Abstract
Fruit waste and by-products have considerable potential for valorization as a source of value-added compounds of industrial interest, and unconventional substrates, including native fruits, are a promising source of pectins. This study investigated the potential of umbu (Spondias tuberosa L.) peel as a raw material for pectin isolation using high-intensity ultrasound technology and organic acid for the development of an eco-friendly extraction method aiming high yields and adequate degree of esterification. After optimization through central composite design (CCD) with three independent variables (2³) and five levels, a high yield close to 22% of low esterified pectin (DE = 46%) was achieved under ultrasound amplitude of 60%, SLR (solid-to-liquid ratio) of 1:33, and pH 1.5. The extraction process was validated, and the effects of different acids on the yield of pectins and DE were evaluated, demonstrating that the use of citric acid allows yields of around 22% of low methoxyl pectin (LMP) to be achieved, confirming the reproducibility of the process, while using oxalic acid, nitric acid, and hydrochloric acid led to the production of around 13% of high methoxyl pectin (HMP). The quality of pectins, assessed through instrumental color, showed significant differences when compared to commercial citrus pectin CCP, which demonstrated the influence of the type of raw material and extraction method on the quality of the product obtained. Therefore, our study describes for the first time the extraction of pectins from umbu peels using ultrasound technology, proving that this material is a promising source of LMP and HMP pectins and that this approach can considered an efficient green method to obtain different food grade pectins with higher yield and quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The search for new ingredients in the food industry consists of a challenging process that includes the stages of prospecting and selecting new sources or alternative substrates, application of efficient extraction methods aligned with current environmental trends, and application of appropriate separation technologies and purification [1,2,3,4,5]. Furthermore, several studies highlight the importance of obtaining new ingredients such as antioxidants, natural sweeteners, pigments, aromas, phytonutrients, and other functional compounds from agro-industrial by-products and sustainable processes, strongly contributing to the circular economy approach [6,7,8,9,10]. In this sense, the biodiversity of various regions around the world can provide new substrates for obtaining compounds of industrial interest.
Caatinga is a dynamic and heterogeneous biome exclusively Brazilian and restricted to the northeast and part of the state of Minas Gerais [11,12,13]. In recent decades, there is a growing interest in characterizing the commercial and bioactive potential of plant matrices from this biome, including medicinal plants and fruits [12, 14]. Fruits from the Caatinga have economic and nutritional importance for the populations of the Brazilian semi-arid region, requiring studies to strengthen production chains, develop new products, and use their by-products.
In this context, the umbu tree (Spondias tuberosa L.) is a plant native from semi-arid northeastern region of Brazil, and its fruits, known as umbu, are highly valued and appreciated due to their characteristic taste and aroma [15]. Umbu is an important food for local communities, especially in times of drought, when the fruit is harvested in large quantities, sold in natura or processed, traditionally been used to produce juices, frozen pulps, nectars, jellies, jams, ice creams, beers, etc. [16,17,18,19]. It should be noted that these products can be prepared using only the pulp or the pulp with the peel, making it possible to verify a high viscosity of these substrates, which can be attributed to the presence of pectic substances.
Pectin is complex heteropolysaccharide widely consumed as dietary fiber in human diet and plays an important role in the maintenance of health [20, 21]. This hydrocolloid is recognized as food additive in industry (E440) and can be used as stabilizer, emulsifier, and texturizer in diverse food products such as jellies, canned fruits, fruit juices, jams, and confectionery products as well as also been considered fat replacer for production of low-calorie products [22, 23].
Structurally, pectins comprise homogalacturonan (HG), rhamnogalacturonan-I (RG-I), rhamnogalacturonan-II (RG-II), arabinogalacturonan (AG), and xylogalacturonan (XGA) regions, and HG is the most abundant pectic polysaccharide, composed of a linear chain of (1,4)-linked α-D-GalA units, which can be partly methyl-esterified at O-6 position and at lower extent also acetyl-esterified at O-2 or O-3 [24, 25]. The commercial pectins are mainly derived from three agro-industrial residues, including citrus peels, apple pomace, and sugar beet pulp, and in the last years, many studies have been proposed novel unconventional substrates (e.g., food processing by-products) as alternative sources of pectins [23, 26, 27].
The conventional extraction of pectin is carried at low pH values mainly by use of mineral acids (e.g., hydrochloric acid, nitric acid) and high temperature, which leads to environmental pollution, a time-consuming process, and low quality and yields due to degradation of pectin [26]. To overcome these problems, the replacement of mineral acids by organic acids (such as citric acid and oxalic acid) as well as the use of new technologies such as ultrasound, microwaves, high pressure, pulsed electric field, among others, has been proposed [23]. In this sense, due to their low dissociation constant, organic acids have a lower hydrolyzing capacity than mineral acids, causing less depolymerization of the pectin structure [24, 27]. Among emerging technologies, high-intensity ultrasound stands out as the most used approach for pectin extraction, providing less time consumption, high yields, and preservation of the pectin structure since it does not require high temperatures.
The ultrasound technology comprises mechanical waves with frequencies between 20 kHz and 10 MHz, higher than audible frequency range of human hearing (20 Hz to 20 kHz) [28]. The main mechanism involved in the ultrasound-assisted extraction (UAE) consists in acoustic cavitation, which involves collapsing cavitation bubbles and the sound waves that may result in fragmentation, localized erosion, pore formation, shear force, increased absorption, and swelling index in the plant matrix. The efficiency of ultrasound-assisted extraction is influenced by different factors including the ultrasound intensity and frequency, the process time and temperature, the solid-to-liquid ratio, the acid type and concentration, pH of the extracting solution, and the sonicator duty cycle (continuous or pulsed) [29,30,31]. This technology has been proposed in pectin extraction processes as well as for the oriented modification of pectins aiming to modify their rheological, chemical, and functional properties. In recent years, many studies have been developed both using ultrasound and combining this approach with other methods, mainly enzymatic and microwave methods [32,33,34]. In general, higher pectin yields are observed using UAE when compared to conventional acid extraction. Panwar et al. [35] developed an optimized ultrasound-assisted extraction process for obtaining pectins from Citrus limetta peels. They achieved a maximum pectin yield of 28.82% after 24 min of sonication at 40 °C, 37% amplitude, and a pH of 1.9. This approach demonstrated significant advantages over conventional acid extraction, even under optimized conditions, where the pectin yield ranged from 3.97 to 22%. Recently, Singhal et al. [31] investigated the feasibility of using new technologies including UAE and MAE for pectin extraction from Citrus limon and comparing with conventional acid extraction. The highest pectin yield observed was close to 32% using UEA, followed by CE and MAE, which allowed 19.61% and 15.56%, respectively. These results clearly demonstrate the great potential of ultrasound technology, and this promising yield was achieved due to the optimization of ultrasound amplitude, sonication time, and solid-to-liquid ratio (SLR). Thus, the effects of the extraction method must always be considered since they can significantly influence the structure and, consequently, the functionality of pectin.
In addition to the use of ultrasound technology and other emerging technologies for the extraction of pectins, it has been observed in recent years that most of these studies use strategies to optimize the extraction process. Statistical tools for experimental design and process optimization have also been employed to determine the best extraction parameters, with the Box-Behnken design (BBD), factorial design (FD), and central composite design (CCD) being the most used for this purpose [36,37,38]. Some of these experimental designs allow the application of the response surface methodology (RSM), which results in the establishment of optimal conditions for each type of substrate from which pectins are intended to be extracted. The independent variables most used in these experimental designs include pH, solid-to-liquid ratio, temperature, time, and variables linked to the technology used such as nominal power (W), frequency (kHz), amplitude (%), and energy density (J/cm3) related to ultrasound methods.
Although many of the pectin extraction studies have used conventional substrates based on agro-industrial by-products and residues, such as citrus peel and apple pomace, the potential of unconventional substrates is notable [20, 23].Thus, considering that several countries have specific fruit production chains, some of them based on native fruits, there is a clear need to investigate the pectin content of by-products from non-conventional vegetable matrices as an alternative for their use and stimulation of biorefinery. In this context, Brazil is one of the largest citrus producers in the world, ranking as the largest producer of oranges in the world according to Food and Agriculture Organization of the United Nations (FAO) database [39]. Furthermore, this country is also recognized for its great biodiversity, including several types and species of native fruits, which have been extensively investigated for their bioactive and functional potential [40,41,42,43,44]. However, fruits from Brazilian biomes and their by-products have been investigated regarding the content, variety of pectins, and the technological and functional potential of these polysaccharides [45,46,47].
Thus, considering that umbu is a fruit native to the caatinga biome, which is economically important for several communities of small farmers and cooperatives, this study aimed to develop and optimize an environmentally friendly process for extracting pectins from peels of this fruit using ultrasound technology and organic acid. Firstly, a univariate study to define the best sonication time was carried out aiming for high yield and lower time and energy consumption. After, a central composite design (CCD) was applied to evaluate the effects of ultrasound amplitude, SLR, and pH on the yield and degree of esterification of the extracted pectins at a fixed sonication time. Finally, the UAE method was validated under optimal conditions for greater yield with evaluation of the influence of the type of acid (organic and mineral) on the quantitative and qualitative aspects of the extracted pectins.
2 Materials and methods
2.1 Sample preparation
The umbu fruits were purchased at the São Joaquim market located in Salvador city, Bahia state, Brazil (S12.9730401; W38.5023040). The fruits were selected and separated according to their stage of ripeness and structural integrity (absence of lesions). The fruits were carefully cleaned, and the peel was separated from the pulp and seeds and then dried in a forced-air oven at 60 °C until constant weight.
2.2 Production of umbu peel flour and alcohol insoluble residue
The dried peel was ground in an industrial blender, and the particle size was standardized to 20-mesh and named as umbu peel flour (UPF). This material was subjected to extraction step for removal of alcohol-soluble compounds (organic acids, sugars, pigments, etc.) with 80% ethanol (1:3 w/v) for 10 min under ultrasonic treatment with an amplitude at 50% of nominal power in pulsed mode with pulse duration of 30 seg, cycle time of 1 min, and duty cycle of 50%. Thereafter, the mixture was filtrated, and the solid fraction was dried in an oven at 40 °C resulting in the alcohol insoluble residue (AIR, 76% w/w of UPF). Then, AIR was characterized according to item 2.6 and used as substrate for pectin extraction using conventional and ultrasound-assisted extraction.
