Abstract
The approach of converting fruit by-products to produce value-added products contributes to sustainable development and environmental protection. Ultrasound assisted extraction technique was applied to extract pectin from waste pomelo peel (PP) using eco-friendly organic acid (citric acid). A Box-Behnken design (BBD) of response surface methodology (RSM) was employed to optimize the extraction conditions and investigate the individual and interactive effect of process variables (citric acid concentration, ultrasound temperature and ultrasound power) on the pectin yield. A second-order polynomial model was developed using multiple regression analysis and it showed higher adequate in predicting the pectin yield. The results showed that the maximum pectin yield of 21.68% was obtained at optimal conditions of citric acid concentration 1.66%, ultrasound temperature 75 °C and ultrasound power 191 W. The optimized pectin was classified as high methoxyl pectin due to its high esterification degree 67.26 ± 0.32%. The galacturonic acid content and ash content of the pectin were 82.89 ± 1.35% and 1.47 ± 0.21%, respectively, indicating its higher degree of purity. In addition, the pectin presented higher emulsion properties, emulsion activity of 54.3% and the values of emulsion stability of 76.9% to 85.7%. The PP pectin presented a smooth, but wrinkled and loosen surface. The pectin degradation was found in the temperature range from about 220 to 280 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pomelo (citrus maxima), as a member of citrus family, is widely cultivated in southern east Asia [1, 2]. The world’s main pomelo growing country include China, United States, Cuba, South Africa, Brazil, Argentina and Japan countries. Only in China, the annual output of pomelo fruit was 5.227 million tons in 2020 and it shows an increasing trend year by year. In the future, the consumption of pomelo products will no longer be limited to the consumption of fresh fruit, and the deep processing will be intensified. All kinds of high value-added products will be favored by the market, and the products will be diversified. In addition to the edible flesh, the pomelo peel also contains great value for development and utilization. The spongy mesocarp and exocarp covered with oil cells account for approximately 40% of the single fruit weight, which has become an important research object for scientists [3, 4]. Pomelo peel contains a lot of effective ingredients such as pectin, flavonoids, dietary fiber and essential oil. If these ingredients are fully utilized, the utilization rate of pomelo peel will be further improved.
Pectin, as a complex mixture of heterogenous polysaccharide, is localized in the middle lamella and primary cell of higher plants [5, 6]. Pectin is basically composed of α-(1–4)-D-galacturonic acid monomers which can be methyl esterified or acetylated [7, 8]. Pectin is widely used in the food and drug industries because it has good stability, viscosity and gelatinicity, and also has some physiological functions such as lowering blood sugar, lowering cholesterol, preventing cancer occurrence and metastasis [7]. It is reported that world’s annual demand for pectin is about 40,000 tons and experts predict that the demand will continue to grow at an annual rate of 5%-10%. At present, citrus peels (85.5%), apple pomace (14.0%) and sugar beet pulp (0.5%) are the main raw materials used in the production of commercial pectin [9]. In addition, there have been extensive studies on the extraction of pectin from other byproducts such as walnut processing wastes [10], sunflower head [11], potato pulp [12], tomato waste [13], hawthorn wine pomace [14], carrot pomace [15], and cocoa husks [16], etc. But these raw materials are limited to basic laboratory studies. However, it is noted that the extracted pectin yield and its physico-chemical properties varies greatly depended on the raw materials and extraction techniques. As for the pectin extraction from pomelo peel, Chen et al. [2] used a hot-solvent microwave extraction for pectin using conventional inorganic acid (HCl). Wandee et al. [6] combined conventional inorganic acid (HCl) solvent and microwave method to extract pectin. However, on the one hand, the mentioned strong mineral acids (hydrochloric acid) tend to corrode equipment and/or pollute water, on the other one hand, they are against the principles of “green” chemistry and “green” technology due to the production of toxic compounds. Recently, organic acids (such as tartaric, malic, and citric acid) have been used for pectin extraction due to their eco-friendly nature [5, 17]. Besides, compared with inorganic acids, organic acids possess lower hydrolyzing abilities ascribed to their lower dissociation constant, which could reduce the depolymerization ability. Therefore, pectin extracted using organic acid always had higher molecular weight, viscosity and degree of esterification as compared to inorganic acids [17]. Liew et al. [3] carried out the research on the extraction of pectin from pomelo peel using citric acid. Conventionally, extraction of pectin by acidic method requires long extraction time, high energy input. Therefore, other non-conventional techniques involving microwave, ultrasound, subcritical water, and enzyme have been applied to recovery pectin from fruit by-products. Among these methods, ultrasound-assisted extraction using cavitation effect has been reported to present some advantages, such as lower energy input, shorter extraction time, less solvent usage, and higher yield [7, 18, 19], and so on. During the ultrasound-assisted extraction process, some processing parameters such as temperature, pH, intensity of ultrasound and time could affect the pectin yields. Furthermore, in order to study the effects of extraction parameters on pectin yield and optimize the parameters, the interaction effects of the process factors should not be neglected. Response surface methodology (RSM) applying Box-Behnken design (BBD) is a powerful tool for designing, statistical modeling, and optimizing the extraction factors, and also to determine the association between one or more process parameters. So, in order to achieve the maximum pectin yield, the optimal processing conditions should be obtained. Response surface methodology (RSM) has been applied extensively to optimize the extraction conditions [11, 20,21,22].
