Abstract
Himalayacalamus falconeri is a socio-economically important temperate woody bamboo of north-western Himalayas, which has been investigated for genetic diversity and population genetic structure across distribution range in western Himalayas using genomic STMS markers. The calculated diversity measures have indicated a high gene diversity in H. falconeri at population level (Ho = 0.637; He = 0.714; Ar = 5.05). Despite the larger proportion of genetic variation (88%) confined within the populations, a moderate level of genetic differentiation (FST = 0.121) was detected with relatively lower gene flow (Nm = 1.891). Furthermore, clustering and STRUCTURE analysis displayed high genetic heterogeneity in a metapopulation, where populations in both the spatially disconnected regions of the Uttarakhand state, Garhwal and Kumaon, were clustered in different groups. Whereas, nested sub clustering and between-population genetic admixing were not correlated to their physical proximity. Also, the Mantel test supports the isolation by distance model showing a significant correlation between genetic and horizontal geographic distances. For conservation implications, diverse hotspots with high allelic richness were also identified in both the geographical regions of Uttarakhand state. To the best of our knowledge, it is a pioneer study presenting in depth knowledge of metapopulation in any Indian temperate bamboo, which will be of paramount importance to the researchers, foresters, and policymakers for guiding future conservation decisions of H. falconeri in the Indian Himalayan Regions (IHRs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bamboos are imperative non-wood forest produce occurring in the forest as well as non-forest areas, and play a significant role in improving livelihoods of people globally (Hogarth and Belcher 2013; Kithan 2014; Khanal 2015). As described by Kelchner and Bamboo Phylogeny Group (2013), Bamboo Genetic Resources (BGRs) in India falls under two lineages, paleotropical (tribe Bambuseae) and temperate (tribe Arundinarieae) woody bamboos. Former occupied tropical and subtropical zone of the country from alluvial plains to hills up to the altitude of 1500 m AMSL. Whereas, later are dominating at high hills above 1500 m AMSL in the subtropical and temperate zone of Indian Himalayan Regions (IHRs). Among the temperate woody bamboos, four shrubby taxa (commonly called “ringal”), namely Drepanostachyum falcatum, Himalayacalamus falconeri, Thamnocalamus spathiflorus, and Yushania anceps, are naturally occurring in the NW Himalayas at the altitudinal range 1800 to 3600 m AMSL. Ringal forms a dense thicket or moderately dense undergrowth in the evergreen forest of the Himalayas, ubiquitously grown on steep slopes of riverbanks, and aids in soil stabilization with their fibrous root system (Kumari and Tewari 2009; Banik 2016). They serve as an important source of fodder in time of scarcity during winter and provide food for wild animals, such as red panda (Ailurus fulgens) and bears (Yonzon 1991).
Among the four hill bamboos of the NW Himalayas, culms of Himalayacalamus falconeri are most preferred by artisans for craft and weaving purposes due to their flexibility, strength, and smoothness. Beside the IHRs, it has also been reported to occur in the temperate zone of the Nepal, western Bhutan, and southwest China or Tibet (Banik 2016). Moreover, it has been introduced in New Zealand which was naturalized later in 1935 (Sykes 1996). In the western Himalayas, it is commonly known as “Dev (meaning God) ringal” due to its traditional utilization for making temple-related basketry or decorative articles. Socio-economically, ringal plays a crucial role in livelihood generation and offers employment opportunities for the people inhabiting at high hills of the Himalayas (Kumar 2009). About 451 villages have been identified in the Uttarakhand where artisans are involved in bamboo or ringal-based occupation. Dejectedly, the communities involved in this profession do not own the resources and about 93% of the raw material is directly harvested from the natural forest (Sundriyal and Sundriyal 2011). As a result, local populations nearby human settlements are severely deteriorating and people are struggling hard to collect the raw material illegally from protected forest areas. Awfully, selective and indiscriminate extraction from the natural forest may put the species in a miserable situation in long term. To ensure the sustainable utilization of this valuable genetic resource, baseline information and genetic characterization need to be derived for its natural populations, which is inevitable to frame a sound conservation and management plan.
Genetic variability among individuals ensures the long-term survival of metapopulation, bestows the evolutionary and adaptive potential against changing environment, and serves as a resource for future breeding programmes (Booy et al. 2000; Porth and El-Kassaby 2014; Godhe and Rynearson 2017; Nonić and Šijačić-Nikolić 2021). Explicitly, the spatial genetic structure is defined as the non-random distribution of genetic variation among individuals within populations which may or may not be shaped as per the geographical proximity of individuals, and some species may display a cryptic spatial structure with noticeable genetic heterogeneity due to unidentified migrants (Manel et al. 2003). In addition, the genetic structure of a metapopulation is also influenced by the extent and amount of gene flow across the spatially disconnected populations, which is controlled by several evolutionary processes and life history traits of a species (Porth and El-Kassaby 2014). Hence, estimation of gene diversity and understanding of the genetic structure is immensely important to elucidate the threat status of the species, vulnerability to extinction, and guiding precise conservation decisions (Andelman and Willig 2002; DeSalle and Amato 2004).
