Abstract
Radiotherapy (RT) is one of the major modalities for the treatment of human cancer and has been established as an excellent local treatment for malignant tumors. However, the existence of radioresistant cells remains one of the most critical obstacles in RT. To know the characteristics of radioresistant cells, clinically relevant radioresistant (CRR) cell lines were established. CRR cells can continue to proliferate in vitro and in vivo after exposure to 2 Gy/day of X-rays for more than 30 days. Daily microscopic observation of the irradiated CRR cells has indicated that the increase in cell death is not observed within 7 days of irradiation with 10 Gy of X-rays, suggesting that cell death is involved in cellular radioresistance. Radiation-induced regulated cell death (RCD) can be classified into three categories: apoptosis, autophagy-dependent cell death and necrosis (necroptosis). This review focuses on an aspect of radiation-induced RCD that has often been neglected: the manner in which the cells are destroyed. In many studies, apoptosis is considered the primary mode of RCD in irradiated cancer cells; however, it is necessary to consider necrosis or necroptosis as one of the modes of radiation-induced RCD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy (RT) is one of the major modalities for the treatment of human cancer and has been established as an excellent local treatment for malignant tumors. The objective of RT is to achieve local control of cancer to prevent invasion, organ dysfunction, and metastases. The efficacy of RT depends on the presence of radioresistant cells, which remains one of the most critical obstacles in RT and radio-chemotherapy. Cellular radiosensitivity has long been a focus area in the field of radiation biology and oncology because it clearly influences the outcome of therapy. To develop more effective RT, it is necessary to understand the characteristics of radioresistant cells. Many studies have attempted to identify the molecules involved in cancer cell radioresistance (Guo et al. 2005; Chang et al. 2007; Ishigami et al. 2007; Ogawa et al. 2007). However, these studies did not necessarily aim to improve conventional fractionated RT. Moreover, it is noteworthy that cellular radioresistance has primarily been studied in cells with different genetic backgrounds and different origins. Therefore, the concordant mechanisms underlying cellular radioresistance have not been clarified yet. Therefore, it is necessary to develop a system to compare radioresistant and radiosensitive cells with isogenic background. On the other hand, published studies about the genes responsible for cellular radioresistance have analyzed surviving cells after exposure to single or several rounds of fractionated radiation. Surviving cells include many senescent cells that lack the ability to undergo mitosis, which suggests that the group of cells surviving irradiation is highly heterogeneous.
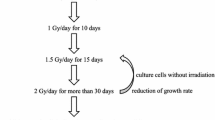
Conventional fractionated RT consists of 2 Gy of X-rays, administered once a day, 5 days a week, for 5–7 weeks, to constitute a total of 60 Gy. The rationale underlying this schedule is to allow easier recovery of normal cells, compared to cancer cells, from the sub-lethal radiation damage occurring between the fractions. The concept of fractionated RT has evolved from the empirical evidence obtained through clinical trials. Recently, the authors have established radioresistant human cancer cells that can continue to proliferate in vitro after exposure to 2 Gy/day of X-rays for more than 30 days (Table 1). These cells have been termed as “clinically relevant radioresistant (CRR)” cells (Kuwahara et al. 2009, 2017). An in vivo experiment using a xenograft tumor model of CRR SAS-R cells showed that the tumors of CRR cells are also radioresistant to 2 Gy/day of X-rays (Fig. 1). Our previous study revealed that CRR cells could escape death caused by X-ray irradiation both in vivo and in vitro (Kuwahara et al. 2011). Therefore, to overcome radioresistance in cancer cells, it is necessary to study the mechanisms underlying radiation-induced cell death. Mammalian cells exposed to unrecoverable perturbations of the intracellular or extracellular microenvironment can activate one of many signal transduction cascades ultimately leading to their demise. Each of such regulated cell death (RCD) modes is initiated and propagated by molecular mechanisms that exhibit a considerable degree of interconnectivity (Galluzzi et al. 2018). Although the underlining molecular mechanisms exhibit considerable overlap, RCD is involved in two diametrical scenarios. On one hand, RCD can occur in the absence of any exogenous environmental perturbation. These completely physiological forms of RCD are generally referred to as programmed cell death (PCD). On the other hand, RCD can originate from perturbations of the intracellular or extracellular microenvironment, when such perturbations are too intense or prolonged for adaptive responses to cope with stress and restore cellular homeostasis (Galluzzi et al. 2016).
