Abstract
To understand the molecular mechanisms responsible for radioresistance in cancer cells, we previously established clinically relevant radioresistant (CRR) cell lines from several human cancer cell lines. These CRR cells proliferate even under exposure to 2 Gy/day of X-rays for more than 30 days, which is a standard protocol for tumor radiotherapy. CRR cells received 2 Gy/day of X-rays to maintain their radioresistance (maintenance irradiation; MI). Interestingly, CRR cells that did not receive MI for more than a year lost their radioresistance, indicating that radiation-induced radioresistance is reversible. We designated these CRR-NoIR cells. Karyotyping of the parental and CRR cells revealed that the chromosomal composition of CRR cells is quite different from that of the parental cells. However, CRR and CRR-NoIR cells were more similar compared with the parental cells because CRR cells repair X-ray-induced DNA damage with higher fidelity. To identify the factor(s) involved in tumor radioresistance, previously published studies including ours have compared radioresistant cells to parental cells. In this review, we conclude that a comparison between CRR and CRR-NoIR cells, rather than parental cells, is the best way to identify factors involved in tumor radioresistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major health concern, and the most common types of cancer treatments include radiotherapy, chemotherapy, immunotherapy, and surgery. Surgery and radiotherapy are local therapies, whereas chemotherapy and radiotherapy are generally noninvasive treatments. Depending on the type of cancer, each treatment may be used alone or as part of a multidisciplinary approach, such as radio-chemotherapy [1,2,3]. Because tumor cells exhibit sensitivity to ionizing radiation (IR), radiotherapy has emerged as the primary type of treatment for solid tumors [4]. Radiotherapy directly induces DNA damage or indirectly results in the production of reactive oxygen species (ROS) in cancer cells [5]. The goal of radiotherapy is to eradicate tumor cells completely, while sparing nearby normal tissues. Radiotherapy takes advantage of the difference in radiation sensitivity between normal and tumor tissue [6]. Therefore, radiotherapy has the great advantage of being able to treat cancer, while preserving the function of the organ in which it was formed. Significant progress has been made in the development of technology to deliver radiation therapy, including stereotactic radiosurgery and intensity-modulated radiotherapy [7, 8]. Radio-chemotherapy has also made rapid progress. Radiotherapy combined with chemotherapy can reverse tumor hypoxia by reducing tumor oxygen consumption, which may lead to considerable clinical improvements for many tumor types [9]; however, some issues still need to be resolved. Among these, the existence and development of cancer cells that are resistant to radiation its overall efficacy. Many studies have attempted to explain this phenomenon and many mechanisms of radioresistance have been suggested over recent decades [10,11,12,13].
Resistance to radiation in cancer cells has been recognized and studied for a long time [11, 14,15,16,17]. Most studies compare cell lines that have the same organ of origin, but exhibit different radiosensitivities [18,19,20]. For example, to screen for novel biomarkers of radioresistance and elucidate the underlying mechanism, transcriptome analysis of a radioresistant and radiosensitive prostate cancer cell lines was performed [21]. To determine the effect of miR-4778-3p on the radiosensitivity of cervical cancer cells, HeLa and SiHa cervical cancer cell lines were used as radioresistant cell lines [22]. Radioresistant SW480 and moderately radioresistant HCT-15 colorectal cancer cells were used to determine whether survivin plays a direct role in mediating radiation resistance [23]. What should be considered, however, is that cancer cells with different genomic backgrounds were analyzed in these studies. Various factors suspected of being involved in cancer cell radioresistance have been reported, such as DOC-2/DAB2 interactive protein (DAB2IP) [24], phosphoprotein associated with glycosphingolipid-enriched microdomains 1 [25], and cyclin D1 [26]. Interestingly, Martin et al. showed that cyclin D1-overexpressing MCF7 cells were more sensitive to IR compared with their nonoverexpressing counterparts [27]. The cyclin D1-overexpresing cells also exhibited a higher induction of apoptosis; however, it was also reported that overexpression of cyclin D1 is responsible for the radioresistance phenotype of long-term fractionated irradiated cells [28]. The PI3K/Akt/mTOR pathway in prostate cancer [29] and the RAF1/ERK/IKK/NFκB pathway in lung cancer [30] may also be involved in radioresistance. Thus, many factors are believed to contribute to cancer cell radioresistance. Recently, cancer stem cells (CSCs) have received attention, because they may be involved in the radioresistance of tumors [31,32,33,34,35]; however, attempts have also been made to establish radioresistant cell lines. Researchers have selected cells that survive radiation treatment and determined whether the radioresistant phenotype persists. In an isogenic study comparing cancer cell lines with the same genetic background, cells that survived relatively high doses of X-rays or gamma rays were considered radioresistant cells [36,37,38,39]. In these studies, the involvement of multiple factors in cancer cell radioresistance was reported [40]. Although these results are of significant importance to radiation biology, the irradiation conditions used in these studies were sometimes different from those of standard tumor radiotherapy (such as fractionated 2 Gy/day of X-rays). Thus, it is doubtful whether the results can be translated to a clinical context. In the future, cross-validation of the results is necessary to establish robust evidence that can be translated into clinical practice.