As phenotypic evaluation in natural populations is more laborious and error-prone, molecular markers are the most favoured tools for performing genetic analysis in wild plant populations (Nadeem et al. 2018). However, due to limited sequence information, earlier genetic studies in most bamboo taxa employed markers based on random primers, such as RAPD, AFLP, and ISSR (Yang et al. 2012; Ma et al. 2013; Nag et al. 2013; Nilkanta et al. 2017). Comparatively, sequence-based markers, such as STMS or SSR markers and SNPs, are more informative, robust, reproducible, and codominant (Nadeem et al. 2018). Unlike random primer-based markers, STMS is derived from the unique flanking sequence of repeat loci which share a high level of homology among taxonomically related taxa (Saha et al. 2004; Sharma et al. 2008). Hence, cross-transferability is a fast and cost-effective approach for identifying STMS markers in related taxa (Barbara et al. 2007), which has been successfully utilized in several bamboo species also (Barkley et al. 2005; Sharma et al. 2009; Mason 2015; Meena et al. 2019). Moreover, microsatellite markers have been developed in several bamboo taxa using different methodologies (Abreu et al. 2011; Peng et al. 2013; Bhandawat et al. 2015; Cai et al. 2019; Meena et al. 2021), which could be further utilized in other bamboo species through cross-transferability. The present study aimed to quantify and map the genetic diversity, and determine the spatial genetic structure of the natural populations of H. falconeri in the western Himalayas using SSR markers.
Materials and methods
Study area, population sampling, DNA extraction and marker genotyping
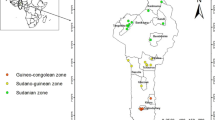
The study was carried out in Uttarakhand (India) state which is represented by biographic zone 2B western Himalaya and 7B Shivalik consisting of Kumaon and Garhwal regions (Rodgers and Panwar 1988). Geographically, a major portion of the state is constituted by the Himalayan Mountain, which is one of the youngest mountain systems of the world (∼40 million years in age compared to peninsular mountains of ∼1500–2500 million years old); hence, ecologically very fragile (Uttarakhand Biodiversity Board, Dehradun; https://sbb.uk.gov.in/). The flora of the state ranges from tropical deciduous to alpine vegetation, and the key dominant tree species occurring in the temperate zones of the state are Quercus leucotrichophora, Q. floribunda, Q. semecarpifolia, Rhododendron arboreum, Myrica esculenta, Lyonia ovalifolia, Ilex dipyrena, Pinus roxburghii, P. wallichiana, Cedrus deodara, Abies pindrow, Picea smithiana, Taxus wallichiana, Betula utilis, etc. Ringal is a group of understory shrubby bamboos that are naturally interspersed with above trees species and connect the different forest types.
Leaf samples were collected from 219 individuals of H. falconeri from nine locations in the Garhwal and Kumaon Himalayas of Uttarakhand (Table 1), and desiccated with silica gel. In accordance with most studies on population genetic analysis, we attempted to maintain the sample size of about 30 individuals per population; however, it could not be accomplished for all due to reduced and deteriorated conditions. Within a population, sampling was conducted in compliance with the assumption followed in the method of McClure (1966), where each population is presumed to maintain the random assortment of genetic material considering individual clump as a potential genet and culms within it as ramets. To minimize the sampling bias, the minimum distance between two sampled individuals per location was kept at 100 to 300 m. Genomic DNA was extracted from silica dried leaf tissues using the protocol given by Doyle and Doyle (1987) with slight modification as per Krizman et al. (2006).
In a recent study, STMS markers were developed in another Himalayan temperate bamboos Drepanostachyum falcatum through genome skimming approach, and some of these have also been verified for their transferability in H. falconeri (Meena et al. 2021). These were re-examined for the level of polymorphism through PCR amplification in 20 random individuals of H. falconeri. The PCR products of each primer pair were subjected to gel electrophoresis with 4% high resolution agarose gel. After visualizing banding pattern, ten highly polymorphic STMS loci were finally selected for further genotyping (Table 2). The PCR reaction mixture was prepared by mixing of 2 µL genomic DNA (25 ng) with 1.5 µL enzyme buffer (10×), 1.8 µL MgCl2 (25 mM), 1.2 µL dNTPs (2.5 mM), 0.15 µL primers (20 µM) and 0.2 µL Taq DNA Polymerase enzyme (3 U µL− 1) (Genei, India). Finally, a total reaction volume of 15 µL was made up of nuclease-free sterile water. The PCR reactions were run in the thermal cycler machine (Eppendorf Mastercycler Nexus) with an initial denaturing step (95 °C for 5 min) followed by 35 cycles of 94 °C for 1 min, 55–62 °C for 1 min and 72 °C for 1 min, and then final elongation at 72 °C for 10 min. The PCR products were loaded into automated capillary electrophoresis system LabChip GX Touch 24 (PerkinElmer, USA), and fragment analysis was carried out with the software LabChip GX reviewer ver. 5.8 (PerkinElmer, USA).
Marker data analysis for deciphering diversity measures and spatial genetic structure
The non-integer allelic data were transformed into the integers by applying the power function, and the allele sizes were binned as per the periodicities of repeat motifs using software TANDEM ver 1.07 (Matschiner and Salzburger 2009). The marker data were analyzed to calculate the various diversity measures, such as polymorphic information content (PIC), number of different alleles (Na), effective number of alleles (Ne), observed heterozygosity (Ho) and expected heterozygosity (He), etc., using software PowerMarker ver 3.25 (Liu and Muse 2005) and GenAlex ver. 6.5 (Peakall and Smouse 2012). Whereas, the software Arlequin ver. 3.1 (Excoffier et al. 2005) was used to perform AMOVA and estimation of Wright’s fixation index (FST). In order to quantify the distribution of variance among different levels, the AMOVA was performed with and without assuming hierarchical structuring. The hierarchical structuring was carried out by considering three levels, i.e., among regions (Garhwal and Kumaon), among populations within groups, and within populations. Further, the measures of allelic diversity, such as allelic richness (Ar) and private allelic richness (PAr), were calculated using software HP-Rare ver. 1.0, which gives an unbiased estimate of allelic richness by compensating sampling disparity using statistical technique of rarefaction (Kalinowski 2005), and spatially overlaid over distribution map after interpolating with IDW algorithm used in ArcGIS (Shepard 1968; Hengl 2009; Chiocchini et al. 2016).