2.3 Proximate composition, physicochemical and physical characterization of UPF and AIR samples
The nutritional composition of UPF and AIR was determined through AOAC Official Methods for fruit and fruit products. The moisture was determined gravimetrically by loss on drying method at 105 °C (AOAC 934.06). The ash content was determined gravimetrically after complete incineration of the sample at 550 °C in a muffle furnace (AOAC 940.26). The fat fraction was determined in an intermittent Soxhlet extractor using petroleum ether as solvent (AOAC 920.39 C). The total nitrogen content was determined by the Kjeldahl method, using a multiplication factor of 6.25, typical for determining proteins (AOAC 920.152). Titratable acidity was measured by titration of the samples with standardized 0.1 N NaOH solution, and the results were expressed as g/100 mL of citric acid (AOAC 942.15). The total carbohydrate content was obtained by difference. The instrumental color was measured in a CR-400 Chroma Meter (Konica Minolta) using the CIELAB as described in item 2.7.5.
2.4 Preliminary study of pectin extraction using UAE and statistical optimization
2.4.1 Selection of sonication time for UAE and its effects in pectin yield
Extraction time is an important variable and widely used in experimental designs to optimize pectin extraction, but it can generate interaction effects with other process variables, making them statistically significant. Furthermore, it is known that long periods of ultrasonic processing can lead to the degradation of biopolymers such as pectins; in this study, we proposed to evaluate the influence of time by fixing other three variables as ultrasound amplitude, pH, and SLR. The extraction was performed at different times, including 0, 6, 10, 18, and 23 min, using 1:30 (w/v) of solid to liquid ratio (SLR) based on AIR (w) and citric acid solution (v) at pH 2.0 and employing 50% of ultrasound amplitude. The extraction was carried out using an Ultrasonic Processor (Sonics Vibra-Cell VC 505), frequency of 20 kHz, and maximum power of 500 W, equipped with a 13-mm-diameter probe, in pulsed mode with pulse duration of 30 seg and cycle time of 1 min, resulting in a duty cycle of 50%. The energy density (ED) was calculated based on the nominal power input (W), processing time (s), and sample volume (mL) (Eq. 1). After the sonication process, the extracts were used for recovery of pectin as described below. Additionally, the temperature was measured before and after the ultrasonic treatment to establish the relationship between the increase of sonication time, ED, and increase in temperature (ΔT). All experiments were carried out in triplicate, and the best time was selected considering the binomial greater extraction yield and lower energy expenditure.
2.4.2 Dual-objective optimization pectin extraction process
A CCD with three independent variables (2³) and five levels was employed to optimize the ultrasound-assisted extraction of pectin from umbu peel aiming to understand the effects of different variables on the quality of pectin and finally establish the best conditions to achieve high yields of pectin with different degrees of esterification. Thus, the target dependent variables in this study were pectin extraction yield (\(Y\)1,%) and degree of esterification (\(Y\)2,%). The levels of independent variables including ultrasound amplitude (\(x\)1), pH (\(x\)2), and solid-to-liquid ratio (\(x\)3) are presented in Table 1. Thus, appropriate content of AIR was mixed with the extracting solution and citric acid at final volume of 200 mL. The extraction was carried out using the ultrasonic processor described previously with the best processing time selected in item 2.3.1. Multiple regression analysis was applied to determine the regression coefficients for the linear, quadratic and interaction terms. Experimental data were fitted to a second-order polynomial mathematical equation to express the relationship between independent variables and responses (dependent variables). The generalized form of second order polynomial equation was given as follows:
where \({Y}_{n}\) is the dependent variable, including \({Y}_{py}\) to pectin yield (%) and \({Y}_{DE}\) to degree of esterification; \(x\)i and \(x\)j are the independent variables (i and j range from 1 to \(k\)); β0 is the model intercept coefficient; \({\beta }_{j}\), \({\beta }_{jj}\), and \({\beta }_{ij}\) are interaction coefficients of linear, quadratic, and the second-order terms, respectively; k is the number of independent parameters (k = 3 in this study).
2.4.3 Statistical analysis of optimization study
The reparameterization of the quadratic model was performed using only the significant terms. The significance of each effect and the regression coefficient were determined considering a significance level of 5% (p < 0.05). In addition, one-way analysis of variance (ANOVA) using the significant coefficients was performed to verify the statistical accuracy of developed mathematical models and included the F-test and obtention of the coefficient of determination (R2). All the statistical analysis was carried out using the Statitica®10 package software (Stat Soft Inc., Tulsa, USA).
2.4.4 Validation and evaluation the effects of different acids on pectin yield and DE
The validation of mathematical models from optimization study was carried out using extraction conditions to achieve high yield and low DE to verify the accuracy of the models. The predicted and experimental of responses were statistically compared, and the percentage error and residual were calculated according to Zaid et al. [48], at which less than 10% of error is relatively desirable [49]. Furthermore, the effects of acid type (organic and mineral) on the extraction yield and DE of pectin were verified as well as their impact in color quality parameters.
2.5 Conventional extraction of pectin
The acidic hot water extraction (AIWE) was performed according to the method described by Gharibzahedia et al. [50] with adaptations. Pectins were extracted from AIR with water acidified with citric acid until stability in a thermostatic water bath under stirring at 70 °C for 30 min, SLR and pH from the optimal conditions for higher yield from 2.4.2.
2.6 Pectin recovery and yield determination
After the extraction process, the extracts obtained were collected and centrifuged at 4.200 rpm for 20 min at 10 °C. The supernatant was then separated by filtration, precipitated with ethanol 96% (1:3 v/v), and left to stand overnight at 4 °C. The coagulated pectin was collected and washed three times with ethanol 96%, and the wet pectin was dried in a forced air circulation oven at 35 °C until constant weight. Finally, the dried sample was ground into powder, and the yield of extracted pectin was determined as follows (Eq. 3):
2.7 Chemical and physical characterization of pectin
2.7.1 Galacturonic acid content
The galacturonic acid content was determined by the metahydroxydiphenyl (MHDP) method developed by Blumenkrantz and Asboe-Hansen [51] with adaptations. An analytical curve was prepared using a standard solution of galacturonic acid (GalA) in concentrations from 5 to 100 µg.mL−1. Pectin solution (0.4 mL) was poured into a tube, and sulfuric acid/sodium tetraborate (2.4 mL) was added and cooled in a cold-water bath. Your tubes were stirred by a vortex mixer, heated in a water bath, and cooled. The abovementioned color reagent was then added and stirred for 5 min before reading the absorption at 520 nm using a UV/Vis spectrophotometer.
2.7.2 Degree of esterification (DE)
The degree of esterification (DE) was determined using the titration method as reported by Panwar et al. [35]. The pectin samples (100 mg) were moistened in ethanol (2 mL), dissolved in distilled water (20 mL), and stirred until completely dissolved. Five drops of phenolphthalein were then added, and the solutions were titrated with 0.1 N sodium hydroxide until a pale pink color appeared (V1). Then 0.1 N sodium hydroxide (10 mL) was added to the titrated samples and stirred for 15 min. Next, 0.1 N hydrochloric acid (10 mL) was added, and the samples were shaken vigorously until the pink color completely disappeared. Finally, the samples were titrated again with 0.1 N sodium hydroxide until a pale pink color appeared (V2). The DE was calculated using the following formula (Eq. 4):
2.7.3 Instrumental color
Instrumental color of pectin samples was measured using a CR-400 Chroma Meter colorimeter (Konica Minolta) using the CIE L. The color coordinates obtained were L* (brightness ranging from 0 (black) to 100 (white)), a* (+ a* indicating tendency for red and −a* tendency for green), and b* (+ b* indicating tendency for yellow and −b* tendency for blue) system. In addition, the hue angle (h0) which represents the qualitative attribute of color was calculated using Eq. (5), while the chroma index (C*) was calculated using Eq. (6). The colorimeter was calibrated in the reflectance mode, using the illuminant D65 and an observation angle of 10° [52].
3 Results and discussion
3.1 Chemical characterization of UPF and AIR
The plant materials used as substrates (UPF and AIR) for pectin extraction were similar in terms of nutritional composition, with high values of total carbohydrates, around 85%, while the content of moisture, total minerals, lipids and proteins were approximately 10%, 3%, 1.1%, and 0.5%, respectively (Table 2). These values are close to those recently reported by Cangussu et al. [53] where these authors evaluated the proximal composition of flours produced from the peels of ripe and semi-ripe umbu fruits collected from the Caatinga biome. The differences can be attributed to geographic and seasonal factors that notably affect the composition of fruits and vegetables, as the umbu fruits in the aforementioned study were collected in the portion of the Caatinga biome in the state of Minas Gerais, in the southeast region, while the fruits of umbu in the present study come from the portion of the Caatinga biome in the state of Bahia, located in the northeast region of Brazil. A significant difference for titratable acidity was observed, where AIR presented a lower value (0.29 ± 0.01) compared to UPF (0.42 ± 0.01), indicating the process of producing insoluble residue by prior extraction with ultrasound was able to remove alcohol-soluble organic acids.
Regarding color analysis, AIR presented a higher value L* coordinate, indicating that this substrate was lighter when compared to UPF, probably due to the removal of natural pigments in the insoluble residue production stage. Significant differences were observed between the h0 values of UPF and AIR, which ranged from 73.27 to 68.93, respectively, indicating a yellowish/beige color for these substrates, with AIR being slightly lighter compared to umbu peel flour. Regarding coordinate a*, no significant difference was observed, with small positive values, close to 4.00, for both substrates. This result is related to the degradation of chlorophyll and carotenoids present in umbu peel, leading to browning reactions during the drying steps employed produce UPF and AIR, resulting in particulate matter with color tending from dark greenish to reddish, characteristic of fruit powders and flours produced after drying processes [54] Regarding the b* coordinate, a significant difference was observed between UPF and AIR, with a higher value for UPF (13.30 ± 0.08), indicating a more yellowish tone for this material, which may probably be related to a higher carotenoid content, which were removed during the extraction step that results in the production of AIR (10.89 ± 0.27).