Establishment of clinically relevant radioresistant (CRR) cell lines
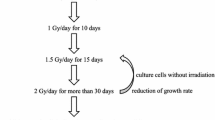
To understand the molecular mechanisms underlying the radioresistance of cancer cells, we previously established CRR cell lines from several human cancer cell lines representing different organs of origin [41]. Generally, the standard X-ray dose for conventional fractionated radiotherapy is 60 Gy with 2 Gy daily fractions over 5 weeks. CRR cells can proliferate despite exposure to 2 Gy/day of X-rays for more than 30 days in vitro [42]. To date, there have been no reports of cancer cells that proliferate following exposure to 2 Gy/day of X-rays for 30 days or longer. For example, although parental HepG2, HeLa, or SAS cells had completely died after exposure to 2 Gy/day X-rays for 30 consecutive days, CRR cells continued to proliferate stably [42, 43]. Over a long period of time, we have succeeded in establishing CRR cell lines from several cancer cell lines, such as the H1299 human non-small cell lung carcinoma cell line, HSC-2 human oral squamous cell carcinoma cell line, and A172 human glioblastoma cell line. Now that the establishment method has been tested, CRR cell lines may be obtained from any cancer cell line in at least 3 months using our method [41]. To identify the factors that contribute to cancer cell radioresistance, several studies using CRR cells have been reported [44,45,46,47] (Table 1). CRR cells are not only resistant to X-rays, but also to docetaxel, an anti-cancer drug that inhibits microtubule depolymerization [48]. Radiation exposure can induce autophagic cell death in cancer cells [49,50,51], but a significant induction of autophagic cell death was not identified in CRR cells following X-ray exposure [52]. CRR cells implanted subcutaneously in the back of nude mice were found to be resistant to fractionated X-rays compared with parental cells, suggesting that the CRR phenotype was also maintained in vivo [53]. Although CRR cells have been the subject of extensive research, the definitive factors involved in X-ray resistance in CRR cells have not yet been identified.
When analyzing CRR cell lines, it should be noted that these cell lines are usually established by exposing cancer cells to stepwise increases of X-rays. Therefore, the characteristics of CRR cells are not exactly the same as the radioresistant cells originally present in human tumors, such as CSCs that have not been irradiated prior to radiotherapy. CRR cells may resemble the properties of acquired radioresistant cells that appear during radiotherapy and should be analyzed to take advantage of their radioresistance to standard radiation therapy (2 Gy/day). CRR cell lines are a useful tool for obtaining basic knowledge as a model of cancer cells that are resistant to fractionated X-rays. For clinical applications, it is necessary to verify in vitro results using in vivo models, such as nude mice. In addition, verification is necessary using clinical samples of human origin that are resistant to radiation.
