Abstract
An endeavour is underway to isolate native cellulose-degrading bacteria from different sources in order to have them used in composting of organic waste. On the basis of cellulase production and growth in different environmental circumstances, five isolates were selected. The 16S rRNA genetic sequence of five isolates was submitted to the National Center for Biotechnology Information (NCBI), which assigned accession numbers ON150745, ON178665, ON725042, ON479186 and ON142173 for CBD4, CBG3, CBG2, CBC9 and CBG4 in accordance. The growth of CBD4, CBG2, CBG3 and CBG4 reached the stationary phase at 24 h and lasted up to 78 h, but in case of CBC9 stationary phase of growth lies between 32 and 50 h of incubation which was less than other bacterial strains. The strains’ optimum CMCase production conditions were 5% inoculum, a pH of 7.5, the temperature of 35° C for CBD4 and CBC9; 4% inoculum, a pH of 6.5 and temperature of 35° C for CBG2, CBG3; and 3% inoculum with same pH and temperature for CBG4. The CMCase production at respective optimum conditions was highest in the case of CBG2 strain. The CMCase reaction for all the strains was highest at 50 °C and decreased in higher or lower temperatures. The reaction of cellulase produced by strain CBG2 at optimum pH and temperature was highest. In this study, the cellulolytic potential of the CBC9 strain (B. xiamenensis) was examined for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulose is composed of three main components: hemicellulose, cellulose and lignin [1]. A plentiful lignocellulosic biomass on earth including agricultural waste such as crop residue, vegetable waste, saw dust and dry leaves [2]. The plant’s dry weight consists of 5–30% lignin, 20–35% hemicellulose and 35–50% cellulose. Cellulose has a water-insoluble highly crystalline structure which makes it difficult to be hydrolyzed into glucose [3]. At present, this plant cellulose is used mainly for animal feed and manure, in the paper industry and to some extent for fuel production. However, this causes considerable environmental pollution at the time of its least utilization. The degradation of lignocellulose materials like agro-waste [4, 5] and hazardous biowastes like swine manure [6] can be utilised by composting and vermicomposting.
Cellulase belongs to glycosyl hydrolase enzyme family and helps in degradation of cellulose into glucose. Endoglucanase (EC 3.2.1.4), exoglucanase or cellobiohydrolase (EC 3.2.1.91) and -glucosidase are the three enzymes that make up the complex known as cellulase (EC 3.2.1.21). Endoglucanases increase the free ends by breaking internal glycosidic linkages in the amorphous portions of cellulose chains, which is further degraded by cellobiohydrolase and β-glucosidase [2, 7].
The successful bioconversion of lignocellulosic biomass requires cellulolytic enzymes source with enormous potential in hydrolysis. The polymer-degrading microorganisms also able to degrade plastics [8]. Fungi, bacteria and actinomycetes are the microorganisms that have been isolated thus far for the investigation of their capacity to break down cellulosic substrates into glucose monomers [9]. Due to their resilience to harsh environments, bacteria have recently received substantial research attention for the enzymatic depolymerization of lignocellulose. In contrast to fungi, bacteria grow quickly, express several enzyme complexes, are stable at high temperatures and pH levels, have less feedback inhibition, can occupy a wide range of environmental niches and can resist a variety of environmental stresses [2, 10]. It is possible to extract and describe cellulase-producing bacteria from a range of sources, including soil, decomposed plant matter, hot springs, organic waste, ruminant faeces and composts [11]. The gut symbionts of termites and beetles, as well as the gut bacterial association in Lepidoptera, have recently received substantial study [12,13,14,15]. Cellulolytic bacteria also used for extraction of lactic acid from cellulose-rich organic wastes [15]. However, the process of searching for a new bacterial strain with higher cellulase activity is continuous with a view to finding an efficient cellulase-producing bacteria with extreme environmental resistance [16]. The thermophilic lignocellulolytic bacteria inoculation enhances the composting rate with increasing enzyme activity [17], whereas the types of cellulose, activity of cellulolytic enzymes and cultivation conditions are the primary determinants of bioconversion of cellulosic materials to value-added products [5]. The metagenomics study is another key to know the microbial diversity [18]. This study aimed to isolate indigenous bacterial strains efficient in cellulose degradation from different natural environment including composting site, dump yards and gut bacteria of Moringa hairy caterpillar and their optimal cultivation condition for carboxymethyl cellulase (CMCase).
In this study, different cellulolytic bacteria were isolated from compost pits, dump yard sites, forest soil, residue incorporated field and gut of actively feeding Moringa hairy caterpillar on the basis of hydrolytic capacity and their cellulase activity and the best of the five isolates were optimized for their enzyme production for their further utilization. Isolation and screening of cellulose-degrading microbes are of immense importance due to their various applications. The goal of the current research is to identify prospective cellulolytic bacterial strains that can more effectively break down various types of organic waste by creating a higher amount of cellulase. The several research groups have shown interest in isolation of cellulose-degrading bacteria such as Bacillus subtilis [3]; Klebsiella sp. [12]; Bacillus pumilis [19] and Bacillus velezensis [20].
2 Material and methods
2.1 Collection of samples
Waste and soil samples were collected randomly from 15-cm depth from the surface of dumping yard of Bhubaneswar, compost pit, residue incorporated field of Department of Soil Science and Agricultural Chemistry, College of Agriculture, Odisha University of Agriculture and Technology (OUAT), Bhubaneswar, India, and human activity area of Chandaka forest of Bhubaneswar. The weight of each representative sample was reduced to 100 g after following quartering process. The actively feeding Moringa hairy caterpillars collected from Moringa oleifera of Department of Soil Science and Agricultural Chemistry, College of Agriculture, Odisha University of Agriculture and Technology (OUAT), Bhubaneswar, India.
