot FWs, simultaneously exhibited low survival rates. The relative growth rate of root FW of the in vitro-propagated ‘M9’ apple plantlets was characterized by dramatically greater increases at 4–6 weeks after transplantation, when compared to that of plantlets at 2–4 weeks after transplantation, which suggests that, during the period of 2–4 weeks after transplantation, plant resources are mainly allocated to acclimation and vascular connection between shoots and roots, rather than to rigorous growth. The apple plantlets used in this study had initially developed roots in the rooting medium, but such root development increases water loss, owing to poor vascular connections between shoots and roots (Fila et al. 1998; Grout and Aston 1977; Hazarika 2003). In addition, James and Thurbon (1979) reported that the ‘M9’ apple plantlets exhibited difficulties in root development and growth after transplantation to ex vitro conditions. With the exception of the H and VH treatments, the M, L2M2H2, and L4H2 treatments were the best in terms of final root FW.
4.3 Photosynthetic rate
In this study, light intensity was observed to affect chlorophyll content, which is closely related to the rate of photosynthesis. The M group exhibited the greatest chlorophyll content, whereas the levels of the L and VH groups were significantly lower. This indicates that either low or excessively high light levels can inhibit chlorophyll biosynthesis. However, a previous study reported that the chlorophyll content of lettuce was reduced under high light intensity (800 μmol m−2 s−1) and was greatest under low light intensity (100 μmol m−2 s−1; Fu et al. 2012). Meanwhile, the photosynthetic rates observed in this study increased with increasing light intensity, and even under the increasing-intensity treatments, the light intensity of 140 μmol m−2 s−1 yielded greater photosynthetic rates at 6 weeks than the light intensity of 100 μmol m−2 s−1 (Fig. 6c). Therefore, light intensities of 100 μmol m−2 s−1 or greater may promote photosynthesis during the acclimatization of in vitro-propagated apple plantlets, at least at 5 weeks after transplantation. The ETR results also suggested that photosynthetic rate increased with increasing light intensity.
4.4 Starch content
The starch content of apple plantlets did not effectively accumulate under low light intensity (60 μmol m−2 s−1) (Fig. 7). In the treatments with a long irradiation period of 60 μmol m−2 s−1, such as L4M2 and L4H2, the starch content was low. This suggests that light intensities greater than 60 μmol m−2 s−1 are required for the normal growth and development of in vitro-propagated ‘M9’ rootstock apple plantlets. A study conducted with wheat showed a similar result as our study. Low light intensity impeded starch production of wheat grains due to an increased sucrose ratio and inhibition of net carbon fixation and sucrose transport by abscisic acid and gibberellin (Mengel et al. 1985; Mengin et al. 2017).
Sufficient light intensity is a crucial element to produce photosynthetic CO2 assimilates for growth and development because light serves as a direct energy source for the process (Shi et al. 2018; Stitt and Zeeman 2012). Starch, together with sucrose and sorbitol, is a major end product of photosynthesis in plants; therefore, a high photosynthetic rate should increase the starch content (Escobar-Gutiérrez and Gaudillère 1997). In our study, photosynthetic rate was closely associated with starch content of apple plantlets; decreased photosynthetic rate was observed in low light intensity (60 μmol m−2 s−1), and subsequently the starch content of apple plantlets was low (Figs. 6, 7), while enhanced photosynthetic rate under higher light intensity improved the starch content.
5 Conclusion
This study demonstrated that moderate (60 μmol m−2 s−1) and gradually increasing (L2M2H2: 60 → 100 → 140 μmol m−2 s−1) light intensity conditions yielded the best results in terms of apple plantlet survival when using a 6-week ex vitro acclimatization period.