Radioresistance in CRR cells is reversible
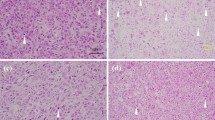
CRR cells are resistant not only to fractionated 2 Gy/day X-rays, but also to higher doses of X-rays, such as single exposure to 10 Gy. Therefore, there is no doubt that CRR cells can acquire radioresistance. Shimura et al. suggested that the acquired radioresistance phenotype in cancer cells through long-term fractionated radiation (FR) exposure was long-lasting and possibly irreversible [54]. To maintain radioresistance, CRR cells are irradiated daily with 2 Gy X-rays (maintenance irradiation; MI). Therefore, some CRR cells, such as HeLa-R or SAS-R, have been irradiated with a total dose of more than 5000 Gy. Based on these findings, we hypothesized that CRR cells may be able to maintain their radioresistant phenotype despite continued culture without MI. To test this hypothesis, CRR cells were cultured for at least 1 year without MI and their radiation sensitivity was examined. CRR cells cultured without MI for 6 months maintained their radioresistance; however, CRR cells cultured without MI for more than 1 year lost radioresistance [55]. The CRR cell lines that lost their radioresistance were designated CRR-NoIR. If there were even a few radioresistant cells among the CRR-NoIR cell population, they should survive and increase in number; however, all CRR-NoIR cells exposed to 2 Gy/day of X-rays daily died within 30 days. A modified high-density survival assay [43] also revealed that the radiosensitivity of CRR-NoIR cells was nearly the same as that of the parental cells. A decreasing mitochondrial membrane potential (Δψm) compared with the parental cells is one hallmark of CRR cells [48]. JC-1 staining, which can detect Δψm, revealed that Δψm of CRR-NoIR cells was elevated to the same level as that of the parental cells (Fig. 1) [55]. The amount of basal mitochondrial ROS (mtROS) was also increased in CRR cells compared with the parental cells. When the amount of basal mtROS was measured in CRR-NoIR cells, it was reduced to the same level as that in the parental cells (Fig. 2). These results strongly indicate that radioresistance in CRR cells is maintained by MI, but it is reversible (Table 2).
The mechanism by which CRR cells “relose” their acquired radioresistance is currently unknown. Live cell imaging of CRR and CRR-NoIR cells irradiated with 10 Gy of X-rays revealed that mitotic catastrophe was less likely occur in CRR cells, but more likely to be induced in CRR-NoIR cells, as in the parental cells. Because the induction of mitotic catastrophe and the DNA repair capacity of cells are closely related, the DNA repair capacity of CRR-NoIR cells is considered to have returned to the same level as that of the parental cells. CRR cells irradiated with 2 Gy of X-rays daily (MI) adopt an adaptive response-like effect, but if this MI is stopped, elevated DNA repair capacity returns to the parental level and radioresistance may be lost. The most interesting mechanism associated with the loss of radioresistance acquired by CRR cells involves the mitochondria. As mentioned above, the mitochondria of CRR cells exhibit a different phenotype compared with the parental cells, whereas the mitochondria of CRR-NoIR exhibit a phenotype similar to that of the parental cells. To determine whether the mitochondria are involved in radioresistance, we plan to transfer mitochondria from CRR cells to CRR-NoIR cells to see if CRR-NoIR cells become radioresistant. The radioresistance of CRR cells may be maintained for 6 months without MI, possibly as a result of epigenetic changes, such as DNA methylation. There are several possibilities to explain how CRR cells lose radioresistance, but we believe that epigenetic changes are the most likely, because it takes several months to establish CRR cells and several months for CRR cells to lose their radioresistance.
Cancer stem cells (CSCs)
Resistance to radiation and chemotherapy is an unsolved problem in cancer treatment. In recent years, the involvement of CSCs in refractory tumors following radiotherapy has been suggested [56]. CSCs are a small subpopulation of cells within tumors that have the capability of self-renewal, differentiation, and tumorigenicity when transplanted into an animal host. The clinical relevance of CSCs has been supported by recent evidence demonstrating that CSCs are resistant to conventional chemotherapy and radiotherapy and are likely the origin of cancer metastasis [57, 58]. Shimura et al. demonstrated that exposure of HepG2 and A172 cells to FR at 0.5 Gy of X-rays every 12 h for 82 days enriched the CSC population (82FR-31NR cells), because of their intrinsic radioresistance [59]. 82FR-31NR cells exhibited radioresistance along with activation of the AKT/cyclin D1 survival signaling pathway. They also reported that HepG2 and HeLa cells acquired radioresistance (31FR–31NR cells) along with cyclin D1 overexpression [54] following exposure to FR at a 0.5 Gy fractionated dose of X-rays every 12 h and 6 days a week, followed by culture without irradiation for more than 31 days. This study suggested that fractionated irradiation confers radioresistance to cancer cells and additional fractionated irradiation results in CSC enrichment. Because CRR cells are exposed to long-term FR, the CSCs may become enriched in the CRR population. If CSCs are present in CRR cells, then they should continue to be present in CRR-NoIR cells cultured without MI. However, we suspect that CSCs are not involved in cancer cell radioresistance, because all CRR-NoIR cells disappear following exposure to 2 Gy/day of X-rays for 30 days [55]. Fukumoto et al. also reported that radioresistant cells are not necessarily CSCs [60]. We do not deny the existence of CSCs that are resistant to radiation; however, we believe that further analysis is required to determine whether the radioresistant cells that appear during cancer radiotherapy are actually CSCs. Therefore, we conclude that it is more reasonable to consider CSCs and acquired radioresistant cells, such as CRR cells, as separate classes of radioresistant cells.