Therefore, the objective of this study is to investigate and optimize the processing parameters on the maximum pectin yield by ultrasound assisted method using organic acid employing Box-Behnken experimental design, as well as to further study the physico-chemical characteristics of the pectin extracted from pomelo peel (PP).
2 Materials and methods
2.1 Sample preparation and reagents
Fresh commercial pomelos (a pomelo is about 1300 g) used in this work were purchased from a local fruit market. The pomelo peels, approximately 8.85% of its fruit weight, were removed from pulp and chopped into small pieces with a sharp knife. Afterwards, the peels with 62.6% moisture content were soaked in 95% ethanol solvent for half an hour. Then, the peels were washed two times with distilled water and dried (50 °C) in an oven with air circulation to a constant weight. Subsequently, the dried peels were powdered and sieved (40-mesh size). The powdered sample was packed in sealed plastic bags and stored in a dry environment for further use, and its proximate analysis is as follows: 3.5% moisture content, 8.1% protein content, 3.0% ash content.
All reagents used in this study were bought from Macklin biochemical Co.,Ltd (Shanghai, China) and were of analytical grade.
2.2 Ultrasound-assisted pectin extraction
The pretreated pomelo peels powder (2.0 g) was poured into a 250 mL glass beaker and blended in a certain concentration of citric acid solution, ranging from 1 to 2%, making the liquid–solid ratio (20:1 mL/g). After stirring the mixture completely, sonication was made with different ultrasonic power (120W, 160W, and 200W) at different temperatures (55, 65, 75 °C) for 30 min. After extraction, the mixture was filtered using four sheets of gauze and then, the filtrate was centrifuged at 4000 rpm for 10 min to remove the solid particles. Then, the supernatant was cooled to room temperature and precipitated with cold ethanol (95%) in a ratio (1:2 v/v). In order to allow the pectin product to better precipitate completely, the prepared mixture was left at 4 °C for 24 h. Then, the cool mixture was centrifuged at 4000 rpm for 10 min. Afterwards, the coagulated pectin was collected, washed with ethanol (95%) for two times using centrifuge. Finally, the wet pectin was dried (50 ℃) in a hot air oven (approximately 12 h) to a constant weight. The pectin yield of extraction was calculated using the following formula (Eq. 1):
- m0:
-
the weight of dried pectin (g)
- m:
-
the weight of dried pomelo peels powder (g)
2.3 Experimental design and statistical analysis
The extraction parameters were optimized by applying RSM. A Box-Behnken design (BBD) was used to optimize and investigate the effect of independent variables on the pectin extraction yield, selected as a response. Based on our previous studies, three major processing parameters, i.e., citric acid concentration (A), ultrasound temperature (B), and ultrasound power (C) were selected in this study. Each variable has three levels presented in Table 1. All the calculations and graphical analysis were conducted using the software Design-Expert 8.0 and origin 8.0.
2.4 Physicochemical characterization
2.4.1 Galacturonic acid content
Galacturonic acid, as the main component of pectin, can reacts with carbazole reagent in sulfuric acid solution and generate purple red complex, which has maximum absorption at wavelength 525 nm. Based on the above principles, the galacturonic acid content of pectin was analyzed by carbazole colorimetry method applying a UV-2501PC UV–Vis spectrophotometer (Shimadzu Corporation, Tokyo, Japan). The content of galacturonic acid was measured by means of the method developed by Florina Dranca and Mircea Oroian with some modifications [23]. In brief, 1 mL of the sample solution (20 μg/mL) was taken into a glass tube, then 6 mL 98% H2SO4 was added under cold water bath conditions. The tube was placed in 85 °C water bath for 25 min in order to make the pectin hydrolysis adequately. After that, the tube was cooled to room temperature and 0.5 mL 0.15% carbazole anhydrous ethanol solution was added. The mixture was shaken for 5 min and was placed in the dark. After about 30 min, the absorbance was read at 525 nm immediately and the reagent blank was applied as reference. The galacturonic acid content was calculated according to a standard curve drawn from a series of standard galacturonic acid solutions (0–100 μg/mL).
2.4.2 Protein content
The protein content of the extracted PP pectin was analyzed according to Kjeldahl method (N × 6.25).
2.4.3 Degree of esterification
The degree of esterification (DE) of the extracted PP pectin was determined using the titration method described previously by other researchers [9, 10, 23] with minor modifications. In brief, dried pectin sample (100 mg) was moistened with ethanol (2 mL) and was then dissolved completely in distillated water free of CO2 (20 mL). Subsequently, the obtained solution was titrated with 0.1 M solution of NaOH (V1) using phenolphthalein as an indicator. Afterwards, 10 mL 0.5 M solution of NaOH was added, and the mixture was set for about 25 min at continuous stirring in order to adequately hydrolyze. After this, 10 mL 0.5 M solution of HCl was added for neutralization, and the obtained solution was stirred constantly until the pink color disappeared. At last, a final titration was made with 0.1 M solution of NaOH (V2). The DE of the pectin was calculated using Eq. (2).