2.2 Isolation and screening of cellulose-degrading bacteria
Immediately after collection of waste samples were serially diluted for the purpose of isolating bacteria that degrade cellulose by spreading 0.1 ml of each dilution on Leuria Bertani (LB) liquid medium (NaCl (10.0 g l−1), tryptone (10.0 g l−1) and yeast powder (5.0 g l−1)) supplemented with 1% carboxymethyl cellulose (CMC) [3] for 24 h in a shaker. The plates were incubated for 15–20 min, then flooded with 1% Congo red, followed by 15–20 min of destaining with 1 M NaCl [3], and the strain with the largest clearing zone was selected for further screening. The caterpillar was starved for 24 h to lessen the possibility of contamination from sporadic bacteria, surface sterilised with ethanol and immobilised on ice for 10 min before dissection. Utilising sterilised tools, the caterpillars were sacrificed in laminar airflow under sterile conditions to expose the insides. Following dissection, the stomach was suspended in 1 ml of sterile saline solution (0.9%) and ground using a micro pestle [12]. For the purpose of isolating cellulose-degrading bacteria, the macerated stomach of the caterpillar was inoculated in a basal salt media (NaNO3 0.25 g, KH2PO4 0.2 g, MgSO4 0.02 g, NaCl 0.02 g and CaCl2.6H2O 0.01 g in 100 ml). These cultures were cultivated for 7 days at 37 °C and 100 rpm in a shaker incubator [21]. After every 2 days, 1 ml of inoculum was taken for serial dilution up to 10−5.
Repeated streaking in LB agar plates with 1% CMC is used to purify strains. By measuring the positive isolates’ hydrolytic capacity (HC) or the ratio of the clearing zone’s diameter to that of the entire colony, it was also possible to qualitatively quantify their capacity to degrade cellulose.
2.3 Secondary screening through estimation of cellulase enzyme activity
2.3.1 Crude enzyme production
The chosen cellulose-degrading bacteria (CDB) isolates were grown in LB broth with 1% CMC at 37 °C and 150 rpm. After 48 h of incubation, the broth culture was centrifuged at 10,000 rpm for 15 min at 4 °C. For subsequent enzyme experiments, supernatant was collected and kept as a crude enzyme preparation at 4 °C.
2.3.2 Enzyme assay
The selected strain’s cellulase activity was estimated using the DNS (3,5-dinitrosalicylic acid) technique [22]. The substrate solution (citric acid buffer with 1% CMC-Na) was dissolved in 0.5 ml of crude enzyme solution before being incubated at 50 °C for 30 min. Following that, 3 ml of DNS reagent was added and heated for 10 min in a water bath at 100 °C. The volume was then cooled to room temperature and well agitated before being adjusted to 25 ml with deionised water. The absorbance value of mixed liquid was determined at 540 nm wavelength by using Systronics UV Visible Spectrophotometer. The standard curve was plotted from different concentrations of glucose. The cellulase activity was calculated by using following formula [20].
2.4 Biochemical characterization
Bergey’s Manual of Determinative Bacteriology was used to identify bacterial isolates. Gram staining, citrate utilisation, nitrate reduction, oxidase, indole, methyl red (MR), Vogas Proskauer and catalase synthesis were the biochemical tests used for the identification. The other biochemical characteristics were obtained by using microbial identification system (VITEK-2).
2.5 Molecular characterization
The Quick-DNATM Fungal/Bacterial Miniprep Kit Catalog No. D6005 from Zymo Research was used to isolate the bacterial DNA. Each DNA sample’s concentration was determined using Nanodrop (Biotech Instruments, USA), and the DNA was then kept at 800 °C for further use. The 16S rDNA gene fragment was amplified using the universal primers (forward primer 5′-AGAGTTTGATCCTGGCTCAG-3′ and reverse primer 5′ CTTGTGCGGGCCCCCGTCAATTC-3′) [13, 14]. Electrophoresis was used to isolate the amplified DNA. On the gel documentation system, the gel was photographed. The ABI 3730XL instrument was used to carry out the sequencing. After collecting the 16 S rRNA sequence, each isolate was put through Basic Local Alignment Search Tool (BLAST); then, Molecular Evolutionary Genetics Analysis-X (MEGA-X) software was used to create the phylogenetic tree [23,24,25].
2.6 Antibiotic sensitivity test
Pure isolates were put in a petri dish with nutrient agar (NA). The paper disc diffusion experiment was used to conduct a susceptibility test. The antibiotics of Himedia was taken for experimentation. An array of eleven antibiotics, including erythromycin (E), amphotericin (Ap50), bacitracin (B10), ciprofloxacin (CIP50), fluconazole (FU10), penicillin-G (P10), neomycin (N30), amikacin (Ak30), streptomycin (S10), vancomycin (VA30) and tetracycline (T), were utilised. Each of the NA plates was prepared with 25 ml of media. One loop of culture was used to inoculate each petri dish, covering the entire plate. Bio discs were placed on the agar medium’s hardened surface. At 28 ± 1 °C, the plates were incubated for 24 h. In mm, the zone creation was recorded.
2.7 Growth curve
One millilitre of a broth culture of a chosen bacterial strain was added to 100 ml of LB-CMC liquid medium before being cultured at 37 °C for 132 h. At various time intervals, the population growth was determined using the serial dilution method (5 h, 10 h, 16 h, 24 h, 32 h, 40 h, 59 h, 64 h, 78 h, 85 h, 96 h, 108 h, 124 h and 132 h). The average value obtained from three replicated samples was used to create the growth curves for the strains.