Karyotype of CRR cells
The exposure of cells to X-rays may induce chromosomal abnormalities [61]. Therefore, it is unlikely that the karyotype of CRR cells exposed to 2 Gy/day of X-rays for more than a year would remain similar to that of the parental cells. A previously published study suggested that DNA double-strand breaks (dsbs) induced by 2 Gy/day of FR were repaired with high fidelity in radioresistant, but not in parental cells [62]. The mutation frequencies at the hypoxanthine phosphoribosyltransferase locus induced by X-rays were lower in HepG2-8960-R cells compared with that in HepG2 cells. Our previous study also showed that DNA dsbs induced in CRR cells by X-ray irradiation were efficiently repaired [42]. These results suggest that chromosomal rearrangement does not occur as frequently following the acquisition of radioresistance in CRR cells. However, the karyotype of CRR cells was quite different compared with that of parental cells. To study the incidence of chromosomal aberrations in CRR cells, such as aneuploidy, translocation, and partial deletion, karyotyping was conducted by Giemsa staining. The G-banding pattern revealed that the chromosomes of the parental HepG2 cells were not markedly altered compared with normal human cells and it was possible to identify each chromosome. In contrast, karyotyping of HepG2-8960-R cells revealed an extremely complex reconstruction of the chromosomes, making it impossible to identify each chromosome [63]. These observations strongly suggested that during the establishment of CRR cell lines, the chromosomes of CRR cells are repeatedly cleaved and recombined. To analyze chromosome reconstruction in more detail, we performed a chromosome comparative genomic hybridization (CGH) analysis of HepG2 and HepG2-8960-R genomes. CGH can be used to detect reduced copy number, excess, and amplification of chromosomes. CGH analysis indicated that the genomes of HepG2-8960-R cells exhibited reduced copy number, excess, and amplification on all chromosomes [63]. These results indicate that although CRR cells have the same genetic background as the corresponding parental cells, their chromosomal composition is completely different. Therefore, parental cells and their resistant counterparts are completely different cells, even though they share the same genetic background.
Future plans to identify the factors involved in cancer cell radioresistance
A number of studies have established and analyzed radioresistant cells, in which the genetic backgrounds were identical to that of their parental cell lines [37, 40]. In some studies, cells were exposed to low daily doses of X-rays, and the exposure dose was gradually increased up to 1.5–2 Gy to establish radioresistant cells. Gray et al. established radioresistant cell lines by exposing MDA-MB-231, MCF-7, and ZR-751 parental cells to increasing weekly doses of radiation [15]. Todorovic et al. obtained a radioresistant cell line by exposing FaDu cells to 2 Gy/day for 5 days/week for 3 weeks [38]. To date, various factors involved in radiation resistance have been reported, such as alterations in Wnt signaling [64], Hedgehog signaling [65], and AKT/cyclin D1/Cdk4 survival signaling [59], but no common factors have been identified. Using miRNA arrays and qPCR, Tomita et al. found that miR-7-5p is involved in cancer cell radioresistance [66]. MiR-7-5p downregulates mitoferrin and reduces Fe2+, which attenuates ferroptosis. In addition, Fukumoto et al. reported that guanine nucleotide-binding protein 1 is involved in cancer cell radioresistance [60]. Shimura et al. demonstrated that a moderate level of long-term FR confers radioresistance to tumor cells, which is caused by DNA-PK/AKT/GSK3β-mediated cyclin D1 overexpression [54]. However, in CRR cells, the overexpression of cyclin D1 was not detected (Fig. 3). We established and analyzed several types of CRR cells from human cancer cell lines derived from various tissues to identify common factors associated with radioresistance. Several laboratories have also used the same approach. Some of these studies included microarray and metabolome analysis; however, no candidate genes or signaling pathways that are commonly silenced/activated in all CRR cells have been found.
CRR cells must be subjected to MI to maintain their radioresistant phenotype. As mentioned above, during the development of CRR cells, genomic rearrangements can occur in these cells. Karyotyping and CGH analysis revealed that parental cells and their corresponding CRR cells are considerably different at the chromosomal level. Moreover, there are many differences in gene copy number. Therefore, when we compared the parental cells with CRR cells undergoing MI, the gene expression changes may be noise, which may prevent us from identifying the factors involved in the radioresistance of CRR cells. It should be noted that CRR cell lines, once established, are completely different from the parental cell lines. In terms of cell morphology, the volume of CRR cells is smaller compared with that of parental cells [67]. We compared the parental cells with CRR cells undergoing MI in order to identify factors involved in clinically relevant radioresistance; however, common factors involved in radiation resistance in several types of CRR cell lines have not been identified. Therefore, it is likely that a comparison between parental and CRR cell lines is not a suitable way to identify the factors involved in cancer cell radioresistance.