2.4.4 Ash content
The ash content of the extracted PP pectin was determined as follows: a certain amount of pectin was selected and its weight is denoted as m. Then the pectin sample was placed in a crucible and a lid was covered on it. Then put them in a Muffle furnace and maintain continuous burning at 700 °C for 4 h. Subsequently, as the temperature dropped to room temperature, the residual was weighed, denoted as m0. The ash content calculation formula (Eq. 3) is as follows:
where m0 (g), the weight of residue; m (g), the weight of dried pectin.
2.4.5 Molecular weight analysis
The molecular weight of the sample was determined on a Gel Permeation Chromatography (GPC, Waters 1515, USA). For GPC analysis, the pectin sample was dissolved in distilled water to prepare solution with a concentration of 1.5 mg/mL. Distilled water was used to be as a mobile phase and the solvent flow rate was 1 mL/min. The molecular weights were calibrated with a set of standard dextran samples.
2.5 Emulsifying properties
Emulsion activity (EA) and emulsion stability (ES) of PP pectin were evaluated using the method previously reported by Jafari et al. [15] with some modifications. Briefly, 5 mL olive oil was mixed with 5 mL aqueous pectin solution (0.5%, w/v) containing 0.02% sodium azide as a bacteriocide. The prepared mixture was mixed thoroughly for 10 min by means of ultrasound method and then centrifuged at 4000 rpm for 10 min. At last, the EA was calculated using Eq. (4) as follows:
where the volume of the whole mixture was recorded as VT (mL) and the volume of the emulsified phase was recorded as VE (mL).
Furthermore, the ES was evaluated using the prepared emulsion as mentioned above. The prepared emulsions were stored at 4 and 23 °C for 1 and 30 days, respectively. The ES was determined according to the formula as follows (Eq. 5):
where the initial volume of the emulsified phase was recorded as VI (mL) and the volume of the remained emulsified phase was recorder as VR (mL).
2.6 Fourier transform infrared (FT-IR) spectroscopy analysis
FT-IR spectroscopy of the pectin was determined by applying an FT-IR spectrometer (Perkin Elmer Co., MA, USA) using KBr method in the frequency of 4000 – 500 cm−1 at a resolution of 4 cm−1.
2.7 Nuclear magnetic resonance (NMR)
The 1H-NMR was collected with a spectrometer operating at 400 MHz (Varian-FT-80A, USA). The main technical parameters were as follows: a flip angle 45°, a repetition rate 3 s. Deuterated water (D2O) was used as solvent.
2.8 Scanning electron microscopy analysis
The morphological properties of the dried pectin particles was observed using scanning electron microscope (SEM, JSM-6610LV, JEOL, Japan). The particles were dispersed uniformly on a conductive sample table and was coated using a thin gold layer under argon atmospheric conditions by an iron sputter coater. The images of the sample were obtained at an accelerating voltage of 15 kV with a magnification of 1000 × ~ 5000 × .
2.9 Thermal analysis
Before the thermal analysis, the sample was dried at room temperature. The thermal behavior (thermogravimetry analysis, TGA) of PP pectin sample was investigated using a STA 449C device (Netzsch, Selb, Germany) according to Einhorn-Stoll et al.[24]. The conditions were as follows: linear heating rate 10 °C /min from 60 to 600 ˚C, nitrogen atmosphere, sample weight approximately 20 mg.
3 Results and discussion
3.1 Effects of different liquid–solid ratio and extraction time on PP pectin yield
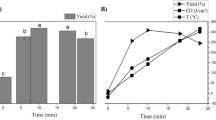
The effect of different liquid–solid ratios (10:1, 15:1, 20:1, 25:1 and 30:1 v/w) on PP pectin yield was presented in Fig. 1a while other parameters were fixed as follows: citric acid concentration 1.5%, ultrasound temperature 65 °C, ultrasound power 200 W and extraction time 30 min. The extraction yields of PP pectin increased from 12.8% to 20.5% with an increase in liquid–solid ratio from 10:1 to 25:1 (v/w). The increase in yield is due to the enhanced contact surface area between the solvent and the plant matrix. However, the pectin yield decreased with further increase in liquid–solid ratio. The reason for this was that with an increase in liquid–solid ratio beyond an optimal value, the solution starts to get saturated with a solute, leading to decrease in mass transfer of pectin into the solution. In addition, higher liquid–solid ratio will need higher energy consumption in the next stage of pectin separation. Therefore, the liquid–solid ratio of 20:1 (v/w) was used as the optimal ratio for all experiments.
The effect of different extraction time (15, 20, 25, 30, 35, 40 min) on PP pectin yield was shown in Fig. 1b, the other conditions were fixed as follows: citric acid concentration 1.5%, ultrasound temperature 65 °C, ultrasound power 200 W and liquid–solid ratio 20:1 (v/w). The results showed that with an increase in the extraction time, pectin yield increased. This might be due to the prolonged reaction time between plant matrix and solvent. However, it could be found that longer time would lead to degradation of the pectin. The results indicated that 30 min was the most favorable time for pectin extraction.
