Abstract
Attempts of clonal propagation of sugar maple through conventional and micropropagation techniques have been largely unsuccessful and the basis for this longstanding problem has remained unknown. Typical symptoms of stress in in vitro cultures of sugar maple and its intrinsic tolerance to highly shaded growing conditions indicated that optimization of light intensity might overcome its recalcitrance. To test this hypothesis we evaluated the growth, morphology, chlorophyll content and various chlorophyll fluorescence parameters of in vitro plantlets cultured under three intensities (4, 16 and 40 µmol m−2 s−1) using two spectra of light (red-blue and full spectrum white). Higher light intensities increased fresh and dry mass, but had no significant effect on the number of nodes or plant height. Anthocyanin accumulation in tissues, reduction in chlorophyll content, and significantly increased physiological stress quantified by chlorophyll fluorescence parameters, indicated increasing levels of light induced damage to the tissues with increasing light intensities. The critical factor limiting sugar maple’s response to in vitro propagation was light intensity and the levels commonly used for in vitro propagation are not suitable for sugar maple. The current study demonstrates the unique low light requirement, between 4 and 16 µmol m−2 s−1, of this species to overcome the recalcitrance to in vitro propagation. Spectral differences in quality of light and its effect on in vitro growth and stress in cultures are also discussed. In general light induced stress symptoms begin to appear at lower intensities with RB spectrum as compared to the full spectrum lights. While this study is limited to maples, requirement of specific light intensity is likely the cause of recalcitrance in other woody species with similar ecological roles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugar maple (Acer saccharum Marsh.) is an important hardwood tree of North American forests. Commercially, it is valued for its dense wood, appealing fall colours, and sugary sap for making maple syrup (Kriebel and Gabriel 1969; Morselli 1989; Godman et al. 1990; Larochelle et al. 1998). It is heterozygous and heterogeneous in nature (Young et al. 1993; Graignic et al. 2013) and there is considerable genetic variation in its lumber quality, growth pattern and sap sugar content (Taylor 1956; Kriebel 1957, 1989, 1990; Wilkinson 1984; Godman et al. 1990; Laing and Howard 1990; Larochelle et al. 1998). Breeding sugar maple for specific traits is challenging because of long generation time, with trees often requiring more than 20 years before they first set seed (Godman et al. 1990). However, previous breeding efforts have been conducted and were successful in producing trees with increased sugar content compared to unselected populations, demonstrating a genetic basis for the trait and producing a series of elite “sweet” trees (Kriebel 1990). The genetic basis has subsequently been confirmed using RAPD markers that found 30% of variation in sap sugar content could be explained by just four markers (Roy et al. 1997). As such, while there are no active breeding programs for this species, superior germplasm from previous breeding efforts and natural stands provide a source of elite planting material.
To utilize superior trees, efficient vegetative propagation systems are needed. Vegetative propagation of this species through cuttings has been largely unsuccessful, with low percentage of rooting, high mortality, non-reproducible results, significant year to year variation, genotype and season dependent responses, and an overall very low rate of success that makes it commercially impractical (Gabriel et al. 1961; Godman et al. 1990; Laing and Howard 1990; Henry and Preece 1997; Tousignant et al. 2003; Richer et al. 2004; Sutanto 2010; Roussy 2014). While grafting has been reported in sugar maple, there is a considerable scion-rootstock interactions related to sap sugar content (Kriebel 1989) and this method is time consuming and expensive (Tousignant et al. 2003). Therefore, conventional propagation methods appear unsuitable for propagating elite sugar maples to improve syrup production. Application of micropropagation techniques to overcome these limitations would be an obvious method of choice, however, micropropagation too has been largely unsuccessful in sugar maple. Hanus and Rohr (1984) reported poor in vitro response and halted growth of microcuttings. In another study, sugar maple produced fewer axillary shoots in vitro compared to red maple and resulted in no rooted plants (Connolly et al. 1998). The problems of in vitro propagation are explicitly described by Brassard et al. (2003), where they report a low overall rate of success and large losses at each step of in vitro propagation. As a result, from over 1417 explants from 208 clones that were initiated with 7 shoot induction treatments, 36 multiplication treatments and 9 rooting treatments, only 7 rooted plantlets from 3 clones were produced and no viable micropropagation system was developed. In this study, they mention that the low survival rate is not due to contamination and is likely a result of “cumulative stress” imposed upon explants, resulting in poor survival and proliferation (Brassard et al. 2003). Similar results were reported by Sutanto (2010), where only a small percentage of embryo explants displayed shoot proliferation and those that survived failed to form multiple shoots. Additionally, the explants developed an excessive amount of anthocyanins resulting in purple coloured tissues, which is generally considered a sign of plant stress (Sutanto 2010).
While it is widely accepted that sugar maple performs poorly in vitro, the basis for this longstanding problem has remained unknown and this promising technology has not be developed for this species. The stress symptoms limiting the in vitro propagation of sugar maple include accumulation of anthocyanins resulting in excessive red/purple pigmentation of tissues, gradual loss of chlorophylls, yellowing of explants and ultimately death of the tissues (Sutanto 2010). Biosynthesis and accumulation of anthocyanins often occurs in response to high light intensity (Vanderauwera et al. 2005) and they play a photo-protective role by shielding photosynthetic apparatus of the cells from excessive light and aiding in reabsorption of foliar nutrients before senescence (Hoch et al. 2003; Schaberg et al. 2008). Bleaching of leaves, reduction of chlorophyll and yellowing of tissues are also typical of high light induced photo-inhibition damage (Powles 1984; Muller et al. 2001). As such, the symptoms described in sugar maple during micropropagation are consistent with light induced stress.