CRR cells can lose their radioresistance after more than a year in culture without MI. CRR cells also exhibit hydrogen peroxide resistance, which can be lost after a year or more in culture without MI [55]. A decreasing mitochondrial membrane potential is the hallmark of CRR cells; however, in CRR-NoIR cells, Δψm increased to almost the same level as in parental cells [55]. These results suggest that CRR cells cultured in the absence of MI for more than 1 year lose their CRR phenotype. In addition, chromosomal aberrations are unlikely to occur as much in CRR cells during 1 year of culture without MI. The genome composition of CRR-NoIR cells, which are not radioresistant, may be closer to CRR cells compared with parental cells. Therefore, to identify the factors involved in cancer cell radioresistance, it may be better to compare CRR cells with CRR-NoIR cells, not the corresponding parental cells. A comparison of CRR cells and CRR-NoIR cells would involve cell lines with similar copy numbers of genes, chromosomes, and genomes but with different radiation sensitivities. We reported that CRR-NoIR cells exhibited lower (like parental) miR7-5p expression compared with CRR cells, and when miR7-5p was down-regulated by siRNA in CRR cells, the radioresistance was lost [66, 67]. We also reported that miR7-5p downregulates Fe2+ levels through its target gene mitoferrin [67]. Knockdown of mitoferrin resulted in radioresistant in parental cells [66]. and knockdown of miR7-5p in CRR cells increase Δψm [55, 67]. Because mitochondria are the primary iron-utilizing intracellular organelle [68], we hypothesize that during the process of CRR to CRR-NoIR conversion, changes in iron metabolism and mitochondrial functional occur, which leads to increased iron-dependent cell death (ferroptosis). To our knowledge, this type of comparison has not been done previously, but it is a simple way to identify factors of radioresistance in cancer cells.
Conclusions
Radioresistant cell lines used in other studies are not CRR cells and are expected to differ considerably in genome organization compared with their parental cells. In such cases, it may have been easier to identify the factors involved in radioresistance if the radioresistant cells had been cultured until they lost their radioresistance and then compared with the radioresistant cells.
Cancer cells are not only forced to change their behavior by direct irradiation, but also receive bystander effects through irradiation of the surrounding cells [69]. When the supernatant from the CRR cell culture was used to culture parental cells, the parental cells did not exhibit radioresistance. To determine the possibility of bystander effects in radioresistance, we are in the process of establishing CRR cells from multiple cells labeled with different fluorescent dyes. We plan to perform live cell imaging by co-culturing fluorescent CRR cells with nonfluorescent parental cells to gain new insight into whether parental cells in contact with CRR cells acquire radioresistance.
Data availability
All data generated during the current study are included in the article.
References
Valentini V, Boldrini L, Mariani S, Massaccesi M. Role of radiation oncology in modern multidisciplinary cancer treatment. Mol Oncol. 2020;14(7):1431–41.
Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: the role of heat in multidisciplinary cancer care. Semin Oncol. 2014;41(6):714–29.
Cheng Y, Weng S, Yu L, Zhu N, Yang M, Yuan Y. The role of hyperthermia in the multidisciplinary treatment of malignant tumors. Integr Cancer Ther. 2019;18:1534735419876345.
Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci. 2014;1:24.
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25: 101084.
Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9(2):134–42.
Maciunas RJ. Stereotactic radiosurgery. Nat Med. 1996;2(6):712–3.
Nakamura K, Sasaki T, Ohga S, Yoshitake T, Terashima K, Asai K, et al. Recent advances in radiation oncology: intensity-modulated radiotherapy, a clinical perspective. Int J Clin Oncol. 2014;19(4):564–9.
Ghattass K, Assah R, El-Sabban M, Gali-Muhtasib H. Targeting hypoxia for sensitization of tumors to radio- and chemotherapy. Curr Cancer Drug Targets. 2013;13(6):670–85.