3.2 Selection of sonication time for UAE of pectin from umbu peel
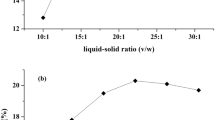
The preliminary and univariate study for selection the best processing time for extraction of pectin from umbu peels using UEA was carried out using 1:30 of SLR at pH 2.0 and 50% of ultrasound amplitude. The results showed that 4.82% pectin yield was achieved from the solubilization of AIR in citric acid solution (pH 2.0) without ultrasonic processing (Fig. 1A), indicating the presence of a considerable content of pectic substances in this substrate. Using pulsed ultrasonic processing for 6 min at 50% amplitude (50 W), the ED was 82.33 J.cm−3 and allowed a pectin yield of 12.20%, approximately. The pectin yield was significantly increased (p < 0.05) to 13.76% and 13.26%, when the UAE method was carried out for 10 min (143.04 J.cm−3) and 18 min (258.30 J.cm−3), respectively. These findings are associated with the greater intensity of the cavitation effects generated by ultrasonic waves on plant material particles of AIR resulting from the increase in sonication time. Additionally, a directly proportional relationship was observed between the sonication time with ED and the temperature in the extracting solution, which together contribute to the improvement of mass transfer, leading to greater amounts of extracted pectin (Fig. 1B).
In contrast, a significant decrease in pectin yield was observed by increasing the extraction time to 23 min, where 11.85% of pectin was obtained. The reduction in pectin yield when extraction was carried out for 23 min can be explained by the high energy density (315.91 J.cm−3) applied, which probably resulted in partial degradation of the polysaccharide chain into low molecular weight oligomers. These degradation products may comprise mixtures of mono-, disaccharides, and mainly pectic oligosaccharides, which are more soluble in the acid extracting solution and are not recovered in the alcohol precipitation step since they are removed in the subsequent filtration and washing steps, reducing the amount of recovered pectin. In pectin extraction processes, this effect must be avoided, but it is known that power ultrasound has been reported as promising approach for oriented degradation and modification of polysaccharides, including pectin, resulting in structural and functional changes [32, 55, 56].
In general, extraction processes of natural compounds of industrial interest (e.g., pectin) must be robust and reproducible, and it is desirable that they be low cost, with reduced time consumption, less energy expenditure, and consequently reduced environmental impact. These factors are crucial for enabling the extraction processes for pilot scale and for scale-up purposes in industrial level [57]. Although the application of other emerging technologies for extracting value-added compounds, such as pectins, from agro-industrial by-products is considered promising, it is known that some of them require pressurized apparatus such as those used in high-pressure techniques or require specific components to generate heat or for strict temperature control such as microwave-based methods [57,58,59,60]. In this context, high-intensity ultrasound has been widely proposed for the extraction of pectins due to its clear advantages in terms of improving extraction and reducing time consumption in the process [34].
Thus, to develop a simple, fast, and efficient extraction process of pectin from umbu peels, we do not propose strict temperature control since ultrasound can generate an increase in temperature during the sonication process. Therefore, UAE can generate heat depending on the intensity and time applied, increasing the temperature of the medium, being desirable in some processes as it leads to improved extraction without the need for thermostatic baths or complex temperature control devices. Figure 1B shows the correlation between ultrasonic processing time (min), energy density (J.cm−3), and temperature and the impact of these variables on pectin yield. It is worth noting that with sonication time of 23 min, a considerable increase in temperature was observed, ΔT23′ = 36 °C, when compared to the other sonication times evaluated (ΔT0 = 0, ΔT6′ = 16 °C, ΔT10′ = 21 °C and ΔT18′ = 31.5 °C). This phenomenon may also have contributed, associated with high ultrasound intensity, to the lower yield as it is reported that depolymerization rate of pectin increases with temperature [61]. On the other hand, we must highlight that this pectin degradation was attenuated by conducting the sonication process in pulsed mode, as if we had opted for continuous mode the extent of the temperature increase, and degradation would probably be greater. In pulsed mode, the sonication is regulated on a periodic basis and through a switch on and off mode, in our study 30 s, that allows a moderate heat in the solution [30]. This approach is interesting because causing lesser destruction of bioactive compounds through such intermittent relaxation when compared to continuous mode. However, variables such as pH, type of acid employed for extraction, and temperature will influence the extent of the effects in pulsed mode and must be considered [32, 62, 63].
The negative effect of prolonged extraction time yield was reported by Sengar et al. [64] in study about extraction of pectin from tomato processing waste using UAE, where a maximum yield of 15.21% was obtained at ultrasound power of 600 W after 8.61 min, while 14.29% was achieved after16 min using same ultrasound power, suggesting that inadequate power input can be affect the efficient extraction of pectin. These authors also associate that the phenomenon of lower yield at higher ultrasound power can be explained by the fact that long exposer time and increasing power input causes degradation of pectin into low molecular weight compounds. A similar finding was found in UEA of pectin from waste custard apple peel (Annona squamosal), where the yields were close to 9.1% with a sonication time of 20 min, while with a time of 30 min, under the same conditions, the pectin yield decreased. to around 8% [65]. Previously, Xu et al. [66] demonstrated that with increasing power density from 0.20 to 0.40 W/mL, the pectin yield increased from 22.67 to 27.27%, and this can be explained by the fact that the cavitation bubble collapse became more energetic with amplitude or power increased. Moreover, these authors observed that power density was higher than 0.40 W/mL which led to a significant decrease of pectin yield, concluding that degradation effect on pectin increased with the increasing of ultrasound intensity.
In this study, the statistical analysis of results revealed no significant difference (p < 0.05) between the pectin yield achieved in 10 min and 18 min of ultrasonic processing, demonstrating that a UAE process conducted by 10 min would be the most advantageous as it allows reduction in process time (8 min) and energy consumption (difference of approximately 115 J.cm−3). Therefore, this sonication time was selected and fixed for the optimization study of ultrasound-assisted extraction of pectin from umbu peel.
3.3 Model fitting and experimental data analysis
In this study, a CCD was successfully applied to optimize the UAE of pectin from umbu peel. The experimental design consisted of a total of 19 experimental runs, and the experimental and predicted values of pectin yield and DE obtained at these different extraction conditions are shown in Table 3. The pectin yield varied between 10.97 and 21.82%, while the DE ranged 46.48–83.54% indicating that by varying the extraction conditions, important differences are observed in the quantity and characteristics of the pectins obtained. The pectin content in umbu peels obtained using UAE is close to the described by Cangussu et al. [53] who extracted 16.69% of pectin from mature umbu peel and 20.41% from semi-mature umbu peel using microwave-assisted method and citric acid solution. However, these authors did not carry out the chemical characterization of obtained pectins. Therefore, our study presented here is the first to extract pectins from semimature umbu peel (called “de vez” maturation stage) using ultrasound technology and the first to describe the chemical characteristics of pectins from this fruit.
Applying multiple regression analysis on the experimental data obtained the regression coefficients and statistical parameters presented in Table 4. These coefficients were employed to generate the quadratic polynomial models which could express the relationship between the evaluated independent variables, including ultrasound amplitude (\(x\)1), pH (\(x\)2), and SRL (\(x\)3), and our responses of interest, in this case, represented pectin yield (\(Y\)1,%) (Eq. 7) and DE (\(Y\)2,%) (Eq. 8).
The effect of independent variables in the studied responses can be predicted by the sign of the regression coefficient of the models, where positive sign suggest that the linked variable has positive impact on the response, whereas those with negative sign lead to an opposite effect [67]. The statistical significance and quality of the constructed quadratic models were evaluated through analysis of variance (ANOVA) shown in Table 5. The results showed that the F-values of the model for yield and DE were 8.42 and 20.90, respectively, while the p-value was less than 0.05 for both models constructed. The calculated F-value was higher than critical F value (F9;9;0.05 = 3.18) obtained from F-distribution table considering α = 0.05, indicating that the models were adequate and could be selected to explain and predict the variation of pectin yield and DE. Although the lack of fit for both models appeared significant (less than 0.05), this can be attributed to the very low value of the pure error calculated from the replicates represented by the central points, where the values are very similar. Furthermore, high correlation coefficients were observed for the pectin yield and DE models, with R2 of 93.80% and 97.41%, while the Adj-R2 were 87.61% and 94.38%, respectively. Similar findings regarding the adjustment of predictive models for pectin yield and DE were described in a study to optimize the conventional extraction of pectin from orange peels, where the lack of fit these responses also proved to be significant [68]. However, high F-values (27.57 for pectin yield model and 6.40 for DE model) were observed as well as correlation coefficients R2 of 96.13% and 85.20%, and Adj-R2 of 92.64% and 71.88%, respectively, indicating that the obtained models are fit to experimental data and suitable to predict the relationship between the dependent variables and responses. From the ANOVA data, the linear terms of all variables were significant, while only the quadratic terms of ultrasound amplitude and pH were significant for the yield predictive model. For the DE predictive model, only the linear term for pH was significant, while all quadratic terms and the interaction between SLR and pH were significant. Thus, the substitution of the coded values relating to the independent variables in the predictive models obtained in our study leads to predicted values (Table 3) very close to those found experimentally. Naturally, variations related to extraction process and even in the pectin recovery stages may cause small variations, but these do not invalidate the robustness and quality of the models.
3.4 Effects of extraction process variables on the response variables
Considering the fitted models described in the previous section, 3D response surfaces graphs and contour plots were constructed for study of the interactive effects of the independent variables on the pectin yield (Fig. 2) and DE (Fig. 3). In each figure, the interactive effects between two independent variables and response could be observed which the remaining independent variable was fixed at the central point level, where in (A) pH = 2.5, (B) SLR = 1:33 (w/v), and (C) ultrasound amplitude = 60%. Thus, the main results of the interactive effects of the independent variables on the yield of pectin and DE will be discussed, understanding how each variable affects the responses to establish the best process conditions.
3.4.1 Effect of process variables on pectin yield
Generally, extraction processes are designed to achieve high yields of the compound of interest. In this context, most studies that propose improving pectin extraction aim to achieve high yields. From the analysis of the response surfaces and contour curves in Fig. 2, it is possible to observe that using moderate ultrasound amplitude, close to 60%, and SLR around 1:50 to 1:30 in acidic conditions with pH less than 2.0, higher yields can be achieved (Fig. 2A). The characteristics of the plant material used as a substrate for pectin extraction as well as its proportion in the extraction medium affect the yield. Considering the analysis of the response surfaces and contour curves A and C (Fig. 2), it is possible to verify that the increase in the solid-liquid proportion negatively affects the yield, with greater amounts of pectin being obtained with SLR up to 1:33 (w/v). The negative effect related to the increase in solute in extractive processes can be explained by the saturation of the extracting solution with a consequent decrease in the mass transfer rate, and this saturation can compromise the efficiency of the cavitation process (e.g., rupture of cavitation bubbles), leading to a decrease in yield. Similarly, Shivamathi et al. [65] reported the influence of SLR on yield in a study that aimed the optimization of pectin extraction from waste curtard apple peel. The yields obtained with 1:10 (6.54%) for example was lower than the most assays employing 1:15 g/mL (6.52–7.94%), 1:20 (6.69–7.73%), 1:25 (7.12–8.22%), and 1:30 g/mL (7.58%) of SLR, which is probably related to the increase in saturation of the acid extracting solution that occurs when there is a large increase in the amount of solid and a decrease in liquid.