The genetic relationship among sampled populations was studied through the neighbour-joining (NJ) clustering and the principal coordinate analysis (PCoA) using software POPTREE2 (Takezaki et al. 2009) and GenALEx, respectively. In order to test the isolation by distance model, the Mantel test (Mantel 1967) was performed between genetic and geographical distances. Herein, genetic distances were correlated with horizontal as well as vertical altitudinal distances between the sampled populations. To unravel the population genetic structure, the Bayesian model-based clustering method was implemented in the software STRUCTURE ver. 2.2 (Pritchard et al. 2000), where the posterior probability of K [Pr(K)] was calculated using an ancestry model with admixture under the assumption of correlated allele frequencies. The model was replicated ten times for each K value (2 to 9) with 3,00,000 MCMC sampling runs after a burn-in period of 3,00,000 iterations. Finally, an optimal number of subpopulations were determined using the web-based program Structure Harvester 0.6.92 (Earl and vonHoldt 2012).
Results
Marker polymorphism and gene diversity in sampled populations
Polymorphism of successfully transferred STMS markers of D. falcatum was reconfirmed in H. falconeri through PCR amplification in 20 random samples. Based on the polymorphism and distinctness of the banding pattern on agarose gel (Supplementary Fig. S1), ten marker loci were selected for further genotyping. A total of 299 alleles were generated by genotyping with ten STMS markers, where highest 52 alleles were displayed by the marker DfStm1461 while lowest seven alleles were obtained with DfStm307. By analysing allelic polymorphism across the populations, a mean of different and effective number of alleles per population were recorded as 9.9 and 5.2, respectively. Accordingly, all marker loci demonstrated a high level of polymorphism with PIC value ranging from 0.418 for DfStm307 to 0.945 for DfStm1461 (Table 2).
Calculated diversity indices revealed a high level of gene diversity in the sampled populations of H. falconeri (Table 3). For instance, observed heterozygosity (Ho) was ranged from 0.540 (HF04_Pinswad) to 0.727 (HF01_Munsyari) with a mean of 0.637, and expected heterozygosity (He) was ranged from 0.591 (HF06_Mornaula) to 0.810 (HF08_Triyuginarayan) with a mean of 0.714. Also, the population HF08 from Triyuginarayan exhibited highest allelic richness (Ar = 5.77), whereas it was recorded as lowest (Ar = 4.43) for the population HF02 from Chopta. Further, spatial overlaying of allelic diversity has enabled to distinguish the populations or regions of conservation importance, where the values of diversity measures are depicted in hypsometric scale (Fig. 1a, b). Decisively, the populations or the regions capturing high allelic richness were designated as diversity hotspots, and one diversity hotspot has been recognized in both the geographical regions of Uttarakhand Himalayas, i.e., HF08 (Triyuginarayan) in Garhwal and HF06 (Mornaula) in Kumaon, for their in-situ conservation. Importantly, most populations distributed in Kumaon region were identified to possess significant number of private alleles, and therefore, entire region can be considered important for conservation.
Genetic relationship, divergence and structuring in sampled populations
The results of AMOVA without hierarchical structure have demonstrated that the major proportion of genetic variance (87.90%) existed within the populations, and only 12.10% was observed among populations (Table 4a). Accordingly, a moderate level of genetic differentiation (FST = 0.121) was recorded with relatively lower gene flow (Nm = 1.891). However, AMOVA with hierarchical structuring showed that the 87% of the total variance was contained by the individuals within populations, 11% was existed among populations and only 3% was between the regions (Table 4b). Based on the pairwise FST and Nm values (Supplementary Table S1), the highest genetic distance was recorded between the population HF06 (from Mornaula) and HF09 (from Darma valley), whereas it was observed as lowest between the population HF01 (from Munsiyari) and HF02 (from Ghes). Plausibly, an inverse trend of gene flow was displayed among the populations, i.e., the gene flow was highest between populations which are least genetically distant, and vice-versa.
The genetic divergence revealed by AMOVA and F-statistics was further validated with the cluster and structure analysis. Unrooted NJ dendrogram showed two major groups, where populations of both the regions, i.e., Garhwal and Kumaon, were clustered in distinct groups in accordance with their geographical distribution (Fig. 2). Similar pattern was also displayed by the spatial clustering in the PCoA plot (Fig. 3), where all the three coordinates cumulatively accounted for a substantial level of genetic variance (69.35%). Individually, first, second, and third coordinates, were accounted for 25.4, 22.57, and 21.36% of the genetic variance, respectively. Conclusively, overall genetic divergence was well supported by the cluster analysis but the structuring among geographical regions was not evident in AMOVA with hierarchical structuring. The genetic divergence among spatially separated populations were further supported by the Mantel test, where the correlation of genetic distance was significant with horizontal geographical distance (R2=0.332; P value = 0.001) (Fig. 4) and non-significant with vertical altitudinal distances (R2 = 0.025; P value = 0.213) (Supplementary Fig. S2).