Xenograft tumor model of parental SAS and clinically relevant radioresistant SAS-R cells. SAS-R cells show in vivo resistance to 2 Gy/day X-rays. a SAS tumor not exposed to X-rays. b SAS tumor exposed to a total of 60 Gy of X-rays (30 fractions). The irradiated SAS tumor showed an increase in connective tissue. Many pyknotic cells are also detected in these tumors. c SAS-R tumor not exposed to X-rays. d SAS-R tumor exposed to a total of 60 Gy of X-rays (30 fractions). Unlike SAS tumor, SAS-R tumor do not show an increase in connective tissue. Arrow heads, pyknotic cells
Elucidation of the molecular mechanisms underlying radiation-induced RCD remains one of the hot topics in the field of RT (Lomax et al. 2013; Kim et al. 2015). It is generally accepted that ionizing radiation kills tumor cells primarily by causing double-strand breaks (dsbs) in the DNA directly and indirectly through the formation of intermediate radicals. Survival of cancer cells mainly depends on their capacity for oxidative defense as well as the efficacy of DNA repair. The level of reactive oxygen species (ROS)-mediated oxidative stress is also linked to cellular radiosensitivity (Dayal et al. 2014). Formerly, the mechanisms responsible for radiation-induced cell death are divided into two distinct forms: interphase death and reproductive death (Shinohara and Nakano 1993). Since they are defined based on the classical radiobiological concepts using the clonogenic cell survival assay, the biochemical and molecular mechanisms involved in the induction of radiation-induced cell death are not fully understood in relation to the modes of cell death. In 1972, Kerr and colleagues classified cell death under two headings (Kerr et al. 1972): apoptosis and necrosis. The former is PCD, which is genetically influenced. On the other hand, the latter is a form of accidental cell death, and not PCD. In 1990, the four types of PCD are reviewed based on the morphological features of dying cells, as observed during the development of both vertebrates and invertebrates (Clarke 1990). Type I PCD corresponds to apoptosis, which proceeds as cell shrinkage, chromatin condensation, nucleosomal DNA degradation, and fragmentation of the cell into apoptotic bodies. Activation of the caspase family gives rise to apoptosis. Type II PCD corresponds to autophagy-dependent cell death, which is characterized by the appearance of double- or multiple-membrane cytoplasmic vesicles engulfing bulk cytoplasm and/or cytoplasmic organelles. Autophagic vesicles and their contents are destroyed by the lysosomal system. Type III PCD is defined as non-lysosomal degradation on the vesicular membrane. This mode of cell death corresponds to necrosis or necroptosis, which can be further subdivided into type IIIA (non-lysosomal degradation) and IIIB (cytoplasmic type of degeneration).
Apoptosis, is considered the main mode of radiation-induced RCD, and autophagy-dependent cell death and necrosis are ignored until recent. On the other hand, advances in molecular biology revealed many modes of RCD (Kroemer et al. 2009; Galluzzi et al. 2018). Recently, several modes of radiation-induced RCD, such as apoptosis (Dewey et al. 1995; Mirzaie-Joniani et al. 2002a, b; Balcer-Kubiczek 2012), autophagy-dependent cell death (Gozuacik and Kimchi 2004; Tsuboi et al. 2009; Jo et al. 2015; Chen et al. 2016), mitotic catastrophe (Roninson et al. 2001), necrosis (Rainaldi et al. 2003), and senescence-like cell death (Galluzzi et al. 2012) have been reported.
Although apoptosis is a critical mode of cell death observed during the normal process (Meier et al. 2000), apoptotic cells are also observed in tumor tissues (Brown and Attardi 2005; Ouyang et al. 2012). The main objective of RT for cancer cells is to eliminate their reproductive potential. One approach to achieve this is by inducing tumor cell apoptosis (Kerr et al. 1994; Dewey et al. 1995) or a state of irreversible growth arrest such as senescence (Sabin and Anderson 2011; Liao et al. 2014). Kerr et al. originally coined the term “apoptosis” to differentiate naturally occurring developmental cell death from necrosis caused by acute tissue injury (1972). They adopted the Greek word for the process of leaves falling from trees or petals falling from flowers (Duque-Parra 2005). In many studies, apoptosis is considered the primary mode of cell death in irradiated cancer cells, and recent advances in tumor biology showed that apoptosis is not the main mechanism underlying the death of cancer cells in response to common treatment regimens, especially RT. Except for the caspase-dependent mechanism, several lines of evidences demonstrated that the caspase-independent mechanism is directly involved in radiation-induced cell death (Chipuk and Green 2005; Sun et al. 2016). Accumulating evidences also suggest that the induction of apoptosis alone is insufficient to account for the therapeutic effect of RT (Abend 2003; Eriksson and Stigbrand 2010). It has become obvious in the last few years that the inhibition of the proliferative capacity of malignant cells following IR, especially in case of solid tumors, can occur via alternative cell death modalities or permanent cell cycle arrest, i.e., mitotic catastrophe or senescence. Previously, the authors reported that apoptosis is not significantly induced in HepG2 cells after X-ray irradiation (Kuwahara et al. 2011). This review focuses on an aspect of radiation-induced RCD that has often been neglected: the manner in which the cells are destroyed. The dying cells, after exposure to IR, might adopt one of the three different morphological types, namely, apoptosis, necrosis and autophagy-dependent cell death. In this review, radiation-induced senescence and mitotic catastrophe, considered as one of the modes of cell death in tumor cells, are not mentioned because the metabolic processes of those cells continued to remain active. These cells finally perish by apoptosis, autophagy-dependent cell death, or necrosis (Fukumoto 2014).