Furthermore, it should be noted that increasing the volume of extracting solution (liquid) allows a greater amount of pectin to be contained in the extraction solvent, thus obtaining a greater yield of pectin. This is due to difference of concentration that is established between the interior of the plant cell and the exterior solvent, thus increasing the mass transference of pectin from the solid matrix to the liquid solvent, resulting in a greater amount of pectin [37, 69]. Throughout extraction, the concentration of pectin in the solvent increases and the extracting liquid becomes viscous, leading to a decrease in the pectin mass transfer rate until it reaches the saturation point [37, 58]. This phenomenon depends on the total amount of pectin available on the substrate to be extracted, the particle size (which affects the surface area), and other process variables. Therefore, SLR is an important independent variable to be considered in pectin extraction optimization studies.
Due to its mechanical wave nature, characteristics of the ultrasonic wave such as frequency, wavelength, and amplitude can influence the acoustic cavitation and therefore extraction [70]. In higher amplitudes, the ultrasound wave contains a high number of cycles of compression and rarefaction, leading to a more intense collapse of bubbles, allowing the targeted compound to be released into the solvent rapidly [71]. This parameter is directly related to power parameters that can be expressed as ultrasonic intensity (UI, W.cm−2), power density (PD, W.mL−1), and energy density (ED, J/cm−3), where the increase of amplitude leads to increase of these parameters, resulting in improvement of sonochemical effects, and many cases lead to an increase of the extraction efficiency [35, 70]. Through the analysis of response surfaces and contour curves A and B (Fig. 2), it is possible to verify that the best ultrasonic amplitude range is between 50 and 60%, leading to higher yields of pectin. These higher yields are achieved under conditions of low pH values and intermediate substrate amounts, between 1:50 and 1:33 of SRL. From the mathematical model for pectin yield (Eq. 8), we infer that there is a negative effect of increasing amplitude, since high amplitude values are related to an increase in energy density in the solution as well as temperature, which can contribute to depolymerization and reduced pectin extraction yield. Therefore, the ultrasound amplitude used in pectin extraction processes must be evaluated and optimized to have an adequate range for processing. Our results show that with the use of 60% amplitude, equivalent to a power density of 0.26 W.mL−1 and ED of 149.08 J/cm−3, the yields were greater than 13.26%, reaching a maximum yield of 21.82%. In contrast, using 90% amplitude, equivalent to 0.45 W.mL−1 and 262.63 J/cm−3, a yield of only 12.01% was observed, probably due to the degradation of the pectin structure into low molecular weight compounds. When 30% amplitude (0.11 W.mL−1 and 60.17 J/cm−3) was used, a yield of 12.46% was achieved, like the yield using 90% amplitude. The lowest yield was observed at 78% amplitude (0.385 W.mL−1 and 221.25 J/cm−3), 1:25 of SLR, and pH of 3.1. Probably, this finding can be related to a greater quantity of AIR used, in addition to the pH of 3.1, a value greater than the best yield range (pH < 2.0). The negative effect related to the increase in solute in extractive processes can be explained by the saturation of the extracting solution with a consequent decrease in the mass transfer rate, and this saturation can compromise the efficiency of the cavitation process (e.g., rupture of cavitation bubbles), leading to a decrease in yield.
Some studies show that depending on the plant matrix used, maximizing amplitude can contribute to higher yields. For example, in recent optimization study of the UAE of pectin from Assam lemon (Citrus limon Burm f.), the ultrasound amplitude was found to have a higher effect on the extraction, where the increase in the amplitude from 20 to 100% resulted in increase of pectin yield from 2.9 to 32.17% [31]. Similarly to our results, an optimization study of UAE of pectin from Citrus limetta peels performed by Panwar et al. [35] demonstrated increase in amplitude till 40%, the yield of pectin increased linearly, but further increase in amplitude beyond 40% reduced the pectin yield, demonstrating that the ultrasound amplitude is a variable that must be controlled appropriately depending on the plant matrix used to extract pectins.
One the most important variables evaluated in optimization studies of conventional and unconventional extraction of pectin is pH or acid concentration [72, 73]. Several studies describe that pectin yield is greater at low pH values or in more acidic conditions. Traditionally, pectin is extracted using strong mineral acids at pH ranging 1 to 3, and acidic conditions are essential to hydrolysis of the complex cross-linked networks of the cell wall in plant material and the insoluble pectin constituents, promoting the release of soluble pectin [72,73,74]. Our results show that when pH 1.5 was used in UAE, the pectin yield was 21.82%, and with the increase in pH to higher values, a reduction in yield was observed. At pH 1.9 and 2.5, pectin yields varied from 14.43 to 16.06%, and from 12.01 to 13.98%, respectively. At a pH of 3.1, the yield varied from 10.97 to 13.12%, and at a pH of 3.5, the yield was 13.67%. According to the mathematical model obtained for yield, a strong negative effect of pH is observed, which indicates and explains the results obtained where, in more acidic conditions, greater yields are obtained. This result corroborates the study reported by Colodel et al. [75], which demonstrated that pH was the most influential variable in the study to optimize the conventional extraction of pectins from grape pomace.
The effect of pH in pectin extraction from orange peel was also observed in recent study performed by Iñiguez-Moreno et al. [76], where the results from Box-Behnken Design showed that at pH 2.0, the pectin yield ranges from 22.77 to 31.20%, while at pH 4.0, the pectin yield varies from 3.00 to 3.99%. The effects of pH may vary depending on the duty cycle applied in the UAE, where pulsed mode and low pH values, in some cases, have been shown to be more useful for achieving higher yields when compared to continuous mode. In this context, the UAE of pectin from pomelo fruit using citric acid at different pH values showed that in continuous mode at pH 1.5, the yield ranged from 22.55 to 33.9%, while in pulsed mode, the yield varied from 33.46 to 46.4% [30]. With the increase in pH to values above 1.5 to 3.5, a decrease in pectin yields was observed, demonstrating that in ultrasound-assisted processes, the duty cycle must be considered to improve the extraction process. The UAE of pectin from navel orange peels in continuous and pulsed mode was reported by Patience et al. [63]. The results showed that in continuous mode, pH 2, and power density of 0.24 W.mL−1, a yield close to 11% can be achieved, with only a 1.3% difference between this yield to achieved at pulse ultrasound mode at the same conditions. These authors concluded that pulsed mode can be considered more efficient due to less energy consuming (80 kJ) when compared to continuous mode (190 kJ).
Therefore, in our study, we proved that high levels of pectin with low and high DE can be extracted from umbu peels, and a higher yield (greater than 21%) of low DE pectin (close to 44%) can be achieved using 60% ultrasound amplitude in pulsed mode, SLR of 1:33, and pH 1.5, while pectins with higher DE (greater than 80%) can be extracted with a yield of around 13% under the same conditions by only varying the pH of 1.5 to 2.5.
3.4.2 Effect of process variables on degree of esterification of pectin
Among the main chemical characteristics of pectins, the degree of esterification (DE), also expressed relatively as the degree of methoxyl (DM), can be considered one of the main chemical guidelines for the applications of this polysaccharide [74]. Other chemical characteristics, including molecular weight (MW), monosaccharide composition (MC), RG-I/HG ratio, and degree of branching, are also reported to be important for the functional properties of pectins, such as modulation of the gut microbiota [77].
The DE has been used mainly as indicator of the gelling and emulsifying properties of pectin. Moreover, this parameter has been used to classify pectins into two groups, high methoxyl pectins (HMP), and low methoxyl pectins (LMP). HMP pectins have a DE value greater than 50% and require solutes such as sucrose and pH lower to 3.5 for effective gelation, which occurs through hydrogen bonds and hydrophobic interactions. LMP pectin, on the other hand, has a DE of less than 50%, and for gelation, it depends on divalent cations, such as calcium ion, which interact with the free carboxyl groups of galacturonic acid residues, forming gels over a broad pH range. Recent studies have reported the interesting applications of pectins with low DE as for increase the water holding capacity and viscoelasticity of gluten proteins [78]. Considering the analysis of the response surfaces and contour curves of Fig. 3, it is possible to verify that all variables have some effect on DE, being pH the most important variable to be considered, with linear, quadratic, and interaction terms significantly. Thus, the positive effect of pH is evident, suggesting that increase of pH close to 3.0 leads to extraction of HMP, while acidic conditions allowing the extraction of LMP. It can be seen in Fig. 3B that regardless of the amplitude and SLR used, the DE tends to increase with increasing pH. Thus, based on Fig. 3A, it was possible to verify that higher DE are achieved using SLR of 1:33 and amplitude of 60%. This observation supporting the experimental data is shown in Table 3, where it is observed that at pH 1.5, higher amounts of LMP were extracted with DE of 46.48%, while at pH of 1.9, the DE ranged from 60.82 to 63.59%, and at pH of 2.5, the DE percentages ranged from 82.33 to 83.61%. DE percentages increases at pH of 3.1 to values varying from 82.12 to 83.39% and at pH 3.5 the DE observed was close to 80%.
Similar effects were observed in optimization study of UAE of pectins in continuous and pulsed mode from pomelo peels at different pH values, where in continuous UAE, the DE ranging from 40.73 to 52.09% was observed when the extractions were carried out at pH of 1.5, while DE ranged from 63.26 to 72.25% and 76.92 to 84.90% at pH of 2.5 and 3.5, respectively [30]. When the UAE was performed in pulsed mode, the DE values at pH of 1.5 were slightly higher than those obtained in continuous mode, ranging from 47.6 to 55%, while at pH of 2.5, the DE varying from 67.8 to 75.2% and DE of 82.43 to 88.6% at pH of 3.5. This small increase in DE by varying the duty cycle is possibly associated with a greater cavitation effect that can be generated in continuous mode when compared to pulsed mode; thus, in continuous mode, there is greater de-esterification of the pectin chain due to interactive effects of ultrasonic waves with a more acidic environment. Acid de-esterification by hydrolysis is a pH-dependent reaction, and it occurs mainly under acidic conditions using strong inorganic acids, and the increase of pH leads to β-elimination as main mechanism of de-esterification of pectin [79]. Another study reported that at pH of 2.0, the DE of extracted pectins from orange peel ranged from 28.93 to 34.76%; at pH 3.0, the pectins showed DE from 62.84 to 84.46%; and at pH 4.0, the DE varied from 72.46 to 90.86%, depending on temperature and time of extraction [76].