2.8 Optimization of cellulase production
The LB-CMC liquid medium (100 ml) with pH ranging from 4.5 to 9.5 were taken in 250-ml conical flasks and inoculated with broth culture of different strains to identify the optimum pH for CMCase production. The 0.1N HCl or 0.1N NaOH was used to adjust the pH of the medium. The flasks were incubated on shaker incubator at 220 rpm for 36 h. A fermentation experiment was conducted to determine the strains’ effective temperature for cellulose production. The strains were incubated for 36 h in LB-CMC liquid medium between the temperatures of 20 and 70 °C, with intervals of 10 °C, to estimate the optimal temperature for the isolated strain to produce cellulase. To explore the effect of various carbon compounds on CMCase production, lactose, CMC, glucose, sucrose and starch were selected for the experiment. Before culture inoculation, a broth containing 1% of each carbon source was prepared. The flasks were then incubated on a shaker for 36 h. To determine the best source of nitrogen for the isolated strain’s cellulase production, the fermentation medium was supplemented at a 1% level with both organic and inorganic substances (peptone, urea, ammonium nitrate, sodium nitrate and yeast extract). To establish the ideal inoculum size, broth media were inoculated with varying inoculum concentrations (1%, 2%, 3%, 4%, 5%, 6% and 7%) in 100 ml LB-CMC liquid medium (starting pH = 7.0) and incubated on a rotary shaker for 36 h at the optimum temperature (35 ± 1 °C). The CMCase activities of were assessed by DNS method to determine the ideal pH, best source of C, N and ideal inoculum size for CMCase production. Each experiment employed three replicated samples, and the average values were plotted for each experiment.
2.9 Effect of temperature and pH on enzyme reaction and stability
Using the proper buffers, the ideal pH for the CMCase reaction was determined to be between 4.5 and 9.5. The 50 mM of sodium acetate (pH 3.0–6.0), sodium phosphate (pH 7.0–8.0) and Tris-base were employed as buffers (9.0–10.0). The appropriate buffer, including 1% CMC-Na, served as the enzyme reaction’s substrate. By incubating the reaction mixture of 0.5 ml of crude enzyme with the same quantity of substrate at 50 °C for 30 min, the CMCase reaction was identified, and the released glucose was quantified using the DNS method. The following procedures were used to determine the ideal temperature for the CMCase reaction: the released glucose was determined using the DNS method after 0.5 ml of crude enzyme and 0.5 ml of substrate were incubated for 30 min at various temperatures (25 °C, 40 °C, 50 °C, 60 °C and 70 °C). Three replicated samples were used in each experiment, and the average values were plotted for each experiment.
2.10 Statistical analysis
Each assay was carried out in replications. Results were presented as the mean of three replicates. The standard error was calculated by taking three replicated data and Duncan’s multiple range (DMRT) was used to compare significant means at a probability of 0.01. The statistical analysis was performed by using “R” software [26].
3 Results and discussion
3.1 Isolation and screening of cellulolytic bacteria
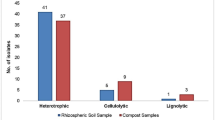
A total 42 cellulose-degrading bacterial isolates were obtained from various sources and grown on LB agar plates with 1% carboxymethyl cellulose sodium salt (CMC-Na) (Supplementary 1). The colonies were identified by their zone of clearance formed due to hydrolysis of cellulose contained in the LB agar plate. Eleven isolates were isolated from the compost pits, 5 isolates from the dump yard, 5 isolates from the waste pits, 6 isolates from Chandaka forest soil, 8 isolates from the residue incorporated field and 8 from the gut of actively feeding Moringa hairy caterpillar. In the isolated colonies, the zone of clearance ranged from 10 to 24 mm. The hydrolytic capacity was used as a criterion for the primary screening in the present study. Hydrolytic capacity of the isolated colonies ranged from 2.20 to 4.25. In the present study, cellulolytic bacteria were isolated from different sources using LB agar media containing 1% CMC which is congruent with the study of Yang et al. [3] and Islam and Roy [27]. Isolates were selected here based on how well they could hydrolyze. The hydrolytic capacity of isolated strains was examined using sodium chloride as a destaining agent and Congo red as a staining agent. Transparent circles were formed around the colony of strains with higher cellulose hydrolytic capacities, due to the breakdown of cellulose into cellulose disaccharide, glucose and organic acid, which alters the pH of the media, a translucent circle forms around the colony [20], due to its powerful interaction with polysaccharides, Congo red was used to identify cellulolytic bacteria and may be useful for identifying other cellulolytic microorganisms. In addition to the hydrolytic capacity, the cellulase activity (CMCase) was examined and the isolates showing higher enzyme activity viz., CBC 09, CBC 10, CBC 11, CBD 01, CBD 02, CBD 03, CBD 04, CBR 02, CBR 05, CBR 06, CBG 01, CBG 02, CBG 03 and CBG 04 were selected for further study. In the current study, 5 isolates showing higher enzyme activity (> 25 Uml−1) were selected for their optimization of the cultivation condition (Table 1). By quantifying the quantity of reducing sugars generated during hydrolysis using the DNS (3,5-dinitrosalicylic acid) technique, the CMCase or Endo—1,4-glucanase activity was determined. By oxidising the aldehyde groups on the glucose molecule, 3,5-DNS was reduced in this process to 3-amino-5-nitro salicylic acid, which was visible by the colour changing from yellow to orange in an alkaline environment [20]. These isolates were further identified and characterised (morphologically and biochemically) before optimization of their cultivation condition.
3.2 Morphological characteristics
The colony morphology of all the isolates in LB agar media is listed in supplementary 2. All the cellulolytic bacteria are circular in shape except strain CBR8 which is irregular. Thirteen isolates were white in pigmentation, whereas the rest among 42 isolates were pale yellowish pigmentation. The margin of 9 cellulolytic bacteria was undulated while the rest were of entire margin. There was a large variation in elevation of the isolated bacteria, where 28 isolates were raised, 10 were convex, 3 isolates were umbonate and 1 among the 42 bacteria was flat.