Effect of X-ray exposure on cancer cells
Many in vitro studies using cancer cell lines showed that radiation-induced cell death usually occurs within 48 h of irradiation. However, daily microscopic observation of the irradiated HepG2 cells has indicated that the increase in cell death is not observed within 48 h of irradiation with 10 Gy of X-rays (Kuwahara et al. 2011). Cell death is not induced in HeLa cells within 48 h of exposure to 10 Gy of X-rays (Fig. 2c), but begins 3 days after the exposure (Fig. 2d). Cells in mitotic catastrophe are also frequently observed around the same time. Dead cells with apoptotic bodies are seldom observed in irradiated cells. At day 5 after the exposure, a significant increase is observed in the number of dying cells (Fig. 2e). At day 7, most of the dying cells are without features of apoptosis or necrosis, suggesting that autophagy-dependent cell death is induced at this time point. During these 7 days, the induction of apoptosis is ≤ not higher than 20% in HepG2, SAS, and HeLa cell lines, as determined by Annexin-V staining. Autophagy-dependent cell death is the predominant mode of cell death in HepG2 (Kuwahara et al. 2011); on the other hand, necroptosis or necrosis also seemed to be the predominant mode of cell death in irradiated cancer cells.
Morphological changes in HeLa cells after exposure to 10 Gy of X-rays. a HeLa cells not exposed to X-rays. b HeLa cells at day 1 after exposure to 10 Gy of X-rays. Cell death is rare. c HeLa cells at day 2 after exposure to 10 Gy of X-rays. Mitotic cells increase in number (white arrow heads). d HeLa cells at day 3 after exposure to 10 Gy of X-rays. e HeLa cells at day 5 after exposure to 10 Gy of X-rays. f HeLa cells at day 7 after exposure to 10 Gy of X-rays. Black arrow heads, necrotic cells; white arrows, dying cells without features of apoptosis or necrosis; black arrows, apoptotic death with apoptotic bodies
Radiation-induced apoptosis
Apoptosis is generally characterized by dynamic morphological changes and energy-dependent biochemical mechanisms (Elmore 2007). Apoptosis is a vital component of various processes, including normal cell turnover, proper development and functioning of the immune system, hormone-dependent atrophy, embryonic development, and chemical-induced cell death (Elmore 2007). Apoptosis is considered the primary mode of cell death following exposure to radiation. Normal tissues such as the thymus, intestine, and testis show significant induction of apoptosis by IR (Ohyama et al. 1985; Hasegawa et al. 1997; Potten and Grant 1998). In the lymphoid and myeloid lineages, apoptosis is the primary death mechanism induced by irradiation (Radford and Murphy 1994). However, radiation-induced apoptosis is not so frequently observed in the cells of epithelial origin compared with cells of the hematological origin. Radiation-induced apoptosis has been characterized and known to include pyknosis, cell shrinkage, and internucleosomal breakage of chromatin, all of which are hallmarks of apoptotic cell death (Shinomiya 2001). Many studies discuss radiation-induced apoptosis in cancer. X-ray irradiation alone is shown to efficiently induce apoptotic bodies in wild-type p53 SAS (Ohnishi et al. 2004). Another study also showed that 4 Gy of X-rays induce apoptosis in about 20% of H23 human non-small cell lung cancer cells (Huang et al. 2010). On the other hand, 10 Gy of X-rays induce apoptosis in about 20% of HepG2 cells about 48 h after exposure to 10 Gy of X-rays (Shimura et al. 2010). Pinar et al. also showed that radiation-induced apoptosis increases with increasing dose of radiation, and that the data fit to a semi-logarithmic mathematical model; in fact, a positive correlation is noted among radiation-induced apoptosis values at different doses of radiation (2010). One of the well-known hallmarks of apoptosis is the induction of apoptotic bodies (Figs. 3b, 5a, b). To determine whether apoptosis is the main mode of radiation-induced cell death or not, morphological changes occurring in irradiated cancer cells are observed daily using phase contrast microscopy. As shown in Fig. 2, dying cells with apoptotic bodies are, at times, observed after irradiation; however, during the 7 days post-irradiation, no drastic induction of apoptosis is observed in HeLa and HepG2 cells (Kuwahara et al. 2011). In three other tumor cell lines, that is, SAS, HeLa, and U2OS-RFP-LC3, X-ray exposure induced significant rate of cell death; however, cells with apoptotic features are seldom encountered. These morphological observations strongly suggest that although X-ray exposure induces apoptosis in cancer cells, apoptosis is not the main mode of cell death induced by X-ray. Time-lapse analysis of irradiated cancer cells also revealed that the extent of induction of apoptosis is very low. In CRR cells, apoptotic cells are seldom observed after irradiation with 10 Gy of X-rays (Kuwahara et al. 2011).