In comparative study performed by Tran et al. [80], pH was the most influencing variable on DE of pectin from jackfruit rags. For both conventional and UAE methods, DE of pectin decreased when pH was decreased, and lowest DE values of 11.71% in conventional method and 8.48% in UAE method were found at pH of 1.5. This can be explained due to increase de-esterification of the polygalacturonic chain under severe pH conditions [81].
Therefore, our results suggest that the use of experimental design would facilitate the establishment of conditions for the extraction of different types of pectin, making it possible to obtain HMP and LMP from umbu peels using UAE in pulsed mode by varying the experimental conditions.
3.5 Comparison between conventional heating extraction and ultrasound-assisted extraction
The conventional methods for the extraction of commercial pectins are based on the use of hot diluted acids. In general, long times of extraction, high temperatures, and low pH leads to high pectin yields but can influence the physicochemical properties. Thus, the exact extraction conditions are adjusted for each raw material to obtain pectins with suitable properties [24, 82].
Many studies use reflux or stirring methods, generally employing temperatures greater than 60 °C under acidic conditions using strong mineral acids. Furthermore, conventional methods are conducted for prolonged periods (several hours) which lead to high costs related to energy consumption [24]. In general, these processes are recognized as being expensive, and there is concern about their alignment with the principles of “Green Chemistry,” as they employ strong mineral acids that result in waste that needs to be adequately treated after the extraction process [83]. In this context, many studies strengthen the trend towards using new technologies such as ultrasound, microwave, high pressure techniques, and ohmic heating, for pectin extraction [84]. Among the proposed technologies, ultrasound appears as the main emerging technology used in the extraction of pectins, with several advantages reported for this method alone or in combination with others, which has resulted in several studies in the last 10 years using this approach. In this study, using the conventional method of hot acid extraction, a yield of 12.16% was achieved, and the extracted pectin presented a DE of 49.63%, while the UAE method proposed in this study allowed a high yield of LMP close to 22%.
Previously, significant differences on pectin yield from dragon fruit peel were reported using conventional and UAE at different times and temperature [85]. Using conventional extraction for 30 min at 45 °C, 60 °C, and 75 °C, the pectin yields were 4.98%, 8.42%, and 10.44%, respectively. On other hand, UAE for 30 min at same temperatures achieved yield of 9.38%, 15.30%, and 16.30%, respectively, demonstrating that ultrasound technology can lead to higher yields using same raw materials. In addition, different extraction methods influenced the DE of pectins, where the conventional extraction for 30 min at 45 °C, 60 °C, and 75 °C leads to DE of pectins of 49.87%, 41.37%, and 46.06%, respectively, while using UAE method, the DE values were 37.64%, 36.80%, and 35.44%, respectively. Recently, the efficiency of an optimized UAE method for extracting pectin from Assam lemon (Citrus limon Burm. f.) was described with yield close to 32%, while conventional extraction approach leads to pectin yield of 19.61% [31].
Therefore, based on our results, UAE can be considered a favorable approach for obtaining pectins from umbu peels when compared to conventional method as it allows higher yields of different pectins (HMP and LMP) in a reduced processing time (10 min of sonication), which results in a process that is economically viable in terms of energy consumption, time, and energy. Furthermore, the use of an organic acid increases the appeal and suitability of the process in environmental terms and in obtaining a product for the food industry.
3.6 Validation and evaluation of the impact of different acids in pectin characteristics
Considering the results obtained finding in the Table 2 and the discussion abovementioned through analysis of surfaces and contour plots, the optimum conditions were considered to be ultrasound amplitude of 60%, 1:33 of SLR, and pH of 1.5 for obtaining a maximum pectin yield. These conditions were set because reducing pH to values lower than 1.5 would result in a highly acidic environment that could, when associated with ultrasound, lead to pectin depolymerization. Furthermore, the reduction in pH would depend on an increase in the concentration of citric acid and the consequent increase in process costs for this substrate. The experiments were carried out in triplicate, and to assess whether the results obtained so far were dependent on citric acid, different acids were evaluated, including oxalic acid, nitric acid, and hydrochloric acid, to investigate the impact on the yield and DE as well as the color parameters, here used as quality parameter, of pectins from umbu peel. Considering the results obtained in Table 6 and comparing with the predicted values in Table 2, percentage errors less than 10% were obtained for both the yield and the DE under the conditions established as optimal and using citric acid, indicating that the process is reproducible and can be considered validated [37]. The results show that citric acid allows higher yields of LMP, while HMP (DE > 50%) were obtained using both organic acid (pectin extracted with oxalic acid, POA) as mineral acids (pectin extracted with nitric acid, PNA, and pectin extracted with hydrochloric acid, PHA), demonstrating that the type of acid is determinant on the yield and DE of pectins (Table 6).
No significant difference was observed (p < 0.05) between the pectin yields obtained with the other acids evaluated, yields varying between 10.20 and 11.93%, while in relation to DE, significant differences were observed, with PHA exhibiting the highest DE (84%), followed by PNA (82.94%), POA (75.82%), and PCA (46.07%). These results demonstrate that LMP can be obtained in high yields using the optimized UAE method proposed in our study and that by varying the type of acid HMP can also be obtained under the same conditions with a yield reduction of around 50% (Fig. 4). The extraction of LMP can be considered an interesting approach, as this pectin type has been pointed as suitable additive in the food industry. In this context, recent study described that LMP increased the water holding capacity (WHC) and viscoelasticity of gluten protein, as well as promoted the conversion of sulfhydryl groups (-SH) to disulfide bonds (-S-S-), inducing gluten aggregation and strengthened gluten network [78]. The addition of LMP led to improvement in technological and sensorial characteristics of low-fat set yoghurt, being able to reduce whey loss, improve firmness, rheology, quality, and overall liking of this dairy product [86].
The different effects of the type of acid, mineral and organic acids, on the pectin yield and DE were previously described for various raw materials, including jackfruit peel, pomelo fruit peel, and grape pomace [30, 87, 88]. The type of acid and pH of the acid used in the extraction process play a critical role in achieving higher pectin yields [89]. In this study, at pH 1.5, important differences were observed in the yield of pectins obtained from umbu peel using UAE. Using citric acid, the weakest acid of those evaluated, a higher yield, and lower DE and GalA contents were obtained, while the other acids led to yields between 10 and 11%, approximately, but with high DE and high GalA content. Probably, the stronger acids associated with the effects of ultrasound at 60% amplitude at pH 1.5 contributed to the hydrolysis of pectin and the consequent reduction in yield. These findings diverge from what is generally expected in relation to acid strength and pectin yield, where many authors report that the greater the acid strength, the greater the pectin yield. However, at extreme acid strength, such as pH 1, severe acid hydrolysis and degradation of pectin is observed [89].
Regarding the galacturonic acid content, it was observed that pectins with a high GalA content, around 70.5%, can be extracted using oxalic acid and hydrochloric acid, without a statistically significant difference (p < 0.05). However, this content can be considered statistically different but close to the GalA content of commercial citrus pectin, which has a content close to 76%. Pectins obtained with nitric acid and citric acid have GalA contents close to 62 and 56%, respectively. These levels are statistically different from each other, from the commercial one, and from the other extracted pectins. The extraction of pectin with high GalA content (74.4–77.9%) from lemon peels through conventional method and using oxalic acid was recently reported [90]. The GalA content in pectins extracted by conventional hot method and UAE may vary depending on the acid type used, according to study performed with onion (Allium cepa L.) waste as raw material [91]. Similar trend to that found for umbu pectins was observed for citric acid and hydrochloric acid, in which in the study with onion waste lower GalA contents were found for pectins extracted with citric acid using the conventional method (37.82%) and UAE (27.03%), while pectins extracted with these methods and using hydrochloric acid showed higher levels, around 55.12% and 50.79%, respectively. In contrast, the effects of organic acids on GalA content of pectin from different pomelo varieties showed that pectin extracted using citric acid contained higher GalA contents (76.5–85.1%) than those extracted by lactic acid (60.4–63.8%) and acetic acid (65.1–68.2%) [92]. According to these authors, the observed variations can be attributed to fact that probably pectin extracted by acetic acid or lactic acid contained high amounts of neutral sugars such as fucose, rhamnose, arabinose, galactose, glucose, xylose, and mannose, resulting in low galacturonic contents.
Color is an important quality parameter for pectins as it affects the appearance of the gel produced and the characteristics of the product to which it will be added, influencing product acceptance [64, 93, 94]. The color of pectin can be affected by the type of extraction process, raw material used as substrate, presence of associated pigments, and the pretreatment and purification steps [64]. For the color analysis of pectins, significant differences were observed for all coordinates evaluated. According to the data in Table 6, significant differences (p < 0.05) were verified for the three parameters (L*, a*, and b*) of the pectins obtained with different acids, indicating that the type of acid used for extraction affects the final color of the extracted pectin. Commercial citrus pectin (CCP) had the highest lightness value (91.34), while the lowest lightness values were observed to pectins obtained with oxalic acid (72.31), hydrochloric acid (71.91), acid nitric acid (69.21), and citric acid (64.43).
Regarding the a* coordinate, positive values indicative of redness palettes was observed for all pectins, with the highest values being found for PNA (8.16%) and PCA (7.42), followed by POA (7.14%), PHA (6.41%), and CCP (0.97). In relation to coordinate b*, the highest value was observed for POA (12.86), followed by the pectins PHA (12.07), PNA (11.23), CCP (10.49), and PCA (7.6), where higher positive values indicates the yellowish color of the pectins. Therefore, establishing commercial pectin as a reference regarding the color quality parameter and that it is desirable that pectins for industrial use are not brownish or darkened, pectins extracted with different acids can be grouped in the following order of color quality: CCP (reference) > POA > PHA > PNA > PCA. Although the acid pectin obtained with citric acid is listed as the most brownish pectin among those obtained with different acids, its L*, a*, and b* values result in a color considered more interesting than that described for pectins extracted by UAE and MAE a from tomato processing waste where L*, a*, and b* values of 54.92, 13.49, and 24.22, and 50.96, 11.07, and 24.9 were observed, respectively, indicating that they are darker brownish pectin with a reddish hue [64]. This reddish color is strictly related to the raw material used for extraction, tomato processing waste, which presents high levels of lycopene that were not properly removed in the pretreatment stages and that remained until the pectin recovery stages, remaining associated with them and affecting its final coloring.