In nature, young sugar maples are shade-tolerant, forest floor/understory plants, well adapted to surviving in deeply shaded low light conditions (Baker 1949; Hinckley et al. 1978; Niinemets and Tenhunen 1997; Beaudet and Messier 1998). Photosynthesis in sugar maple can saturate even at low light intensities (Wuenscher and Kozlowski 1970; Veeranjaneyulu et al. 1991). Since sugar maple prefers shade in its natural habitat (Logan 1965) and the stress symptom reported in literature are typical of plants in growing in excessive light, we evaluated the effect of light intensities and spectra on in vitro growth and development. Our results show that the light intensity most commonly used in most micropropagation systems (~40 µmol m−2 s−1), is too high for sugar maple and in vitro multiplication could be achieved by reducing the light intensity, laying the groundwork for in vitro propagation of this species.
Materials and methods
Plant material and general culture conditions
Mature seeds of Acer saccharum procured from Ontario Tree Seed facility, Angus, ON, were surface sterilized using 10% Bleach (Clorox® Regular Bleach (5–10% Sodium hypochlorite solution), The Clorox Company of Canada Ltd., ON, Canada) for 10 min followed by three washes with sterile distilled water. The embryonic axes were aseptically excised as described previously (Janerette 1978; Sutanto 2010) and inoculated onto DKW (Driver and Kuniyuki 1984) basal medium with vitamins (PhytoTechnology Laboratories®, Shawnee Mission, KS, US) and 3% sucrose with the pH adjusted to 5.7 prior to autoclaving at 121 °C temperature, 118 kpa pressure for 20 min. The resulting plantlets were subcultured and multiplied on DKW medium with vitamins supplemented with 2 µM 6-Benzylamino Purine (BA), 1 µM Gibberellic acid (GA3), 1 µM α-(p-Chlorophenoxy)isobutyric acid (PCIB) and 3% sucrose, pH adjusted to 5.7, solidified using phytagel™ at 2.2 g L−1, for multiplication of cultures in magenta boxes (GA-7; Caisson Laboratories, Inc., US). All growth regulators and phytagel were procured from Sigma-Aldrich Co. LLC.
Initial attempts at culturing the explants under approx. 40 µmol m−2 s−1 light intensity from 30 W cool while fluorescent lamps (Sylvania, Mississauga, ON) resulted in yellowing and death of tissues, therefore, to multiply the tissues for further experimentation, the cultures were maintained at approx. 5 µmol m−2 s−1 diffused light intensity and 16 h light and 8 h dark photoperiod at 24 ± 2 °C. For the experiments, only the apical bud (~2 mm tip) with any opened leaves removed were used (Supplementary Fig. 1). The apical buds were separately cultured onto DKW medium with vitamins supplemented with 2 µM BA, 1 µM GA3 and 3% sucrose, pH adjusted to 5.7, solidified using phytagel™ at 2.2 g L−1. New clear polycarbonate culture vessels and lids (Magenta™ boxes—GA-7; Caisson Laboratories, Inc., US) were used for uniformity of light reaching the explants. All of the observations were recorded 6 weeks after inoculation.
Light treatments
Custom-made growth chambers fitted with dimmable LED lamps of two different spectra of lights were used in this study (Supplementary Fig. 2a, b). A spectrum composed mainly of red and blue (RB) wavelengths of light was selected as these wavelengths are known to have highest photosynthetic efficiency (McCree 1972; Inada 1976). A full spectrum light providing light across the complete visible wavelengths of light as well as far-red was also selected so that the phytochrome responses similar to sunlight could be achieved and to study if this spectral difference of light had any effect on plant growth. The RB spectrum had about 14, 7 and 79% of light in blue, green-yellow and red spectrum, respectively, with major peaks at 450 and 660 nm and an additional peak at 625 nm wavelength (Supplementary Fig. 2c). The full spectrum light had broadly distributed light with 25, 37 and 38% light in blue, green-yellow and red spectrum, respectively, with additional 8.5% light in the far-red wavelengths (Supplementary Fig. 2c). Twelve-inch long LED lamps, each with a combination of 15 diodes producing light of different wavelengths, of GrowMax™ and SolarMax™ lamp series (BML Horticulture, Texas, USA), were used to generate the RB spectrum and full spectrum lights, respectively. The in vitro plantlets were grown in the dark as well as under three light intensities separately in each chamber from each of the selected sources of light at 4 (±2), 16 (±1) and 40 (±1) µmol m−2 s−1, hereinafter referred to as low, intermediate and high light intensities, respectively. The uniformity of light intensity in each chamber was evaluated by plotting the intensity values across the area of each growth chamber and the culture vessels were kept for treatment only in the central area showing uniform intensity. All measurements on light intensity and spectrum were made using a light spectrometer (model: USB 2000+, Ocean Optics, Inc., FL, USA). In summary, there were three light intensity treatments from each of the two different spectra of light in addition to no light (dark treatment), forming seven treatments in each replication. The experiment was laid out as a randomised complete block design with two replications, with each replication in a separate set of chambers and separated over a time. Each treatment was applied to six magenta boxes per replication.
Morphological observations
Observations on shoot height (cm), number of nodes, total fresh weight of the tissue (including callus) (g), fresh weight of the shoots (g), dry weight of the shoots (g) were recorded after measuring the chlorophyll fluorescence and chlorophyll content. All observations are reported as mean ± standard error.