SAS cells exposed to 10 Gy of X-rays. a SAS cells not exposed to X-rays. b Apoptotic cells with apoptotic bodies (black arrow) at day 3 after exposure to 10 Gy of X-rays. c Dying cells without features of apoptosis or necrosis (white arrows) at day 5 after exposure to 10 Gy of X-rays. d SAS cells at day 7 after exposure to 10 Gy of X-rays. Dying cells without features of apoptosis or necrosis are found to increase in number
Radiation-induced autophagy-dependent cell death
A relatively new discovery among the different modes of RCD is the autophagy-dependent cell death. This type of cell death is characterized by an ultrastructurally intact nucleus and an increase in the number of autophagosomes in the cytoplasm (Tsujimoto and Shimizu 2005; Motyl et al. 2007). Autophagy is a dynamic process of protein degradation, which is typically observed during a state of nutrient deprivation (Sato et al. 2007). Cells undergoing autophagy-dependent cell death utilize the autophagic/lysosomal compartment to auto-digest proteins and damaged organelles as well as to recycle amino and fatty acids. Autophagy is characterized by the sequestration of targeted cytoplasmic components and organelles from the rest of the cell within a double-membrane vesicle called the autophagosome (Mizushima 2007). The autophagy pathway is negatively regulated by the PI3K/Akt/mTOR pathway (Chang et al. 2015). Hyperactivation of this pathway contributes to cell death, whereas controlled activation has a pro-survival effect. Recently, the interest in autophagy has been renewed among oncologists, because different types of cancer cells undergo autophagy-dependent cell death after various anticancer therapies (Moretti et al. 2007; Janku et al. 2011). This type of non-apoptotic cell death has been documented primarily by observing morphological changes such as numerous autophagic vacuoles in the cytoplasm of dying cells. Thus, autophagy-dependent cell death is considered one of RCD processes. Autophagy-dependent cell death is suggested to be one of the modes of radiation-induced cell death (Moretti et al. 2007; Kuwahara et al. 2011; Jo et al. 2015). Autophagy-dependent cell death is involved in in vitro radioresistance because the extent of induction of autophagy-dependent cell death is lower in radioresistant HepG2-8960-R cells compared to parental HepG2 cells after exposure to fractionated radiation of X-rays. These observations are also confirmed in SAS and SAS-R tumors (Fig. 4). After exposure to a total of about 30 Gy of X-rays (15 fractions), an increase in the number of cytoplasmic autophagosomes is observed in the SAS tumor, but not in the SAS-R tumor. After exposure to 60 Gy of X-rays (30 fractions), autophagosome-filled SAS cells are observed, suggesting that autophagy-dependent cell death is induced in the SAS tumor. Induction of autophagy in radioresistant cells by rapamycin treatment is an effective in vitro approach to overcome radioresistance in tumors. The morphological changes during autophagy-dependent cell death are characterized by non-apoptotic bodies and increase in the number of cytoplasmic autophagosomes (Fig. 5). Daily microscopic observation of cancer cells revealed that this type of cell death is observed after 5 days of exposure to 10 Gy of X-rays (Kuwahara et al. 2011). Dying cells without any apoptotic bodies are filled with autophagosomes positive for an autophagosome marker, LC3 (Fig. 5c, d), suggesting that X-ray exposure induces autophagy-dependent cell death. On the other hand, autophagosomes are seldom observed in dying cells containing apoptotic bodies (Fig. 5a, b). It should be emphasized that the frequency of autophagy-dependent cell death is higher than that of apoptotic death after irradiation.