Rahimi S, Roushandeh AM, Ahmadzadeh E, Jahanian-Najafabadi A, Roudkenar MH. Implication and role of neutrophil gelatinase-associated lipocalin in cancer: lipocalin-2 as a potential novel emerging comprehensive therapeutic target for a variety of cancer types. Mol Biol Rep. 2020;47(3):2327–46.
Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–25.
Kim W, Lee S, Seo D, Kim D, Kim K, Kim E, et al. Cellular stress responses in radiotherapy. Cells. 2019;8(9):1105.
Joiner MC. Induced radioresistance: an overview and historical perspective. Int J Radiat Biol. 1994;65(1):79–84.
Kim BM, Hong Y, Lee S, Liu P, Lim JH, Lee YH, et al. Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci. 2015;16(11):26880–913.
Gray M, Turnbull AK, Ward C, Meehan J, Martinez-Perez C, Bonello M, et al. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat Oncol. 2019;14(1):64.
Petragnano F, Pietrantoni I, Camero S, Codenotti S, Milazzo L, Vulcano F, et al. Clinically relevant radioresistant rhabdomyosarcoma cell lines: functional, molecular and immune-related characterization. J Biomed Sci. 2020;27(1):90.
Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F, et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. 2018;37(1):87.
Zang C, Zhao F, Pu Y. LMX1B involved in the radioresistance, proliferation and migration of esophageal cancer cells. Biomed Pharmacother. 2019;118: 109358.
Guo WF, Lin RX, Huang J, Zhou Z, Yang J, Guo GZ, et al. Identification of differentially expressed genes contributing to radioresistance in lung cancer cells using microarray analysis. Radiat Res. 2005;164(1):27–35.
Asanuma K, Kobayashi D, Furuya D, Tsuji N, Yagihashi A, Watanabe N. A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res. 2002;93(9):1057–62.
Young A, Berry R, Holloway AF, Blackburn NB, Dickinson JL, Skala M, et al. RNA-seq profiling of a radiation resistant and radiation sensitive prostate cancer cell line highlights opposing regulation of DNA repair and targets for radiosensitization. BMC Cancer. 2014;14:808.
Zhang Y, Li P, Hu J, Zhao LN, Li JP, Ma R, et al. Role and mechanism of miR-4778-3p and its targets NR2C2 and Med19 in cervical cancer radioresistance. Biochem Biophys Res Commun. 2019;508(1):210–6.
Rodel F, Hoffmann J, Distel L, Herrmann M, Noisternig T, Papadopoulos T, et al. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005;65(11):4881–7.
Yun EJ, Lin CJ, Dang A, Hernandez E, Guo J, Chen WM, et al. Downregulation of human DAB2IP gene expression in renal cell carcinoma results in resistance to ionizing radiation. Clin Cancer Res. 2019;25(14):4542–51.
Shen L, Ke Q, Chai J, Zhang C, Qiu L, Peng F, et al. PAG1 promotes the inherent radioresistance of laryngeal cancer cells via activation of STAT3. Exp Cell Res. 2018;370(1):127–36.
Marampon F, Gravina G, Ju X, Vetuschi A, Sferra R, Casimiro M, et al. Cyclin D1 silencing suppresses tumorigenicity, impairs DNA double strand break repair and thus radiosensitizes androgen-independent prostate cancer cells to DNA damage. Oncotarget. 2016;7(5):5383–400.
Coco Martin JM, Balkenende A, Verschoor T, Lallemand F, Michalides R. Cyclin D1 overexpression enhances radiation-induced apoptosis and radiosensitivity in a breast tumor cell line. Cancer Res. 1999;59(5):1134–40.
Shimura T. Acquired radioresistance of cancer and the AKT/GSK3beta/cyclin D1 overexpression cycle. J Radiat Res. 2011;52(5):539–44.
Chang L, Graham PH, Ni J, Hao J, Bucci J, Cozzi PJ, et al. Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol. 2015;96(3):507–17.
Tsolou A, Liousia M, Kalamida D, Pouliliou S, Giatromanolaki A, Koukourakis M. Inhibition of IKK-NFkappaB pathway sensitizes lung cancer cell lines to radiation. Cancer Biol Med. 2017;14(3):293–301.
Atashzar MR, Baharlou R, Karami J, Abdollahi H, Rezaei R, Pourramezan F, et al. Cancer stem cells: A review from origin to therapeutic implications. J Cell Physiol. 2020;235(2):790–803.
Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer stem cells and radioresistance: DNA repair and beyond. Cancers (Basel). 2019;11(6):862.