Chlorophyll content
The chlorophyll content of the in vitro leaves was estimated using a modulated ratio fluorescence chlorophyll fluorometer (CCM-300, Opti-Sciences, Hudson, NH, USA) based on the method developed by Gitelson et al. (1999). Five measurements were taken from each plantlet to represent the chlorophyll content of each treated plantlet. The results are expressed as chlorophyll content (mg m−2) and reported as mean ± standard error.
Chlorophyll fluorescence kinetics imaging
Chlorophyll fluorescence kinetics assays were performed on intact dark adapted (>48 h) plantlets using a chlorophyll fluorescence imaging system (Z200 Open FluorCam, Qubit Systems Inc., Kingston, ON, Canada). A saturating pulse of ~1600 µmol m−2 s−1 of light for 960 ms was used to generate FM values. The measuring pulses were less than 1.65 µmol m−2 s−1 of flickering red light. The fluorescence kinetics values were averaged over time for each treatment for plotting the fluorescence kinetics graphs.
The following parameters were measured/calculated from the fluorescence values:
-
F0 (minimum fluorescence in dark adapted state)
-
FM (maximum fluorescence in dark adapted state)
-
Fv = FM − F0 (variable fluorescence in dark adapted state)
-
QY max = Fv/FM (maximum PSII quantum yield)(Genty et al. 1989).
-
NPQ = (FM − FM_Lss)/FM_Lss (steady-state non-photochemical quenching)(Horton and Ruban 1992).
-
Rfd = (FP − Ft_Lss)/Ft_Lss (fluorescence decline ratio in steady state also known as vitality index) (Lichtenthaler and Miehe 1997).
-
Fo Lss (steady state minimum fluorescence in light)
-
Fv Lss (steady state variable fluorescence in light)
-
Fp (peak fluorescence during initial phase of the Kautsky effect)
-
Fv/FM_Lss = (FM_Lss − F0_Lss)/FM_Lss (PSII quantum yield of light adapted sample at steady-state)(Oxborough 2004).
-
qP Lss (coefficient of photochemical quenching in steady state)
The results are expressed as mean ± standard error for each of the parameters reported.
Statistics
The experiment was conducted as a randomised complete block design with two replications, each in a separate set of chambers and separated over a time. Each treatment was applied to six magenta boxes per replication. The data was analysed using proc glimmix procedure in SAS University Edition software (SAS Institute Inc, Cary, NC, USA). Residuals for each treatment were analysed separately for each parameter to check the fulfilment of assumptions of analysis of variance. Appropriate transformation of data and alternate covariance structures were entertained to meet the assumptions of variance analysis. Briefly, log transformation was used for number of nodes, weight measurements, Fo, Fm, Fv, Fo Lss, and Fp, whereas, arcsine square-root transformation was used for QY max, Rfd and QY Lss data. For presentation of the data, the mean estimates and the corresponding standard errors were converted to the data scale by back-transformation and delta method, respectively (Bowley 2015). No transformation was needed for shoot height, chlorophyll content, NPQ, Fv/Fm Lss and qP Lss data. The means were compared using Tukey’s test at α = 0.05 significance level.
Results
Morphological observations
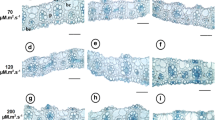
The visual appearance of the plants indicated increasing levels of photo-inhibition symptoms with increasing light intensity (Fig. 1a). Production and accumulation of anthocyanins, evident by red pigmentation of the tissues, was clearly seen in plants cultured under 40 µmol m−2 s−1 of light for both spectra of light. In contrast, the leaves of plants cultured in lower light intensities (4 µmol m−2 s−1) were dark green. The leaves of plants cultured under 16 µmol m−2 s−1 of light showed intermediate greenness and exhibited some stress symptoms. While anthocyanin and general appearance of the plants are qualitative visual observations, the symptoms of stress increased with increasing light intensity under both sources of light.
Effect of light intensity and spectra on growth of in vitro plantlets. Typical in vitro plantlets developed under two different spectra of light each at three intensities (bar 1 cm) (a). False colour images of chlorophyll fluorescence parameters viz., F 0 minimum fluorescence in dark adapted state, F M maximum fluorescence in dark adapted state, QY max maximum PSII quantum yield, NPQ steady-state non-photochemical quenching, Rfd fluorescence decline ratio in steady state, of the same plantlets (b)
No significant differences were observed among the treatments for shoot height or number of nodes. The total fresh weight of the tissue, fresh weight of the shoot with callus removed, and dry weight of the shoot increased with increasing light intensity with both spectra of light. There were significant differences among low light treatments compared to the intermediate or high light treatments for total fresh weight. The treatments differed markedly for shoot dry weight among all the light intensity treatments with RB spectrum, whereas, only the low light treatment differed significantly from the intermediate and high light intensity treatments with full spectrum light (Fig. 2). In general, higher levels of light increased fresh and dry mass of the in vitro plants, but not the multiplication rate based on the number of nodes or plant height.