Surprisingly, structure analysis has revealed five optimum numbers of subpopulations (Fig. 5a, b), indicating considerable amount of heterogeneity among the geographically disconnected populations (Fig. 2). The proportional membership coefficient of each population was calculated for five inferred clusters, and pattern of genotypic admixing is displayed in the form of a bar plot (Fig. 5c). As per the population Q-matrix, seven sampled populations were clearly defined by one of the five inferred clusters with a proportional membership coefficient of more than 0.70. Whereas, two populations of the Garhwal region, namely HF04 (from Pinswad) and HF08 (from Triyuginarayan), exhibited admixed ancestry from other inferred clusters (Supplementary Table S2). As evident in supplementary table S1, the high genotypic admixing in both the populations would have been resulted due to significantly high gene flow with other populations.
Graphical representation of the estimated Ln probability of data (a) and ΔK (b) for each K value, Bar plot (c) illustrated the proportional membership coefficient for all the genotypes at K = 5, where each population is separated by a vertical line and individual samples are represented by coloured bars
Discussion
The fitness of individual population and spatial distribution of the genetic variation are mainly controlled by the gene flow, i.e., exchange of genetic material between the populations (Porth and El-Kassaby 2014; Xie et al. 2019; Bontrager and Angert 2019; Luo et al. 2021; Morente-López et al. 2021), which itself controlled by multitude of factors like topography, environmental, geographical, biological characteristics of species, etc. (Wu et al. 2015; Gharehaghaji et al. 2017; Bontrager and Angert 2018; De Kort et al. 2021;). Among biological attributes, breeding behaviour and life cycle characteristics are the major factors affecting genetic composition of the populations. In general, an open-pollinated species with widespread distribution tends to capture more genetic diversity than a self-pollinated and narrowly distributed species (Hamrick et al. 1992; Hamrick and Godt 1996). For instance, a high level of genetic differentiation and heterogeneity were recorded in a metapopulation of Ochlandra travancorica, an endemic bamboo of Western Ghat, India (Nag et al. 2013). Being the open-pollinated breeding behaviour and wide distribution in the Himalayan ranges, high gene diversity with low genetic differentiation was expected in H. falconeri metapopulation. In congruence, this study has recorded high gene diversity (He = 0.714; Ar = 5.05) in the sampled population of H. falconeri from the western Himalayas. However, high genetic heterogeneity detected herein has indicated the noticeable hindrance in gene flow and substantial genetic changes adopted by the populations.
Though the overall gene diversity is high, populations HF06 from Mornaula in Kumaon and HF07 from Chopta in Garhwal region have exhibited relatively lesser gene diversity. Population HF06 is located in the reserve forest at Champawat forest division, which has demonstrated lesser gene diversity and high allelic richness. This disparity in both the diversity measures could have been aroused due to suboptimal sample size. Whereas, the population HF07 belongs to the Kedarnath wildlife sanctuary, one of most healthy and intact reserve forests of the state, showed relatively low allelic diversity. The reason for poor allelic richness in this region could be the excessive extraction of ringal culms, as this is one of the most popular sites among ringal artisans, where all four species are adequately found. However, the population of Triyuginarayan (HF08) showed the highest gene diversity. Further, diversity hotspots demarcated in the diversity maps could be prioritized for conservation and management. For instance, the populations with high allelic richness, namely HF08 (Triyuginarayan) and HF06 (Mornaula) are designated as diversity hot spots and the populations exhibiting lower allelic diversity HF07 (Chopta) and HF09 (Darma Valley) are considered as genetically fragile. Interestingly, most populations of the Kumaon region contained substantial number of private alleles, and therefore, entire region is important from the conservation point of view. Based on the current diversity statistics, the metapopulation of the studied species is observed to be genetically healthy with sufficient evolutionary or adaptive potential. The diversity measures observed in the present study were found consistent with the Sri Lankan temperate woody bamboo Kuruna debilis (He = 0.708; Ar = 4.58; FST=0.113), characterized with twelve microsatellite loci (Attigala et al. 2017). However, the population genetic studies in other bamboo taxa, viz., Dendrocalamus membranaceus (Yang et al. 2012), O. travancorica (Nag et al. 2013), Melocanna baccifera (Nilkanta et al. 2017), P. edulis (Jiang et al. 2017), D. sinicus (Yang et al. 2018), D. hamiltonii (Meena et al. 2019; Bhandawat et al. 2019), Oxytenanthera abyssinica (Oumer et al. 2020), etc., displayed relatively lower genetic diversity with high genetic differentiation. Recently, high genetic diversity and differentiation were also detected in three native Mexican woody bamboo species of genus Guadua (Pérez-Alquicira et al. 2021).
Furthermore, unique life cycle characteristics of bamboo such as the long vegetative phase of 3 to 150 years and strong capability of asexual reproduction (Zheng et al. 2020), make them different from other forestry species where general principles of population genetics may not be obeyed. When the conditions of Hardy-Weinberg Equilibrium (HWE) do not fulfil, populations may undergo genetic differentiation over the period. In the case of bamboo in general, a key HWE condition, i.e., random mating, not always be ensured in the metapopulation due to their indistinct breeding behaviour. Though the floral biology and breeding behaviour is not studied in H. falconeri, most woody bamboo taxa are reproduced through open pollination (Ruiz-Sanchez et al. 2017; Chen et al. 2017), and flowered either, sporadically or gregariously or both, at different time intervals (Banik 2016; Zheng et al. 2020). The ringal bamboo species flowers gregariously with a periodicity of 28 to 35 years (Troup 1921; Campbell 1988). However, few flowering culms may be spotted in a population almost every year. Both sporadic and gregarious flowering has also been reported in H. falconeri, and a recent event of gregarious flowering in this taxon was recorded in 2002 at some cohorts in Uttarakhand Himalayas (Naithani et al. 2003). The longer and unsynchronized flowering cycle within and across the population act as a temporal barrier, and only the simultaneously flowered populations get a chance of intermating. In an open-pollinated plant taxon, the flowering synchrony increases reproductive success while asynchronous or sporadic flowering negatively affects the population’s fitness (Rodríguez-Pérez 2016; Bogdziewicz et al. 2020; Pérez-Alquicira et al. 2021). As a result, a high level of genetic heterogeneity was observed in structure analysis, where the entire variability was divided into five subpopulations.