Ultrastructural analysis of parental SAS and clinically relevant radioresistant SAS-R tumors unexposed or exposed to 2 Gy/days of X-rays. a SAS tumor not exposed to X-rays. b SAS-R tumor not exposed to X-rays. c SAS tumor exposed to a total of 30 Gy (15 fractions). d SAS-R tumor exposed to a total of 30 Gy (15 fractions). e SAS tumor exposed to a total of 60 Gy (30 fractions). The typical features of autophagy-dependent cell death, including an increase in the number of autophagosomes in the cytoplasm and intact nucleus (white arrow heads). f SAS-R tumor exposed to a total of 60 Gy (30 fractions)
U2OS cells expressing RFP-LC3. a, b X-ray-induced apoptotic cells. Autophagosomes are seldom observed in apoptotic bodies. c, d X-ray-induced dying cells without morphological features of apoptosis or necrosis. A drastic increase is observed in the number of autophagosomes, suggesting that this type of dying cells are autophagy-dependent cell death. e, f Necrotic cell death induced by X-rays. No increase is observed in the number of autophagosomes in the cytoplasm
Radiation-induced necroptosis
Necrosis plays an important role in multiple physiological and pathological processes (Vakkila and Lotze 2004). Generally, necrosis is considered to be a form of un-controlled cell death, observed after exposure to relatively higher doses of radiation. Recently, a new form of necrosis has been characterized as “necroptosis” (Wu et al. 2012). Necroptosis is considered as one of the modes of RCD (Jiang et al. 2011; Wu et al. 2012; Fulda 2013, 2014; Giampietri et al. 2014; Galluzzi et al. 2018). Morphologically, necroptosis exhibits the feature of necrosis; however, necroptosis includes a unique signaling pathway involving the receptor interaction protein kinases 1 and 3 (RIP1 and RIP3) and can be specifically inhibited by necrostatins (Degterev et al. 2008; Vandenabeele et al. 2010; Silke et al. 2015). Necroptosis has been found to contribute to the regulation of the immune system, cancer development, as well as cellular response to different types of stress (Fulda 2013). Recent advances in radiation biology have shown that necroptosis plays an important role in radiation-induced cell death in cancer cells, including endocrine cancer (Nehs et al. 2011; Su et al. 2016). Traditionally, necrosis and apoptosis after mitotic catastrophe are the principal mechanisms of cellular death caused by RT (Mansilla et al. 2006). Improperly repaired double-strand breaks in DNA can cause chromosomal abnormalities that interfere with normal segregation during mitosis, which culminates in programmed or spontaneous cell death (Castedo et al. 2004; Eriksson and Stigbrand 2010). Necrosis was previously thought to be a passive process of cellular death involving morphological changes to cells, including oncosis (increased cell volume), chromatin condensation, and swelling of organelles (Vandenabeele et al. 2010). Apoptosis, however, is a coordinated process involving the activation of specific proteases called caspases in response to the binding of specific ligands to the so-called ‘‘death receptors’’ on the plasma membrane. The morphological changes occurring during apoptosis, in contrast to necrosis, include pyknosis (decreased cellular volume), nuclear fragmentation (karyorrhexis), and blebbing of the plasma membrane (Elmore 2007). For years, these two processes were thought to represent categorically different cellular death mechanisms. However, more recent evidence has revealed coordinated caspase-independent programmed cellular death, which has been termed as necroptosis (Galluzzi and Kroemer 2008; Tait and Green 2008; Declercq et al. 2009).
A pan-caspase inhibitor, namely, Z-VAD-FMK, does not reduce the number of dying cells after exposure to X-rays (Kuwahara et al. 2011). Inhibition of caspase does not change the radiosensitivity of HepG2 cells. The inhibition of apoptosis has little or no effect on clonogenic survival after treatment with drugs or radiation in several tumor cell lines (Roninson et al. 2001). These observations suggest that the contribution of apoptosis to radiation-induced cell death is lower than that assumed previously. Daily microscopic observation of X-ray-irradiated cancer cells show the existence of swelling cells without apoptotic bodies (Fig. 2b). The number of swelling cells increases from day 3 after irradiation (Fig. 2c), in a manner dependent on the dose of X-rays. This can be considered as necroptosis. Necroptosis is a recently described mechanism of RCD (Galluzzi et al. 2018), and is a RIP-kinase-dependent mode of cell death (Walsh 2014). This mode is commonly observed in HepG2, SAS, HeLa, and U2OS cells (Figs. 2, 3, 5). Notably, after exposure to 10 Gy of X-rays, the frequency of induction of necroptosis is different among the four cell lines, suggesting that the dying cells with swelling cytoplasm are induced by genetic, not physical, factors. HepG2 cells are the most radiosensitive, however, the extent of induction of necroptosis is the highest among HepG2, SAS, and HeLa cell lines. These indicate that necroptosis is one of the modes of RCD induced by X-rays.
