Abstract
Pelvic floor dysfunction (PFDs), which include pelvic organ prolapse (POP), stress urinary incontinence (SUI) and anal incontinence (AI), are common degenerative diseases in women that have dramatic effects on quality of life. The pathology of PFDs is based on impaired pelvic connective tissue supportive strength due to an imbalance in extracellular matrix (ECM) metabolism, the loss of a variety of cell types, such as fibroblasts, muscle cells, peripheral nerve cells, and oxidative stress and inflammation in the pelvic environment. Fortunately, exosomes, which are one of the major secretions of mesenchymal stromal cells (MSCs), are involved in intercellular communication and the modulation of molecular activities in recipient cells via their contents, which are bioactive proteins and genetic factors such as mRNAs and miRNAs. These components modify fibroblast activation and secretion, facilitate ECM modelling, and promote cell proliferation to enhance pelvic tissue regeneration. In this review, we focus on the molecular mechanisms and future directions of exosomes derived from MSCs that are of great value in the treatment of PFD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic floor dysfunction (PFD) describes a series of clinical diseases such as include pelvic organ prolapse (POP), stress urinary incontinence (SUI) and anal incontinence (AI), which have dramatic effects on the well-being and satisfaction of millions of adult women [1].
In addition to the reduction in various types of cells, such as fibroblasts, smooth muscle cells and neural cells, in pelvic tissues, PFD develops as a result of qualitative or quantitative defects in pelvic connective tissues, including ligaments, fascia, and the levator ani, urethra and anal sphincter, which cannot provide sufficient support for the pressure from the abdominal cavity [2, 3]. Therefore, any biological event that interferes with the functional capacity of connective tissue or its repair process, as well as the depletion of any pelvic component without adequate supplementation, may promote the occurrence and development of PFD [4, 5].
Currently, the treatments for PFD can be divided into nonsurgical and surgical therapies, which are still conservative and based on symptoms. The former mainly includes pelvic floor muscle physiotherapy (PFMT), biofeedback (BF) and electronic stimulation, which can alleviate symptoms but fail to restore anatomic structures and have a high recurrence rate of up to 20–30% [6, 7]. The latter could help to achieve better anatomical and functional restoration but is concomitant with many medical complications [7,8,9,10], such as foreign body response (FBR), scarring, chronic pain and especially erosion in the mesh implantation site [11,12,13,14]. Because of the warnings of the Food and Drug Administration (FDA) and European Medicines Agency (EMA), many types of meshes have been banned from gynaecologic surgical operations [12]. Thus, alternative treatments to promote the regeneration and repair of damaged tissue are urgently needed.
Recently, the use of mesenchymal stromal cells (MSCs) has become a promising therapeutic approach for PFD and regenerative medicine and has attracted much interest because of the various capacities of these cells, such as self-renewal, multipotency, immunoregulation and secretory functions [5, 15, 16]. Under specific conditions, MSCs can migrate to the damaged site and differentiate into certain local cells to replace the lost, senescent, apoptotic and diseased cells and accelerate the repair progress. Furthermore, MSCs have powerful antiapoptotic, anti-inflammatory and neuroprotective effects. Although it has been gradually studied and appreciated (Table 1), stem cell therapy has many limitations, such as the heterogeneity of progeny stem cells, cell ageing, potential tumorigenesis, immune rejection and thrombosis [17, 18], which hinders its use in human regenerative medicine [19, 20]. Therefore, finding a new biological product to replace MSC therapy is an essential research subject due to these limitations.
MSCs promote tissue repair and regulate immunity primarily via paracrine factors instead of differentiation. Exosomes, which are one of the major secretory products of MSCs, are considered a cell-free approach that is superior to stem cell transplantation [21,22,23]. Accumulating evidence suggests that stem cells can mediate tissue regeneration and functional improvements via paracrine effects, rather than undergoing de novo differentiation, as confirmed by the administration of conditioned medium containing the secreted factors inducing equivalent beneficial effects. Compared to local exosome administration, MSC administration without exosomes delayed wound healing and decreased M2 macrophage polarization [24]. Exosomes reduce the risk of cell-based therapies, such as tumorigenesis, thrombosis and malformation, as mentioned previously. In addition, exosomes are convenient to collect and store and have low immunogenicity. Furthermore, they are enriched with various kinds of molecular cargo (such as mRNAs, miRNAs, and proteins) that affect numerous biological processes in target cells [25]. In summary, exosomes provide a safer and more efficient novel treatment with broader prospects than MSCs.
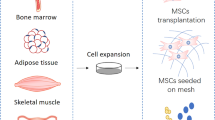
This review is intended as an overview of the effects and mechanisms of MSC-derived exosomes (MSC-Exs) in PFD, as well as future directions (Fig. 1).
Exosome-mediated reconstruction of the pelvic floor environment. MSCs from various sources (bone marrow, adipose tissue, endometrial biopsy, umbilical cord blood, skeletal muscle, etc.) can promote the function of fibroblasts and regulate immunity by releasing exosomes. These extracellular vesicles contain lipids, proteins and RNAs, which could promote the proliferation of fibroblasts and modulate the metabolism of ECM, as well as regulate immunity
The mechanisms of PFD
Pelvic floor connective tissues mainly consist of fibroblasts and extracellular matrix (ECM), which is the product of fibroblasts and contains type I and III collagen fibres, elastin, fibulins, fibronectin, laminins, hyaluronic acid and a variety of glycoproteins [26, 27]. The major components of the ECM are collagen I and collagen III; the former provides tension, while the latter is important for the elasticity and resilience of connective tissues. Fibroblasts can remodel the ECM to maintain the pelvic microenvironment through the production of collagens and the activation of catabolic enzymes (e.g. matrix metalloproteinases (MMPs)) [28].