3.2 Model fitting and statistical analysis
BBD is a collection of mathematical and statistical technique and could be employed to examine the process conditions for a maximum pectin yield. Factorial BBD of type 33 was applied to evaluate the effect of citric acid concentration (1 ~ 2%), ultrasound temperature (55 ~ 75 °C), and ultrasound power (120 ~ 200 W) on extracted pectin yield. The experimental and predicated values of PP pectin yield obtained from 17 different experiments were given in Table 2. By applying multiple regression analysis on the achieved experimental data, a second-order polynomial equation (Eq. 6) was developed which could express the relationship between variables and the response (pectin yield).
where Yield is PP pectin yield (%), and A, B, C are coded independent factors (A = citric acid concentration, B = ultrasound temperature, C = ultrasound power).
The statistical significance of the constructed quadratic model was evaluated using the results of analysis of variance (ANOVA) shown in Table 3. The ANOVA results showed that, the higher model F- value (80.40) for pectin yield, lower p-value (< 0.0001) less than 0.05 implied that this developed model was significantly. Moreover, F-value (0.7420) and p-value (0.5802) of the lack-of-fit indicated that the model was not significantly relative to the pure error. All these results indicated that this model was adequate and could be selected to explain and predict the variation of pectin yield. In addition, the values of the determination coefficient R2 for pectin yield, adjusted R2 and predicted R2 were 0.990, 0.978 and 0.936, respectively. Furthermore, the value of coefficient of variation was low (C.V. = 2.14%). These results further confirmed the higher confidence for the proposed model in predicting the pectin yield [19, 25, 26]. The significance of each coefficient was also analyzed by contrasting their p-values and the results were as follows: most variables in each coefficient including linear (A, B, C), interactive (AB, AC) and quadratic (A2) were lower than 0.05, indicating their significant effect on the pectin yield [5].
3.3 Effect of process variables on PP pectin yield
The pectin yield extracted from PP in Table 2 ranged from 13.84% (citric acid concentration 1%, ultrasound temperature 55 °C, ultrasound power 160 W) to 20.67% (citric acid content 1.5%, ultrasound.temperature 65 °C, ultrasound power 160 W). Generally, F and p values analyzed using ANOVA can be used to determine the degree of influence of each variable on response value. Based on this, the results could be obtained that, among all the process parameters the most influential factor was citric acid concentration. Also the interaction between citric acid concentration and ultrasound temperature had great effect on the pectin yield. The effects of all the process parameters and their interactions on pectin yield response could also be studied using three-dimensional (3D) response surface plots and two-dimensional (2D) contour plots, as depicted in Fig. 2. It was noted that, in each figure, the plots were obtained by keeping the variables not shown at constant level 0, whereas the two tested variables were varied in the testing ranges.
The response surfaces showing the effects of citric acid concentration on the pectin yield were shown in Fig. 2a, c. It was observed that the citric acid concentration factor had a significant effect on pectin yield. The results showed that the extracted pectin yield increased with an increase in acid concentration. According to the experimental results listed in Table 2, the lowest pectin yield extracted from PP was obtained under the lowest acid concentration. High acidic condition was useful for extraction solvent to hydrolyze the insoluble pectin constituents into the soluble form. Furthermore, the high acidity extraction solvent could release the pectin from plant matrix and in consequence, increased the pectin yield. These results were in close accordance with the results obtained from melon peel [25], sugar beet pulp [27] and sour orange peel [28].
Ultrasound temperature was also an important factor that influenced the pectin extraction efficiency. According to the Fig. 2a, e, it was observed that the pectin yield increased with an increase of ultrasound temperature. This was probably due to that high temperature processing had stronger disruptive influence on the plant cell wall, thereby increasing solvent diffusivity and pectin solubility from plant matrix hence giving a higher pectin extraction rate [29]. However, pectin extraction at higher temperature beyond the optimum limit would result in a decrease in pectin extraction. This was because at higher temperature the pectin was hydrolyzed into short-chain molecules through the breakdown of a-1, 4 linked units of galacturonic acid or methyl ester which couldn’t be precipitated in ethanol [7]. Furthermore, high temperature processing would promote substantial energy consumption resulting in higher production costs.
The ultrasound power was another important parameter influencing pectin extraction yield, as observed in Fig. 2c, e. It was seen that the extracted pectin yield increased with increasing of the ultrasound power. As reported by Wang et al. [19], increased ultrasound power would enhance the extractability of pectin by disrupting plant cell which was related to the cavitation effects of ultrasound waves in the solvent medium, thereby improving pectin yield. However, it was also indicated that high ultrasound power would cause an adverse effect, i.e., a decrease in pectin yield. This was attributed to “saturation effect” which might reduce energy efficiency of bubbles collapsing [30].