3.3 Secondary screening of bacterial colony
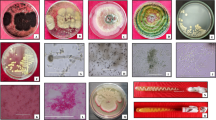
The cellulase activity of the selected isolates was taken as the standard for the secondary screening. The isolates were cultured in LB broth media containing 1% CMC and the cellulase activity was measured after 48 h using DNS as coupling agent. Among the 14 isolates grown in LB broth medium, strain CBC9, CBD4, CBG2, CBG3 and CBG4 showed high CMCase activity and were selected for further study (Table 1). The strain CBG2 isolated from gut bacteria showed maximum activity (46.17 Uml−1) followed by strain CBD4 isolated from dump yard having enzyme activity of 37.61 Uml−1 after 48 h of incubation. The enzyme activity of strain CBC9 selected from compost pit was 31.39 Uml−1 and the rest two strains CBG3, CBG4 from gut bacteria showed lower enzyme activity. The size of the isolates (Fig. 1) ranged from 1.26 to 1.41 μm (CBC9), from 1.08 to 1.38 μm (CBD4), from 1.16 to 3.36 μm (CBG2), from 1.17 to 1.31 μm (CBG3) and from 1.16 to 1.49 μm (CBG4). The morphological character showed that one (CBC9) among the five isolates was umbonate, circular and undulate margin with pale yellowish appearance, whereas others were convex circles and regular in margin with a whitish appearance. Similarly, Ferbiyanto et al. [28] reported that colony of cellulolytic bacteria isolated from gut of worker Macrotermes gilvus were rounded, smooth and convex.
3.4 Biochemical characteristics
The biochemical characteristics of the 5 selected isolates are depicted in Supplementary 3. The selected bacterial strains were found to be Gram-positive rods (Fig. 1). All 5 isolates showed negative reactions to biochemical assay of Indole and Phenylalanine Deaminase, whereas they showed positive reactions to catalase, gelatin liquefaction and citrate utilization. Strain CBC9 was non-motile and showed positive reaction to methyl red and negative reaction to nitrate utilization, while others being motile in nature showed negative reaction to methyl red and positive to nitrate utilization. Sugar fermentation and hydrogen sulphide production analyses showed that all the 5 strains could ferment glucose but could not produce hydrogen sulphide when cultured on Triple Sugar Iron Agar.
3.5 Molecular characteristics
The PCR amplification product resulted from agarose gel electrophoresis of all the 5 selected strains is shown in Fig. 2. All of the DNA fragments amplified by PCR were single bands, each measuring around 1500 bp. The 16S rDNA of the selected bacteria were submitted in the GeneBank for their accession number, and all were found to have similarities with Bacillus sp. The strains CBD4 and CBG3 were found to be Bacillus subtilis CBG2 was Bacillus sp., CBC9 was found to be Bacillus xiamenensis and CBG4 was found to be Bacillus velezensis with accession no ON150745, ON178665, ON725042, ON479186 and ON142173 respectively. MEGA 11.0 cluster analysis was used to create the phylogenetic tree [29], and the strains were classified as shown in Fig. 3. The results of morphological and biochemical identification and 16S rRNA gene analysis showed that the homology between CBC9 and Bacillus xiamenensis was 99.54%, CBD4 and Bacillus subtilis was 99.55%, CBG2 and Bacillus sp. was 95.20%, CBG3 and Bacillus subtilis was 99.60% and CBG4 and Bacillus velezensis was 99.85%. The evolutionary relationships of isolated strains were determined by using the phylogenetic tree. The present study showed that the Bacillus subtilis strains produce highly active cellulase, which was also suggested from previous work like Bacillus subtilis isolated from Tibetan pig’s intestine [3]; gut of rice weevil (Sitophilus oryzae) [13]; Bacillus velezensis from faeces of piglets [30]; from Min pigs’ manure [20]. Bacillus xiamenensis from the intestinal tract contents of a flathead mullet, Mugil cephalus [31].
3.6 Antibiotic sensitivity of bacterial strains
Antibiotic sensitivity of 5 bacterial strains using 11 different antibiotic discs was depicted in Table 2 and Fig. 4. All the selected strains showed resistance to fluconazole (FU10), amphotericin B (AP50) and penicillin-G (P10), while they showed susceptibility to ciprofloxacin (CIP5), neomycin (N30) and tetracycline (TE30). All the bacterial strains showed susceptibility to erythromycin (E) except CBC9 which showed moderate resistance. CBC9 and CBG4 showed susceptibility to streptomycin (S10), whereas CBD4, CBG2 and CBG3 showed moderate resistance reaction. CBG4 showed susceptibility to bacitracin (B10), whereas other showed resistance in reaction. Three of the 5 strains Bacillus xiamenensis, Bacillus sp and Bacillus velezensis showed susceptibility to vancomycin (VA30) while Bacillus subtilis showed resistance in reaction. The Bacillus subtilis and Bacillus sp. showed susceptibility to amikacin (Ak30), whereas others showed resistance in reaction.