Defects in the number and function of pelvic fibroblasts
Unfortunately, several studies have confirmed that the morphology and essential functions of the fibroblasts of PFD patients are severely damaged, which results in the gradual weakening of supportive tissues. Chen found that POP patient fibroblasts may be more likely to have fewer organelles, more irregular shapes [29], higher apoptosis rates [30, 31] and large declines in adhesion capacity, collagen gel shrinkage, mechanical reactivity and collagen secretion [28, 32,33,34]. There have been insights into the overexpression of apoptotic proteins, including cytochrome c, bax, bad, caspase 3, caspase 9 [35,36,37,38] and hypoxia-inducible factor 1a (HIF-1a), which can mediate fibroblast apoptosis through death receptor and mitochondrial apoptotic pathways [39]. Chen provided direct evidence of an increase in advanced glycation end products (AGEs) in POP sites, which showed that the activation of AGE receptors could trigger the downstream MAPK/NF-κB signalling pathway to suppress cell proliferation [40].Similarly, there is reduced cellularity and less muscle content in prolapsed pelvic tissue than in women with normal pelvic support [41,42,43]. The main reasons for SUI may be injuries to the pubococcygeal muscle [44] and pudendal nerve [45].
Disorganized ECM proteins
Because of the decreased number of fibroblasts with normal functions, collagen fibres and elastin have great quantitative and qualitative defects in the tissues of PFD patients [27, 29, 31, 32, 46,47,48,49,50]. ECM degradation is precisely regulated by MMPs and their endogenous suppressors, tissue inhibitors of matrix metalloproteinases (TIMPs) [51, 52], and these processes involve a variety of signalling pathways [53, 54]. Studies have shown that MMP1 and MMP3 expression are increased [31, 55, 56] and TIMP1 and TIMP2 expression are decreased [50, 57, 58] in PFD patient tissues.
Pelvic oxidative stress (OS) and inflammatory imbalance
Many chronic diseases share the feature of complex interactions between OS and inflammatory imbalance. Increasing lines of evidence have demonstrated that OS and inflammation are widespread in the pelvic floor. For instance, antioxidant proteins, including mitochondrial superoxide dismutase (MnSOD) and glutathione peroxidases (GPX1, GPX3) [59, 60], are downregulated, whereas there is an opposite trend in molecules related to inflammation (cyclooxygenase 2 and prostaglandin E2) [58, 61] and OS (8-OHdG and 4-HNE) [39]. It was previously suggested that changes in the synthesis of ECM occur in fibroblasts treated with hydrogen via the TGF-β signalling pathway [62]. Moreover, Hong showed that mechanical stress can promote intracellular reactive oxygen species (ROS) levels and decrease mitochondrial membrane potential, which indicates that mechanical stress leads to the development of PFD via intracellular OS [63]. Wu demonstrated that t high concentrations of COX-2 could improve the level of PGE2, resulting in the upregulation of MMP1 expression [64]. Thus, OS participates in PFD by influencing the activities of fibroblasts and collagen metabolism [39, 59, 60, 62, 63, 65].
These findings support the connective tissue injury theory that impaired fibroblasts and muscles and an imbalance in ECM metabolism result in supportive connective tissue weakness [28], eventually causing PFD [66].
Exosomes
Extracellular vesicles (EVs) were first reported by E Chargaff in 1946 [67], and secretory membrane fragments were shown to be a universal phenomenon in 1977 [68]. Harding and Johnstone pronounced that transferrin receptors were released into the ECM during reticulocyte maturation via vesicles (50 nm in diameter), which were later named exosomes [69,70,71]. To date, EVs have been divided into three types: apoptotic bodies (800–5000 nm in diameter), microvesicles (200–1000 nm in diameter) and exosomes (30–150 nm in diameter) depending on size, contents, and mechanism of formation [72]. The first two vesicle types originate from the plasma, and the third is endogenous [73, 74]. Exosome contents can not only indicate their origins but also be passed to other cells as messengers to change cell functions [21,22,23, 75]. Exosomes were regarded as cellular waste in the past; however, the contents carried in exosomes play major roles in cellular activities and pathological processes, including the immune response, angiogenesis, cell death, neurodegenerative diseases and cancers [76, 77]. Exosomes are also one of the major secretory products of MSCs and are considered a cell-free approach that is superior to stem cell transplantation.
The formation of exosomes
The identification of exosomes is of great importance and could indicate their sources and biological functions. Well-recognized methods vary from physical properties to biochemical characteristics, such as transmission electronic microscopy (TEM), nanoparticle tracking analysis (NTA) and Western blotting, to confirm exosome morphology, size, porosity and surface markers [78, 79]. NTA, one of the key biophysical technologies, can measure exosome concentrations and size distribution by tracking the Brownian motion of individual vesicles. Moreover, NTA can also determine exosome phenotype through the association of fluorescent measurements [80]. Transmembrane proteins (such as CD9 and CD63) and some molecules associated with biological functions, including heat shock protein 70 (HSP70) and tumour-susceptibility gene 101 (TSG101), are common components used to identify exosomes, which can be detected by Western blot analysis [81].
Exosome biogenesis, which is tightly regulated, consists of three main stages: (1) endocytic vesicle generation via invagination of the plasma membrane; (2) formation of multivesicular bodies (MVBs) via inward budding of the endosomal membrane; and (3) fusion of these MVBs with the plasma membrane to release internal vesicles as exosomes [82,83,84,85]. Exosomes are protected from degradation by ribonucleases by their lipid bilayer and communicate with other cells with the help of soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and the endosomal sorting complex required for transport (ESCRT) [85, 86]. As a type of messenger, exosomes play a significant role in intercellular communication, which is mainly mediated by three distinct mechanisms. First, exosomes can activate intracellular signalling pathways through the interactions of exosome membrane proteins and receptors. For example, after the binding of TNF on the surface of dendritic cell-derived exosomes (DCexs) and its specific receptor on NK cells, the Based on the data in Table 2, latter could be stimulated to secrete interferon-γ (IFNγ) [87]. Secondly, exosomes can be actively transported along the cytoskeleton, actin filaments or microtubules in a rapid and directed pattern after internalization, which leads to signal transfer to targeted organelles such as lysosomes [88]. Thirdly, the exosome membrane can fuse with the target cell, leading to the release of its contents, including proteins, mRNAs and microRNAs, into the cytoplasm. Both the vesicle and the target cell express proteins and glycoproteins, by which they can complete exosome uptake [89]. Compared with traditional gene therapies, MSC-Exs, which are a type of nanocarrier, transfer specific molecules to recipients via endocytosis and membrane fusion, achieving therapeutic effects with safety and precision [90, 91].