The significance of interaction effect between the two studied variables can be estimated by the shape of contour lines. The elliptical contour plots shown in Fig. 2b, d indicated that the interactions between ultrasound temperature/ultrasound power and citric acid concentration were significant, whereas the circular contour plot in Fig. 2f indicated that the interactions between ultrasound temperature and ultrasound power were insignificant (p > 0.05). These results indicated that the effect of ultrasound temperature or ultrasound power on the pectin yield was significantly influenced by the varying of citric acid concentration, and vice versa.
3.4 Optimization and Validation of optimized experimental conditions
Based on the above-mentioned findings and discussions, the optimal conditions for obtaining maximum pectin yield were determined using the Derringer’s desirability function method [31]. An optimization study was performed using the selected experimental range of process variables by means of the design of expert program. The obtained optimal conditions were as follows, citric acid concentration 1.66%, ultrasound temperature 75 °C and ultrasound power 191 W and the highest predicted pectin yield was 22.24%. According to Wandee et al., the highest pectin yield extracted from pomelo peel using combined conventional inorganic acid (HCl) solvent and microwave method was 20.5% [6]. Chen et al. [2] used a hot-solvent microwave extraction for pectin from oil-free pomelo peelusing conventional inorganic acid (HCl) and the yield was lower 3.29%. To validate the developed model, three repeated experiments were carried out under these optimal conditions, and the mean pectin yield was 21.68%, which was very close to the predicted value. This result demonstrated the validation of the optimized conditions and also the adequate of the developed model.
3.5 Physicochemical characterization
A set of physicochemical properties of PP pectin extracted at the obtained optimal conditions were evaluated in Table 4. It could be seen that the ash content of PP pectin was 1.47 ± 0.21%, which was lower than the reported data about pectin extracted from sour orange peel by ultrasound assisted method (1.89 ± 0.51) [32], and also the pectin microwave-assisted extracted from pistachio hull (5.16% ± 0.19) [33]. The result revealed the purity of PP pectin. It’s worth noting that the different contents are probably attributed to plant sources, extracting methods and processing conditions. According to the results in Table 4, the average molecular weights of PP pectin were 8.86 × 104 Da and 7.75 × 104 Da for Mw and Mn, respectively. Compared with average molecular weights of the pectin extracted from pomelo peels [4], these values were lower probably ascribed to ultrasonic degradation. In addition, the polydispersity (PD) represented the distribution of molecular weight of pectin was 1.14. It was narrower.
The degree of esterification is a vital property of pectin, reflecting the molar ratio of methyl-ester to galacturonic acid, is used to classify pectin. Commercially, the pectin is classified into two groups based on the degree of esterification, namely high methoxyl pectin (DE > 50%) and low methoxyl pectin (DE < 50%) [7]. The two groups have different applications because of their different physicochemical properties. According to the result obtained in our study, the measured degree of esterification was 67.26 ± 0.32% (Table 4), indicating that the pectin extracted from PP can be categorized as high methoxyl pectin. Therefore, it can be used at relatively strong acidic conditions in the presence of soluble solids and is suitable in jam and jellies preparation.
The galacturonic acid content is another vital property indicating the purity of extracted pectin. According to Food and Agriculture Organization (FAO) guidelines, the galacturonic acid content of pectin at least 65% is one of the prerequisite for use in food ingredient [9, 21]. The galacturonic acid content of PP pectin was found to be 82.89 ± 1.35%, which was higher than most of other pectin sources such as sugar beet pulp (60.2–77.8%) [34], Snot apple (6.03–67.6%) [35], citrus limetta (67.81%) [5], and also the same source of pomelo peel (76.62%) [36]. The higher degree of purity for PP pectin might be related to ultrasound irradiation treatment during the process of extraction, what could break the some covalent linkages between pectin and non-pectic polysaccharides [37]. As reported, pectin with higher galacturonic acid content is effective on diverse functional characteristics such as emulsifying properties and radical-scavenging activity [38].
3.6 Emulsifying properties
The determined values of emulsion activity (EA) and emulsion stability (ES) of PP pectin extracted at optimal conditions were listed in Table 4. According to the results, the value of EA for the pectin 54.3% was higher than the reported commercial citrus pectin (44.87%) and apple pectin extracted from potato pectin (45.34%) [12], and was close to the reported pectin extracted from sour orange peel (53.42% ± 2.14%) [32]. In addition, the emulsion stability were 85.7% and 80.2% at 4 °C and 23 °C for 1 day, respectively. Also, the emulsions were 78.4% and 76.9% at 4 °C and 23 °C after 30 days of storage. The results indicated that the emulsions were more stable stored in lower temperature. Furthermore, compared with other reported results, these values were higher than the pectins extracted from sugar beet [34] and Citrus limetta peels [5]. Presumably, its higher emulsion properties were related to the higher galacturonic acid content [32]. Therefore, it could be inferred that PP pectin was suitable for emulsifier and stabilizer in food industries.