3.7 Growth pattern of cellulolytic bacterial strains
The growth curve of all the bacterial isolates at 37 °C in LB broth medium at pH 7.0 was shown in Fig. 5. The exponential growth starts from 5 h and lasts up to 24 h for CBC9, but for other bacterial strains, it lasts up to 16 h, where they attain a stationary phase. The population count of CBD4, CBG2, CBG3 and CBG4 reached the stationary phase at 24 h and lasted up to 78 h, but in the case of CBC9 stationary phase of growth lies between 32 and 50 h of incubation which was less than other bacterial strains. The decline in population count starts after 40 h of incubation and lasts for 96 h. The population curve of all the strains attain plateau after 85 h except in case of CBC9 where it occurs after 96 h. The population at a respective time is too high for CBC9 followed by CBG2 > CBD4 > CBG3 > CBG4. The growth characteristics of most of the previous work were based on the OD600 value of the incubated broth culture. The OD value showed the absorbance of the medium at 600 nm wavelength, which was related to the bacterial growth in the medium irrespective of their viability. In this study, the bacterial count showed that the logarithmic phase of the strains started after 5 h of incubation which was faster than previously reported Bacillus spp. [3, 32]. The growth phase of the strains continued up to 40 h of incubation and the population count of all the Bacillus strains was maximum at 40th h. This was congruent with the previous study of Yang et al. [3], and Li et al. [20] reported that the maximum growth of B. subtilis (BY-2) and B. velezensis (M2) were up to 20 to 39 h and 20 h of incubation respectively. B. xiamenensis CBC9 which was studied first time for the cellulolytic activity having logarithmic phase over 5 h to 24 h and growth period up to 50 h of incubation; thereafter, the population count declined due to lack of nutrition.
3.8 Optimization of cellulase production condition
The strains were incubated under various conditions, including pH, temperature, carbon supply, N supplement and inoculum size. The CMCase activity of the cellulase generated by the bacteria was measured using the DNS calorimetric method. According to growth characteristics of the strains (Fig. 1), the population count was maximum around 40 h of incubation for all the strains. Since this was the most active phase of the strains being in the logarithmic phase, we conducted cellulase production cultivation experiment for 36 h in order to examine the optimum cultivation condition for all the strain and to figure out the best three for application in composting.
3.9 Study of some abiotic factors affecting cellulase activity
The entire fibre degrading enzyme influenced by growth parameters like carbon and nitrogen sources, inoculum concentration, temperature and pH of the medium, aeration and on the presence of various metal ions as activators and inhibitors [33].
3.10 pH
The cellulase production of different isolates as influenced by pH of the medium which was implied by CMCase activity was depicted in Fig. 6. The optimum cellulase production of the strains isolated from gut bacteria of hairy caterpillar (CBG2, CBG3 and CBG4) were at pH 6.5, whereas other two strains (CBC9 and CBD4) showed optimum CMCase production at pH 7.5. CMCase activity was determined to be at its peak in strain CBG2 (38.39 Uml−1) which was followed by CBD4 (32.47 Uml−1) and CBC9 (24.51 Uml−1). The CMCase production by CBG3 was 22.56 Uml−1 and CBG4 was 23.19 Uml−1. Through the experiment, it can be said that different strains isolated from various sources are capable of producing enzyme in a wide pH range (4.5 to 9.5), while the incubation condition for maximum activity was at the pH of 6.5 and 7.5 for different strains. The result of the current study was congruent with previous work on Bacillus pumilus MGB05 [14]. This result contradicts the study of Yang et al. [3] which showed that the optimum production condition for Bacillus subtilis (BY-2) isolated from intestine of Tibetan pig was at pH 5.5. While study of Dar et al. [12] indicated that a Klebsiella sp. MD21 isolated from gut of Helicoverpa armigera showed highest enzyme production at pH 9.0. The optimum CMCase activity was observed at different pH for different isolated strains because the pH of the fermentation medium is reported to impact the growth of any microbial strain [34]. In the current study, strains were isolated from various sources which differentiate their habitat; this might be responsible for their differential enzyme production under different pH range. The Bacillus sonorensis (HSC7) isolated from water samples of Gorooh hot spring [35] and Bacillus velezensis [M2] from Min pigs’ manure [20] produced highest CMCase at pH 6.0 and 4.5 respectively. The present results, however, concur with previous research that most bacterial enzymes produce CMCase at their highest levels between pH 6 and 8 [36].
3.11 Temperature
The enzyme activity of cellulolytic bacterial strain produced at different temperature was shown in Fig. 7. The optimum temperature for enzyme production was 35 °C for all the selected strain. The strain CBG2 isolated from gut bacteria of hairy caterpillar showed higher enzyme production (30.32 Uml−1) among all the selected strain followed by strain CBD4 (38.51 Uml−1) isolated from dump yard and strain CBC9 (41.66 Uml−1) isolated from composting unit. The enzyme productions of the rest of the two strains (CBG3 and CBG4) were at lower levels in all the respective temperatures. The extracellular enzyme secretion was found to be influenced by temperature, possibly due to the change in physical properties of the cell membrane [16]. The results of the current study showed that all strains produced CMCase best at a temperature of 35 °C and that production declined as temperature increased or dropped. The current findings substantiated by the previous studies, i.e., optimum temperature for CMCase production of Bacillus pumilus (MGB05) isolated from midgut of muga silkworm [14] and strain Bacillus velezensis (M2) from mini-pigs’ manure were found to be 35 °C [20]. The Paenibacillus sp. had maximum cellulose activity at 40 °C [27]; however, there was no activity at higher temperatures (i.e., 50 and 60 °C) for Klebsiella sp. MD21 isolated from gut of Helicoverpa armigera [12]. The decrease in enzyme production at lower or higher temperatures may be caused due to the fact that the growth of the organisms is inhibited at these temperatures, resulting in a reduction in the synthesis of the enzymes [37], whereas maximum CMCase production at the ideal temperature is due to higher metabolic activity resulting in increasing protein content and extracellular enzyme production in culture. The reduction in enzyme production was also due to the denaturation of enzyme transport system carrying proteins and the membranes hardening at low temperature which damage the microorganism and results in reduced enzyme activity [38]. It was discovered that other Bacillus enzymes isolated from various settings shared these pH and temperature properties.