The role of MSC-Exs in PFD
Crucial changes in PFD pathology are deficiencies in fibroblast functions and ECM structure, OS in the pelvic floor and impaired muscle and nerves [41,42,43, 45]. MSC-Exs can stimulate the proliferation of fibroblasts, smooth muscle cells (SMCs) and Schwann cells and enhance fibroblast functions [106, 107]. Studies have shown that exosome therapy can reduce mesh exposure and promote hyperplasia in tissue and capillaries in mesh implantation sites in vivo [104, 108]. Exosomes tagged with the fluorescent dye PKH67 were internalized into target cells in vitro. Therefore, exosomes purified from MSCs may be a promising treatment for PFD via the consistent release of miRNAs and proteins to regulate targets (Table 2) [109].
Based on the data in Table 2, we can see various sources of MSCs that have been studied as cell-based therapies, and Fig. 2 shows the study designs of the references in Table 2. However, it is unclear which exosomes were more applicable and efficient. Given the concern regarding immunologic rejection, using autologous would be an ideal treatment option that could reverse the underlying pathologic condition. Adult MSCs are mainly isolated from adipose tissue, bone marrow or skeletal muscle, which can be more applicable than cells from other sources. Liposuction from the hip and thigh regions of the patient exemplifies an easy approach to ADSC isolation. The majority of MSC-Exs show powerful anti-inflammatory effects and promote proliferation-. However, we cannot determine which is the best choice for the treatment of PFD because each of the types has unique advantages and disadvantages. Moreover, there are apparent limitations in current studies: almost none of these studies used large animals. Finally, the effective exosome dose and injection sites vary in the current studies. As a result, the heterogeneity of the reviewed studies makes it difficult to draw firm conclusions.
Schematic presentation of the study design according to the references in Table 2
Promoting cell proliferation
PFD patients exhibit impairments in function and decreased numbers of fibroblasts, myocytes, and nerve cells [110] in the pelvic floor environment [28]. The main cause of SUI is injuries to the pubococcygeal muscle, which is important for supporting pelvic organs [44, 111]. MSC-Exs exhibit obvious beneficial effects in rescuing these essential cells, which makes treatment based on MSC-Exs a viable therapeutic strategy [21, 112]. Increasing lines of evidence suggest a time- and dose-dependent increase in cell proliferation and migration and a decrease in apoptosis [21, 22, 113, 114], which can restore cell and nerve fibre density and involves the TGFβ/SMAD [115], PI3K/AKT, Jak-STAT, Wnt, and Ras/ERK pathways. Ni indicated that local injection of exosomes promoted functional and histological recovery after SUI, which involved the PI3K-Akt, Jak-STAT, and Wnt pathways, as revealed by proteomic analysis [21]. Activation of the AKT pathway by MSC-Exs was related to a decrease in fibroblast apoptosis in vivo [107]. The PI3K/AKT/eNOS pathway mediates the biological events of MSC-Exs [116]. The Ras/ERK signalling pathway is crucial for the activation and proliferation of SCs, which is the first step in muscle regeneration and promotes the recovery of pubococcygeal muscle. MSC-Exs could enhance the phosphorylation of ERK1/2 and mediate significant improvements in urodynamic parameters and the function of injured muscle tissue [22, 117]. Wnt4 was contained in MSC-Exs, which promoted β-catenin nuclear translocation to enhance the proliferation and migration of cells in vivo, and this phenomenon could be reversed by the β-catenin inhibitor ICG001 or knockdown of Wnt4 in MSC-Exs in vitro [107]. Mechanistically, Wnt signalling targets the c-Myc gene, which regulates the cell cycle via the transition from G1 to S phase and shortens the cell cycle [118]. Similarly, Jagged 1, which is detected in MSC-Exs, is a ligand of the Notch pathway that shows the same effect on cell proliferation [119]. Zhao showed that MSC-Ex treatment could suppress cell apoptosis through another approach in vivo and in vitro, which inhibited nuclear translocation of apoptosis-inducing factor (AIF) and upregulated poly ADP-ribose polymerase 1 (PARP-1) and poly ADP-ribose (PAR), which play predominant roles in H2O2-induced cell death [120]. Furthermore, crosstalk between pathways could provide a foundation for MSC-Ex therapy. For example, Jagged1 (JAG1), the direct agonist of Notch, was recognized as an evolutionarily conserved target of the WNT/beta-catenin signalling pathway [121]. Wnt/β-catenin signalling was notably increased in response to the expression of JAG1 in vivo [118].
Regulating ECM metabolism
The major components of pelvic ECM, especially collagen I and III, constitute the supportive strength for pelvic organs. MMPs, especially MMP2 and MMP9, and TIMPs play fundamental roles in the balance of collagen synthesis and degradation, greatly contributing to ECM homeostasis [52]. However, severe disruption to this balance in the pelvic environment in PFD patients contributes to the weakness of pelvic connective tissues [5]. Luckily, accumulating evidence has suggested that MSC-Exs could enhance the supportive ability of pelvic connective tissues and obtain ideal therapeutic effects by overexpressing type I and III collagen and MMPs and downregulating TIMPs [106, 107, 122,123,124,125], resulting in increased collagen production [126] and ordered mature collagen [127]. Guo reported that the enhancement in ECM was achieved by activating TGF-β, which increased the phosphorylation level of SMAD and AKT [128]. However, MSC-Exs not only accelerate collagen production to repair ECM but also control these processes by delivering 14–3-3ζ to recruit YAP and p-LATS. As a result, the complex of YAP and p-LATS restricts excessive collagen deposition and subsequently inhibits Wnt/β-catenin signalling [129].