3.7 Chemical structure analysis by FT-IR
FT-IR is always used to analyze the primary functional groups. The FT-IR spectrum of PP pectin obtained under optimal conditions was illustrated in Fig. 3. For PP pectin, the broad and strong peaks at around 3200 ~ 3500 cm−1 were due to the stretching vibrations of inter and intramolecular hydrogen of O–H [19]. The peaks in the range of 3000 ~ 2800 cm−1 and 1500 ~ 1300 cm−1 were the bending and stretching vibrations of saturated C-H groups, including CH, CH2 and CH3 [9]. Furthermore, the peaks at 1641 and 1718 cm−1 were related to the free and esterified carboxyl groups, respectively. The adsorption bands between 1250 and 1000 cm−1 were mainly related to glycosidic linkages (C–O–C functional groups) in galacturonic acid molecules. Additionally, the absorption signals in the ranges of 1200 ~ 500 cm−1 were considered as the “finger print” region of carbohydrates which was unique for every kind of pectin [39].
3.8 1H-NMR spectrum analysis
The 1H-NMR spectrum of PP pectin extracted under optimal conditions was illustrated in Fig. 4. The sharp peak at 3.7 ppm was assigned to the methoxyl group protons from the esterified units of galacturonic acid [32]. The proton signals of non-esterified galacturonic acid units were observed at anomeric H-1 (5.1 ppm) and H-5 (4.9 ppm). The signals around 1.0 ppm were probably indicative of the presence of methyl group links of rhamnose. Further, proton signals at 3.4, 3.8 and 1.1 ppm were related to H-4, H-5, and H-6 of rhamnose units, respectively. Moreover, the region at around 3.7—5.28 ppm contained typical signals of protons originating from arabinose units. The signals of H-1 (4.8 ppm), H-2 (3.6 ppm), H-3 (3.9 ppm), H-4 (4.4 ppm) and H-5 (4.6 ppm) were ascribed to esterified galacturonic acid monosaccharide [40]. Thus, the above-mentioned results further confirmed the presence of pectin structure in the sample.
3.9 SEM analysis
The surface morphology of the pectin obtained from PP using the optimal conditions was analyzed by SEM seen in Fig. 5a, c, e. In order to further compare with the conventional method, the pectin was extracted from PP by using mineral hydrochloric acid (c-pectin), mainly according to the steps described in 2.2 (pH 2.0, temperature 85, liquid–solid ratio 20: 1, time 60 min), the images with different magnifications in Fig. 5b, d, f. SEM images with different magnifications (Fig. 5) indicated that ultrasonic treatment influenced the microstructures of pectin. As shown in Fig. 5b, d, the PP c-pectin exhibited a flat, but rough surface, while the PP pectin in Fig. 5a, c exhibited a smooth, but wrinkled surface. Moreover, the PP c-pectin in Fig. 5f showed a impact surface, but the PP pectin in Fig. 5e appeared very loosen. This phenomenon is similar to the results reported by Wang et al. [19]. The different microstructures indicated that ultrasound plays an important role in breaking up the vegetal tissue or disrupting the crosslinks between pectin molecules.
3.10 Thermal analysis
As one typical of diagram of the thermal degradation, the TG curves records the weight loss during heating. The thermal investigation of PP pectin was shown in Fig. 6. The result showed that the first weight loss transition at 120 °C could be due to evaporation of free and bound water in pectin. This water content was found to be 4%. The second considerable weight loss was about 50%, which was ascribed to pectin degradation. It started at temperatures of about 230 °C and ended at about 280 °C. The thermal degradation features of PP pectin were similar to the citrus pectin described in Einhorn-Stoll et al. [41].
4 Conclusions
In this study, ultrasound assisted extraction of pomelo peel (PP) pectin using organic citric acid was investigated under different processing parameters. Box-Behnken design (BBD) of response surface methodology (RSM) was used for extraction optimization. The second-order polynomial model developed using multiple regression analysis showed higher adequate in predicting the pectin yield. The highest pectin yield 21.68% was achieved in the optimal conditions, citric acid concentration of 1.66%, ultrasound temperature of 75 °C and ultrasound power of 191 W. The extracted pectin at the optimal conditions was categorized as high methoxyl pectin according to its esterification degree of 67.26 ± 0.32%, which was confirmed by FTIR and 1H-NMR results. In addition, the pectin had higher purity because of its higher galacturonic acid content of 82.89 ± 1.35%, also lower ash content of 1.47 ± 0.21% and lower protein content of 1.82 ± 0.26. SEM analysis indicated that ultrasonic treatment was conducive to break up the vegetal tissue or disrupt the crosslinks between pectin molecules. Thermal analysis indicated that PP pectin showed better thermal stability. Thus, the proposed method of ultrasound assisted extraction using citric acid can be an eco-friendly process for extraction of pectin from pomelo peel. The eco-friendly organic citric acid can be substituted for traditional inorganic acids in pectin extraction from pomelo peel.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Guo X, Zhao W, Liao X, Hu X, Wu J, Wang X (2017) Extraction of pectin from the peels of pomelo by high-speed shearing homogenization and its characteristics. LWT-Food Sci Technol 79:640–646. https://doi.org/10.1016/j.lwt.2016.12.001