4 Conclusion
In this study, ultrasound-assisted extraction of pectin from umbu peels using organic citric acid was investigated under different processing parameters, and central composite design associated with response surface methodology was used for extraction optimization. Two second-order polynomial models were developed using multiple regression analysis for prediction the pectin yield and degree of esterification. Preliminarily, the effect of sonication time on the pectin yield was evaluated, demonstrating that the highest yield, around 13.7%, was achieved after 10 min, and no statistically significant difference was observed (p > 0.05) when compared with the yield obtained after 18 min. After optimization study, the highest yield, approximately 22%, of low esterification pectin (DE = 46%) was achieved under ideal conditions, established under ultrasound amplitude of 60%, SLR of 1:33, and pH of 1.5. Process validation was carried out under established optimal conditions, and the effects of different acids on the yield of pectins and DE were evaluated, demonstrating that the use of citric acid allows yields of around 22% of LMP pectin to be achieved, while using oxalic acid, nitric acid, and hydrochloric acid led to the production of around 13% of HMP pectin. Furthermore, LMP pectins have a lower galacturonic acid content (56%) when compared to HMP pectins extracted with other acids, where the content varied from 61.9 to 70.8%. In terms of quality, assessed through instrumental color, pectins extracted with different acids under optimized UAE conditions showed significant differences when compared to commercial citrus pectin CCP, which demonstrated the influence of the type of raw material and extraction method on the quality of the product obtained. Therefore, our study describes for the first time the extraction of pectins from umbu peels using ultrasound technology, proving that this material is a promising source of LMP and HMP pectins, which can be obtained in yields of 13 to 22% depending on the type of acid using. Thus, this study demonstrates the potential of high-intensity ultrasound to obtain compounds of industrial interest from native Brazilian fruits, particularly from the Caatinga biome, thus contributing to the valorization of biodiversity, preservation of the environment, and innovation in the food sector.
Moreover, the development of new pectin extraction processes faces important challenges such as the feasibility of the technology used, the yield and quality of the pectin obtained, and the associated general cost. Therefore, it is believed that studies that determine the costs related to extraction as well as scale-up approaches will become more frequent. Another perspective is that unconventional substrates from production chains are among the most popular for new industrial pectin extraction processes. New studies are necessary in order to evaluate the impacts of different emerging technologies, such as ultrasound, microwaves, high pressure, ohmic heating, pulsed electric field, among others, on the technological and functional properties of pectins. Finally, simultaneous or dual-objective optimizations tend to consolidate to obtain not only high pectin yields but also to modulate the type of biopolymer obtained.
References
Castro-Muñoz R, Boczkaj G, Gontarek E, Cassano A, Fíla V (2020) Membrane technologies assisting plant-based and agro-food by-products processing: a comprehensive review. Trends Food Sci Technol 95:219–232. https://doi.org/10.1016/j.tifs.2019.12.003
Castro-Muñoz R, Ahmad MZ, Cassano A (2023) Pervaporation-aided processes for the selective separation of aromas, fragrances and essential (AFE) solutes from agro-food products and wastes. Food Rev Int 39(3):1499–1525. https://doi.org/10.1080/87559129.2021.1934008
Ferreyra-Suarez D, Paredes-Vargas L, Jafari SM, García-Depraect O, Castro-Muñoz R (2024) Extraction pathways and purification strategies towards carminic acid as natural-based food colorant: a comprehensive review. Adv Colloid Interface Sci 323:103052. https://doi.org/10.1016/j.cis.2023.103052
Valencia-Arredondo JA, Hernández-Bolio GI, Cerón-Montes GI, Castro-Muñoz R, Yáñez-Fernández J (2020) Enhanced process integration for the extraction, concentration and purification of di-acylated cyanidin from red cabbage. Sep Purif Technol 238:116492. https://doi.org/10.1016/j.seppur.2019.116492
Siddiqui SA, Azmy Harahap I, Suthar P, Wu YS, Ghosh N, Castro-Muñoz R (2023) A comprehensive review of phytonutrients as a dietary therapy for obesity. Foods 12(19):3610. https://doi.org/10.3390/foods12193610
Castro-Muñoz R, Díaz-Montes E, Cassano A, Gontarek E (2020) Membrane separation processes for the extraction and purification of steviol glycosides: an overview. Crit Rev Food Sci Nutr 61(13):2152–2174. https://doi.org/10.1080/10408398.2020.1772717
Hernández-Pinto FJ, Miranda-Medina JD, Natera-Maldonado A, Vara-Aldama Ó, Ortueta-Cabranes MP, Vázquez del Mercado-Pardiño JA, El-Aidie SAM, Siddiqui SA, Castro-Muñoz R (2024) Arabinoxylans: a review on protocols for their recovery, functionalities and roles in food formulations. Int J Biol Macromol 259: 129309. https://doi.org/10.1016/j.ijbiomac.2024.129309
Scarano P, Sciarrillo R, Tartaglia M, Zuzolo D, Guarino C (2022) Circular economy and secondary raw materials from fruits as sustainable source for recovery and reuse. A review. Trends Food Sci Technol 122:157–170. https://doi.org/10.1016/j.tifs.2022.02.003
Castro-Muñoz R, Díaz‐Montes E, Gontarek‐Castro E, Boczkaj G, Galanakis CM (2021) A comprehensive review on current and emerging technologies toward the valorization of bio‐based wastes and by products from foods. Compr Rev Food Sci Food Saf 21(1):46–105. https://doi.org/10.1111/1541-4337.12894
Ferreira SF, Buller LS, Maciel-Silva FW, Sganzerla WG, Berni MD, Forster‐Carneiro T (2020) Waste management and bioenergy recovery from açaí processing in the Brazilian amazonian region: a perspective for a circular economy. Biofuel Bioprod Biorefin 15(1):37–46. https://doi.org/10.1002/bbb.2147
Silva JMC, Leal IR, Tabarelli ME (2017) Caatinga. The largest tropical dry forest region in South America. https://doi.org/10.1007/978-3-319-68339-3
Luna EM, Lopes HTO, Rodrigues FAÁ, Coutinho HDM, Oliveira LCC (2022) Antioxidant potential of the Caatinga flora. Phytomed Plus 2(2):100240. https://doi.org/10.1016/j.phyplu.2022.100240
Araujo HFP, Garda AA, Girão e Silva WA, Nascimento NFF, Mariano EF, Silva JMC (2022) The Caatinga region is a system and not an aggregate. J Arid Environ 203:104778. https://doi.org/10.1016/j.jaridenv.2022.104778
Vieira ÉA, Coêlho JGS, Grisi CVB, Santos BS, Silva Júnior JC, Alcântara MA, Meireles BRLA, Santos NA, Cordeiro AMTM (2022) Correlation and influence of antioxidant compounds of peels and pulps of different species of cacti from Brazilian Caatinga biome using principal component analysis. S Afr J Bot 147:434–442. https://doi.org/10.1016/j.sajb.2022.02.005
Gouvêa RF, Ribeiro LO, Souza ÉF, Penha EM, Matta VM, Freitas SP (2017) Effect of enzymatic treatment on the rheological behavior and vitamin C content of Spondias tuberosa (umbu) pulp. J Food Sci Technol 54(7):2176–2180. https://doi.org/10.1007/s13197-017-2630-8
De Oliveira APD, De Oliveira Almeida TJ, Santos TMB, Dias FS (2021) Symbiotic goat milk ice cream with umbu fortified with autochthonous goat cheese lactic acid bacteria. LWT 141:110888. https://doi.org/10.1016/j.lwt.2021.110888
De Oliveira RL, Dias JL, Da Silva OS, Porto TS (2018) Immobilization of pectinase from aspergillus aculeatus in alginate beads and clarification of apple and umbu juices in a packed bed reactor. Food Bioprod Process 109:918. https://doi.org/10.1016/j.fbp.2018.02.005
Vidigal MCTR, Minim VPR, Carvalho NB, Milagres MP, Gonçalves ACA (2011) Effect of a health claim on consumer acceptance of exotic Brazilian fruit juices: Açaí (Euterpe oleracea Mart.), Camu-Camu (Myrciaria dubia), Cajá (Spondias lutea L.) and Umbu (Spondias tuberosa Arruda). Food Res Int 44(7):19881996. https://doi.org/10.1016/j.foodres.2010.11.028
De Lima MAC, Silva SM, De Oliveira VR (2018) Umbu Spondias tuberosa. Exotic Fruits 427433. https://doi.org/10.1016/b978-0-12-803138-4.00057-5
Reichembach LH, Petkowicz CLO (2021) Pectins from alternative sources and uses beyond sweets and jellies: an overview. Food Hydrocoll 118:106824. https://doi.org/10.1016/j.foodhyd.2021.106824
Blanco-Pérez F, Steigerwald H, Schülke S, Vieths S, Toda M, Scheurer S (2021) The dietary fiber pectin: health benefits and potential for the treatment of allergies by modulation of gut microbiota. Curr Allergy Asthma Rep 21(10). https://doi.org/10.1007/s11882-021-01020-z
Sharma P, Osama K, Gaur VK, Farooqui A, Varjani S, Younis K (2023) Sustainable utilization of Citrus limetta peel for obtaining pectin and its application in cookies as a fat replacer. J Food Sci Technol 60(3):975–986. https://doi.org/10.1007/s13197-022-05424-1
Roman-Benn A, Contador CA, Li M-W, Lam H-M, Ah-Hen K, Ulloa PE, Ravanal MC (2023) Pectin: an overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem Adv 2:100192. https://doi.org/10.1016/j.focha.2023.100192
Marić M, Grassino AN, Zhu Z, Barba FJ, Brnčić M, Rimac Brnčić S (2018) An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci Technol 76:28–37. https://doi.org/10.1016/j.tifs.2018.03.022