Conclusion
Radiation-induced cell death can be classified into three categories: apoptosis, autophagy-dependent cell death and necroptosis. Many studies have shown that apoptosis is one of the primary modes of radiation-induced cell death; however, microscopic analysis of irradiated cancer cells do not show significant induction of apoptosis. Autophagy-dependent cell death is one of the main modes of X-ray-induced RCD; however, daily microscopic observation of irradiated cells in the author’s laboratory have suggested that it is necessary to consider necroptosis as one of the modes of radiation-induced RCD. To establish more efficient cancer radiotherapy, experiments are underway to clarify how and which pathway cancer cells select after radiation exposure.
References
Abend M (2003) Reasons to reconsider the significance of apoptosis for cancer therapy. Int J Radiat Biol 79:927–941. https://doi.org/10.1080/09553000310001632958
Balcer-Kubiczek EK (2012) Apoptosis in radiation therapy: a double-edged sword. Exp Oncol 34:277–285
Brown JM, Attardi LD (2005) The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 5:231–237. https://doi.org/10.1038/nrc1560
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825–2837. https://doi.org/10.1038/sj.onc.1207528
Chang JT et al (2007) Differentially expressed genes in radioresistant nasopharyngeal cancer cells: gp96 and GDF15. Mol Cancer Ther 6:2271–2279. https://doi.org/10.1158/1535-7163.MCT-06-0801
Chang L, Graham PH, Ni J, Hao J, Bucci J, Cozzi PJ, Li Y (2015) Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol 96:507–517. https://doi.org/10.1016/j.critrevonc.2015.07.005
Chen Z, Wang B, Yu F, Chen Q, Tian Y, Ma S, Liu X (2016) The roles of mitochondria in radiation-induced autophagic cell death in cervical cancer cells. Tumour Biol 37:4083–4091. https://doi.org/10.1007/s13277-015-4190-8
Chipuk JE, Green DR (2005) Do inducers of apoptosis trigger caspase-independent cell death? Nat Rev Mol Cell Biol 6:268–275. https://doi.org/10.1038/nrm223910.1038/nrm1573
Clarke PG (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat Embryol (Berl) 181:195–213
Dayal R, Singh A, Pandey A, Mishra KP (2014) Reactive oxygen species as mediator of tumor radiosensitivity. J Cancer Res Ther 10:811–818. https://doi.org/10.4103/0973-1482.146073
Declercq W, Vanden Berghe T, Vandenabeele P (2009) RIP kinases at the crossroads of cell death and survival. Cell 138:229–232. https://doi.org/10.1016/j.cell.2009.07.006
Degterev A et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321. https://doi.org/10.1038/nchembio.83
Dewey WC, Ling CC, Meyn RE (1995) Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys 33:781–796. https://doi.org/10.1016/0360-3016(95)00214-8
Duque-Parra JE (2005) Note on the origin and history of the term “apoptosis”. Anat Rec B New Anat 283:2–4. https://doi.org/10.1002/ar.b.20047
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Eriksson D, Stigbrand T (2010) Radiation-induced cell death mechanisms. Tumour Biol 31:363–372. https://doi.org/10.1007/s13277-010-0042-8
Fukumoto M (2014) Radiation pathology: from thorotrast to the future beyond radioresistance. Pathol Int 64:251–262. https://doi.org/10.1111/pin.12170
Fulda S (2013) The mechanism of necroptosis in normal and cancer cells. Cancer Biol Ther 14:999–1004. https://doi.org/10.4161/cbt.26428
Fulda S (2014) Therapeutic exploitation of necroptosis for cancer therapy. Semin Cell Dev Biol 35:51–56. https://doi.org/10.1016/j.semcdb.2014.07.002
Galluzzi L, Kroemer G (2008) Necroptosis: a specialized pathway of programmed necrosis. Cell 135:1161–1163. https://doi.org/10.1016/j.cell.2008.12.004
Galluzzi L et al (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19:107–120. https://doi.org/10.1038/cdd.2011.96
Galluzzi L, Bravo-San Pedro JM, Kepp O, Kroemer G (2016) Regulated cell death and adaptive stress responses. Cell Mol Life Sci 73:2405–2410. https://doi.org/10.1007/s00018-016-2209-y
Galluzzi L et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Giampietri C, Starace D, Petrungaro S, Filippini A, Ziparo E (2014) Necroptosis: molecular signalling and translational implications. Int J Cell Biol 2014:490275. https://doi.org/10.1155/2014/490275