Chen Q, Hu Z, Yao FY-D, Liang H (2016) Study of two-stage microwave extraction of essential oil and pectin from pomelo peels. LWT-Food Sci Technol 66:538–545. https://doi.org/10.1016/j.lwt.2015.11.019
Liew SQ, Ngoh GC, Yusoff R, Teoh WH (2018) Acid and Deep Eutectic Solvent (DES) extraction of pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Biocatal Agric Biotechnol 13:1–11. https://doi.org/10.1016/j.bcab.2017.11.001
Methacanon P, Krongsin J, Gamonpilas C (2014) Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocolloids 35:383–391. https://doi.org/10.1016/j.foodhyd.2013.06.018
Panwar D, Panesar PS, Chopra HK (2022) Green extraction of pectin from Citrus limetta peels using organic acid and its characterization. Biomass Conversion Biorefinery. https://doi.org/10.1007/s13399-021-02127-z
Wandee Y, Uttapap D, Mischnick P (2019) Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocolloids 87:237–244. https://doi.org/10.1016/j.foodhyd.2018.08.017
Picot-Allain MCN, Ramasawmy B, Emmambux MN (2020) Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev Intl 38:282–312. https://doi.org/10.1080/87559129.2020.1733008
Dranca F, Oroian M (2018) Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res Int 113:327–350. https://doi.org/10.1016/j.foodres.2018.06.065
Hosseini SS, Khodaiyan F, Yarmand MS (2016) Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohyd Polym 140:59–65. https://doi.org/10.1016/j.carbpol.2015.12.051
Asgari K, Labbafi M, Khodaiyan F, Kazemi M, Hosseini SS (2020) High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int J Biol Macromol 152:1274–1282. https://doi.org/10.1016/j.ijbiomac.2019.10.224
Ma X, Yu J, Jing J, Zhao Q, Ren L, Hu Z (2021) Optimization of sunflower head pectin extraction by ammonium oxalate and the effect of drying conditions on properties. Sci Rep 11:106–116. https://doi.org/10.1038/s41598-021-89886-x
Yang JS, Mu TH, Ma MM (2018) Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem 244:197–205. https://doi.org/10.1016/j.foodchem.2017.10.059
Sengar AS, Rawson A, Muthiah M, Kalakandan SK (2020) Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason Sonochem 61:104812. https://doi.org/10.1016/j.ultsonch.2019.104812
Sun D, Chen X, Zhu C (2020) Physicochemical properties and antioxidant activity of pectin from hawthorn wine pomace: A comparison of different extraction methods. Int J Biol Macromol 158:1239–1247. https://doi.org/10.1016/j.ijbiomac.2020.05.052
Jafari F, Khodaiyan F, Kiani H, Hosseini SS (2017) Pectin from carrot pomace: Optimization of extraction and physicochemical properties. Carbohyd Polym 157:1315–1322. https://doi.org/10.1016/j.carbpol.2016.11.013
Chan SY, Choo WS (2013) Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem 141:3752–3758. https://doi.org/10.1016/j.foodchem.2013.06.097
Cho EH, Jung HT, Lee BH, Kim HS, Rhee JK, Yoo SH (2019) Green process development for apple-peel pectin production by organic acid extraction. Carbohyd Polym 204:97–103. https://doi.org/10.1016/j.carbpol.2018.09.086
Matsuo Y, Akita K, Taguchi H, Fujii S, Yoshie-Stark Y, Araki T (2022) Utilization and evaluation of Citrus natsudaidai peel waste as a source of natural food additives. Food Chem 373:131464. https://doi.org/10.1016/j.foodchem.2021.131464
Wang W, Ma X, Xu Y, Cao Y, Jiang Z, Ding T, Ye X, Liu D (2015) Ultrasound-assisted heating extraction of pectin from grapefruit peel: optimization and comparison with the conventional method. Food Chem 178:106–114. https://doi.org/10.1016/j.foodchem.2015.01.080
Peng X, Yang G, Shi Y, Zhou Y, Zhang M, Li S (2020) Box-Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci Rep 10:3595. https://doi.org/10.1038/s41598-020-60339-1
Kamal MM, Ali MR, Hossain A, Shishir MRI (2020) Optimization of microwave-assisted extraction of pectin from Dillenia indica fruit and its preliminary characterization. J Food Process Preserv 44:1–11. https://doi.org/10.1111/jfpp.14466
Fakayode OA, Abobi KE (2018) Optimization of oil and pectin extraction from orange (Citrus sinensis) peels: a response surface approach. J Anal Sci Technol 9:1–18. https://doi.org/10.1186/s40543-018-0151-3
Dranca F, Oroian M (2019) Ultrasound-assisted extraction of pectin from Malus domestica ‘Fălticeni’ Apple pomace. Processes 7:488. https://doi.org/10.3390/pr7080488
Einhorn-Stoll U, Kunzek H (2009) Thermoanalytical characterisation of processing-dependent structural changes and state transitions of citrus pectin. Food Hydrocolloids 23:40–52. https://doi.org/10.1016/j.foodhyd.2007.11.009