Data on morphology of in vitro plantlets of sugar maple raised under different light quality treatments, shoot height (a), number of nodes (b), total fresh weight (c), shoot fresh weight (d), shoot dry weight (e), callus fresh weight (f). Data represents mean ± standard error from about six plantlets per treatment and two replications. Means with the same letter in the graphs are not significantly different using Tukey’s test at α = 0.05 significance
Chlorophyll content measurement
The plants in complete dark were etiolated and exhibited significantly lower chlorophyll than any of the treatments (Fig. 1a). The chlorophyll content (mg m−2) of the plantlets decreased with increasing light intensity under both spectra of light (Fig. 3). In general, the plantlets under light intensities of 40 µmol m−2 s−1 show significantly lower chlorophyll content with both sources of lights, and these results corroborate well with the visual appearance of the plants indicating photo-bleaching of the in vitro leaf tissues under high light intensity treatments.
Chlorophyll content of in vitro leaves raised under different light quality treatments. The data is represented as mean ± standard error of two replications with about six plants per treatment per replication. Means with the same letter in the graphs are not significantly different using Tukey’s test at α = 0.05 significance
Chlorophyll a fluorescence kinetics and imaging assay
Chlorophyll a fluorescence Kinetics
The average values of chlorophyll fluorescence kinetics revealed differences in fluorescence among the treatments (Fig. 4). The fluorescence intensity reduced with increasing light intensity of RB spectrum. With full spectrum light the fluorescence intensity was higher for intermediate light intensity treatment compared to other treatments. The fluorescence from the plantlets grown in complete darkness were markedly lower in intensity indicating extremely poor physiological condition of these plantlets due to complete absence of light. The rate of decline of fluorescence indicated by the kinetics slope between 30 and 100 s indicated that though the initial fluorescence levels are higher in the low light treatments, the photosynthetic ability of leaves under intermediate and high light intensity is better. The fluorescence signal intensity from the plantlets under high light intensity were quite lower than other treatments where light was provided indicating manifestation of physiological stress in these tissues. Overall, the plantlets growing under intermediate intensity full spectrum light showed kinetics typical of optimal growth conditions.
Chlorophyll a fluorescence parameters
No significant differences were observed among the treatment in the base fluorescence levels under dark adapted state (F0) except in the dark treatment (Figs. 1b, 5). Considerable differences were observed in other fluorescence parameters indicating stress with increasing levels of light intensity. The maximum fluorescence (FM), variable fluorescence (Fv), were significantly lower in plantlets grown under 40 µmol m−2 s−1 light intensity with both sources of light, indicating manifestation of light intensity induced stress.
Data on various chlorophyll a fluorescence parameters from in vitro plantlets developed under different light quality treatments. Fo-minimum fluorescence in dark adapted state (a), Fm maximum fluorescence in dark adapted state (b), Fv variable fluorescence in dark adapted state (c), QY max maximum quantum yield of photosystem II (d), NPQ non-photochemical quenching in steady state (e), Rfd fluorescence decline ratio or vitality index (f). The data is represented as mean ± standard error of two replications with about six plants per treatment per replication. Means with the same letter in the graphs are not significantly different using Tukey’s test at α = 0.05 significance
A maximum quantum yield (QY Max, or Fv/Fm ratio) value of 0.83 or lower is considered an indicator of stress, specifically indicating photoinhibition (Maxwell and Johnson 2000). The maximum quantum yield of plants growing under low intensity of RB spectrum light was significantly higher than the plants grown under intermediate and high light intensity of the same light. With the RB spectrum light, the plantlets grown under low light had an Fv/Fm ratio of 0.84, whereas the plantlets developed under intermediate and high light had values of 0.81 and 0.79, respectively. In case of full spectrum light, the maximum quantum yield values from plants grown under low and intermediate light intensity were significantly higher than under high light intensity treatment. Under the full spectrum light, the maximum quantum yield values were 0.85, 0.83 and 0.77 for low, intermediate and high light intensity treatments, respectively (Fig. 5). With both the sources of light, the light intensity at 40 µmol m−2 s−1 was stressful for the plantlets and exhibited considerable photoinhibition. A similar trend was observed for Fv/Fm ratios under the light steady state (Fv/Fm Lss), which showed significant differences among the plants grown under low and high light intensities with both spectra of light (Supplementary Fig. 3).
Non photochemical quenching (NPQ) in leaves developed under low light intensity was significantly lower than the NPQ of leaves grown under intermediate and high intensity light with both spectra of lights used in the study, indicating increased NPQ with increasing light intensities. Occurrence of significantly higher NPQ with light intensity of 16 and 40 µmol m−2 s−1 with both spectra of light, also indicated low light requirement of the plantlets and a considerable increase of physiological stress under intermediate and high light intensities.
Compared to other fluorescence parameters, the fluorescence decline ratio/vitality index values of plantlets grown under intermediate and high light intensities were significantly higher than for plantlets raised under low light with both sources of light (Figs. 1b, 5). This is also evident from the rate of decline of fluorescence between 30 and 100 s from the fluorescence kinetics from the plantlets grown under different light intensities (Fig. 4), indicating suitability of intermediate light intensities for in vitro growth of plantlets over the low and high light intensity treatments.
The other fluorescence parameters viz., base fluorescence in light adapted state (Fo Lss), steady state variable fluorescence (Fv Lss), peak fluorescence during initial phase of the Kautsky effect (Fp), steady state photosystem II quantum yield (QY Lss), quantum yield of photosystem II (light adapted) (Fv/Fm Lss), coefficient of photochemical quenching in steady state (qP Lss) is presented in the Supplementary Fig. 3. In general, these chlorophyll a fluorescence parameters too indicated elevated stress conditions in the plants under high light intensity.