Remarkably, the genetic clustering derived through NJ dendrogram and PCoA plot revealed two major clusters, where the overall clustering was appeared in accordance to their spatial distribution, i.e., the populations of Garhwal and Kumaon regions of Uttarakhand Himalayas were categorised into different groups (Fig. 2). Further, Mantel test also supports the isolation by distance model, signifying the imperative role of physical distance in distribution of the genetic diversity across the range. As per the surveyed area in this study, species distribution has been recorded from 29° 26′ to 30° 55′ in north and 78° 22′ to 80° 37′ in east, and altitudinally from 1950 m at Ranachatti to 2600 m AMSL at Sunderdhunga. Thus, both horizontal and vertical distances were analysed against the genetic distances, where only horizontal distance displayed significant relationship. It suggests that the geneflow is adequate across the altitudinal gradient but limited on longitudinal range due to various topographical, environmental or biological constraints. A similar clustering pattern and correlation were also observed in D. hamiltonii, where the populations of different regions were clustered in different groups (Meena et al. 2019). However, the nested sub clustering, i.e., clustering among the populations of smaller areas was not appeared to be correlated with their physical proximity, possibly due to asynchronous flowering among populations.
Conclusions and conservation implications
The present study has demonstrated a high gene diversity in the natural populations of H. falconeri of Uttarakhand Himalayas with a moderate level of genetic differentiation, indicating substantial evolutionary and adaptive potential of the species. However, the populations with suboptimal size and diversity like DH06 need special conservation attention. If the natural habitat continues to deteriorate, the alleles of such populations could be rescued by infusing into a large healthy population with a wide genetic base, such as protected areas. As evident by the diversity map of private alleles, the populations located in the Kumaon region harboured most private alleles, and require suitable conservation measures. Viewing the pattern of genetic clustering and structure analysis, it is appeared that the ecological or geographical factors played a crucial role in the structuring of metapopulation at a large distribution range. Whereas, the genetic make-up of closely located subpopulations in smaller geographical area, is appears to be controlled by synchronized flowering episodes. Further understanding of genetic structure demands an up-to-date record of past flowering data, and therefore, the recording of flowering episodes is very crucial in bamboo. Also, the detailed analysis of phenology, mating system, and contemporary gene flow, is important to understand the reason behind high genetic heterogeneity.
Considering a high level of genetic heterogeneity in H. falconeri metapopulation, conservation of any one population alone would not serve the purpose, and for ex-situ conservation, germplasm must be pooled from all the major clusters depicted in structure analysis. In addition, populations, namely HF04 (Pinswad) and DH08 (Triyuginarayan) displaying significant genetic admixture may be treated as natural gene banks and recommended for in-situ conservation. The knowledge base generated here will be of paramount importance to the researchers, foresters, and policymakers for guiding future conservation and management plans of H. falconeri in IHRs.
Data availability
All the data required to understand the manuscript are provided in original manuscript and supplementary material.
Abbreviations
- AFLP:
-
Amplified fragment length polymorphism
- AMOVA:
-
Analysis of molecular variance
- AMSL:
-
Above mean sea level
- Ar:
-
Allelic richness
- BGRs:
-
Bamboo genetic resources
- FIS :
-
Inbreeding coefficient
- FST :
-
Fixation index
- He:
-
Expected heterozygosity
- Ho:
-
Observed heterozygosity
- IDW:
-
Inverse distance weighted
- IHRs:
-
Indian Himalayan regions
- ISSR:
-
Inter simple sequence repeat
- MCMC:
-
Markov chain monte carlo
- Na:
-
Number of different alleles
- Ne:
-
Effective number of alleles
- NJ:
-
Neighbour-joining
- Nm:
-
Number of migrants per generation
- NW:
-
North West
- PAr:
-
Private allelic richness
- PCoA:
-
Principal coordinate analysis
- PCR:
-
Polymerase chain reaction
- PIC:
-
Polymorphism information content
- Pr(K):
-
Posterior probability of K
- RAPD:
-
Random amplified polymorphic DNA
- SNPs:
-
Single nucleotide polymorphisms
- SSRs:
-
Simple sequence repeats
- STMS:
-
Sequence-tagged micro satellite
References
Abreu AG, Grombone-Guaratini MT, Monteiro M, Pinheiro JB, Tombolato AFC, Zucchi MI (2011) Development of microsatellite markers for Aulonemia aristulata (Poaceae) and cross-amplification in other bamboo species. Am J Bot 98:90–92. https://doi.org/10.3732/ajb.1000511
Andelman SJ, Willig MR (2002) Alternative conservation reserve configurations for paraguayan bats: considerations of spatial scale. Conserv Biol 16:1352–1363