3.12 Carbone sources
The cellulase activity of strains influenced by C supplements (Lactose, dextrose, sucrose, starch and CMC) shown in Fig. 8 concludes that the enzyme activity was highest on supplementation of CMC as a carbon source followed by lactose and dextrose. CBG2 strain’s CMCase activity was greater than rest of the strain in all the carbon supplementation except dextrose where CBD4 showed higher enzyme activity. Cellulases are inducible enzymes and their activation and repression mechanisms depend upon different types of substrate as well as their availability in the vicinity [14]. CMC was the ideal carbon source for all five strains in this study to produce the most cellulase, whereas Hussain et al. [36] reported that best carbon source for optimum cellulose production was sucrose. However, Sreena and Sebastian [39] reported that CMC plays a significant role in cellulase production by Bacillus sp. Yang et al. [3] further observed the maximum CMCase activity when mixture of 1% corn powder and 1% CMC was used as the sole carbon source. The best carbon sources differed across research, which might be explained by the examined strain’s predilection for a particular substrate, the carbon sources used in these investigations or the isolation environment.
3.13 Nitrogen sources
The CMCase activity of the strains in the presence of different organic and inorganic N supplements was depicted in Fig. 9 which showed that the strains expressed higher enzyme activity when there was organic supplement. The enzyme activity was the highest in all the strains where yeast extract was used as N supplement except CBG4 which showed highest activity on urea supplementation. The enzyme activity of bacterial strains decreased in presence of inorganic supplement and the lowest activity was found in case of NH4NO3 supplementation. In order to build amino acids, nucleic acids and proteins and to assist bacteria produce enzymes, nitrogen is a necessary ingredient. This source of this required nitrogen for the bacteria varied according to their habitat or isolation environment. In this study, four among the five bacteria strains performed well in presence of organic nitrogen, i.e., yeast and peptone. Our study is substantiated by Ire et al. [38] who stated that yeast extract was the preferred nitrogen source for cellulase production and their activity. Current study showed that the strains can utilize organic nitrogen efficiently, whereas in presence of inorganic nitrogen the activity reduced by manifold. The lower level of enzyme activity in the inorganic nitrogen supplementation might be due to the metabolism of inorganic nitrogen contributing to the acidification of the growth medium. Similar results were found by Yang et al. [3], where enzyme activity was found almost zero in presence of inorganic nitrogen in the medium. In contrast to previous study, one strain showed maximum enzyme activity in presence of urea. These findings demonstrate how different Bacillus strains’ enzymatic characteristics and productivities are closely related to their initial environments and medium conditions [20].
3.14 Inoculum size
The enzyme activities of cellulolytic bacterial strains depend on their inoculum concentration. The CMCase activity of strains as influenced by inoculum concentration was shown in Fig. 10. Strain CBG4 showed highest enzyme activity at 3% inoculum concentration, whereas CBG2 and CBG3 perform to their highest potential at 4% inoculum concentration. The strains CBC9 and CBD4 produce highest CMCase enzyme where they are inoculated with 5% inoculum. The potential activity of strains decreased on further increasing the inoculum concentration. Strain CBG3 and CBG4 showed less CMCase production than other three strains at their maximum potential. The findings revealed that, depending on the strain, the ideal inoculum size for enzyme synthesis ranged from 3 to 5%. This result was similar to the diverse reporting about the inoculum concentration for the optimum cellulase production by different authors. Inoculum size for optimum enzyme activity was 2% for Paenibacillus terrae ME27-1 isolated from soil [40] and Bacillus velezensis M2 isolated from mini-pigs’ manure [20]. In another study, Yang et al. [3] reported 4% inoculum concentration for optimum CMCase activity. Thereafter, the enzyme activity decreased with further increasing in inoculum concentration. This may be because of the inadequate nutrients and dissolved oxygen caused by the excessive bacterial density, which ultimately inhibits bacterial development and lowers the enzyme production capability [13].
3.15 Properties of CMCase produced by selected strains
The crude enzyme generated by the strain was incubated under various pH and temperature ranges, and activity was evaluated using the DNS calorimetric method to establish the ideal pH and temperature for the CMCase reaction. The CMCase activities of bacterial isolates under different pH (4.5 to 9.5) showed in Fig. 11 states that the activity was highest at pH 6.5 for all the isolates. The enzyme activity of CBG2 strain was the highest (53.92 Uml−1) among all the isolates and was stable (80% of the maximal level) in between pH 5.5 and 7.5 while strain CBC9 and CBD4 showed less stability (> 70%) in the similar pH range with maximum activity being 37.60 and 53.83 Uml−1 respectively. Strain CBG3 showed stability (> 70%) at pH 5.5 to 7.5, whereas CBG4 showed stability (> 70%) at pH 5.5 to 6.5.