References
Ali MB, Hahn EJ, Paek KY (2005) Effects of light intensities on antioxidant enzymes and malondialdehyde content during short-term acclimatization on micropropagated Phalaenopsis plantlet. Environ Exp Bot 54:109–120. https://doi.org/10.1016/j.envexpbot.2004.06.005
Baťková P, Pospíšilová J, Synkova H (2008) Production of reactive oxygen species and development of antioxidative systems during in vitro growth and ex vitro transfer. Biol Plant 52:413–422. https://doi.org/10.1007/s10535-008-0085-5
Campbell AI (1962) Apple virus inactivation by heat therapy and tip propagation. Nature 195:520. https://doi.org/10.1038/195520a0
Carvalho LC, Osório ML, Chaves MM, Amâncio S (2001) Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and chestnut plantlets under ex vitro acclimatization. Plant Cell Tissue Organ Cult 67:271–280
Carvalho LC, Amâncio S (2002) Antioxidant defense system in plantlets transferred from in vitro to ex vitro: effects of increasing light intensity and CO2 concentration. Plant Sci 162:33–40. https://doi.org/10.1016/s0168-9452(01)00524-6
Cembali T, Folwell RJ, Wandschneider P, Eastwell KC, Howell WE (2003) Economic implications of a virus prevention program in deciduous tree fruits in the US. Crop Prot 22:1149–1156. https://doi.org/10.1016/s0261-2194(03)00156-x
Chatenet M, Delage C, Ripolles M, Irey M, Lockhart BEL, Rott P (2001) Detection of Sugarcane yellow leaf virus in quarantine and production of virus-free sugarcane by apical meristem culture. Plant Dis 85:1177–1180. https://doi.org/10.1094/pdis.2001.85.11.1177
Dami I, Hughes HG (1997) Effects of PEG-induced water stress on in vitro hardening of ‘Valiant’grape. Plant Cell Tissue Organ Cult 47:97–101. https://doi.org/10.1007/bf02318944
Escobar-Gutiérrez AJ, Gaudillère JP (1997) Carbon partitioning in source leaves of peach, a sorbitol-synthesizing species, is modified by photosynthetic rate. Physiol Planta 100:353–360. https://doi.org/10.1034/j.1399-3054.1997.1000218.x
Estrada-Luna AA, Davies FT, Egilla JN (2001) Physiological changes and growth of micropropagated chile ancho pepper plantlets during acclimatization and post-acclimatization. Plant Cell Tissue Organ Cult 66:17–24
Faisal M, Anis M (2009) Changes in photosynthetic activity, pigment composition, electrolyte leakage, lipid peroxidation, and antioxidant enzymes during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets. Plant Cell Tissue Organ Cult 99:125–132. https://doi.org/10.1007/s11240-009-9584-0
Faisal M, Anis M (2010) Effect of light irradiations on photosynthetic machinery and antioxidative enzymes during ex vitro acclimatization of Tylophora indica plantlets. J. Plant Interact 5:21–27. https://doi.org/10.1080/17429140903137652
Fila G, Ghashghaie J, Hoarau J, Cornic G (1998) Photosynthesis, leaf conductance and water relations of in vitro cultured grapevine rootstock in relation to acclimatisation. Physiol Plant 102:411–418. https://doi.org/10.1034/j.1399-3054.1998.1020309.x
Fu W, Li P, Wu Y (2012) Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci Hortic 135:45–51. https://doi.org/10.1016/j.scienta.2011.12.004
Grout BWW, Aston MJ (1977) Transplanting of cauliflower plants regenerated from meristem culture. I. Water loss and water transfer related to changes in leaf wax and to xylem regeneration. Hortic Res 17:1–7
Guan QZ, Guo YH, Sui XL, Li W, Zhang ZX (2008) Changes in photosynthetic capacity and antioxidant enzymatic systems in micropropagated Zingiber officinale plantlets during their acclimation. Photosynthetica 46:193. https://doi.org/10.1007/s11099-008-0031-y
Hansen AJ, Lane WD (1985) Elimination of apple chlorotic leafspot virus from apple shoot cultures by ribavirin. Plant Dis 69:134–135
Hayashi M, Nakayama M, Kozai T (1988) An application of the acclimatization unit for growth of carnation explants, and for rooting and acclimatization of the plantlets. Acta Hortic 230:189–194. https://doi.org/10.17660/actahortic.1988.230.22
Hazarika BN (2003) Acclimatization of tissue-cultured plants. Curr Sci 85:1704–1712
Hu G, Dong Y, Zhang Z, Fan X, Ren F, Zhou J (2015) Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue Organ Cult 121:435–443. https://doi.org/10.1007/s11240-015-0714-6
James DJ, Thurbon IJ (1979) Rapid in vitro rooting of the apple rootstock M.9. J Hortic Sci 54:309–311. https://doi.org/10.1080/00221589.1979.11514887
Jeon MW, Ali MB, Hahn EJ, Paek KY (2006) Photosynthetic pigments, morphology and leaf gas exchange during ex vitro acclimatization of micropropagated CAM Doritaenopsis plantlets under relative humidity and air temperature. Environ Exp Bot 55:183–194. https://doi.org/10.1016/j.envexpbot.2004.10.014
Kim JH, Kim CC, Ko KC, Kim KR, Lee JC (1998). The particular of pomology, Ed 4. Hyangmunsa, Korea, pp 41–45