Attigala L, Gallaher T, Nason J, Clark LG (2017) Genetic diversity and population structure of the threatened temperate woody bamboo Kuruna debilis (Poaceae: Bambusoideae: Arundinarieae) from Sri Lanka based on microsatellite analysis. J Natl Sci Found Sri 45(1):53–65. https://doi.org/10.4038/jnsfsr.v45i1.8038
Banik RL (2016) Ringal bamboos of the Himalayas. Silviculture of South Asian priority bamboos. Springer, Singapore, pp 277–291. https://doi.org/10.1007/978-981-10-0569-5
Barbara T, Palma-silva C, Paggi GM, Bered F, Fay MF, Lexer C (2007) Cross-species transfer of nuclear microsatellite markers: potential and limitations. Mol Ecol 16:3759–3767. https://doi.org/10.1111/j.1365-294X.2007.03439.x
Barkley NA, Newman ML, Wang ML, Hotchkiss MW, Pederson GA (2005) Assessment of the genetic diversity and phylogenetic relationships of a temperate bamboo collection by using transferred EST-SSR markers. Genome 48(4):731–737. https://doi.org/10.1139/g05-022
Bhandawat A, Sharma V, Sharma H, Sood A, Sharma RK (2015) Development and crosstransferability of functionally relevant microsatellite markers in Dendrocalamus latiflorus and related bamboo species. J Genet 94:48–55. https://doi.org/10.1007/s12041-014-0377-9
Bhandawat A, Sharma V, Singh P, Seth R, Nag A, Kaur J, Sharma RK (2019) Discovery and utilization of EST-SSR marker resource for genetic diversity and population structure analyses of a subtropical bamboo, Dendrocalamus hamiltonii. Biochem Genet 57(5):652–672. https://doi.org/10.1007/s10528-019-09914-4
Bogdziewicz M, Pesendorfer M, Crone EE, PérezIzqui-erdo C, Bonal R (2020) Flowering synchrony drives re-productive success in a windpollinated tree. Ecol Lett 23:1820–1826. https://doi.org/10.1111/ele.13609
Bontrager M, Angert AL (2018) Genetic differentiation is determined by geographic distance in Clarkia pulchella. bioRxiv 374454. https://doi.org/10.1101/374454
Bontrager M, Angert AL (2019) Gene flow improves fitness at a range edge under climate change. Evol Lett 3(1):55–68. https://doi.org/10.1002/evl3.91
Booy G, Hendriks RJJ, Smulders MJM, Van Groenendael JM, Vosman B (2000) Genetic diversity and the survival of populations. Plant Biol (Stuttg) 2(4):379–395. https://doi.org/10.1055/s-2000-5958
Cai K, Zhu L, Zhang K, Li L, Zhao Z, Zeng W, Lin X (2019) Development and characterization of EST-SSR markers from RNA-Seq data in Phyllostachys violascens. Front Plant Sci 10:50. https://doi.org/10.3389/fpls.2019.00050
Campbell JJN (1985) Bamboo flowering patterns: a global view with special reference to east Asia. J Am Bamboo Soc 6:17–35
Chen LN, Cui YZ, Wong KM, Li DZ, Yang HQ (2017) Breeding system and pollination of two closely related bamboo species. AoB Plants 9(3):plx021. https://doi.org/10.1093/aobpla/plx021
Chiocchini F, Mattioni C, Pollegioni P, Lusini I, Martín MA, Cherubini M, Lauteri M, Villani F (2016) Mapping the genetic diversity of Castanea sativa: exploiting spatial analysis for biogeography and conservation studies. J Geogr Inf Syst 8(02):248. https://doi.org/10.4236/jgis.2016.82022
De Kort H, Prunier JG, Ducatez S, Honnay O, Baguette M, Stevens VM, Blanchet S (2021) Life history, climate and biogeography interactively affect worldwide genetic diversity of plant and animal populations. Nat Commun 12:516. https://doi.org/10.1038/s41467-021-20958-2
DeSalle R, Amato G (2004) The expansion of conservation genetics. Nat Rev Genet 5(9):702 – 12. https://doi.org/10.1038/nrg1425. PMID: 15372093
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochem Bull 19:11–15
Earl DA, vonHoldt BM (2012) Structure harvester: a website and program for visualizing structure output and implementing the Evanno method. Conserv Genet Resour 4(2):359–361. https://doi.org/10.1007/s12686-011-9548-7
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50. https://doi.org/10.1177/117693430500100003
Gharehaghaji M, Minor ES, Ashley MV, Abraham ST, Koenig WD (2017) Effects of landscape features on gene flow of valley oaks (Quercus lobata). Plant Ecol 218:487–499. https://doi.org/10.1007/s11258-017-0705-2
Godhe A, Rynearson T (2017) The role of intraspecific variation in the ecological and evolutionary success of diatoms in changing environments. Phil Trans R Soc B 372:20160399. https://doi.org/10.1098/rstb.2016.0399
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans Royal Soc B 351:1291–1298. https://doi.org/10.1098/rstb.1996.0112
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. New For 6:95–124. https://doi.org/10.1007/BF00120641
Hengl T (2009) A practical guide to geostatistical mapping. University of Amsterdam, Amsterdam
Hogarth NJ, Belcher B (2013) The contribution of bamboo to household income and rural livelihoods in a poor and mountainous county in Guangxi, China. Int For Rev 15(1):71–81. https://doi.org/10.1505/146554813805927237
Jiang W, Bai T, Dai H, Wei Q, Zhang W, Ding Y (2017) Microsatellite markers revealed moderate genetic diversity and population differentiation of moso bamboo (Phyllostachys edulis)—a primarily asexual reproduction species in China. Tree Genet Genomes 13(6):1–14. https://doi.org/10.1007/s11295-017-1212-2