Inhibiting OS and the inflammatory response
An increase in OS activates the PARP pathway, which is responsible for regulating proinflammatory cytokine expression [60]. The involvement of inflammation and OS accelerates the disease process and has direct toxic effects on fibroblasts and Schwann cells [130]. MSC-Exs have been shown to play significant roles in the treatment of PFD because of their antioxidative effects against ROS and inflammation in fibroblasts [131, 132], which can be resolved by the induction of M1-M2 macrophage polarization [133]. There are several different mechanisms to explain this effect. In MSC-Ex, the level of the anti-inflammatory factor IL-10 was increased, while proinflammatory factors such as IL-1β, IL-8, IL-2 and IFN-γ were significantly decreased [134]. Liu reported that this effect was mediated by inhibiting the phosphorylation of AKT and overexpression of PTEN [135]. Another study showed that miR-223 in MSC-Exs played a fundamental role in the regulation of macrophage polarization by binding to homeobox domain PBX/Knotted 1 [136].
Taken together, these studies demonstrate the notable reparative function of MSC-Exs, such as improving the proliferation and activity of cells that are important for pelvic tissues and the release of inflammatory mediators via different signal pathways, suggesting that the administration of MSC-Exs is a potentially efficacious therapeutic strategy for PFD and could be of tremendous value in clinical settings.
Challenges in the use of MSC-Ex therapy for PFD
Despite the undeniable benefits of MSC-Exs in regeneration, great challenges still exist that impair their clinical application. It remains unclear which molecule in exosomes plays a key role in regulating the function of cells and the microenvironment in the pelvic floor. Therefore, more basic research should be conducted to explain the mechanism. At present, common methods in labs cannot meet these requirements, such as classical differential ultracentrifugation, commercial precipitation kits and physical separation approaches (e.g. ultrafiltration, concentration and size-exclusion chromatography [137,138,139], and while differential ultracentrifugation is the gold standard for obtaining exosomes, with demands for operators and equipment, commercial precipitation kits can more easily obtain targets with smaller diameters; moreover, high costs and with the use of chemicals affect follow-up studies. Certain physical methods can avoid chemical contaminants; however, these methods may destroy exosome membrane structure and morphology, altering outcomes. Therefore, a suitable separation method should be developed to efficiently obtain purified exosomes, which is important for carrying out experiments and clinical applications. At present, local injection of exosomes is mainly used, but these particles rapidly dissipate. It is impossible to perform repeat injections in the clinic, which urges the search for better delivery approaches with good tissue receptivity, safety and ease of degeneration. Thus, to promote research on the application of exosomes, promising methods should be developed to produce exosomes on a large scale and establish efficient delivery systems and recognized administration protocols.
Future directions for MSC-Ex therapy in PFD
Currently, some methods are being developed to attain more exosomes with higher quality, which can help researchers obtain optimal results. It is acceptable and feasible to use technologies including preconditioning of MSCs, various types of biomaterial scaffolds and improved modification approaches to purify exosomes.
Preconditioning parental MSCs
Preconditioning, which enhances the function of exosomes, is a promising strategy to improve transplantation efficacy in vitro and in vivo [140]. Some studies have shown that pretreating or modifying parental MSCs can change exosome contents, including proteins and noncoding RNAs, which influences key downstream signalling pathways and improves treatment efficacy [25, 141, 142]. For example, it has been demonstrated that exosomes derived from MSCs infected by a lentivirus overexpressing TSG-6 can decrease the secretion of inflammatory molecules and ameliorate collagen deposition in vivo much more robustly than conventional exosomes [141]. Similarly, SIRT1-overexpressing human BMSCs showed improved effects via their exosomes by augmenting the proliferation, differentiation and activation of SCs via ERK signalling, resulting in the repair of urethral function in vivo [23]. The ability of MSC-Exs to augment the levels of growth factor expression and decrease the levels of proteins related to inflammation and OS can be increased by overexpressing nuclear factor-E2-related factor 2 (Nrf2), a transcription factor [132]. Interestingly, changes in the noncoding RNA levels in exosomes after preconditioning can greatly enhance their curative effects. MSCs preconditioned with lithium produce exosomes with more miR-1906, a new regulator of Toll-like receptor 4 (TLR4) signalling, which inhibits the NF-κB signalling pathway and enhances neuroprotective effects [113]. Coincidentally, other studies have shown that exosomes from MSCs that were pretreated with deferoxamine [142] or transfected with lncRNA H19 [143] were enriched in miR-126 and lncRNA H19, which contribute to ECM remodelling, proliferation, migration and the inhibition of inflammation [23].
Combination with tissue-engineered repair material
In addition to carrying out research on the contents, therapeutic mechanisms and effects of MSC-Exs, scholars have also focussed on safer and more effective delivery methods to replace injections. Tissue-engineered products are excellent candidates for delivery with outstanding biocompatibility, low cytotoxicity and immunogenicity and include but are not limited to hydrogels [144], decellularized scaffold ECM (dECM) [145] and combinations with other emerging technologies. These biological scaffolds have several advantages. First, these scaffolds can retain loaded exosomes, hydrogels can highly control exosomes by timed release for a long, continuous duration [109, 146], and the fibres of dECM can easily be combined with exosomes [109]. Furthermore, these materials can enhance therapeutic outcomes by providing a good environment in which cells obtain structure to activate biological events, such as adhesion, invasion, proliferation and the regeneration of neurons [109, 146, 147]. Surprisingly, the incorporation of MSC-Exs into hydrogels could effectively promote collagen deposition and remodelling and neuronal ingrowth [148].
Moreover, these delivery systems promote MSC secretion of more effective molecules (e.g. growth factors and cytokines) into exosomes to regulate MMP activity, the local inflammatory environment, and cell proliferation [109, 149]. It is also worth mentioning that these platforms can deliver other necessary cargoes in addition to exosomes. Yuan Xiong produced HA@MnO2/FGF-2/Exs that could provide rapid haemostasis and protection for wounds by concurrently releasing exosomes, oxygen and FGF-2 growth factor [150]. Thus, functional delivery approaches for exosomes offer emerging strategies for regenerative medicine, which can be applied to a variety of degenerative diseases [109].