Reid PA, Wilson P, Li Y, Marcu LG, Bezak E. Current understanding of cancer stem cells: review of their radiobiology and role in head and neck cancers. Head Neck. 2017;39(9):1920–32.
Diehn M, Clarke MF. Cancer stem cells and radiotherapy: new insights into tumor radioresistance. J Natl Cancer Inst. 2006;98(24):1755–7.
Rycaj K, Tang DG. Cancer stem cells and radioresistance. Int J Radiat Biol. 2014;90(8):615–21.
de Llobet LI, Baro M, Figueras A, Modolell I, Da Silva MV, Munoz P, et al. Development and characterization of an isogenic cell line with a radioresistant phenotype. Clin Transl Oncol. 2013;15(3):189–97.
McDermott N, Meunier A, Lynch TH, Hollywood D, Marignol L. Isogenic radiation resistant cell lines: development and validation strategies. Int J Radiat Biol. 2014;90(2):115–26.
Todorovic V, Prevc A, Zakelj MN, Savarin M, Brozic A, Groselj B, et al. Mechanisms of different response to ionizing irradiation in isogenic head and neck cancer cell lines. Radiat Oncol. 2019;14(1):214.
Tang WY, Chau SP, Tsang WP, Kong SK, Kwok TT. The role of Raf-1 in radiation resistance of human hepatocellular carcinoma Hep G2 cells. Oncol Rep. 2004;12(6):1349–54.
Oike T, Ohno T. Molecular mechanisms underlying radioresistance: data compiled from isogenic cell experiments. Ann Transl Med. 2020;8(6):273.
Kuwahara Y, Roudkenar MH, Urushihara Y, Saito Y, Tomita K, Roushandeh AM, et al. Clinically relevant radioresistant cell line: a simple model to understand cancer radioresistance. Med Mol Morphol. 2017;50(4):195–204.
Kuwahara Y, Li L, Baba T, Nakagawa H, Shimura T, Yamamoto Y, et al. Clinically relevant radioresistant cells efficiently repair DNA double-strand breaks induced by X-rays. Cancer Sci. 2009;100(4):747–52.
Kuwahara Y, Mori M, Oikawa T, Shimura T, Ohtake Y, Mori S, et al. The modified high-density survival assay is the useful tool to predict the effectiveness of fractionated radiation exposure. J Radiat Res. 2010;51(3):297–302.
Saito Y, Miura H, Takahashi N, Kuwahara Y, Yamamoto Y, Fukumoto M, et al. Involvement of APOBEC3B in mutation induction by irradiation. J Radiat Res. 2020;61(6):819–27.
Miyashita H, Fukumoto M, Kuwahara Y, Takahashi T, Fukumoto M. ISG20 is overexpressed in clinically relevant radioresistant oral cancer cells. Int J Clin Exp Pathol. 2020;13(7):1633–9.
Tomita K, Takashi Y, Ouchi Y, Kuwahara Y, Igarashi K, Nagasawa T, et al. Lipid peroxidation increases hydrogen peroxide permeability leading to cell death in cancer cell lines that lack mtDNA. Cancer Sci. 2019;110(9):2856–66.
Tomita K, Kuwahara Y, Takashi Y, Igarashi K, Nagasawa T, Nabika H, et al. Clinically relevant radioresistant cells exhibit resistance to H2O2 by decreasing internal H2O2 and lipid peroxidation. Tumour Biol. 2018;40(9):1010428318799250.
Kuwahara Y, Roudkenar MH, Suzuki M, Urushihara Y, Fukumoto M, Saito Y, et al. The involvement of mitochondrial membrane potential in cross-resistance between radiation and docetaxel. Int J Radiat Oncol Biol Phys. 2016;96(3):556–65.
Cui L, Song Z, Liang B, Jia L, Ma S, Liu X. Radiation induces autophagic cell death via the p53/DRAM signaling pathway in breast cancer cells. Oncol Rep. 2016;35(6):3639–47.
Chen Z, Wang B, Yu F, Chen Q, Tian Y, Ma S, et al. The roles of mitochondria in radiation-induced autophagic cell death in cervical cancer cells. Tumour Biol. 2016;37(3):4083–91.
Jo GH, Bogler O, Chwae YJ, Yoo H, Lee SH, Park JB, et al. Radiation-induced autophagy contributes to cell death and induces apoptosis partly in malignant glioma cells. Cancer Res Treat. 2015;47(2):221–41.
Kuwahara Y, Oikawa T, Ochiai Y, Roudkenar MH, Fukumoto M, Shimura T, et al. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011;2: e177.