The CMCase activity under different temperature ranges (25 to 70 °C) was depicted in Fig. 12 showed that enzyme activity of all the strains was highest at 50 °C temperature and decreased in higher or lower temperature ranges. The enzyme activity at 50 °C was highest in case of CBG2 followed by CBD4, CBC9, CBG3 and CBG4 but at extreme temperature (70 °C) the highest enzyme activity was found in CBD4 followed by CBG2 and CBC9. All the strains except CBD4 maintained stability (> 80%) in temperatures between 40 and 50 °C, whereas CBD4 maintained stability (> 70%) in the range of 40 to 60 °C. Strain CBG2 and CBG3 showed stability (> 70%) at 25 to 50 °C temperature while CBG4 showed more than 70% stability at broader 25–60 °C temperature ranges. The strains’ CMCase production optimal pH and temperature were discovered to be 6.5 and 50 °C, respectively. There is diversified reporting on the CMCase properties of different cellulolytic strains, i.e., pH 5.5 and 50 °C were the ideal values for CMCase activity for Paenibacillus terrae ME27-1 [40]; 7.0 and 50 °C for Bacillus cereus JD0404 [41] and 6.0 and 50 °C for Bacillus pumilus MGB05 [14]. In a recent study, Prasad et al. [13] observed that as temperature rises above 50 °C and pH 4.5, the − 1,4-endoglucanase activity decreases because the three-dimensional shape of the enzyme’s active site is maintained by an ideal pH and temperature of the medium, which is necessary for the adsorption of substrate to the active site, and because a change in the ideal environment causes changes in the enzyme’s ionic bonding, which causes the enzyme to lose its functional shape [16]. With respect to other Bacillus enzymes isolated from various settings, the strains in the current investigation maintain their CMCase stability (70–80%) over a wide pH and temperature range. Similar results were found in some of the previous studies like the temperature and pH stability for CMCase activity of Bacillus cereus JD0404 isolated from the muddy sediments of mangrove swamps was found to be 50 to 60 °C and pH 5.0–8.0 [41]. In another study by Li et al. [20] isolated a strain M2 similar to Bacillus velezensis from mini-pigs’ manure and concluded that the strain shows physical stability for CMCase reaction at 4.5 to 7.0 pH and 55 to 70 °C temperature. In the current research, the properties of CMCase produced by Bacillus xiamenensis was examined for the first time which showed stability (> 80%) in the pH range of 6.5 to 7.5 and 40–50 °C temperature with maximum enzyme reaction at 6.5 and 50 °C.
4 Conclusion
From the aforementioned experiment, it can be inferred that, out of the five cellulolytic bacteria, strain CBG2 (Bacillus sp.) produced the most cellulolytic enzyme. The growth and cellulolytic enzyme production of CBG2 was greater than that of other isolates in terms of inoculum size, pH, carbon and nitrogen source concentration, and temperature. This study shown that when the enzymatic conditions are ideal, cellulose strongly absorbs cellulases using the CBG2 strain. The initial concentration of cellulose influences the amount of adsorption. According to our knowledge, the current work is the first to characterise the strain CBC 9’s cellulolytic capabilities (Bacillus xiamenensis).
References
Hemati A, Nazari M, Lajayer BA, Smith DL, Astatkie T (2022) Lignocellulosics in plant cell wall and their potential biological degradation. Folia Microbiol 67(5):671–681. https://doi.org/10.1007/s12223-022-00974-5
Menshawy MN, Abdel-Hamid AM, Mohamed SK, Mo’men H, (2022) Isolation and molecular identification of cellulose/hemicellulose degrading bacteria from agricultural compost and determination of their hydrolytic potential. S Afr J Bot 149:617–621
Yang W, Meng F, Peng J, Han P, Fang F, Ma L, Cao B (2014) Isolation and identification of a cellulolytic bacterium from the Tibetan pig’s intestine and investigation of its cellulase production. Electron J Biotechnol 17(6):262–267
Pandit L, Sethi D, Pattanayak SK, Nayak Y (2020) Bioconversion of lignocellulosic organic wastes into nutrient rich vermicompost by Eudrilus eugeniae. Bioresour Technol Rep 12:100580
Patra RK, Behera D, Mohapatra KK, Sethi D, Mandal M, Patra AK, Balasubramani R (2022) Juxtaposing the quality of compost and vermicompost produced from organic waste amended with cow dung. Environ Res 214:114119. https://doi.org/10.1016/j.envres.2022.114119
Zhang T, Wu X, Shaheen SM, Abdelrahman H, Ali EF, Bolan NS, Ok YS, Li G, Tsang DCW, Rinklebe J (2022) Improving the humification and phosphorus flow during swine manure composting: a trial for enhancing the beneficial applications of hazardous biowastes. J Hazard Mater 425:127906. https://doi.org/10.1016/j.jhazmat.2021.127906
Vodovnik M, Marinsek-Logar R (2010) Cellulosomes - promising supramolecular machines of anaerobic cellulolytic microorganisms. Acta Chim Slov 57(4):767–774
Delangiz N, Aliyar S, Pashapoor N, Nobaharan K, Lajayer BA, Rodríguez-Coutoe S (2022) Can polymer-degrading microorganisms solve the bottleneck of plastics’ environmental challenges? Chemosphere 294:133709. https://doi.org/10.1016/j.chemosphere.2022.133709
Yu H, Zeng G, Huang H, Xi X, Wang R, Huang D, Huang G, Li J (2007) Microbial community succession and lignocellulose degradation during agricultural waste composting. Biodegradation 18(6):793–802
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5(5):500–516
Doi RH (2008) Cellulases of mesophilic microorganisms: cellulosome and noncellulosome producers. Ann N Y Acad Sci 1125(1):267–279
Dar MA, Shaikh AA, Pawar KD, Pandit RS (2018) Exploring the gut of Helicoverpa armigera for cellulose degrading bacteria and evaluation of a potential strain for lignocellulosic biomass deconstruction. Process Biochem 73:142–153
Prasad RK, Chatterjee S, Mazumder PB, Sharma S, Datta S, Vairale MG, Dwivedi SK (2019) Study on cellulase (Β-1, 4-endoglucanase) activity of gut bacteria of Sitophilus oryzae in cellulosic waste biodegradation. Bioresour Technol Rep 7:100274
Bhuyan PM, Sandilya SP, Nath PK, Gandotra S, Subramanian S, Kardong D, Gogoi DK (2018) Optimization and characterization of extracellular cellulase produced by Bacillus pumilus MGB05 isolated from midgut of muga silkworm (Antheraea assamensis Helfer). J Asia Pac Entomol 21(4):1171–1181
Saha S, Khatun F, Nahiduzzaman M, Mahmud MP, Rahman MM, Yasmin S (2022) A greener way of lactic acid production from cellulose-rich waste materials utilising cellulolytic bacteria population from insect gut. Bioresour Technol Rep 18:101021
Mmango-Kaseke Z, Okaiyeto K, Nwodo UU, Mabinya LV, Okoh AI (2016) Optimization of cellulase and xylanase production by Micrococcus species under submerged fermentation. Sustainability 8(11):1168
Hemati A, Aliasgharzad N, Khakvar R, Khoshmanzar E, Lajayer BA, van Hullebusch ED (2021) Role of lignin and thermophilic lignocellulolytic bacteria in the evolution of humification indices and enzymatic activities during compost production. Waste Manage 119:122–134. https://doi.org/10.1016/j.wasman.2020.09.042
Yang Y, Dou Y, Wang B, Xue Z, Wang Y, An S, Chang SX (2022) Deciphering factors driving soil microbial life-history strategies in restored grassland. iMeta 2022: 66. https://doi.org/10.1002/imt2.66.