Discussion
Recalcitrance and poor growth of in vitro tissues of sugar maple has been a long standing problem in developing an efficient micropropagation system for this species (Hanus and Rohr 1984; Connolly et al. 1998). Although considerable efforts were made to develop in vitro multiplication protocols, little success has been achieved using a number of different plant growth regulator combination or modified sources and concentration of sugars in the medium (Brassard et al. 2003; Sutanto 2010). The failure in achieving acceptable micropropagation of sugar maple in previous studies has been associated with a cumulative stress imposed upon cultured explants, which resulted in poor culture initiation, gradual loss of cultures at each stage of micropropagation, and extremely poor survival and proliferation. Visual symptoms of stress in these investigations included excessive red or purple pigmentation of tissues, gradual loss of chlorophyll, yellowing of explants and ultimately death of the tissues (Brassard et al. 2003; Sutanto 2010). Although these symptoms could result from a variety of stresses, the current study demonstrates that light intensity is the primary source. Reducing the light intensity alone mitigated the stress symptoms completely and very good survival of cultures was achieved. Continued culturing of in vitro plantlets at intensities between 4 and 16 µmol m−2 s−1 subsequently resulted in very efficient multiplication of in vitro cultures, which has not previously been reported.
Sugar maple is known to be one of the most shade tolerant plant species (Baker 1949; Hinckley et al. 1978; Niinemets and Tenhunen 1997; Beaudet and Messier 1998). The low light requirement of sugar maple in its natural growing conditions is reflected in the light requirements for its in vitro propagation. Based on the morphology of the in vitro plants, chlorophyll content and chlorophyll fluorescence parameters, in vitro plantlets that developed under the commonly used light intensity of 40 µmol m−2 s−1 are considerably stressed. A light intensity of 80 µmol m−2 s−1 was used by Brassard et al. 2003, and use of light intensity at this level likely resulted in the reported losses. Similar results of saturation of photosynthesis in sugar maple leaves at relatively low light in vivo are previously reported from plants grown in growth chambers (Wuenscher and Kozlowski 1970; Veeranjaneyulu et al. 1991). Our initial efforts on in vitro multiplication using shoot tips from mature trees under 40 µmol m−2 s−1 light from cool while fluorescent lamps exhibited similar bleached leaves, yellowing, and death of tissues. Our further attempts to multiply in vitro plantlets from embryonic axis under this light intensity also exhibited similar stress symptoms and the cultures could only be multiplied under low light intensity.
The evaluation of morphological parameters like plant height and the number of nodes, indicated no significant differences among any of the treatments. However, there was a significant increase in the fresh and dry weight with increasing light intensities with both sources of light. These results are similar to the growth habit of sugar maple in vivo, where no increase in the height of seedlings is reported with increasing light intensities despite significant increases in mass of the tissues (Logan and Krotkov 1969). Similar results of increased biomass production are also reported under in vitro conditions in Castanea sativa (Saez et al. 2012), Schisandra chinensis (Szopa and Ekiert 2016) and strawberry (Nhut et al. 2003). In nature, when shade tolerant species start receiving more light, they do not generally increase their vertical growth; rather, they allocate resources to develop other attributes that favour long-term survival under low light (Beaudet and Messier 1998). This enables them to have higher long term survival in deeply shaded environments. Likewise, in the present study reduced light intensity did not increase the in vitro multiplication in terms of number of nodes, but it alleviated the cumulative stress that would have occurred over repeated subcultures. This observation facilitated our efforts to attain repeated multiplication leading to a practical micropropagation system for sugar maple.
Red pigmentation of plantlets cultured under 40 µmol m−2 s−1 light was among the most obvious indicators of light stress. Similar observations have been observed in other species in response to light stress (Rabino et al. 1977; Sousa Paiva et al. 2003; Vanderauwera et al. 2005; Das et al. 2011; Lu et al. 2015; Wang et al. 2016). The anthocyanins that accumulate in stressed tissues are believed to absorb harmful wavelengths of light and thereby shield or protect the tissues from further damage by high light intensity (Hoch et al. 2003; Van Den Berg and Perkins 2007; Schaberg et al. 2008; Van Den Berg et al. 2009; Chen et al. 2013).
The chlorophyll content of the in vitro tissues was observed to decline with increasing light intensity. Chronic exposure to a photon flux higher than the photon utilizing capacity of green tissues results in photoinhibition and destruction of chlorophyll (Powles 1984). The results corroborate well with similar studies on in vitro plants (Nhut et al. 2003). Improved in vitro propagation was also achieved by altering the intensity of light in several other species (Pierik et al. 1975; Hughes 1981; Navarro et al. 1994; Kitaya et al. 1995; Nhut et al. 2003). Further, similar results were reported in sugar maple seedlings grown in shelter-house as well as under natural forest shade conditions, where an increase in light intensity had no effect on plant height, but resulted in an increased dry weight and a decreased chlorophyll content of leaves (Logan and Krotkov 1969; Messier and Nikinmaa 2000). The photoinhibitory damage and reduction in chlorophyll, however, is not reported to occur in the range used in the present study. This extreme light sensitivity of in vitro tissues of sugar maple might in part be a result of thin leaf anatomy, poor rates of transpiration due to high humidity and poor stomatal development, in addition to its extremely shade tolerant nature.