Improving the yield of MSC-Exs
The quality and quantity of exosomes depend the status and number of MSCs. Future studies should determine the detailed environmental parameters (pH and temperature) for MSC culture to obtain effective and homogeneous exosomes. The inherent secretory ability of MSCs is impaired after several generations because of replicative senescence [151]. Some physical approaches have been shown to increase the yields of exosomes, such as the tangential flow filtration (TFF) system-based method [152] and ultrasonic shearing of MSCs for 1 min before conventional centrifugation and filtration [153]. Pretreatment of stem cells with lithium [113], cytochalasin B, and antifungal agents [154] can promote the production of exosomes.
Compared to conventional culture conditions, the hollow fibre 3D culture system was reported to enhance total exosome production up to 19.4-fold [155]. Moreover, the combination of 3D MSC cultures and TFF [156] or other biological materials can further promote the yields of exosomes. For example, 45S5 Bioglass® (BG), a well-known biomaterial, influences the formation and release of exosomes via the overexpression of neutral sphingomyelinase-2 (nSMase2) and Rab27a, which have been shown to activate the nSMase and Rab GTPase pathways, respectively [157].
Conclusion
PFD significantly compromises quality of life and confers great burdens on families and society. None of the current interventions provide satisfactory effects; therefore, studies are examining promising therapeutic approaches to escape this predicament. MSC-Exs have been reported to powerfully regulate various biological events associated with tissue regeneration, including cell proliferation, migration, ECM homeostasis and anti-inflammatory effects, and these methods are safe because of the lack of cell transplantation. Numerous findings have exciting therapeutic potential in PFD and other diseases. It is worth noting that these MSC-Exs could be tailored to maximize clinical effects through MSC preconditioning or certain delivery systems.
To date, the use of MSC-Exs is still in its infancy and has many limitations. Additional basic studies should be conducted to obtain a full understanding of the properties of exosomes, which include but are not limited to sources, biomarkers and biological functions. In addition, standardized treatment regimens and operating procedures should be established to ensure the effectiveness and safety of these interventions. Last but not least, larger reliable clinical studies will be required to validate previous findings and assess whether the balance between cost and benefits is reasonable.
Availability of data and materials
Not applicable.
Abbreviations
- PFD:
-
Pelvic floor disorders
- POP:
-
Pelvic organ prolapse
- SUI:
-
Stress urinary incontinence
- AI:
-
Anal incontinence
- ECM:
-
Extracellular matrix
- MSC:
-
Mesenchymal stromal cell
- AI:
-
Anal incontinence
- PFMT:
-
Pelvic floor muscle physiotherapy
- BF:
-
Biofeedback
- FBR:
-
Foreign body response
- FDA:
-
Food and drug administration
- EMA:
-
European medicines agency
- HIF-1α:
-
Hypoxia-inducible factor 1α
- AGEs:
-
Advanced glycation end products
- MMPs:
-
Matrix metalloproteinases
- TIMPs:
-
Tissue inhibitors of matrix metalloproteinases
- OS:
-
Oxidative stress
- MnSOD:
-
Mitochondrial superoxide dismutase
- GPX:
-
Glutathione peroxidase
- EVs:
-
Extracellular vesicles
- TEM:
-
Transmission electron microscopy
- NTA:
-
Nanoparticle tracking analysis
- HSP70:
-
Heat shock protein 70
- TSG101:
-
Tumour-susceptibility gene 101
- MVBs:
-
Multivesicular bodies
- SNAREs:
-
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- ESCRT:
-
The endosomal sorting complex required for transport
- MSCs-Ex:
-
Mesenchymal stromal cell-derived exosomes
- HUCMSCs:
-
Human umbilical cord mesenchymal stromal cells
- LPP:
-
Leak point pressure
- eMSCs:
-
Human endometrial mesenchymal stromal cells
- BMSCs:
-
Bone marrow mesenchymal stromal cells
- ADSC:
-
Adipose-derived stromal cell
- USC:
-
Urine-derived stromal cell (USC)
- SCs:
-
Satellite cell
- ERK:
-
Extracellular-regulated protein kinases
- SIRT1:
-
Silent mating type information regulation 2 homologue 1 (SIRT1)
- DRG:
-
Dorsal root ganglion
- SMC:
-
Smooth muscle cell
- AIF:
-
Apoptosis-inducing factor
- PARP-1:
-
Upregulating poly ADP-ribose polymerase 1
- PAR:
-
Poly ADP-ribose
- JAG1:
-
Jagged1
- ROS:
-
Reactive oxygen species
- Nrf2:
-
Nuclear factor-E2-related factor 2
- TLR4:
-
Toll-like receptor 4
- dECM:
-
Decellularized scaffold ECM
- TFF:
-
Tangential flow filtration
References
Dieter AA, Wilkins MF, Wu JM. Epidemiological trends and future care needs for pelvic floor disorders. Curr Opin Obstet Gynecol. 2015;27(5):380–4.
Kim S, Harvey MA, Johnston S. A review of the epidemiology and pathophysiology of pelvic floor dysfunction: do racial differences matter? J Obstet Gynaecol Can. 2005;27(3):251–9.
Campeau L, et al. Pelvic floor disorders: linking genetic risk factors to biochemical changes. BJU Int. 2011;108(8):1240–7.
Norton PA, et al. Genitourinary prolapse and joint hypermobility in women. Obstet Gynecol. 1995;85(2):225–8.
Cheng J, et al. Status, challenges, and future prospects of stem cell therapy in pelvic floor disorders. World J Clin Cases. 2020;8(8):1400–13.
Arnouk A, et al. Physical, complementary, and alternative medicine in the treatment of pelvic floor disorders. Curr Urol Rep. 2017;18(6):47.
Fitz FF, et al. Biofeedback for the treatment of female pelvic floor muscle dysfunction: a systematic review and meta-analysis. Int Urogynecol J. 2012;23(11):1495–516.