Gozuacik D, Kimchi A (2004) Autophagy as a cell death and tumor suppressor mechanism. Oncogene 23:2891–2906. https://doi.org/10.1038/sj.onc.1207521
Guo WF, Lin RX, Huang J, Zhou Z, Yang J, Guo GZ, Wang SQ (2005) Identification of differentially expressed genes contributing to radioresistance in lung cancer cells using microarray analysis. Radiat Res 164:27–35
Hasegawa M, Wilson G, Russell LD, Meistrich ML (1997) Radiation-induced cell death in the mouse testis: relationship to apoptosis. Radiat Res 147:457–467
Huang G, Wang H, Yang LX (2010) Enhancement of radiation-induced DNA damage and inhibition of its repair by a novel camptothecin analog. Anticancer Res 30:937–944
Ishigami T et al (2007) Genes and molecular pathways related to radioresistance of oral squamous cell carcinoma cells. Int J Cancer 120:2262–2270. https://doi.org/10.1002/ijc.22561
Janku F, McConkey DJ, Hong DS, Kurzrock R (2011) Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol 8:528–539. https://doi.org/10.1038/nrclinonc.2011.71
Jiang YG, Peng Y, Koussougbo KS (2011) Necroptosis: a novel therapeutic target for glioblastoma. Med Hypotheses 76:350–352. https://doi.org/10.1016/j.mehy.2010.10.037
Jo GH et al (2015) Radiation-induced autophagy contributes to cell death and induces apoptosis partly in malignant glioma cells. Cancer Res Treat 47:221–241. https://doi.org/10.4143/crt.2013.159
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73:2013–2026
Kim BM et al (2015) Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci 16:26880–26913. https://doi.org/10.3390/ijms161125991
Kroemer G et al (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11. https://doi.org/10.1038/cdd.2008.150
Kuwahara Y et al (2009) Clinically relevant radioresistant cells efficiently repair DNA double-strand breaks induced by X-rays. Cancer Sci 100:747–752. https://doi.org/10.1111/j.1349-7006.2009.01082.x
Kuwahara Y et al (2011) Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis 2:e177. https://doi.org/10.1038/cddis.2011.56
Kuwahara Y et al (2017) Clinically relevant radioresistant cell line: a simple model to understand cancer radioresistance. Med Mol Morphol 50:195–204. https://doi.org/10.1007/s00795-017-0171-x
Liao EC et al (2014) Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis 5:e1255. https://doi.org/10.1038/cddis.2014.220
Lomax ME, Folkes LK, O’Neill P (2013) Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol) 25:578–585. https://doi.org/10.1016/j.clon.2013.06.007
Mansilla S, Priebe W, Portugal J (2006) Mitotic catastrophe results in cell death by caspase-dependent and caspase-independent mechanisms. Cell Cycle 5:53–60. https://doi.org/10.4161/cc.5.1.2267
Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407:796–801. https://doi.org/10.1038/35037734
Mirzaie-Joniani H, Eriksson D, Johansson A, Lofroth PO, Johansson L, Ahlstrom KR, Stigbrand T (2002a) Apoptosis in HeLa Hep2 cells is induced by low-dose, low-dose-rate radiation. Radiat Res 158:634–640
Mirzaie-Joniani H, Eriksson D, Sheikholvaezin A, Johansson A, Lofroth PO, Johansson L, Stigbrand T (2002b) Apoptosis induced by low-dose and low-dose-rate radiation. Cancer 94:1210–1214
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873. https://doi.org/10.1101/gad.1599207
Moretti L, Yang ES, Kim KW, Lu B (2007) Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist Updat 10:135–143. https://doi.org/10.1016/j.drup.2007.05.001
Motyl T, Gajewska M, Zarzynska J, Sobolewska A, Gajkowska B (2007) Regulation of autophagy in bovine mammary epithelial cells. Autophagy 3:484–486
Nehs MA et al (2011) Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery 150:1032–1039. https://doi.org/10.1016/j.surg.2011.09.012
Ogawa K, Murayama S, Mori M (2007) Predicting the tumor response to radiotherapy using microarray analysis. Oncol Rep 18:1243–1248
Ohnishi K, Inaba H, Yasumoto J, Yuki K, Takahashi A, Ohnishi T (2004) C-terminal peptides of p53 molecules enhance radiation-induced apoptosis in human mutant p53 cancer cells. Apoptosis 9:591–597. https://doi.org/10.1023/B:APPT.0000038044.40337.35