In addition to the morphological symptoms and chlorophyll content, the evaluation of stress parameters using several chlorophyll fluorescence parameters indicated a positive relationship between stress and light intensities. These signals are typical of stress in the photosynthetic electron transport chain (Akhtar et al. 2010). Though the chlorophyll fluorescence signals from plants cultured under low levels of light in either spectra is quite high, the rate of decline of fluorescence between 30 and 100 s is lower, indicating suboptimal capacity of the photosynthetic electron transport chain to utilize light. The lower signal intensity of chlorophyll fluorescence from leaves developed under high light intensity indicated photo-damage in the photosynthetic machinery. The fluorescence kinetics of the plantlets under intermediate light intensity appear to have closer to ideal characteristics. The data from various chlorophyll fluorescence based parameters indicates that, while the light intensity of full spectrum at 4 µmol m−2 s−1 appears to be least stressful for the plants, a light intensity between 4 and 16 µmol m−2 s−1 might be optimum for in vitro propagation of sugar maple. Differences in the fluorescence kinetics due to spectral differences of light used in culturing were also observed. The fluorescence signal intensity reduced with light intensity in case of RB spectrum, whereas, in case of full spectrum light the signal intensity from plants under intermediate light intensity was highest. This difference is likely due to lower photosynthetic efficiency of full spectrum light compared to the RB spectrum light. The full spectrum light used in the present study had about 37% of light in green spectrum, which is known to have relatively lower photosynthetic efficiency (McCree 1972; Inada 1976). Therefore, the lowest light intensity of full spectrum light might have been too low for the proper growth and development of in vitro plantlets, while the intermediate light intensity of full spectrum light appears to be close to optimum light intensity.
Photoinhibition is typically indicated by maximum quantum yield (QY Max or Fv/Fm ratio) value of 0.83 or lower (Maxwell and Johnson 2000). In the present study, the maximum quantum yield of plantlets under 16 µmol m−2 s−1 of RB spectrum and 40 µmol m−2 s−1 of both spectra, displayed characteristic low values of Fv/Fm ratio values indicating considerable photoinhibition in the tissues at these intensities. The plantlets grown under low intensity of RB spectrum light as well as low and intermediate light intensity of full spectrum light did not have significant photoinhibition and had a Fv/Fm ratio higher than 0.83. Under intermediate and high light intensities of both spectra, the photosynthetic capacity of the in vitro leaves tended to saturation and resulted in considerably higher NPQ values in plantlets. There was no statistically significant increase in NPQ between the intermediate and high light intensity treatments indicating saturation of the light utilizing capacity of the tissues beyond 16 µmol m−2 s−1 of light. Further, continued exposure to photon flux greater than the photon utilizing capacity of the thylakoid membrane is reported to results in oxidative damage to the photosynthetic machinery (Barber and Andersson 1992; Aro et al. 1993). The present data trends corroborate well with the reported mechanisms of photoinhibition. The fluorescence decline ratio (Rfd) values match well with the decline in fluorescence between 30 and 100 s observed in the chlorophyll fluorescence kinetics. The Rfd values indicate that a light intensity of 4 µmol m−2 s−1 or lower is too low and an intensity between 4 and 16 µmol m−2 s−1 might be optimal for in vitro propagation of sugar maple. The differences in the maximum quantum yield and fluorescence decline ratio among the different sources of light at similar intensities is likely due to the lower photosynthetic efficiency of full spectrum light.
The results on stress parameters measured using chlorophyll fluorescence corroborate well with the trends of chlorophyll fluorescence based stress measurements reported in green and senescing leaves of various stages sugar maple in vivo (Junker and Ensminger 2016), except that in the present study the stress levels in red pigmented leaves in vitro was considerably less, probably because the in vitro plantlets do not encounter the higher levels of stresses that are experienced by plants under natural conditions. Overall the data suggests that an intensity between 4 and 16 µmol m−2 s−1 might be optimum in vitro propagation of sugar maple.
Conclusions
Light has profound effect on in vitro growth of sugar maple. Light intensities used in tissue culture labs are generally around 40 µmol m−2 s−1 or higher, which is not suitable for micropropagation of sugar maple. Use of different spectra of light exhibited differences in growth and stress experienced by the plants, however, the differences due to light intensity were more marked. Plants cultured under low intensity RB spectrum and intermediate intensity of full spectrum light exhibited least stressed conditions. Reducing the light intensity used in in vitro propagation overcame the bottleneck of cumulative stress that persisted over all stages of micropropagation in sugar maple and will facilitate the development of a practical system for this species. The unique low light requirements of in vitro cultures of sugar maple may also provide opportunities to use it as a model system for understand development of fall colours, production of anthocyanins and photobiology of shade tolerant plants under easily reproducible controlled in vitro conditions. Further, while this study is limited to sugar maple, the requirement of specific light intensity is likely the cause of recalcitrance in other woody species with similar ecologically important attributes.