Ko SM, Lee JH, Oh MM (2018a) Control of relative humidity and root-zone water content for acclimation of in vitro-propagated ‘M9’ apple rootstock plantlets. Hortic Environ Biotechnol 59:303–313. https://doi.org/10.1007/s13580-018-0038-7
Ko SM, Lee JH, Oh MM (2018b) Development of nutrient solution for in vitro propagation of ‘M9’apple rootstock plantlets. Hortic Sci Technol 36:202–214. https://doi.org/10.12972/kjhst.20180021
Kumar R, Kumar N (2003) High irradiance-induces changes in carotenoids compositions and increase in non-photochemical quenching of Chl fluorescence in primary wheat leaves. J Plant Physiol 160:1141–1146. https://doi.org/10.1078/0176-1617-01069
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304. https://doi.org/10.1093/jexbot/53.372.1283
Miller GL (1959) Modified DNS method for reducing sugars. Anal Chem 31:426–428
Mengel K, Friedrich B, Judel GK (1985) Effect of light intensity on the concentrations of phytohormones in developing wheat grains. J Plant Physiol 120:255–266. https://doi.org/10.1016/s0176-1617(85)80112-7
Mengin V, Pyl ET, Alexandre Moraes T, Sulpice R, Krohn N, Encke B, Stitt M (2017) Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant Cell Environ 40:2608–2627. https://doi.org/10.1111/pce.13000
Müller P, Li XP, Nigogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566. https://doi.org/10.1104/pp.125.4.1558
Osório ML, Osório J, Romano A (2010) Chlorophyll fluorescence in micropropagated Rhododendron ponticum subsp. baeticum plants in response to different irradiances. Biol Plant 54:415–422. https://doi.org/10.1007/s10535-010-0076-1
Paprstein F, Sedlak J, Polak J, Svobodova L, Hassan M, Bryxiova M (2008) Results of in vitro thermotherapy of apple cultivars. Plant Cell Tissue Organ Cult 94:347–352. https://doi.org/10.1007/s11240-008-9342-8
Pinto G, Silva S, Loureiro J, Costa A, Dias MC, Araújo C, Neves L, Santos C (2011) Acclimatization of secondary somatic embryos derived plants of Eucalyptus globulus Labill.: an ultrastructural approach. Trees 25:383–392. https://doi.org/10.1007/s00468-010-0513-y
Pospíšilová J, Wilhelmová NA, Synková H, Čatský J, Krebs D, Tichá I, Hanáčková B, Snopek J (1998) Acclimation of tobacco plantlets to ex vitro conditions as affected by application of abscisic acid. J Exp Bot 49:863–869. https://doi.org/10.1093/jxb/49.322.863
Pospís̆ilová J, Synková H, Haisel D, Čatský J, Wilhelmová NA, Šrámek F (1999) Effect of elevated CO2 concentration on acclimation of tobacco plantlets to ex vitro conditions. J Exp Bot 50:119–126
Pospóšilová J, Tichá I, Kadleček P, Haisel D, Plzáková Š (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497. https://doi.org/10.1093/jxb/50.330.119
Rodríguez-López NF, Martins SC, Cavatte PC, Silva PE, Morais LE, Pereira LF, Reis JV, Ávila RT, Godoy AG, Lavinski AO, DaMatta FM (2014) Morphological and physiological acclimations of coffee seedlings to growth over a range of fixed or changing light supplies. Environ Exp Bot 102:1–10. https://doi.org/10.1016/j.envexpbot.2014.01.008
Saez PL, Bravo LA, Sáez KL, Sánchez-Olate M, Latsague MI, Ríos DG (2012) Photosynthetic and leaf anatomical characteristics of Castanea sativa: a comparison between in vitro and nursery plants. Biol Plant 56:15–24. https://doi.org/10.1007/s10535-012-0010-9
Shi K, Gu X, Lu W, Lu D (2018) Effects of weak-light stress during grain filling on the physicochemical properties of normal maize starch. Carbohydr Polym 202:47–55. https://doi.org/10.1016/j.carbpol.2018.08.114
Stitt M, Zeeman SC (2012) Starch turnover: pathways, regulation and role in growth. Curr Opin Plant Biol 15:282–292. https://doi.org/10.1016/j.pbi.2012.03.016
Van Huylenbroeck JM, Piqueras A, Debergh PC (1998) Photosynthesis and carbon metabolism in leaves formed prior and during ex vitro acclimatization of micropropagated plants. Plant Sci 134:21–30. https://doi.org/10.1016/s0168-9452(98)00043-0
Walkey DGA (1968) The production of virus-free rhubarb by apical tip-culture. J Hortic Sci 43:283–287. https://doi.org/10.1080/00221589.1968.11514255
Acknowledgements
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the Agri-Bio Industry Technology Development Program, which is funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA; Grant No. 315003051SB020).
Author information
Authors and Affiliations
Contributions
GJ conducted the measurements and data analysis and drafted the manuscript. JH helped with some of the measurements. MM provided substantial guidance regarding experiment design and critically revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Heakeun Yun, Ph.D.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, GJ., Lee, JH. & Oh, MM. Growth and acclimation of in vitro-propagated ‘M9’ apple rootstock plantlets according to light intensity. Hortic. Environ. Biotechnol. 61, 501–510 (2020). https://doi.org/10.1007/s13580-020-00247-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-020-00247-5