Ciriminna R, Fidalgo A, Scurria A, Ilharco LM, Pagliaro M (2022) Pectin: new science and forthcoming applications of the most valued hydrocolloid. Food Hydrocoll 127:107483. https://doi.org/10.1016/j.foodhyd.2022.107483
Adimas MA, Abera BD (2023) Valorization of fruit and vegetable by-products for extraction of pectin and its hydrocolloidal role in low-fat yoghurt processing. LWT 189:115534. https://doi.org/10.1016/j.lwt.2023.115534
Gavahian M, Mathad GN, Pandiselvam R, Lin J, Sun D-W (2021) Emerging technologies to obtain pectin from food processing by-products: a strategy for enhancing resource efficiency. Trends Food Sci Technol 115:42–54. https://doi.org/10.1016/j.tifs.2021.06.018
Kumar K, Srivastav S, Sharanagat VS (2021) Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem 70:105325. https://doi.org/10.1016/j.ultsonch.2020.105325
Del Hierro JN, Herrera T, García-Risco MR, Fornari T, Reglero G, Martin D (2018) Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: quinoa, lentil, fenugreek, soybean and lupin. Food Res Int 109:440–447. https://doi.org/10.1016/j.foodres.2018.04.058
Wani KM, Uppaluri RVS (2022) Continuous and pulsed ultrasound-assisted extraction of pectin from pomelo fruit peel using citric acid. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-03513-x
Singhal S, Deka SC, Koidis A, Hulle NRS (2024) Standardization of extraction of pectin from Assam lemon (Citrus limon Burm f.) peels using novel technologies and quality characterization. Biomass Convers Biorefin:. https://doi.org/10.1007/s13399-024-05367-x
Wang W, Chen W, Zou M, Lv R, Wang D, Hou F, Feng H, Ma X, Zhong J, Ding T, Ye X, Liu D (2018) Applications of power ultrasound in oriented modification and degradation of pectin: a review. J Food Eng 234:98–107. https://doi.org/10.1016/j.jfoodeng.2018.04.016
Chen T-T, Zhang Z-H, Wang Z-W, Chen Z-L, Ma H, Yan J-K (2021) Effects of ultrasound modification at different frequency modes on physicochemical, structural, functional, and biological properties of citrus pectin. Food Hydrocoll 113:106484. https://doi.org/10.1016/j.foodhyd.2020.106484
Gerschenson LN, Fissore EN, Rojas AM, Idrovo Encalada AM, Zukowski EF, Higuera Coelho RA (2021) Pectins obtained by ultrasound from agroindustrial by-products. Food Hydrocoll 118:106799. https://doi.org/10.1016/j.foodhyd.2021.106799
Panwar D, Panesar PS, Chopra HK (2023) Ultrasound-assisted extraction of pectin from Citrus limetta peels: optimization, characterization, and its comparison with commercial pectin. Food Biosci 51:102231. https://doi.org/10.1016/j.fbio.2022.102231
Ke J, Jiang G, Shen G, Wu H, Liu Y, Zhang Z (2020) Optimization, characterization and rheological behavior study of pectin extracted from chayote (Sechium edule) using ultrasound assisted method. Int J Biol Macromol 147:688–698. https://doi.org/10.1016/j.ijbiomac.2020.01.055
Jong SH, Abdullah N, Muhammad N (2023) Optimization of low-methoxyl pectin extraction from durian rinds and its physicochemical characterization. Carbohydr Polym Technol Appl 5:100263. https://doi.org/10.1016/j.carpta.2022.100263
Kamal MM, Akhtaruzzaman Md, Sharmin T, Rahman M, Mondal SC (2023) Optimization of extraction parameters for pectin from guava pomace using response surface methodology. J Agric Food Res 11:100530. https://doi.org/10.1016/j.jafr.2023.100530
Food and Agriculture Organization of the United Nations (FAO) database -FAOSTAT (2024) https://www.fao.org/faostat/en/#data/QCL. Accessed in 20/03/2024
Pereira APA, Lauretti LBC, Alvarenga VO, Paulino BN, Angolini CFF, Neri-Numa IA, Orlando EA, Pallone JAL, Sant’Ana AS, Pastore GM (2020) Evaluation of fruta-do-lobo (Solanum lycocarpum St. Hill) starch on the growth of probiotic strains. Food Res Int 133:109187. https://doi.org/10.1016/j.foodres.2020.109187
Pereira APA, Angolini CFF, Adani HB, Usberti FCS, Paulino BN, Clerici MTPS, Neri-numa IA, Moro TM, de Eberlin MA, Pastore MN GM (2021) Impact of ripening on the health-promoting components from fruta-do-lobo (Solanum lycocarpum St. Hill). Food Res Int 139:109910. https://doi.org/10.1016/j.foodres.2020.109910
Araújo FF, de Paulo Farias D, Neri-Numa IA, Dias-Audibert FL, Delafiori J, de Souza FG, Catharino RR, do Sacramento CK, Pastore GM (2021) Chemical characterization of Eugenia Stipitata: a native fruit from the Amazon rich in nutrients and source of bioactive compounds. Food Res Int 139:109904. https://doi.org/10.1016/j.foodres.2020.109904
Souza FG, de Araújo FF, Orlando EA, Rodrigues FM, Chávez DWH, Pallone JAL, Neri-Numa IA, Sawaya ACHF, Pastore GM (2022) Characterization of Buritirana (Mauritiella armata) fruits from the Brazilian Cerrado: biometric and physicochemical attributes, Chemical Composition and antioxidant and antibacterial potential. Foods 11(6):786. https://doi.org/10.3390/foods11060786
Sviech F, Ubbink J, Prata AS (2022) Potential for the processing of Brazilian fruits - a review of approaches based on the state diagram. LWT 156:113013. https://doi.org/10.1016/j.lwt.2021.113013
Barbieri SF, da Costa Amaral S, Ruthes AC, de Oliveira Petkowicz CL, Kerkhoven NC, da Silva ERA, Silveira JLM (2019) Pectins from the pulp of gabiroba (Campomanesia Xanthocarpa Berg): structural characterization and rheological behavior. Carbohydr Polym 214:250–258. https://doi.org/10.1016/j.carbpol.2019.03.045
Colodel C, de Petkowicz CL O (2019) Acid extraction and physicochemical characterization of pectin from cubiu (Solanum sessiliflorum D.) fruit peel. Food Hydrocoll 86:193–200. https://doi.org/10.1016/j.foodhyd.2018.06.013
Santos MR, Mendes RML, Ribeiro E (2023) Effects of pulp and pectin concentrations of passion fruit from Caatinga (Passiflora cincinnata mast.) On the production of symbiotic beverages. Food Chem Adv 3:100450. https://doi.org/10.1016/j.focha.2023.100450
Zaid RM, Mishra P, Siti Noredyani AR, Tabassum S, Ab Wahid Z, Mimi Sakinah AM (2020) Proximate characteristics and statistical optimization of ultrasound-assisted extraction of high-methoxyl-pectin from Hylocereus polyrhizus peels. Food Bioprod Process 123:134–149. https://doi.org/10.1016/j.fbp.2020.06.011
Abu Bakar FI, Abu Bakar MF, Abdullah N, Endrini S, Fatmawati S (2020) Optimization of extraction conditions of phytochemical compounds and anti-gout activity of Euphorbia hirta L. (Ara Tanah) using response surface methodology and liquid chromatography-mass spectrometry (LC-MS) analysis. Evid Based Complement Alternat Med 2020: 1–13. https://doi.org/10.1155/2020/4501261
Gharibzahedi SMT, Smith B, Guo Y (2019) Pectin extraction from common fig skin by different methods: the physicochemical, rheological, functional, and structural evaluations. Int J Biol Macromol 136:275–283. https://doi.org/10.1016/j.ijbiomac.2019.06.040
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54(2):484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Guandalini BBV, Rodrigues NP, Marczak LDF (2019) Sequential extraction of phenolics and pectin from mango peel assisted by ultrasound. Food Res Int 119:455–461. https://doi.org/10.1016/j.foodres.2018.12.011
Cangussu LB, Fronza P, Franca AS, Oliveira LS (2021) Chemical characterization and bioaccessibility assessment of bioactive compounds from Umbu (Spondias tuberosa A.) fruit peel and pulp flours. Foods 10(11):2597. https://doi.org/10.3390/foods10112597
Alkandari D, Sarfraz H, Sidhu JS (2019) Amla fruit powder addition influences objective color and instrumental texture of pan bread. J Food Sci Technol 56(5):2750–2757. https://doi.org/10.1007/s13197-019-03766-x
Yue Y, Wang B, Xi W, Liu X, Tang S, Tan X, Li G, Huang L, Liu Y, Bai J (2023) Modification methods, biological activities and applications of pectin: a review. Int J Biol Macromol 253:127523. https://doi.org/10.1016/j.ijbiomac.2023.127523
Thirunavookarasu N, Kumar S, Shetty P, Shanmugam A, Rawson A (2024) Impact of ultrasound treatment on the structural modifications and functionality of carbohydrates – a review. Carbohydr Res 535:109017. https://doi.org/10.1016/j.carres.2023.109017
Garcia-Garcia G, Rahimifard S, Matharu AS, Dugmore TIJ (2019) Life-cycle assessment of microwave-assisted pectin extraction at pilot scale. ACS Sustainable Chem Eng 7(5):5167–5175. https://doi.org/10.1021/acssuschemeng.8b06052
Adetunji LR, Adekunle A, Orsat V, Raghavan V (2017) Advances in the pectin production process using novel extraction techniques: a review. Food Hydrocoll 62:239–250. https://doi.org/10.1016/j.foodhyd.2016.08.015
Dias IP, Barbieri SF, Fetzer DEL, Corazza ML, Silveira JLM (2020) Effects of pressurized hot water extraction on the yield and chemical characterization of pectins from Campomanesia Xanthocarpa Berg fruits. Int J Biol Macromol 146:431–443. https://doi.org/10.1016/j.ijbiomac.2019.12.261
Mao Y, Robinson JP, Binner ER (2023) Current status of microwave-assisted extraction of pectin. Chem Eng J 473:145261. https://doi.org/10.1016/j.cej.2023.145261
Zhang L, Ye X, Ding T, Sun X, Xu Y, Liu D (2013) Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason Sonochem 20(1):222–231. https://doi.org/10.1016/j.ultsonch.2012.07.021
Qiu W-Y, Cai W-D, Wang M, Yan J-K (2019) Effect of ultrasonic intensity on the conformational changes in citrus pectin under ultrasonic processing. Food Chem 297:125021. https://doi.org/10.1016/j.foodchem.2019.125021
Patience NA, Schieppati D, Boffito DC (2021) Continuous and pulsed ultrasound pectin extraction from navel orange peels. Ultrason Sonochem 73:105480. https://doi.org/10.1016/j.ultsonch.2021.105480