Kalinowski ST (2005) HP-Rare: a computer program for performing rarefaction on measures of allelic diversity. Mol Ecol Notes 5:187–189. https://doi.org/10.1111/J.1471-8286.2004.00845.X
Kelchner SA, Bamboo Phylogeny Group (2013) Higher level phylogenetic relationships within the bamboos (Poaceae: Bambusoideae) based on five plastid markers. Mol Phylogenet Evol 67:404–413. https://doi.org/10.1016/j.ympev.2013.02.005
Khanal M (2015) Importance of bamboo in poors’ livelihood in Nepal. In: Proceedings of the 10th world bamboo congress, 17–22 September 2015, Damyang, Korea
Kithan LN (2014) Socio-economic importance of bamboo among the Nagas of Nagaland. J Hum Ecol 48(3):393–397. https://doi.org/10.1080/09709274.2014.11906809
Krizman M, Jakse J, Baricevic D, Javornik B, Prosek M (2006) Robust CTAB-activated charcoal protocol for plant DNA extraction. Acta Agric Slov 87:427–433
Kumar B (2009) Conservation of Ringal (a dwarf bamboo) through economic development in Rudraprayag district Garhwal (Uttarakhand), India. J Am Sci 5(6):23–26
Kumari P, Tewari LM (2009) Biodiversity in Uttrakhand Himalaya region. Nat Sci 7(3):113–125
Liu K, Muse SV (2005) PowerMarker: integrted analysis environment for genetic marker data. Bioinformatics 21:2128–2129. https://doi.org/10.1093/bioinformatics/bti282
Luo MX, Lu HP, Chai MW, Chang JT, Liao PC (2021) Environmental heterogeneity leads to spatial differences in genetic diversity and demographic structure of Acer caudatifolium. Plants 10:1646. https://doi.org/10.3390/plants10081646
Ma QQ, Song HX, Zhou SQ, Yang WQ, Li DS, Chen JS (2013) Genetic structure in dwarf bamboo (bashania fangiana) clonal populations with different genet ages. PLoS ONE 8(11):e78784. https://doi.org/10.1371/journal.pone.0078784
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18:189–197. https://doi.org/10.1016/S0169-5347(03)00008-9
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Mason AS (2015) SSR genotyping. In: Batley J (ed) Plant genotyping. Springer, New York, pp 77–89
Matschiner M, Salzburger W (2009) TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25(15):1982–1983. https://pubmed.ncbi.nlm.nih.gov/19420055/
McClure FA (1966) The Bamboos, a fresh perspective. Harvard University Press, Cambridge
Meena RK, Bhandhari MS, Barhwal S, Ginwal HS (2019) Genetic diversity and structure of Dendrocalamus hamiltonii natural metapopulation: a commercially important bamboo species of Northeast Himalayas. 3 Biotech 9:60. https://doi.org/10.1007/s13205-019-1591-1
Meena RK, Negi N, Uniyal N, Bhandhari MS, Shama R, Ginwal HS (2021) Genome skimming based STMS marker discovery and its validation in temperate hill bamboo, Drepanostachyum falcatum. J Genet 100:28. https://doi.org/10.1007/s12041-021-01273-7
Morente-López J, Lara-Romero C, García-Fernández A, Teso MLR, Prieto-Benítez S, Iriondo JM (2021) Gene flow effects on populations inhabiting marginal areas: origin matters. J Ecol 19:139–153. https://doi.org/10.1111/1365-2745.13455
Nadeem MA, Nawaz MA, Shahid MQ, Doğan Y, Comertpay G, Yıldız M, Hatipoğlu R, Ahmad F, Alsaleh A, Labhane N, Özkan H, Chung G, Baloch FS (2018) DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol Biotechnol Equip 32(2):261–285. https://doi.org/10.1080/13102818.2017.1400401
Nag A, Gupta P, Sharma V, Sood A, Ahuja SP, Sharma KR (2013) AFLP and RAPD based genetic diversity assessment of industrially important reed bamboo (Ochlandra travancorica Benth). J Plant Biochem Biot 22:144–149. https://doi.org/10.1007/s13562-012-0114-5
Naithani HB, Pal M, Lepcha STS (2003) Gregarious flowering of Thamnocalamus spathiflorus and Thamnocalamus falconeri, bamboos from Uttaranchal, India. Indian For 129:517–526
Nilkanta H, Amom T, Tikendra L, Rahaman H, Nongdam P (2017) ISSR marker based population genetic study of Melocanna baccifera (Roxb.) Kurz: a commercially important bamboo of Manipur, North-East India. Scientifica 2017:1–9. https://doi.org/10.1155/2017/3757238
Nonić M, Šijačić-Nikolić M (2021) Genetic diversity: sources, threats, and conservation. In: Leal Filho W, Azul AM, Brandli L, Lange Salvia A, Wall T (eds) Life on land. Encyclopedia of the UN sustainable development goals. Springer, Cham. https://doi.org/10.1007/978-3-319-95981-8_53
Oumer OA, Dagne K, Feyissa T, Tesfaye K, Durai J, Hyder MZ (2020) Genetic diversity, population structure, and gene flow analysis of lowland bamboo [Oxytenanthera abyssinica (A. Rich.) Munro] in Ethiopia. Ecol Evol 10(20):11217–11236. https://doi.org/10.1002/ece3.6762
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetics software for teaching and research-an update. Bioinformatics 28(19):2537–2539. https://doi.org/10.1093/bioinformatics/bts460
Peng Z, Lu Y, Li L, Zhao et al (2013) The draft genome of the fast-growing non-timber forest species moso bamboo (Phyllostachys heterocycla). Nat Genet 45(4):456–461. https://doi.org/10.1038/ng.2569
Perez-Alquicira J, Aguilera-Lopez S, Rico Y, Ruiz-Sanchez E (2021) A population genetics study of three native mexican woody bamboo species of Guadua (Poaceae: Bambusoideae: Bambuseae: Guaduinae) using nuclear microsatellite markers. Bot sci 99(3):542–559. https://doi.org/10.17129/botsci.2795