Ohyama H, Yamada T, Ohkawa A, Watanabe I (1985) Radiation-induced formation of apoptotic bodies in rat thymus. Radiat Res 101:123–130
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45:487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Pinar B et al (2010) Radiation induced apoptosis and initial DNA damage are inversely related in locally advanced breast cancer patients. Radiat Oncol 5:85. https://doi.org/10.1186/1748-717X-5-85
Potten CS, Grant HK (1998) The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer 78:993–1003
Radford IR, Murphy TK (1994) Radiation response of mouse lymphoid and myeloid cell lines. Part III. Different signals can lead to apoptosis and may influence sensitivity to killing by DNA double-strand breakage. Int J Radiat Biol 65:229–239
Rainaldi G, Ferrante A, Indovina PL, Santini MT (2003) Induction of apoptosis or necrosis by ionizing radiation is dose-dependent in MG-63 osteosarcoma multicellular spheroids. Anticancer Res 23:2505–2518
Roninson IB, Broude EV, Chang BD (2001) If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat 4:303–313. https://doi.org/10.1054/drup.2001.0213
Sabin RJ, Anderson RM (2011) Cellular Senescence—its role in cancer and the response to ionizing radiation. Genome Integr 2:7. https://doi.org/10.1186/2041-9414-2-7
Sato K et al (2007) Autophagy is activated in colorectal cancer cells and contributes to the tolerance to nutrient deprivation. Cancer Res 67:9677–9684. https://doi.org/10.1158/0008-5472.CAN-07-1462
Shimura T et al (2010) Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene 29:4826–4837. https://doi.org/10.1038/onc.2010.238
Shinohara K, Nakano H (1993) Interphase death and reproductive death in X-irradiated MOLT-4 cells. Radiat Res 135:197–205
Shinomiya N (2001) New concepts in radiation-induced apoptosis: ‘premitotic apoptosis’ and ‘postmitotic apoptosis’. J Cell Mol Med 5:240–253
Silke J, Rickard JA, Gerlic M (2015) The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol 16:689–697. https://doi.org/10.1038/ni.3206
Su Z, Yang Z, Xie L, DeWitt JP, Chen Y (2016) Cancer therapy in the necroptosis era. Cell Death Differ 23:748–756. https://doi.org/10.1038/cdd.2016.8
Sun H, Yang S, Li J, Zhang Y, Gao D, Zhao S (2016) Caspase-independent cell death mediated by apoptosis-inducing factor (AIF) nuclear translocation is involved in ionizing radiation induced HepG2 cell death. Biochem Biophys Res Commun 472:137–143. https://doi.org/10.1016/j.bbrc.2016.02.082
Tait SW, Green DR (2008) Caspase-independent cell death: leaving the set without the final cut. Oncogene 27:6452–6461. https://doi.org/10.1038/onc.2008.311
Tsuboi Y et al (2009) Induction of autophagic cell death and radiosensitization by the pharmacological inhibition of nuclear factor-kappa B activation in human glioma cell lines. J Neurosurg 110:594–604. https://doi.org/10.3171/2008.8.JNS17648
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programmed cell death. Cell Death Differ 12 Suppl 2:1528–1534. https://doi.org/10.1038/sj.cdd.4401777
Vakkila J, Lotze MT (2004) Inflammation and necrosis promote tumour growth. Nat Rev Immunol 4:641–648. https://doi.org/10.1038/nri1415
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714. https://doi.org/10.1038/nrm2970
Walsh CM (2014) Grand challenges in cell death and survival: apoptosis vs. necroptosis. Front Cell Dev Biol 2:3. https://doi.org/10.3389/fcell.2014.00003
Wu W, Liu P, Li J (2012) Necroptosis: an emerging form of programmed cell death. Crit Rev Oncol Hematol 82:249–258. https://doi.org/10.1016/j.critrevonc.2011.08.004
Acknowledgements
This work is supported by JSPS KAKENHI Grant Numbers 24659174, 25340026, 26670184, 18K07727, 16K11513 and 16K15571.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuwahara, Y., Tomita, K., Urushihara, Y. et al. Association between radiation-induced cell death and clinically relevant radioresistance. Histochem Cell Biol 150, 649–659 (2018). https://doi.org/10.1007/s00418-018-1728-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-018-1728-z