Sengar AS, Rawson A, Muthiah M, Kalakandan SK (2020) Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason Sonochem 61:104812. https://doi.org/10.1016/j.ultsonch.2019.104812
Shivamathi CS, Moorthy IG, Kumar RV, Soosai MR, Maran JP, Kumar RS, Varalakshmi P (2019) Optimization of ultrasound assisted extraction of pectin from custard apple peel: potential and new source. Carbohydr Polym 225:115240. https://doi.org/10.1016/j.carbpol.2019.115240
Xu Y, Zhang L, Bailina Y, Ge Z, Ding T, Ye X, Liu D (2014) Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J Food Eng 126:72–81. https://doi.org/10.1016/j.jfoodeng.2013.11.004
Zahari NAAR, Chong GH, Abdullah LC, Chua BL (2020) Ultrasonic-assisted extraction (UAE) process on thymol concentration from Plectranthus Amboinicus leaves: Kinetic modeling and optimization. Processes 8(3):322. https://doi.org/10.3390/pr8030322
Duwee YS, Kiew PL, Yeoh WM (2022) Multi-objective optimization of pectin extraction from orange peel via response surface methodology: yield and degree of esterification. J Food Meas Charact 16:1710–1724. https://doi.org/10.1007/s11694-022-01305-5
Chaharbaghi E, Khodaiyan F, Hosseini SS (2017) Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr Polym 173:107–113. https://doi.org/10.1016/j.carbpol.2017.05.047
Chemat F, Rombaut N, Sicaire A-G, Meullemiestre A, Fabiano-Tixier A-S, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. Rev Ultrason Sonochemistry 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Suhaimi SH, Hasham R, Hafiz Idris MK, Ismail HF, Mohd Ariffin NH, Abdul Majid FA (2019) Optimization of ultrasound-assisted extraction conditions followed by solid phase extraction fractionation from Orthosiphon Stamineus Benth (Lamiace) leaves for Antiproliferative Effect on prostate Cancer cells. Molecules 24(22):4183. https://doi.org/10.3390/molecules24224183
Sun Z, Wang S, Zhou C, Ma Z, Qian F (2023) Optimization of ultrasound assisted organic acid extraction of pectin from pomelo peel by response surface methodology and its preliminary characterization. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-023-04200-1
Vakilian K, Nateghi L, Javadi A, Anarjan N (2023) Optimization of conventional and ultrasound-assisted extraction of pectin from unripe grape pomace: extraction yield, degree of esterification, and galacturonic acid content. J Food Meas Charact 17(6):5777–5793. https://doi.org/10.1007/s11694-023-02085-2
Cui J, Zhao C, Feng L, Han Y, Du H, Xiao H, Zheng J (2021) Pectins from fruits: relationships between extraction methods, structural characteristics, and functional properties. Trends Food Sci Technol 110:39–54. https://doi.org/10.1016/j.tifs.2021.01.077
Colodel C, Vriesmann LC, Teófilo RF, de Petkowicz O, C. L (2020) Optimization of acid-extraction of pectic fraction from grape (Vitis vinifera Cv. Chardonnay) pomace, a Winery Waste. Int J Biol Macromol 161:204–213. https://doi.org/10.1016/j.ijbiomac.2020.05.272
Iñiguez-Moreno M, Pizaña-Aranda JJP, Ramírez-Gamboa D, Ramírez-Herrera CA, Araújo RG, Flores-Contreras EA, Iqbal HMN, Parra-Saldívar R, Melchor-Martínez EM (2024) Enhancing pectin extraction from orange peel through citric acid-assisted optimization based on a dual response. Int J Biol Macromol 263:130230. https://doi.org/10.1016/j.ijbiomac.2024.130230
Larsen N, Bussolo de Souza C, Krych L, Barbosa Cahú T, Wiese M, Kot W, Hansen KM, Blennow A, Venema K, Jespersen L (2019) Potential of pectins to beneficially modulate the gut microbiota depends on their structural properties. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.00223
Cui Y, Chen J, Zhang S (2023) The effect of degree of esterification of pectin on the interaction between pectin and wheat gluten protein. Food Hydrocoll 136:108272. https://doi.org/10.1016/j.foodhyd.2022.108272
Fraeye I, Deroeck A, Duvetter T, Verlent I, Hendrickx M, Vanloey A (2007) Influence of pectin properties and processing conditions on thermal pectin degradation. Food Chem 105(2):555–563. https://doi.org/10.1016/j.foodchem.2007.04.009
Tran NTK, Nguyen VB, Tran TV, Nguyen TTT (2023) Microwave-assisted extraction of pectin from jackfruit rags: optimization, physicochemical properties and antibacterial activities. Food Chem 418:135807. https://doi.org/10.1016/j.foodchem.2023.135807
Hosseini SS, Khodaiyan F, Kazemi M, Najari Z (2019) Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int J Biol Macromol 125:621–629. https://doi.org/10.1016/j.ijbiomac.2018.12.096
Caroço RF, Kim B, Santacoloma PA, Abildskov J, Lee JH, Huusom JK (2019) Analysis and model-based optimization of a pectin extraction process. J Food Eng 244:159–169. https://doi.org/10.1016/j.jfoodeng.2018.09.016
Singhal S, Hulle NRS (2022) Citrus pectins: structural properties, extraction methods, modifications and applications in food systems – a review. Appl Food Res 2(2):100215. https://doi.org/10.1016/j.afres.2022.100215
Roy S, Priyadarshi R, Łopusiewicz Ł, Biswas D, Chandel V, Rhim JW (2023) Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: a review. Int J Biol Macromol 239:124248. https://doi.org/10.1016/j.ijbiomac.2023.124248
Nguyen BMN, Pirak T (2019) Physicochemical properties and antioxidant activities of white dragon fruit peel pectin extracted with conventional and ultrasound-assisted extraction. Cogent Food Agric 5(1):1633076. https://doi.org/10.1080/23311932.2019.1633076
Khubber S, Chaturvedi K, Thakur N, Sharma N, Yadav SK (2021) Low-methoxyl pectin stabilizes low-fat set yoghurt and improves their physicochemical properties, rheology, microstructure and sensory liking. Food Hydrocoll 111:106240. https://doi.org/10.1016/j.foodhyd.2020.106240
Saurabh V, Vathsala V, Yadav SK, Sharma N, Varghese E, Saini V, Singh SP, Dutta A, Kaur C (2023) Extraction and characterization of ultrasound assisted extraction: improved functional quality of pectin from jackfruit (Artocarpus heterophyllus Lam.) Peel waste. J Food Meas Charact 17(6):6503–6521. https://doi.org/10.1007/s11694-023-02126-w
Megías-Pérez R, Ferreira-Lazarte A, Villamiel M (2023) Valorization of grape pomace as a renewable source of techno-functional and antioxidant pectins. Antioxidants 12(4):957. https://doi.org/10.3390/antiox12040957
Nadar CG, Arora A, Shastri Y (2022) Sustainability challenges and opportunities in pectin extraction from fruit waste. ACS Eng Au 2(2):61–74. https://doi.org/10.1021/acsengineeringau.1c00025
Huang Y, Qi J, Liao J, Jiang W, Cao Y, Xiao J, Yang X (2021) Oxalic extraction of high methoxyl pectin and its application as a stabiliser. Int J Food Sci Technol 56(10):5220–5229. https://doi.org/10.1111/ijfs.15135
Şen E, Göktürk E, Hajiyev V, Uğuzdoğan E (2023) Comparisons of pulsed ultrasound-assisted and hot‐acid extraction methods for pectin extraction under dual acid mixtures from onion (Allium cepa L.) waste. Food Sci Nutr 11(11):7320–7329. https://doi.org/10.1002/fsn3.3657
Van Hung P, Anh MNT, Hoa PN, Phi NTL (2020) Extraction and characterization of high methoxyl pectin from Citrus maxima peels using different organic acids. J Food Meas Charact 15(2):1541–1546. https://doi.org/10.1007/s11694-020-00748-y
Kute AB, Mohapatra D, Kotwaliwale N, Giri SK, Sawant BP (2019) Characterization of pectin extracted from orange peel powder using microwave-assisted and acid extraction methods. Agric Res 9(2):241–248. https://doi.org/10.1007/s40003-019-00419-5
Forouhar A, Hamdami N, Djelveh G, Gardarin C, Pierre G, Ursu AV, Michaud P (2023) The effect of ultrasound pretreatment on pectin extraction from watermelon rind using microwave-assisted extraction. Appl Sci (Basel) 13(9):5558. https://doi.org/10.3390/app13095558
Funding
This work was supported by the National Council for Scientific and Technological Development - CNPq (Grant Number 408049/2023-5), Coordination for the Improvement of Higher Education Personnel (CAPES) (Finance Code 001 and Grant Number 88887.690875/2022-00), “Programa de Desenvolvimento da Pós-Graduação (PDPG) – Emergencial de Consolidação Estratégica dos Programas de Pós-Graduação stricto sensu acadêmicos” (Grant Number: 88881.708195/2022-01), and PRPPG and PROEXT Federal University of Bahia (PIBIC 2022–2023, Project number 23533; PIBIC 2023–2024, Project number 26135; SIATEX Project number 20557).
Author information
Authors and Affiliations
Contributions
Joseane Cardoso Gomes de Alencar: investigation, experimental performance, formal analysis, data analysis, manuscript preparation; Denise Nathiele Santos Souza Batista, Jacqueline Carvalho de Souza, Isabelle Palma Patricio Santos: experimental performance, formal analysis; Juliano Lemos Bicas: investigation, data analysis, manuscript preparation; Maria Eugênia de Oliveira Mamede: data analysis, Bruno Nicolau Paulino: investigation, data analysis, supervision.
Corresponding author
Ethics declarations
Ethical approval
The authors claim that none of the material in the paper has been published or is under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Alencar, J.C.G., Batista, D.N.S.S., de Souza, J.C. et al. Dual-objective optimization of ultrasound-assisted organic acid extraction of pectin from umbu (Spondias tuberosa L.): a promising Brazilian native fruit from Caatinga biome. Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05720-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05720-0