References
Akhtar TA, Lees HA, Lampi MA, Enstone daryl, Brain RA, Greenberg BM (2010) Photosynthetic redox imbalance influences flavonoid biosynthesis in Lemna gibba. Plant Cell Environ 33:1205–1219
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Baker FS (1949) A revised tolerance table. J For 47:179–181
Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17:61–66
Beaudet M, Messier C (1998) Growth and morphological responses of yellow birch, sugar maple, and beech seedlings growing under a natural light gradient. Can J For Res 28:1007–1015
Bowley S (2015) A hitchhiker’s guide to statistics in plant biology: generalized linear mixed model edition. Plants et al. Inc, Kincardine
Brassard N, Richer C, Tousignanat D, Rioux JA (2003) Multiplication vegetative de l’Acer saccharum: contribution a la micropropagation. Can J For Res 33:682–690
Chen C, Li H, Zhang D, Li P, Ma F (2013) The role of anthocyanin in photoprotection and its relationship with the xanthophyll cycle and the antioxidant system in apple peel depends on the light conditions. Physiol Plant 149:354–366
Connolly DA, Preece JE, Sambeek JW Van (1998) Micropropagation of adult red maple and sugar maple. In: 95th Annual International Conference of the American Society for Horticultural Science. American society for horticultural science, Charlotte, pp 477–478
Das PK, Geul B, Choi S-B, Yoo S-D, Park Y-I (2011) Photosynthesis-dependent anthocyanin pigmentation in Arabidopsis. Plant Signal Behav 6:23–25
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortScience 19(4):507–550
Gabriel WJ, Marvin JW, Taylor FH (1961) Rooting greenwood cuttings of sugar maple, effect of clone and medium. Northeastern Forest Experiment Station, Upper Darby
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92. doi:10.1016/S0304-4165(89)80016-9
Gitelson AA, Buschmann C, Lichtenthaler HK (1999) The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens Environ 69:296–302
Godman RM, Yawney HW, Tubbs CH (1990) Acer saccharum Marsh. Sugar maple. In: Burns RM, Honkala BH (eds) Silvics of North America, vol 2. United States Department of Agriculture, Forest Service, Hardwoods, pp 78–91
Graignic N, Tremblay F, Bergeron Y (2013) Development of polymorphic nuclear microsatellite markers in sugar maple (Acer saccharum Marsh.) using cross-species transfer and SSR-enriched shotgun pyrosequencing. Conserv Genet Resour 5:845–848
Hanus D, Rohr R (1984) Micropropagation de I’erable par bouturage in vitro de germination de trois especes. Can J Bot 63:277–280
Henry PH, Preece JE (1997) Production of rooting of shoots generated from dormant stem sections of maple species. HortScience 32:1274–1275
Hinckley TM, Aslin RG, Aubuchon RR, Metcalf CL, Roberts JE (1978) Leaf conductance and photosynthesis in four species of the oak-hickory forest type. For Sci 24:73–84
Hoch WA, Singsaas EL, McCown BH (2003) Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiol 133:1296–1305
Horton P, Ruban AV (1992) Regulation of Photosystem II. Photosynth Res 34:375–385
Hughes W (1981) In vitro ecology: exogenous factors affecting growth and morphogenesis in plant culture systems. Environ Exp Bot 21:281–288
Inada K (1976) Action spectra for photosynthesis in higher plants. Plant Cell Physiol 17:355–365
Janerette CA (1978) An in vitro study of seed dormancy in sugar maple. For Sci 24:43–49
Junker LV, Ensminger I (2016) Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol, tpv148
Kitaya Y, Fukuda O, Kozai T, Kirdmanee C (1995) Effects of light intensity and lighting direction on the photoautotrophic growth and morphology of potato plantlets in vitro. Sci Hortic 62:15–24
Kriebel HB (1957) Patterns of genetic variation in sugar maple. Ohio Agricultural Experimentation Station, Ohio. Res Bull 761:1–56
Kriebel HB (1989) Genetic improvement of sugar maple for high sap sugar content. I. Clone selection and seed orchard development. Can J For Res 19:917–923
Kriebel HB (1990) Genetic improvement of sugar maple for high sap sugar content. II. Relative effectiveness of maternal and biparental selection. Can J For Res 20:837–844
Kriebel HB, Gabriel WJ (1969) Genetics of sugar maple. United States Forest Service, USDA, Washington
Laing FM, Howard DB (1990) Sap sweetness consistency vs. growth rates in young sugar maples. North J Appl For 7:5–9
Larochelle F, Forget E, Rainville A, Bousquet J (1998) Sources of temporal variation in sap sugar content in a mature sugar maple (Acer saccharum) plantation. For Ecol Manage 106:307–313
Lichtenthaler HK, Miehe JA (1997) Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci 2:316–320
Logan KT (1965) Growth of tree seedlings as affected by light intensity I. In: White birch, yellow birch, sugar maple and silver maple, vol 1121. Department of Forestry, Ottawa, pp 5–16
Logan K, Krotkov G (1969) Adaptations of the photosynthetic mechanism of sugar maple (Acer saccharum) seedlings grown in various light intensities. Physiol Plant 22:104–116
Lu Y, Zhang M, Meng X, Wan H, Zhang J, Tian J, Hao S, Jin K, Yao Y (2015) Photoperiod and shading regulate coloration and anthocyanin accumulation in the leaves of malus crabapples. Plant Cell Tissue Organ Cult 121:619–632
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659–668
McCree KJ (1972) The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric Meteorol 9:191–216
Messier C, Nikinmaa E (2000) Effects of light availability and sapling size on the growth, biomass allocation, and crown morphology of understory sugar maple, yellow birch, and beech. Ecoscience 7:345–356
Morselli MF (1989) Morselli. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry 5. Trees II. Springer-Verlag, Heidelberg, pp 246–286