Alvarez J, Cvach K, Dwyer P. Complications in pelvic floor surgery. Minerva Ginecol. 2013;65(1):53–67.
Olsen AL, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6.
Sima Y, Chen Y. MSC-based therapy in female pelvic floor disorders. Cell Biosci. 2020;10:104.
Dällenbach P. To mesh or not to mesh: a review of pelvic organ reconstructive surgery. Int J Womens Health. 2015;7:331–43.
Marinaro F, et al. Meshes in a mess: Mesenchymal stem cell-based therapies for soft tissue reinforcement. Acta Biomater. 2019;85:60–74.
Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: A systematic review. Indian J Urol. 2012;28(2):129–53.
Zhang L, et al. Tension-free polypropylene mesh-related surgical repair for pelvic organ prolapse has a good anatomic success rate but a high risk of complications. Chin Med J. 2015;128(3):295–300.
Kariminekoo S, et al. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44(3):749–57.
Hassan WU, Greiser U, Wang W. Role of adipose-derived stem cells in wound healing. Wound Repair Regen. 2014;22(3):313–25.
Li JJ, et al. Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis. Nanomaterials (Basel). 2019;9(2):261.
Ribeiro-Rodrigues TM, et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res. 2017;113(11):1338–50.
Bowers DT, Brown JL. Nanofiber curvature with Rho GTPase activity increases mouse embryonic fibroblast random migration velocity. Integr Biol (Camb). 2021;13(12):295–308.
Wang LT, et al. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: Use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10(9):1288–303.
Ni J, et al. Therapeutic potential of human adipose-derived stem cell exosomes in stress urinary incontinence - an in vitro and in vivo study. Cell Physiol Biochem. 2018;48(4):1710–22.
Wu R, et al. Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res Ther. 2019;10(1):80.
Li Q, et al. Exosomes derived by SIRT1-overexpressing bone marrow mesenchymal stem cells improve pubococcygeus muscle injury in rats. Int J Stem Cells. 2021. https://doi.org/10.15283/ijsc21065.
Marofi F, et al. MSCs and their exosomes: a rapidly evolving approach in the context of cutaneous wounds therapy. Stem Cell Res Ther. 2021;12(1):597.
Bian D, et al. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13(1):24.
Abramowitch SD, et al. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S146–58.
Kieserman-Shmokler C, et al. From molecular to macro: the key role of the apical ligaments in uterovaginal support. Am J Obstet Gynecol. 2020;222(5):427–36.
Ruiz-Zapata AM, et al. Functional characteristics of vaginal fibroblastic cells from premenopausal women with pelvic organ prolapse. Mol Hum Reprod. 2014;20(11):1135–43.
陈义松 and 华克勤, 2008 盆底器官脱垂子宫主韧带和阴道壁的超微结构.. 上海医学 31(7):490–492.
Zhu Y, et al. Mechanical stress influences the morphology and function of human uterosacral ligament fibroblasts and activates the p38 MAPK pathway. Int Urogynecol J. 2021;33(8):2023–212.
Zhu YP, et al. Evaluation of extracellular matrix protein expression and apoptosis in the uterosacral ligaments of patients with or without pelvic organ prolapse. Int Urogynecol J. 2021;32(8):2273–81.
Ruiz-Zapata AM, et al. Extracellular matrix stiffness and composition regulate the myofibroblast differentiation of vaginal fibroblasts. Int J Mol Sci. 2020;21(13):4762.
Poncet S, et al. The expression and function of the endothelin system in contractile properties of vaginal myofibroblasts of women with uterovaginal prolapse. Am J Obstet Gynecol. 2005;192(2):426–32.
Meyer S, et al. The contractile properties of vaginal myofibroblasts: is the myofibroblasts contraction force test a valuable indication of future prolapse development? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(10):1399–403.
Zhao Y, et al. Transforming growth factor beta 1 and p44/42 expression in cardinal ligament tissues of patients with pelvic organ prolapse. Med Sci Monit. 2021;27: e930433.
Wen Y, et al. Expression of apoptotic factors in vaginal tissues from women with urogenital prolapse. Neurourol Urodyn. 2011;30(8):1627–32.
Saatli B, et al. Alteration of apoptosis-related genes in postmenopausal women with uterine prolapse. Int Urogynecol J. 2014;25(7):971–7.
Sun ZJ, et al. Proteomic analysis of the uterosacral ligament in postmenopausal women with and without pelvic organ prolapse. Chin Med J (Engl). 2015;128(23):3191–6.
Kim EJ, et al. Involvement of oxidative stress and mitochondrial apoptosis in the pathogenesis of pelvic organ prolapse. J Urol. 2013;189(2):588–94.
Chen YS, et al. Advanced glycation end products decrease collagen I levels in fibroblasts from the vaginal wall of patients with POP via the RAGE, MAPK and NF-κB pathways. Int J Mol Med. 2017;40(4):987–98.
Takacs P, et al. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod Sci. 2009;16(5):447–52.
Kökçü A, et al. Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet. 2002;266(2):75–8.
Cole EE, et al. Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int. 2006;97(2):345–8.
Lien KC, et al. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103(1):31–40.
Huang G, et al. Protective effect and potential mechanism of Schwann cell-derived exosomes on mechanical damage of rat dorsal root ganglion cells. J Obstet Gynaecol Res. 2021;47(10):3691–701.
Budatha M, et al. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. J Clin Invest. 2011;121(5):2048–59.
Han L, et al. Association between pelvic organ prolapse and stress urinary incontinence with collagen. Exp Ther Med. 2014;7(5):1337–41.
Hu Y, et al. Expression and significance of metalloproteinase and collagen in vaginal wall tissues of patients with pelvic organ prolapse. Ann Clin Lab Sci. 2017;47(6):698–705.
Li Y, et al. Structural, functional and molecular pathogenesis of pelvic organ prolapse in patient and Loxl1 deficient mice. Aging (Albany NY). 2021;13(24):25886–902.