Porth I, El-Kassaby YA (2014) Assessment of the genetic diversity in forest tree populations using molecular markers. Diversity 6:283–295. https://doi.org/10.3390/d6020283
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155(2):945–959
Rodgers WA, Panwar HS (1988) Planning a wildlife protected area network in India, vol 2. FAO, Rome
Rodríguez-Pérez J, Traveset A (2016) Effects of flowering phenology and synchrony on the reproductive success of a long-flowering shrub. AoB Plants 8:plw007. https://doi.org/10.1093/aobpla/plw007
Ruiz-Sanchez E, Peredo LC, Santacruz JB, Ayala-Barajas R (2017) Bamboo flowers visited by insects: do insects play a role in the pollination of bamboo flowers? Plant Syst Evol 303:51–59. http://www.jstor.org/stable/44854154
Saha MC, Mian MAR, Eujayl I, Zwonitzer JC, Wang L, May GD (2004) Tall fescue EST-SSR markers with transferability across several grass species. Theor Appl Genet 109:783–791. https://doi.org/10.1007/s00122-004-1681-1
Sharma RK, Gupta P, Sharma V, Sood A, Mohapatra T, Ahuja PS (2008) Evaluation of rice and sugarcane SSR markers for phylogenetic and genetic diversity analyses in bamboo. Genome 51:91–103. https://doi.org/10.1139/g07-101
Sharma V, Bhardwaj P, Kumar R, Sharma RK, Sood A, Ahuja PS (2009) Identification and cross-species amplification of EST derived SSR markers in different bamboo species. Conserv Genet 10(3):721–724. https://doi.org/10.1007/s10592-008-9630-1
Shepard D (1968) A two-dimensional interpolation function for irregularly-spaced data. In: Proceedings of the 1968 ACM national conference, pp 517–524. https://doi.org/10.1145/800186.810616
Sundriyal M, Sundriyal RC (2011) Bamboo trade and future prospects in the central Himalaya: a case study of the traditional artisans of Uttarakhand, India. Ethnobot Res Appl 9:445–454. https://doi.org/10.17348/era.9.0.445-454
Sykes WR (1996) Checklist of bamboos (Poaceae) naturalised in New Zealand. N Z B Botany 34:153156. https://doi.org/10.1080/0028825X.1996.10410679
Takezaki N, Nei M, Tamura K (2009) POPTREE2: software for constructing population trees from allele frequency data and computing other population statistics with windows interface. Mol Biol Evol 27(4):747–752. https://doi.org/10.1093/molbev/msp312
Troup RS (1921) The Silviculture of Indian Trees. Oxford University Press, New York
Wu Z, Yu D, Wang Z, Li X, Xu X (2015) Great influence of geographic isolation on the genetic differentiation of Myriophyllum spicatum under a steep environmental gradient. Sci Rep 5:15618. https://doi.org/10.1038/srep15618
Xie N, Chen L, Dong Y, Yang H (2019) Mixed mating system and variable mating patterns in tropical woody bamboos. BMC Plant Biol 19:418. https://doi.org/10.1186/s12870-019-2024-3
Yang HQ, An MY, Gu ZJ, Tian B (2012) Genetic diversity and differentiation of Dendrocalamus membranaceus (Poaceae: Bambusoideae), a declining bamboo species in Yunnan, China, as based on inter-simple sequence repeat (ISSR) analysis. Int J Mol Sci 13:4446–4457. https://doi.org/10.3390/ijms13044446
Yang JB, Dong YR, Wong KM, Gu ZJ, Yang HQ, Li DZ (2018) Genetic structure and differentiation in Dendrocalamus sinicus (Poaceae: Bambusoideae) populations provide insight into evolutionary history and speciation of woody bamboos. Sci Rep 8:16933. https://doi.org/10.1038/s41598-018-35269-8
Yonzon PB, Hunter ML Jr (1991) Conservation of the red panda Ailurus fulgens. Biol Conserv 57(1):1–11. https://doi.org/10.1016/0006-3207(91)90104-H
Zheng X, Lin S, Fu H et al (2020) The Bamboo Flowering cycle sheds light on flowering diversity. Front Plant Sci 11:381. https://doi.org/10.3389/fpls.2020.00381
Acknowledgements
The work was financially supported by Indian Council of Forestry Research and Education (ICFRE), Dehradun as a research project [OG-49/CR-19]. We thank to the Director, Forest Research Institute, Dehradun and the Director, Himalayan Forest Research Institute, Shimla for providing laboratory and field facilities. The officials of forest departments of Uttarakhand and Himachal Pradesh are also duly acknowledged for their assistance and permissions in surveying and sample collection from their jurisdictional forest area.
Funding
The work was financially supported by Indian Council of Forestry Research and Education (ICFRE) as Research Project [OG-49/CR-19].
Author information
Authors and Affiliations
Contributions
RKM, MSB, and RS involved in study conceptualization, execution, sample collection, data analysis, interpretation and manuscript writing; NN conducted the laboratory work; RS contributed in generation of diversity maps.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meena, R., Negi, N., Shankhwar, R. et al. Population genetic analysis illustrated a high gene diversity and genetic heterogeneity in Himalayacalamus falconeri: a socio-economically important Indian temperate woody bamboo taxon. J. Plant Biochem. Biotechnol. 32, 438–450 (2023). https://doi.org/10.1007/s13562-022-00824-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-022-00824-y