Kuwahara Y, Mori M, Kitahara S, Fukumoto M, Ezaki T, Mori S, et al. Targeting of tumor endothelial cells combining 2 Gy/day of X-ray with Everolimus is the effective modality for overcoming clinically relevant radioresistant tumors. Cancer Med. 2014;3(2):310–21.
Shimura T, Kakuda S, Ochiai Y, Nakagawa H, Kuwahara Y, Takai Y, et al. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene. 2010;29(34):4826–37.
Kuwahara Y, Tomita K, Roudkenar MH, Roushandeh AM, Urushihara Y, Igarashi K, et al. Decreased mitochondrial membrane potential is an indicator of radioresistant cancer cells. Life Sci. 2021;286: 120051.
Colak S, Medema JP. Cancer stem cells–important players in tumor therapy resistance. FEBS J. 2014;281(21):4779–91.
Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer. 2008;8(7):545–54.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–51.
Shimura T, Noma N, Oikawa T, Ochiai Y, Kakuda S, Kuwahara Y, et al. Activation of the AKT/cyclin D1/Cdk4 survival signaling pathway in radioresistant cancer stem cells. Oncogenesis. 2012;1: e12.
Fukumoto M, Amanuma T, Kuwahara Y, Shimura T, Suzuki M, Mori S, et al. Guanine nucleotide-binding protein 1 is one of the key molecules contributing to cancer cell radioresistance. Cancer Sci. 2014;105(10):1351–9.
Natarajan AT, Palitti F. DNA repair and chromosomal alterations. Mutat Res. 2008;657(1):3–7.
Kuwahara Y, Roudkenar M, Urushihara Y, Saito Y, Tomita K, Roushandeh A, Sato T, Kurimasa A, Fukumoto M. X-ray induced mutation frequency at the hypoxanthine phosphoribosyltransferase locus in clinically relevant radioresistant cells. Int J Med Phys Clin Eng Radiat Oncol. 2017;6:377–91.
Kuwahara Y, Tomita K, Takabatake T, Urushihara U, Igarashi K, Sato T, et al. Clinically relevant radioresistant cells; past history and future plans. J Tohoku Med Pharm Univ. 2019;66:19–24.
Zhao Y, Tao L, Yi J, Song H, Chen L. The role of canonical wnt signaling in regulating radioresistance. Cell Physiol Biochem. 2018;48(2):419–32.
Liu C, Wang R. The roles of hedgehog signaling pathway in radioresistance of cervical cancer. Dose Response. 2019;17(4):1559325819885293.
Tomita K, Fukumoto M, Itoh K, Kuwahara Y, Igarashi K, Nagasawa T, et al. MiR-7-5p is a key factor that controls radioresistance via intracellular Fe(2+) content in clinically relevant radioresistant cells. Biochem Biophys Res Commun. 2019;518(4):712–8.
Tomita K, Nagasawa T, Kuwahara Y, Torii S, Igarashi K, Roudkenar MH, Roushandeh AM, Kurimasa A, Sato T. MiR-7-5p is involved in ferroptosis signaling and radioresistance thru the generation of ROS in radioresistant HeLa and SAS cell lines. Int J Mol Sci. 2021;22:8300.
Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and iron: current questions. Expert Rev Hematol. 2017;10(1):65–79.
Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9(5):351–60.
Sakata J, Hirosue A, Yoshida R, Matsuoka Y, Kawahara K, Arita H, Nakashima H, Yamamoto T, Nagata M, Kawaguchi S, Gohara S, Nagao Y, Yamana K, Toya R, Murakami R, Kuwahara Y, Fukumoto M, Nakayama H. Enhanced expression of IGFBP-3 reduces radiosensitivity and is associated with poor prognosis in oral squamous cell carcinoma. Cancers (Basel). 2020;12(2):494.
Saito Y, Abiko R, Kishida A, Kuwahara Y, Yamamoto Y, Yamamoto F, Fukumoto M, Ohkubo Y. Loss of EGF-dependent cell proliferation ability on radioresistant cell HepG2-8960-R. Cell Biochem Funct. 2015;33(2):73–9.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers 16K00538, 19K12326, and 22K12379.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuwahara, Y., Tomita, K., Habibi Roudkenar, M. et al. The reversibility of cancer radioresistance: a novel potential way to identify factors contributing to tumor radioresistance. Human Cell 36, 963–971 (2023). https://doi.org/10.1007/s13577-023-00871-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-023-00871-0