Jackson SR, et al. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347(9016):1658–61.
Van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015;44–46:224–31.
Kerkhof MH, Hendriks L, Brölmann HA. Changes in connective tissue in patients with pelvic organ prolapse–a review of the current literature. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(4):461–74.
Vetuschi A, et al. Immunolocalization of advanced glycation end products, mitogen activated protein kinases, and transforming growth factor-β/smads in pelvic organ prolapse. J Histochem Cytochem. 2018;66(9):673–86.
Li BS, et al. Role of mechanical strain-activated PI3K/Akt signaling pathway in pelvic organ prolapse. Mol Med Rep. 2016;14(1):243–53.
Dviri M, et al. Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):113–7.
Usta A, et al. Expression of matrix metalloproteinase-1 in round ligament and uterosacral ligament tissue from women with pelvic organ prolapse. J Mol Histol. 2014;45(3):275–81.
Li L, et al. The polymorphisms of extracellular matrix-remodeling genes are associated with pelvic organ prolapse. Int Urogynecol J. 2022;33(2):267–74.
Lin T, et al. Expression of COX-2 and Nrf2/GPx3 in the anterior vaginal wall tissues of women with pelvic organ prolapse. Arch Gynecol Obstet. 2021;303(5):1245–53.
Hong S, et al. The role of GPX1 in the pathogenesis of female pelvic organ prolapse. PLoS ONE. 2017;12(8): e0181896.
Fang G, et al. Oxidative status of cardinal ligament in pelvic organ prolapse. Exp Ther Med. 2018;16(4):3293–302.
Kovács P, et al. Lithocholic acid, a metabolite of the microbiome, increases oxidative stress in breast cancer. Cancers (Basel). 2019;11(9):1255.
Liu C, et al. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol Med Rep. 2016;13(4):2999–3008.
Hong S, et al. Oxidative damage to human parametrial ligament fibroblasts induced by mechanical stress. Mol Med Rep. 2015;12(4):5342–8.
Wu K, et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–54.
Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29.
Drewes PG, et al. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170(2):578–89.
Chargaff E, West R. The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166(1):189–97.
De Broe ME, et al. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977;81(3):237–45.
Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35(2):256–63.
Pan BT, et al. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942–8.
Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–51.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83.
Camussi G, et al. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1(1):98–110.
Livshits MA, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319.
Zhang H, et al. Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 2019;10:1464.
Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–81.
Chang YH, et al. Exosomes and stem cells in degenerative disease diagnosis and therapy. Cell Transpl. 2018;27(3):349–63.
Kowal EJK, et al. Extracellular vesicle isolation and analysis by western blotting. Methods Mol Biol. 2017;1660:143–52.
Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp. 2018. https://doi.org/10.3791/56482.
Dragovic RA, et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine. 2011;7(6):780–8.
Su T, et al. Bone marrow mesenchymal stem cells-derived exosomal MiR-29b-3p regulates aging-associated insulin resistance. ACS Nano. 2019;13(2):2450–62.
Moghadasi S, et al. A paradigm shift in cell-free approach: the emerging role of MSCs-derived exosomes in regenerative medicine. J Transl Med. 2021;19(1):302.
Gruenberg J, van der Goot FG. Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol. 2006;7(7):495–504.
Heijnen HF, et al. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9.
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208.
Jadli AS, et al. Inside(sight) of tiny communicator: exosome biogenesis, secretion, and uptake. Mol Cell Biochem. 2020;467(1–2):77–94.
Munich S, et al. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology. 2012;1(7):1074–83.
Tian T, et al. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228(7):1487–95.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. https://doi.org/10.3402/jev.v3.24641.
Zhou Y, et al. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip Rev RNA. 2016;7(6):758–71.
van den Boorn JG, et al. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29(4):325–6.
Mao M, et al. Human umbilical cord mesenchymal stem cells reconstruct the vaginal wall of ovariectomized Sprague-Dawley rats: implications for pelvic floor reconstruction. Cell Tissue Res. 2021;386(3):571–83.
Ma Y, et al. Mesenchymal stem cell-based bioengineered constructs enhance vaginal repair in ovariectomized rhesus monkeys. Biomaterials. 2021;275: 120863.
Edwards SL, et al. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater. 2015;13:286–94.
Kinebuchi Y, et al. Autologous bone-marrow-derived mesenchymal stem cell transplantation into injured rat urethral sphincter. Int J Urol. 2010;17(4):359–68.
Corcos J, et al. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodyn. 2011;30(3):447–55.
Huang X, et al. Core-shell poly(l-lactic acid)-Hyaluronic acid nanofibers for cell culture and pelvic ligament tissue engineering. J Biomed Nanotechnol. 2021;17(3):399–406.
Jin M, et al. Transplantation of bone marrow-derived mesenchymal stem cells expressing elastin alleviates pelvic floor dysfunction. Stem Cell Res Ther. 2016;7(1):51.
Jin M, et al. MicroRNA-29 facilitates transplantation of bone marrow-derived mesenchymal stem cells to alleviate pelvic floor dysfunction by repressing elastin. Stem Cell Res Ther. 2016;7(1):167.
Wu X, et al. Acceleration of pelvic tissue generation by overexpression of basic fibroblast growth factor in stem cells. Connect Tissue Res. 2022;63(3):256–68.
Zhuang G, et al. Secretomes of human pluripotent stem cell-derived smooth muscle cell progenitors upregulate extracellular matrix metabolism in the lower urinary tract and vagina. Stem Cell Res Ther. 2021;12(1):228.
Zhou M, et al. M2 Macrophage-derived exosomal miR-501 contributes to pubococcygeal muscle regeneration. Int Immunopharmacol. 2021;101(Pt B): 108223.
Liu X, et al. Exosomes secreted by adipose-derived mesenchymal stem cells regulate type I collagen metabolism in fibroblasts from women with stress urinary incontinence. Stem Cell Res Ther. 2018;9(1):159.
Kisby CK, et al. Impact of repeat dosing and mesh exposure chronicity on exosome-induced vaginal tissue regeneration in a porcine mesh exposure model. Female Pelvic Med Reconstr Surg. 2021;27(3):195–201.