Muller P, Li X, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Navarro C, Teisson C, Côte F, Ganry J (1994) Effects of light intensity and CO2 concentration on growth of banana plants (Musa AAA, cultivar ‘Petite Naine’) in vitro and subsequent growth following acclimatization. Sci Hortic 60:41–54
Nhut DT, Takamura T, Watanabe H, Okamoto K, Tanaka M (2003) Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult 73:43–52
Niinemets U, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Oxborough K (2004) Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J Exp Bot 55:1195–1205
Pierik RLM, Jansen JLM, Maasdam A, Binnendiijk C (1975) Optimalization of gerbera plantlet production from excised capitulum explants. Sci Hortic 3:351–357
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:15–44
Rabino I, Mancinelli AL, Kuzmanoff K (1977) Photocontrol of anthocyanin synthesis VI. Spectral sensitivity, irradiance dependence and reciprocity relationships. Plant Physiol 59:569–573
Richer C, Rioux JA, Tousignant D, Brassard N (2004) Improving vegetative propagation of sugar maple (Acer saccharum Marsh.). Acta Hortic 630:167–175
Roussy A (2014) The sexual and vegetative propagation of sugar maple and its threat from Norway maple. Dissertation, University of Guelph
Roy A, Forget É, Bousquet J (1997) Amélioration génétique de la teneur en sucre de la sève des érables à sucre (Acer saccharum Marsh.) par sélection assistée par marqueurs (Genetic improvement of the sugar content of the sap of the sugar maple (Acer saccharum Marsh.) by marker-assisted selection). Ministry of Agriculture, Quebec, pp 88–96
Saez PL, Bravo LA, Latsague MI, Sanchez ME, Rios DG (2012) Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci Hortic 138:7–16
Schaberg PG, Murakami PF, Turner MR, Heitz HK, Hawley GJ (2008) Association of red coloration with senescence of sugar maple leaves in autumn. Trees 22:573–578
Sousa Paiva EA, Mary DSIR, Aguiar Vale FH, De Senna Queiroz CG (2003) The influence of light intensity on anatomical structure and pigment contents of Tradescantia pallida (Rose) Hunt. cv. purpurea boom (commelinaceae) leaves. Braz Arch Biol Technol 46:617–624
Sutanto TA (2010) Propagation studies of sugar maple (Acer saccharum Marsh.). Dissertation, University of Manitoba
Szopa A, Ekiert H (2016) The importance of applied light quality on the production of lignans and phenolic acids in Schisandra chinensis (Turcz.) Baill. cultures in vitro. Plant Cell Tissue Organ Cult 127:115–121
Taylor FH (1956) Variation in sugar content of maple sap of maple sap. Bulletin 587. University of Vermont and State Agricultural College Burlington, Vermont
Tousignant D, Richer C, Rioux JA, Brassard N, Mottard JP (2003) Vegetative propagation of sugar maple: relating stem water content and terminal bud developmental stage to adventitious rooting of stem cuttings. Can J Plant Sci 83:859–867
Van Den Berg AK, Perkins TD (2007) Contribution of anthocyanins to the antioxidant capacity of juvenile and senescing sugar maple (Acer saccharum) leaves. Funct Plant Biol 34:714–719
Van Den Berg AK, Vogelmann TC, Perkins TD (2009) Anthocyanin influence on light absorption within juvenile and senescing sugar maple leaves do anthocyanins function as photoprotective visible light screens? Funct Plant Biol 36:793–800
Vanderauwera S, Zimmermann P, Rombauts S, Vandenabeele S, Langebartels C, Gruissem W, Inzé D, Van Breusegem F (2005) Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol 139:806–821
Veeranjaneyulu K, Charland M, Charlebois D, Leblanc RM (1991) Photosynthetic energy storage of photosystem-I and photosystem-II in the spectral range of photosynthetically active radiation in intact sugar maple leaves. Photosynth Res 30:131–138
Wang N, Zhang Z, Jiang S, Xu H, Wang Y, Feng S, Chen X (2016) Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult 127:217–227
Wilkinson RC (1984) Site and age differences in family heritability estimates for bole diameter and sugar concentration of sap from open-pollinated progeny tests of sugar maple. USDA Forest Service, Northeastern Forest Experiment Station, Burlington, pp 154–161
Wuenscher J, Kozlowski TT (1970) Carbon dioxide transfer resistance as a factor in shade tolerance of tree seedlings. Can J Bot 48:453–456
Young AG, Merriam HG, Warwick SI (1993) The effects of forest fragmentation on genetic variation in Acer saccharum Marsh. (sugar maple) populations. Heredity 71:277–289
Acknowledgements
The authors acknowledge motivation from Todd Leuty, Specialist—Agroforestry and Speciality Crops, Ontario Ministry of Agriculture and Food (OMAFRA), Ontario; Ray Fortune, Fortune Farms, Almonte, ON and the Ontario Maple Syrup Producers Association. The authors also acknowledge the help of Bob Nichols, Scott Belton, Shuping Li and Sarita Khanal for their assistance in the lab and field work.
Author contributions
AS conducted the experiments, collected the in vitro growth data, collected chlorophyll fluorescence imaging data, and analyzed the data. AS and AMPJ, conceptualized and designed the experiments. MS provided guidance and inputs at various stages of in vitro culturing and experiments. AS and AMPJ prepared the manuscript. AMPJ conceived the project. PKS managed, organized, acquired funding, supervised and guided the whole study. All authors read and approved the final manuscript.
Funding
The authors acknowledge the Gosling Research Institute for Plant Preservation (GRIPP), University of Guelph, ON, Canada and Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, A.S., Jones, A.M.P., Shukla, M.R. et al. High light intensity stress as the limiting factor in micropropagation of sugar maple (Acer saccharum Marsh.). Plant Cell Tiss Organ Cult 129, 209–221 (2017). https://doi.org/10.1007/s11240-017-1170-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1170-2