Balasubramanian N, Simões N (2014) Bacillus pumilus S124A carboxymethyl cellulase; a thermo stable enzyme with a wide substrate spectrum utility. Int J Biol Macromol 67:132–139
Li F, Xie Y, Gao X, Shan M, Sun C, Niu YD, Shan A (2020) Screening of cellulose degradation bacteria from Min pigs and optimization of its cellulase production. Electron J Biotechnol 48:29–35
Gupta P, Samant K, Sahu A (2012) Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int J Microbiol 2012: 578925. doi:https://doi.org/10.1155/2012/578925.
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Verma H, Patra RK, Sethi D, Pattanayak SK (2022) Isolation and characterization of native Rhizobium from root nodules of Raikia french bean growing area of Odisha. Indian J Biochem Biophys 59:918–926. https://doi.org/10.56042/ijbb.v59i9.61519
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547
Stecher G, Tamura K, Kumar S (2020) Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol 37:1237
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Islam F, Roy N (2018) Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC res Notes 11(1):1–6
Ferbiyanto A, Rusmana I, Raffiudin R (2015) Characterization and identification of cellulolytic bacteria from gut of worker Macrotermes gilvus. HAYATI J of Biosc 22:197–200
Subudhi S, Sethi D, Pattanayak SK (2020) Characterization of Rhizobium sp (SAR-5) isolated from root nodule of Acacia mangium L. Indian J Biochem Biophys 57:327–333
Ye M, Tang X, Yang R, Zhang H, Li F, Tao F, Li F, Wang Z (2018) Characteristics and application of a novel species of Bacillus: Bacillus velezensis. ACS Chem Biol 13(3):500–505
Lai Q, Liu Y, Shao Z (2014) Bacillus xiamenensis sp. nov., isolated from intestinal tract contents of a flathead mullet (Mugil cephalus). Antonie Leeuwenhoek 105:99–107
Peixoto SB, Florencia CO, Daniel JD, Adriano B (2011) Cellulase-producing Bacillus strains isolated from the intestine of Amazon basin fish. Aquac Res 42:887–891
Raza A, Bashir S, Tabassum R (2019) Evaluation of cellulases and xylanases production from Bacillus spp isolated from buffalo digestive system Kafkas. Univ Vet Fak Derg 25(1):39–46
Okaiyeto K, Nwodo UU, Okoli SA, Mabinya LV, Okoh AI (2016) Implications for public health demands alternatives to inorganic and synthetic flocculants: bioflocculants as important candidates. Microbiol Open 5(2):177–211
Azadian F, Badoei-Dalfard A, Namaki-Shoushtari A, Karami Z, Hassanshahian M (2017) Production and characterization of an acido-thermophilic, organic solvent stable cellulase from Bacillus sonorensis HSC7 by conversion of lignocellulosic wastes. J Genet Eng Biotechnol 15(1):187–196
Hussain AA, Abdel-Salam MS, Abo-Ghalia HH, Hegazy WK, Hafez SS (2017) Optimization and molecular identification of novel cellulose degrading bacteria isolated from Egyptian environment. J Genet Eng Biotechnol 15(1):77–85
Simões MLG, Tauk-Tornisielo MS, Tapia DMT (2009) Screening of culture condition for xylanase production by filamentous fungi. Afr J Biotechnol 8(22):6317–6326
Ire FS, Ezebuiro V, Ogugbue CJ (2016) Production of bioethanol by bacterial co-culture from agro-waste-impacted soil through simultaneous saccharification and co-fermentation of steam-exploded bagasse. Bioresour Bioprocess 3:26. https://doi.org/10.1186/s40643-016-0104-x
Sreena CP, Sebastian D (2018) Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J Genet Eng Biotechnol 16:9–16
Liang YL, Zhang Z, Wu M, Wu Y, Feng JX (2014) Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27–1. Bio Med Res Int 2014:512497. https://doi.org/10.1155/2014/512497
Chantarasiri A (2015) Aquatic Bacillus cereus JD0404 isolated from the muddy sediments of mangrove swamps in Thailand and characterization of its cellulolytic activity. Egypt J Aquat Res 41(3):257–264
Acknowledgements
The infrastructure support of Odisha University of Agriculture and Technology is duly acknowledged.
Funding
The financial support of Odisha University of Agriculture and Technology is duly acknowledged.
Author information
Authors and Affiliations
Contributions
Kshitipati Padhan, Ranjan Kumar Patra: conceptualization, writing—original draft, review and editing; Akshaya Kumar Senapati: conceptualization, writing—review and editing; Debadatta Sethi: writing—original draft, writing—review and editing; Narayan Panda: writing—review and editing; Sanjib Kumar Sahoo: writing—review and editing; Sushanta Kumar Pattanayak: conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Ethical approval
No human/animal studies were involved in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Padhan, K., Patra, R.K., Sethi, D. et al. Isolation, characterization and identification of cellulose-degrading bacteria for composting of agro-wastes. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04087-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04087-y