Raji Z, Khodaiyan F, Rezaei K, Kiani H, Hosseini SS (2017) Extraction optimization and physicochemical properties of pectin from melon peel. Int J Biol Macromol 98:709–716. https://doi.org/10.1016/j.ijbiomac.2017.01.146
Li Y, Zhu CP, Zhai XC, Zhang Y, Duan Z, Sun JR (2018) Optimization of enzyme assisted extraction of polysaccharides from pomegranate peel by response surface methodology and their anti-oxidant potential. Chin Herb Med 10:416–423. https://doi.org/10.1016/j.chmed.2018.08.007
Lv C, Wang Y, Wang LJ, Li D, Adhikari B (2013) Optimization of production yield and functional properties of pectin extracted from sugar beet pulp. Carbohyd Polym 95:233–240. https://doi.org/10.1016/j.carbpol.2013.02.062
Hosseini SS, Khodaiyan F, Yarmand MS (2016) Aqueous extraction of pectin from sour orange peel and its preliminary physicochemical properties. Int J Biol Macromol 82:920–926. https://doi.org/10.1016/j.ijbiomac.2015.11.007
Karbuz P, Tugrul N (2021) Microwave and ultrasound assisted extraction of pectin from various fruits peel. J Food Sci Technol 58:641–650. https://doi.org/10.1007/s13197-020-04578-0
Xu Y, Zhang L, Bailina Y, Ge Z, Ding T, Ye X, Liu D (2014) Effects of ultrasound and/or heating on the extraction of pectin from grapefruit peel. J Food Eng 126:72–81. https://doi.org/10.1016/j.jfoodeng.2013.11.004
Moorthy IG, Maran JP, Surya SM, Naganyashree S, Shivamathi CS (2015) Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. Int J Biol Macromol 72:1323–1328. https://doi.org/10.1016/j.ijbiomac.2014.10.037
Hosseini SS, Khodaiyan F, Kazemi M, Najari Z (2019) Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int J Biol Macromol 125:621–629. https://doi.org/10.1016/j.ijbiomac.2018.12.096
Kazemi M, Khodaiyan F, Labbafi M, Saeid Hosseini S, Hojjati M (2019) Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem 271:663–672. https://doi.org/10.1016/j.foodchem.2018.07.212
Ma S, Yu SJ, Zheng XL, Wang XX, Bao QD, Guo XM (2013) Extraction, characterization and spontaneous emulsifying properties of pectin from sugar beet pulp. Carbohyd Polym 98:750–753. https://doi.org/10.1016/j.carbpol.2013.06.042
Chawafambira A (2021) Extraction and characterization of pectin from Snot Apple (Azanza garckeana) fruits with potential use in zimbabwe. Int J Fruit Sci 21:791–803. https://doi.org/10.1080/15538362.2021.1932693
Liew SQ, Teoh WH, Tan CK, Yusoff R, Ngoh GC (2018) Subcritical water extraction of low methoxyl pectin from pomelo (Citrus grandis (L.) Osbeck) peels. Int J Biol Macromol 116:128–135. https://doi.org/10.1016/j.ijbiomac.2018.05.013
Wang W, Ma X, Jiang P, Hu L, Zhi Z, Chen J, Ding T, Ye X, Liu D (2016) Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocolloids 61:730–739. https://doi.org/10.1016/j.foodhyd.2016.06.019
Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C, Attia H (2011) Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem 124:411–421. https://doi.org/10.1016/j.foodchem.2010.06.077
Ghoshal G, Negi P (2020) Isolation of pectin from kinnow peels and its characterization. Food Bioprod Process 124:342–353. https://doi.org/10.1016/j.fbp.2020.09.008
Sharma R, Kamboj S, Khurana R, Singh G, Rana V (2015) Physicochemical and functional performance of pectin extracted by QbD approach from Tamarindus indica L. pulp. Carbohyd Polym 134:364–374. https://doi.org/10.1016/j.carbpol.2015.07.073
Einhorn-Stoll U, Kunzek H, Dongowski G (2007) Thermal analysis of chemically and mechanically modified pectins. Food Hydrocolloids 21:1101–1112. https://doi.org/10.1016/j.foodhyd.2006.08.004
Funding
This work was supported by the Scientific Research Program funded by Natural Science Foundation of Shaanxi Province (No. 2021JQ-814), Xianyang City Qinchuangyuan science and technology innovation special project (No. L2022-QCYZX-GY-006), Xianyang Science and Technology project (No. 2022KJJ03), the key research and development (R&D) plan of Shaanxi Province (No. 2023-YBSF-595) and Ningxia Natural Science foundation Project (No. 2022AAC03309).
Author information
Authors and Affiliations
Contributions
Zhihui Sun: investigation, experimental performance, manuscript preparation; Shan Wang: experimental performance, formal analysis; Caihua Zhou: data analysis; Zhanying Ma: manuscript preparation; Fang Qian: supervision.
Corresponding author
Ethics declarations
Ethical approval
The authors claim that none of the material in the paper has been published or is under consideration for publication elsewhere.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Z., Wang, S., Zhou, C. et al. Optimization of ultrasound assisted organic acid extraction of pectin from pomelo peel by response surface methodology and its preliminary characterization. Biomass Conv. Bioref. 14, 20129–20141 (2024). https://doi.org/10.1007/s13399-023-04200-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04200-1