Zhou M, et al. Effects of RSC96 schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit. 2018;24:7841–9.
Zhang J, et al. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49.
Zhang B, et al. HucMSC-exosome mediated-wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33(7):2158–68.
Kisby CK, et al. Exosome-induced vaginal tissue regeneration in a porcine mesh exposure model. Female Pelvic Med Reconstr Surg. 2021;27(10):609–15.
Xing H, et al. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology. 2021;19(1):264.
Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193–203.
Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014;49(1):59–68.
Sugimura-Wakayama Y, et al. Peripheral nerve regeneration by secretomes of stem cells from human exfoliated deciduous teeth. Stem Cells Dev. 2015;24(22):2687–99.
Haupt M, et al. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med. 2021;10(3):357–73.
Jun EK, et al. Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci. 2014;15(1):605–28.
Eirin A, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64.
Hu Y, et al. Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnology. 2021;19(1):150.
Kim S, et al. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int J Mol Sci. 2018;19(10):3119.
Shi Y, et al. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther. 2015;6(1):120.
Wang X, et al. Fetal dermal mesenchymal stem cell-derived exosomes accelerate cutaneous wound healing by activating notch signaling. Stem Cells Int. 2019;2019:2402916.
Zhao G, et al. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res Ther. 2020;11(1):174.
Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17(4):681–5.
Monroe MN, et al. Extracellular vesicles influence the pulmonary arterial extracellular matrix in congenital diaphragmatic hernia. Pediatr Pulmonol. 2020;55(9):2402–11.
Lee YI, et al. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. J Cosmet Dermatol. 2021;20(6):1774–81.
Cabral J, et al. Extracellular vesicles as modulators of wound healing. Adv Drug Deliv Rev. 2018;129:394–406.
Kwon TR, et al. Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice. Photodermatol Photoimmunol Photomed. 2016;32(3):120–8.
Chamberlain CS, et al. Exosome-educated macrophages and exosomes differentially improve ligament healing. Stem Cells. 2021;39(1):55–61.
Xu J, et al. Infrapatellar fat pad mesenchymal stromal cell-derived exosomes accelerate tendon-bone healing and intra-articular graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2022;50(3):662–73.
Guo Z, et al. Exosomal MATN3 of urine-derived stem cells ameliorates intervertebral disc degeneration by antisenescence effects and promotes NPC proliferation and ECM synthesis by activating TGF-β. Oxid Med Cell Longev. 2021;2021:5542241.
Zhang B, et al. HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34(10):2485–500.
De Gregorio C, et al. Human adipose-derived mesenchymal stem cell-conditioned medium ameliorates polyneuropathy and foot ulceration in diabetic BKS db/db mice. Stem Cell Res Ther. 2020;11(1):168.
Villatoro AJ, et al. Canine colostrum exosomes: characterization and influence on the canine mesenchymal stem cell secretory profile and fibroblast anti-oxidative capacity. BMC Vet Res. 2020;16(1):417.
Li X, et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50(4):1–14.
Dalirfardouei R, et al. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–68.
Sung SE, et al. Mesenchymal stem cell exosomes derived from feline adipose tissue enhance the effects of anti-inflammation compared to fibroblasts-derived exosomes. Vet Sci. 2021;8(9):182.
Liu W, et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):259.
da Silva Meirelles L, et al. Improving the therapeutic ability of mesenchymal stem/stromal cells for the treatment of conditions influenced by immune cells. Stem Cells Int. 2019;2019:6820395.
Li P, et al. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804.
Tian Y, et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles. 2020;9(1):1697028.
Théry C, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006. https://doi.org/10.1002/0471143030.cb0322s30.
Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22(3):1428–42.
Jiang L, et al. Exosomes derived from TSG-6 modified mesenchymal stromal cells attenuate scar formation during wound healing. Biochimie. 2020;177:40–9.
Ding J, et al. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. Biomed Res Int. 2019;2019:9742765.
Li B, et al. The MSC-derived exosomal lncRNA H19 promotes wound healing in diabetic foot ulcers by upregulating PTEN via MicroRNA-152-3p. Mol Ther Nucleic Acids. 2020;19:814–26.
Khayambashi P, et al. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. 2021;22(2):684. https://doi.org/10.3390/ijms22020684.
Chen P, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–59.
Kim YG, Choi J, Kim K. Mesenchymal stem cell-derived exosomes for effective cartilage tissue repair and treatment of osteoarthritis. Biotechnol J. 2020;15(12): e2000082.
Qazi TH, et al. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials. 2017;140:103–14.
Shi Q, et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904.
Yang J, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed. 2020;15:5911–26.
Xiong Y, et al. All-in-one: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18(1): e2104229.
Kim HY, et al. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243: 119942.
Kim JY, et al. Defined MSC exosome with high yield and purity to improve regenerative activity. J Tissue Eng. 2021;12:20417314211008624.
Wang L, et al. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS Omega. 2019;4(27):22638–45.
Joo HS, et al. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21(3):727. https://doi.org/10.3390/ijms21030727.
Cao J, et al. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11(1):206.
Haraszti RA, et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol Ther. 2018;26(12):2838–47.
Wu Z, He D, Li H. Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact Mater. 2021;6(3):823–35.
Acknowledgements
Not applicable.
Funding
This work was supported by the Science and Technolog Commission of Shanghai Municipality (No.21Y11906700 to Yising Chen). Science Commission of Shangha and Technology Municipality (No.20Y11907300 to Yisong Chen).
Author information
Authors and Affiliations
Contributions
LMX drafted the manuscript; CZX contributed to the analysis of the results; and YZSM and YSC reviewed and modified the manuscript. All authors agreed on the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Sima, Y., Xiao, C. et al. Exosomes derived from mesenchymal stromal cells: a promising treatment for pelvic floor dysfunction. Human Cell 36, 937–949 (2023). https://doi.org/10.1007/s13577-023-00887-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00887-6